Abstract
Sthenoteuthis oualaniensis is an important biological resource in the South China Sea. However, the microbiological characteristics of this squid, especially those of the dwarf-form, are poorly understood. This study was conducted to analyze the microbial community structure and metabolic characteristics of the intestinal and gill tissues of dwarf-form populations of S. oualaniensis. The dwarf-form squids of different sexes and gonadal maturities were collected from South China Sea in spring 2020. Results showed that Mycoplasma was the most dominant group of bacteria in the intestinal samples of the females with immature gonads (FN), females at sexual maturity (FY), and males at sexual maturity (MY) and the second-highest relative abundance group in males with immature gonads (MN). The microbial community structure in squid gills differed from that of intestinal flora. The BD1-7 clade was the dominant genus in gill samples of all groups. Furthermore, the microbial community activities in gills were higher than in intestinal groups, especially FYG. The larger dwarf-form populations had microbial communities with more robust utilization of carbon sources, assessed via average well color development (AWCD). Correlation and redundancy analysis determined that AWCD significantly positively correlated with the relative abundance of BD1-7 clade (p < 0.05). The results indicated that the dominant group of bacteria and microbial community structure were different between the intestinal and gill microbial communities in the dwarf-form S. oualaniensis populations of different sexes and maturities. Moreover, the metabolic potential of the gill microbial community was higher than that of the intestinal microbial community in the dwarf-form populations.
1. Introduction
Sthenoteuthis oualaniensis, or the purpleback squid, is a warm-water oceanic cephalopod mainly distributed in the subtropical and equatorial waters of the Indian and Pacific Oceans, especially abundant in the South China Sea and northwestern Indian Ocean [1]. Based on characteristics such as mantle length, dorsal photophore, and statolith morphology, squids from South China Sea have been divided into several categories: dwarf-form population, medium-form population, and so on [2]. The dwarf-form populations are one of the main squid groups in the South China Sea [1]. At present, research on dwarf-form populations has mainly focused on fishery biology [3], growth and feeding [4], morphological variations and discrimination [5], and characterizing fecundity [6]. Few reports on microbial community characteristics of the intestine and gills of this squid exist.
Intestinal microbes play an important role in host food digestion and metabolic regulation. In invertebrates, the intestinal microbiota commposition is influenced by host lifestyle, diet, habitat and so on [7]. In general, invertebrate microbial communities are relatively simple [8]. Although invertebrates are often exposed to many microorganisms in their habitats, few species of bacteria are found in their digestive tracts [7]. The gill is an essential organ for gaseous exchange. Studies have revealed that the microbial composition of fish intestines and gills is susceptible to changes such as feeding habits, bait, and various environmental factors [9,10]. However, little is known about the microbiomes of invertebrates, particularly S. oualaniensis.
At present, the microbiological characteristics of the South China Sea squid, especially the different sexes and gonadal maturities of the dwarf-form, are poorly understood. Therefore, we aimed to analyze the bacterial community characteristics of the intestinal and gill tissues of females and males with different gonadal maturities in dwarf-form populations of squid collected from the South China Sea in the spring of 2020. This research aims to reveal the (a) differences in the intestinal and gill microbial communities in the dwarf-form S. oualaniensis populations, and (b) metabolic characteristics of microbial communities of intestinal and gill tissue in the dwarf-form squid populations of S. oualaniensis in response to carbon sources. These results will shed valuable light on the biological characteristics of the microbial communities of dwarf-form populations of S. oualaniensis in the South China Sea.
2. Materials and Methods
2.1. Sample Collection and Processing
From 21–27 May 2020, South China Sea squids were caught using a light cover net at stations N1 (11.00° N, 114.00° E) and N2 (9.00° N, 114.00° E) in the Nansha area of China.
Male and female purpleback squids can be distinguished by stemmed left fourth wrist and difference in gonadal structure [1,11]; samples from females with immature gonads (FN), females at sexual maturity (FY), males with immature gonads (MN), and males at sexual maturity (MY) from the dwarf-form populations of the squid were randomly selected. Four groups (FN, FY, MN and MY) of squid samples were collected from sites N1 and N2, with three replicates per group at each site. As such, a total of 24 squid samples were collected.
The mantle length of S. oualaniensis was measured with a measuring plate with an accuracy of 1 mm, and the body mass was weighed using a level. The surface of S. oualaniensis was then sterilized using 75% alcohol, and intestine and gill samples of different sexes with different gonadal maturities were collected. Four groups (FN, FY, MN and MY) included six replicates of intestinal and gill samples, respectively.
The intestinal and gill samples of each squid were divided into two parts. One part was put into the preservation solution at −20 °C for the analysis of the structural characteristics of bacterial community. The component of the preservation solution was 750 mL/L absolute alcohol, 10 mL/L 0.5 M EDTA and 240 mL/L sterile water. In addition, the other was put into the bacteria preservation solution at −20 °C for the analysis of metabolic characteristics of culturable bacterial community. The bacteria preservation solution consists of 25 g/L NaCl and 150 mL/L glycerol.
2.2. DNA Extraction and PCR Amplification
The bacterial DNA from intestinal and gill samples stored in preservation solution was extracted using a MoBio PowerFecal DNA Isolation Kit (MoBio, Carlsbad, CA, USA). The primers 515 F (5′-GTGCCAGCMGCCGCGG-3′) and 806 R (5′-GGACTACHV GGGTWTCTAAT-3′) were used to amplify the V4 region of the 16 S ribosomal RNA. The PCR mixture comprised of a 20-μL reaction volume containing 10 ng of template DNA, 0.4 μL of FastPfu Polymerase, 0.8 μL of each primer (5 μM), 2 μL of 2.5 mM dNTPs, 4 μL of 5× FastPfu Buffer, and distilled water. The PCR program conditions were as follows: 95 °C for 2 min; 25 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA).
2.3. Library Construction and Sequencing
Purified PCR products were quantified by Qubit®3.0 (Life Invitrogen). The pooled DNA products were used to construct Illumina’s paired-end library according to Illumina’s genomic DNA library preparation procedure. Paired-end sequencing was performed on an Illumina HiSeq 2500 platform (Mingke Biotechnology (Hangzhou, China) Co., Ltd.).
2.4. 16S rRNA Sequence Analysis
UPARSE was used to cluster operational taxonomic units (OTUs) with 97% similarity [12]. Rarefaction analysis was conducted using the Mothur software to calculate the diversity indices, including the Chao1, ACE, Simpson, and Shannon diversity indices [13]. A Venn diagram was drawn to reveal the unique and shared OTUs in the different samples [14]. Then, using the Ape package, the beta diversity was analyzed using principal coordinate analysis (PCoA) [15]. The microbial community composition in the intestinal and gill tissues of the squid samples was determined at the phylum and genus levels. Correlation and differential networks were constructed using Cytoscape software according to the relative abundance of individual OTUs (http://www.cytoscape.org/). The linear discriminant analysis (LDA) effect size (LEfSe) was used for quantitative analysis [16]. The PICRUSt software was used to predict the functional abundance of Kyoto Encyclopedia of Genes and Genomes (KEGG) based on 16 S sequencing data.
2.5. Accession Number
The raw data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database. The accession number is PRJNA856085, accessed on 6 July 2022.
2.6. Community-Level Physiological Profiling
According to the sole carbon source utilization, community-level physiological profiling (CLPP) is used to characterize microbial community function [17]. Using EcoPlates (Biolog Inc., Hayward, CA, USA), the functional diversity in each sample was analyzed. A Biolog EcoPlate contains three replicate of a control and 31 carbon substrates.
2.7. Inoculation of Biolog EcoPlate
The intestinal and gill samples from four groups (FN, FY, MN and MY) stored in bacteria preservation solution were took out and ground in the sterile grinding rods. After grinding, the samples were put into 50 mL 2216 E culture medium with sterile dilution 10 times of for resuscitation culture [18]. The culture condition was 30 °C micro aerobic environment. After 72 h of incubation, vigorous bacterial sample was obtained. Then the sample was inoculated into an EcoPlate with 150 μL/well. Then, EcoPlate with samples were incubated at 30 °C. Using the Biolog Microstation (Biolog Inc., Hayward, CA, USA), the absorbance at 590 nm was measured every 24 h for 168 h of incubation.
2.8. Analysis of CLPP
The average well color development (AWCD) represents the potential utilization of carbon sources by microbial communities. AWCD value reflects the metabolic activity of the culturable bacteria [19]. To compare controls and samples, AWCD was calculated based on the optical density at 590 nm (OD590) of each EcoPlate well according to Equation (1).
C is the OD590 of each cultured well, R is the OD590 of the control well, and n is the number of carbon substrates.
2.9. Carbon Source Utilization Analysis of Microbial Community
The standardized absorbance (Rs) was used to better compare the differences for a single type of carbon utilization, indicating the carbon source utilization of microbial community [19]. Rs was calculated according to Equation (2).
Rs = (C − R)/AWCD
According to the classification of six types of carbon sources of EcoPlate, the total absorbance value of each microbial community to different types of carbon sources was calculated with Rs to analyze the difference in the use of the same carbon source by different microbial communities. These six types of carbon sources included polymers, carboxylic acids, amino acids, carbohydrates, amines, and others, respectively.
2.10. Statistical Analysis
The correlation between mantle length of the dwarf-form populations of S. oualaniensis and the AWCD in the intestinal and gill bacterial community was analyzed using Pearson correlation analysis in SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). Then, the correlation between the AWCD and the relative abundance of dominant bacteria was also examined. Statistical significance was set at p < 0.05.
2.11. Redundancy Analysis
To analyze the relationship between the relative abundance of the top ten dominant bacteria and the utilization of six types of carbon sources, a multivariate ordination method was used in CANOCO version 4.5 (Biometris, Wageningen, The Netherlands). Then, to test whether weighted-averaging techniques or linear methods were appropriate, detrended correspondence analysis (DCA) was performed. The longest gradient resulting from DCA was 2.906. Therefore, redundancy analysis (RDA) was performed [19].
3. Results
3.1. Mantle Length and Body Mass of Sthenoteuthis oualaniensis
The mantle length and body mass of FY were significantly (p < 0.05) higher than those of the other types, with an average mantle length and body mass of 112.7 mm and 50.5 g, respectively (Figure 1 and Figure 2). The average mantle length and body mass of FN were 89.5 mm and 23.7 g, and those of MY were 88.7 mm and 22.8 g, respectively. The mantle lengths and body mass of FN and MY were significantly (p < 0.05) higher than those of MN. The MN group’s average mantle length and body mass were 78.5 mm and 17.7 g, respectively.
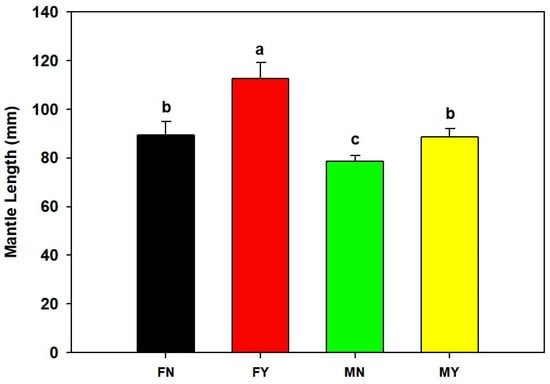
Figure 1.
Mantle lengths of dwarf-form populations of Sthenoteuthis oualaniensis. Significant differences are indicated by different letters (p < 0.05).
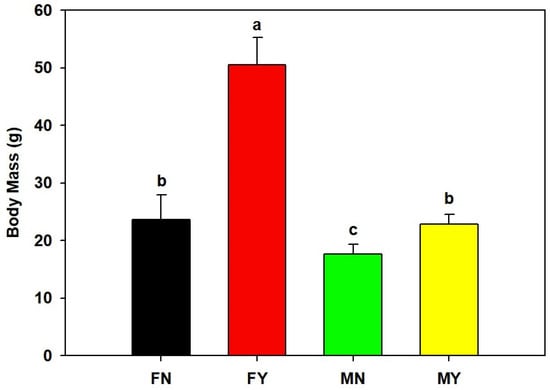
Figure 2.
Body masses of dwarf-form populations of Sthenoteuthis oualaniensis. Significant differences are indicated by different letters (p < 0.05).
3.2. Microbial Richness and Diversity
Alpha diversity indices were calculated, including the Chao1, ACE, Simpson, and Shannon diversity indices (Figure 3). No significant differences were observed in ACE and Chao1 indices among samples (p > 0.05). The Simpson indices of MNI were significantly lower than those of FNI, FYG, and MYG (p < 0.05). The Shannon index of MNI was significantly higher than those of FYG and MYG (p < 0.05).
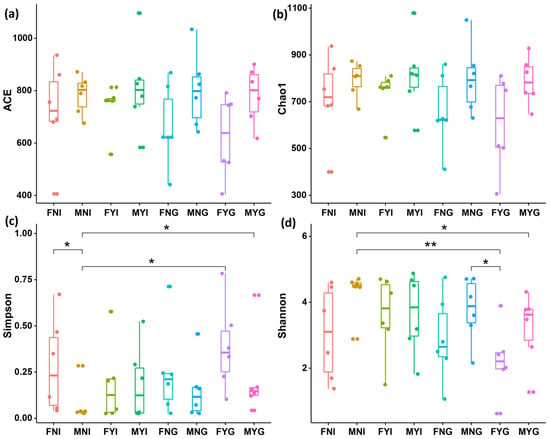
Figure 3.
Microbial diversity indices in the intestine and gill microbial samples of dwarf-form populations of Sthenoteuthis oualaniensis. (a) Observed, (b) Chao1 indices, (c) Simpson indices, and (d) Shannon indices. FNI: intestine samples of females with immature gonads, MNI: intestine samples of males with immature gonads, FYI: intestine samples of females at sexual maturity, MYI: intestine samples of males at sexual maturity; FNG: gill samples of females with immature gonads, MNG: gill samples of males with immature gonads, FYG: gill samples of females at sexual maturity, and MYG: gill samples of males at sexual maturity. * Indicates the significant difference between two samples (p < 0.05) and ** indicates the extremely significant difference between two samples (p < 0.01).
A Venn diagram indicated that 684 OTUs shared in the eight groups. The FNI group had the highest number (238 OTUs) of unique OTUs, while those of other groups were lower (Figure 4).
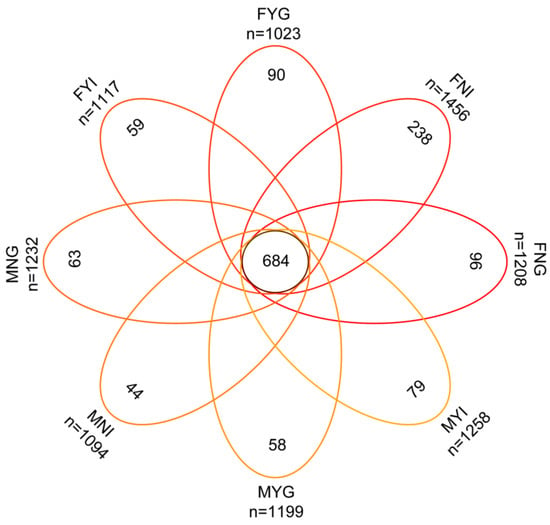
Figure 4.
Venn diagram of the intestine and gill microbial samples of dwarf-form populations of Sthenoteuthis oualaniensis. n represents the total OTU number of each sample.
3.3. Microbial Community
A total of 40 phyla and 840 genera were identified in the intestinal and gill microbial samples from the dwarf-form populations of S. oualaniensis. Proteobacteria, Tenericutes, Firmicutes, and Bacteroidetes were the dominant phyla in FNI, MNI, FYI and MYI, whereas Proteobacteria, Firmicutes, and Bacteroidetes were the dominant phyla in FYG, FNG, MYG, and MNG (Figure 5a).
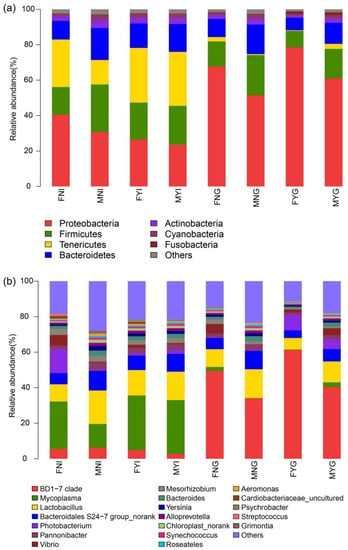
Figure 5.
Dominant bacterial community composition in intestinal and gill tissues of dwarf-form squid. (a) Phylum level, (b) genus level.
In the FNI group, Proteobacteria (40.58 ± 28.26%), Tenericutes (relative abundance of 26.83 ± 34.88%), Firmicutes (15.55 ± 9.01%), and Bacteroidetes (10.49 ± 5.89%) were the dominant phyla. In addition, at the genus level, the dominant bacteria were Mycoplasma (relative abundance of 26.58 ± 34.97%), Photobacterium (13.46 ± 30.03%), Lactobacillus (9.66 ± 5.59%), and Bacteroidales S24-7 group (6.28 ± 3.80%), Vibrio (5.93 ± 12.53%) and BD1-7 clade (5.60 ± 8.27%) (Figure 5b). In the MNI group, Proteobacteria (30.72 ± 8.73%), Firmicutes (26.86 ± 7.15%), Bacteroidetes (18.08 ± 4.69%), and Tenericutes (relative abundance of 13.80 ± 20.11%) were the dominant phyla. The dominant genera in the MNI group were Lactobacillus (18.75 ± 4.80%), Mycoplasma (relative abundance of 13.25 ± 20.18%), Bacteroidales S24-7 group (11.01 ± 2.79%), and BD1-7 clade (6.24 ± 5.89%). In the FYI group, Tenericutes (30.89 ± 29.63%), Proteobacteria (relative abundance of 26.45 ± 10.51%), Firmicutes (20.89 ± 10.32%), and Bacteroidetes (13.79 ± 5.80%) were the dominant phyla. In addition, at the genus level, the dominant bacteria were Mycoplasma (relative abundance of 30.64 ± 29.71%), Lactobacillus (14.29 ± 6.74%), Bacteroidales S24-7 group (8.21 ± 3.51%) and BD1-7 clade (4.93 ± 6.58%). In the MYI group, Tenericutes (relative abundance of 30.49 ± 30.76%), Proteobacteria (23.56 ± 8.96%), Firmicutes (21.95 ± 11.00%), and Bacteroidetes (15.72 ± 7.72%) were the dominant phyla. The dominant genera were Mycoplasma (relative abundance of 30.19 ± 30.93%), Lactobacillus (15.97 ± 8.13%), and Bacteroidales S24-7 group (9.99 ± 5.04%).
Proteobacteria (relative abundance of 67.74 ± 25.06%), Firmicutes (14.13 ± 12.14%), and Bacteroidetes (10.21 ± 9.00%) were the main bacteria in the FNG group. The dominant genera were BD1-7 clade (relative abundance of 49.36 ± 29.45%), Lactobacillus (10.01 ± 8.73%), Bacteroidales S24-7 group (6.30 ± 5.65%), and Vibrio (5.40 ± 12.73%). In the MNG groups Proteobacteria (relative abundance of 51.45 ± 16.50%), Firmicutes (22.72 ± 7.67%), and Bacteroidetes (16.69 ± 5.64%) were the dominant bacteria. In addition, at the genus level, the dominant bacteria were BD1-7 clade (relative abundance of 33.86 ± 23.63%), Lactobacillus (16.24 ± 5.54%), and Bacteroidales S24-7 group (10.29 ± 3.39%). The bacterial population of the FYG group was composed of Proteobacteria (relative abundance of 78.60 ± 15.86%), Firmicutes (9.26 ± 7.94%), and Bacteroidetes (6.99 ± 6.06%) as the dominant bacteria. The dominant genera were BD1-7 clade (relative abundance of 61.27 ± 27.13%), Photobacterium (8.86 ± 19.68%), Lactobacillus (6.45 ± 5.84%), and Bacteroidales S24-7 group (4.32 ± 3.85%). The dominant bacterial phyla in the MYG group were Proteobacteria (relative abundance of 61.02 ± 19.33%), Firmicutes (16.65 ± 8.79%), and Bacteroidetes (11.86 ± 6.49%). At the genus level, the dominant bacteria were BD1-7 clade (relative abundance of 40.39 ± 25.78%), Lactobacillus (11.73 ± 6.35%), Bacteroidales S24-7 group (7.00 ± 3.76%), and Photobacterium (5.49 ± 11.65%).
3.4. Differential Analysis
Differential abundance analysis of the bacterial taxa in the intestines and gills of dwarf-form squid was performed the differences in the eight groups are shown in Figure 6a. In the intestinal bacterial community, 8, 50, 11, and 2 bacterial groups were abundantly enriched in the FNI, MNI, FYI, and MYI groups, respectively. In contrast, 3, 8, 5 and 2 bacterial groups were enriched in the FNG, MNG, FYG, and MYG groups, respectively.
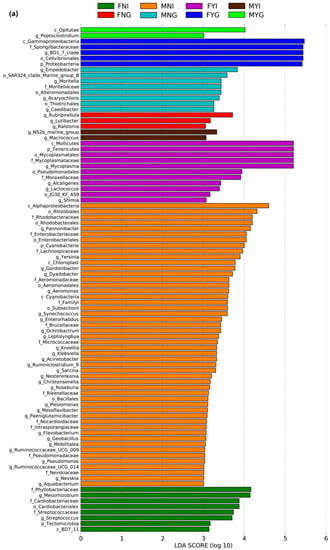
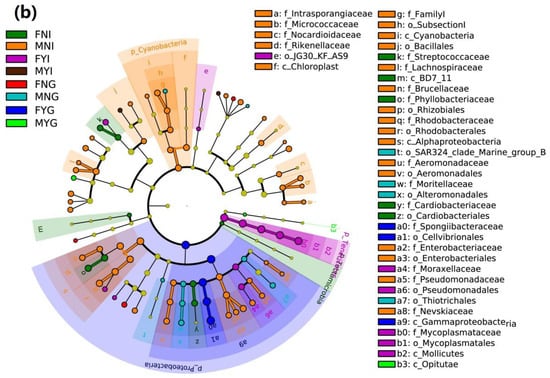
Figure 6.
Inter-group variation of intestinal and gill tissues of dwarf-form squid. (a) LDA score; (b) LEfSe cladogram.
Based on the LEfSe analysis, one class, one order, and three families were enriched in the FNI group, including Streptococcaceae, Phyllobacteriaceae, and Cardiobacteriaceae. In the MNI group, three classes, six orders, and twelve families were enriched, including Intrasporangiaceae, Micrococcaceae, Nocardioidaceae, Rikenellaceae, Familyl, Lachnospiraceae, Brucellaceae, Rhodobacteraceae, Aeromonadaceae, Enterobacteriaceae, Pseudomonadaceae, and Nevskiaceae. In the FYI group, one class, three orders, and two families were enriched, including Moraxellaceae and Mycoplasmataceae. Moreover, three orders and the family Moritellaceae were enriched in the MNG group. However, in the FYG group, the class Gammaproteobacteria, order Cellvibrionales, and family Spongiibacteriaceae were enriched. The MYG group exhibited enrichment in class Opitutae (Figure 6b).
3.5. Network Analyses
Comparing the microbiological characteristics of the medium forms of different sexes with different gonadal maturities showed several variations in the relative abundances of the genus. The dominant genus was the BD1-7 clade belonging to Proteobacteria. The second one was Lactobacillus, belonging to Firmicutes. Furthermore, the BD1-7 clade was negatively correlated with 27 genera of bacteria, including Pseudomonas, Rheinheimera, Ideonella, Rhizobium, Aquabacterium, Roseateles, Yersinia, Aeromonas, Alloprevotella, Plesiomonas, Stenotrophomonas, Pannonibacter, Klebsiella, Sulfitobacter, Gemmobacter, Ochrobactrum, Duganella, Alistipes, Enterorhandus, Lachnoclostridium, Christensenellaceae R-7 group, Aeromicrobium, Paeniglutamicibacter, Candidatus Actinomarina, Lactobacillus, Ruminococcaceae UCG-014, and Lachnospiraceae NK4A136 group. Furthermore, there were positive correlations between most other bacteria (Figure 7).
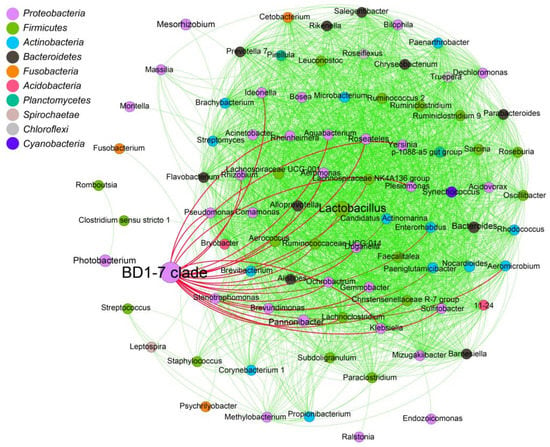
Figure 7.
Correlation network analyses of the microbial communities of dwarf-form squid at the bacterial phyla level. The node size represents the abundance of each phylum. The green lines between phyla indicate a positive relationship, while the red lines indicate a negative relationship.
3.6. Functional Prediction
According to KEGG classification in level 1, the relative abundance of “Metabolism” was the highest, and that of four groups of gill samples were significantly higher than that of intestinal samples (p < 0.05) (Table 1). In the order of relative abundance, the second was “genetic information processing”, and then “environmental information processing”. Among them, the relative abundance of “environmental information processing” of intestinal samples were significantly higher than that of gill samples (p < 0.05).

Table 1.
The predicted KEGG function classification of intestinal and gill tissues of dwarf-form squid.
3.7. Metabolic Characteristics of Microbial Communities by CLPP
To characterize the metabolic function of microbial communities, Biolog EcoPlate was used based on the utilization pattern of the carbon source. After 168 h of incubation, AWCD value of each group was significantly different (Figure 8). The activities in intestinal bacterial communities were lower than in gill groups. Among them, the AWCD of FYG were significantly higher than other samples after 72 h of incubation (p < 0.05). At 168 h of incubation, the AWCD of FNG, FYG, MNG, MYG, FNI, FYI, MNI, and MYI were 0.63, 0.92, 0.55, 0.41, 0.14, 0.30, 0.12, and 0.23, respectively.
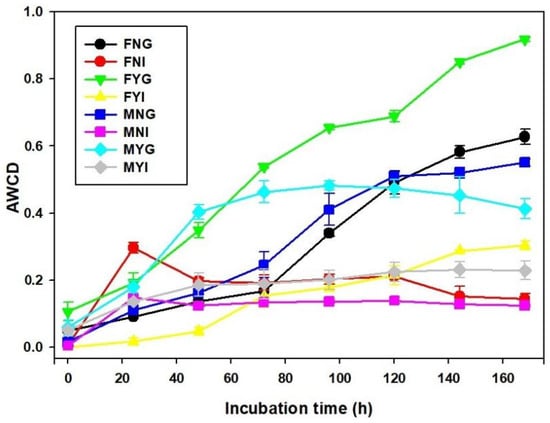
Figure 8.
Average well color development (AWCD) variation in EcoPlates.
The utilization of six types of carbon sources is shown in Figure 9. The utilization of carbohydrates, amino acids, carboxylic acids, and polymers in gill bacterial communities was higher than in intestinal groups. After 24 h, the utilization rates of polymers in FYG and MYG were higher than that in other samples. At 168 h of incubation, the polymer-utilization rates of gill groups were 3.49 (FNG), 4.59 (FYG), 3.48 (MNG), and 2.66 (MYG). These values were significantly higher than that of other intestinal bacterial communities (p < 0.05). At 48–120 h, microbial communities had higher utilization rates of carboxylic acids in MYG than in other groups. Furthermore, the highest utilization rate of carboxylic acids in MYG reached 6.69 at 96 h. The utilization rates of amino acids in FNG, FYG, and MYG were higher than in other groups after 96 h. While the microbial communities had higher carbohydrate utilization rates in FYG and MNG. Compared with other carbon sources, the utilization rates of amines in all samples were low. After 72 h, the utilization rates of other carbon sources in FYG were higher than in other samples.
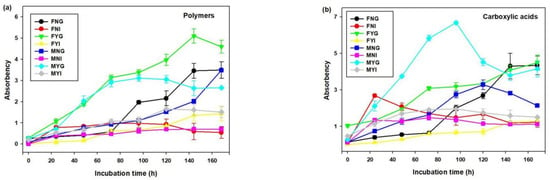
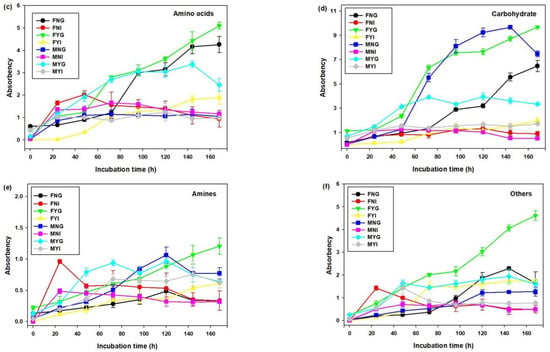
Figure 9.
Variations in carbon source utilization by microbial communities of intestinal and gill tissues of dwarf-form squid. (a) Polymers, (b) Carboxylic acids, (c) Amino acids, (d) Carbohydrate, (e) Amines and (f) Others.
3.8. Correlation Analysis
Correlation analysis showed that the mantle length of the dwarf-form populations of S. oualaniensis was significantly (p < 0.01) positively correlated with the AWCD in the intestinal and gill bacterial community (Table 2). Moreover, the AWCD was significantly (p < 0.05) positively correlated with those of Proteobacteria (Table 3). At the genus level, it was found that AWCD had a significant (p < 0.01) positive correlation with the relative abundance of the BD1-7 clade. At the same time, it was significantly (p < 0.05) negatively correlated with Mesorhizobium (Table 4).

Table 2.
Correlation analysis between mantle length of the dwarf-form populations of Sthenoteuthis oualaniensis and the AWCD in the intestinal and gill bacterial community.

Table 3.
Correlation analysis between the AWCD and the relative abundance of dominant intestinal bacteria at the phylum level.

Table 4.
Correlation analysis between the AWCD and the relative abundance of dominant intestinal bacteria at the genus level.
3.9. Redundancy Analysis
RDA ordination was performed to correlate the utilization of six types of carbon sources with microbial communities (Figure 10). For the species data, the first axis explained 75.9% of the total variation, the first and the second axes explained 95.1%, and all four axes explained 100%. The utilization of six carbon sources, including polymers, carboxylic acids, amino acids, carbohydrates, amines, and others, positively correlated with the abundance and distribution of the BD1-7 clade.
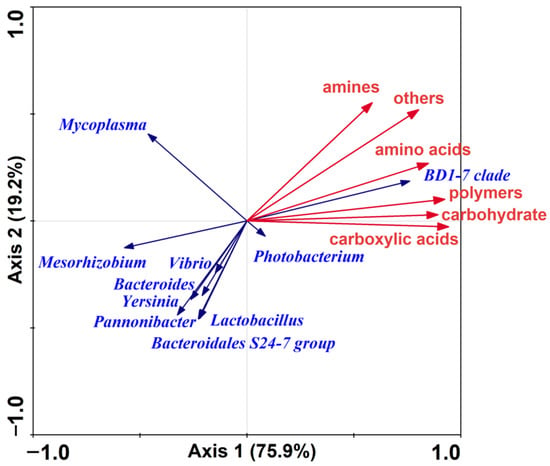
Figure 10.
Redundancy analysis ordination of data.
4. Discussion
4.1. Microbial Community Characteristics in the Intestine and Gills
Mycoplasma was the most dominant group of bacteria in the intestinal samples of FN, FY, and MY and showed the second-highest relative abundance in MN samples in dwarf-form S. oualaniensis. Mycoplasma does not have a cell wall [20]. Different species of Mycoplasma colonize different tissues, such as the intestinal tract [21] and gills [22]. Mycoplasma spp. are pathogens responsible for some human respiratory diseases and seem to play a symbiotic role in the intestines of various fish [20,23,24]. Bano et al. [25] found that Mycoplasma mobile could colonize the intestine of fish without causing disease. Kang et al. [7] suspected that cephalopods may also have symbiotic relationships with gut Mycoplasma through ammonia metabolism, as in the case of salmonoids, because cephalopods are both carnivorous and ammonotelic. In this study, Mycoplasma was the most dominant group of bacteria in the intestinal samples of dwarf-form squids regardless of sexes and gonadal maturities. In addition, the function of Mycoplasma in the intestine of dwarf-form S. oualaniensis needs to be further studied.
Microbial community structure in the gills of dwarf-form squid was different from that of the intestinal bacterial community. The gut will exchange substances with the environment, especially food. While the gill tissue mainly exchanges water and gas with the external environment. So the bacterial community structure of the gill was similar to that of the aquatic environment [26]. In this study, Proteobacteria, Firmicutes, and Bacteroidetes were the dominant phyla in gill samples of S. oualaniensis, regardless of sex and gonadal maturity. The dominant genus was the BD1-7 clade, with a relative abundance of 33.86–61.27% in gill samples of all groups. The BD1-7 clade belongs to the group of oligotrophic marine Gammaproteobacteria (OMG) [27], which is one kind of dominant bacteria in marine environments associated with the gorgonians of the Gorgoniidae family. These data suggest that BD1-7 is crucial for the health of these holobionts [28,29]. In addition, we found that Lactobacillus and the S24-7 Bacteroidales group were present in the microbial communities of the gills and intestines of squid. Zhang et al. [30] considered these groups to be intestinal probiotics for fish and shrimp, which is also a part of the normal intestinal bacterial community [31].
4.2. Metabolic Characteristics of Intestinal and Gill Bacterial Communities
Marine organisms in the South China Sea, such as cephalopod, crustaceans, fish and so on, are not only the good fishery resources, but also the better source of microbial strains. Su et al. [32] found that the abundances of Antibiotic resistance genes (ARGs) in the offshore zone were 2.39–2.66-fold higher than those in the open sea and reef zones in the South China Sea (p < 0.05). Taking deep and open sea and marine organisms as the object, mining microbial resources is one of the future research directions. Therefore, we not only pay attention to the composition of microbial communities in deep and far sea environment and marine organism environment, but also pay attention to the metabolic function of culturable microorganisms.
By analyzing the utilization pattern of the sole carbon source, the metabolic characteristics of a cultured microbial community could be represented [19,33]. Additionally, the differences in metabolic capacity could be derived from the amounts, the composition, and the function of cultivated communities [17]. In this study, we find that the microbial community activities in intestinal bacterial communities of dwarf-form S. oualaniensis populations were lower than that in gill groups. Moreover, the predicted expression of “metabolism” related genes also showed the same rule. This disparity may be related to the short intestinal length of dwarf-form populations of S. oualaniensis [4]. Kang et al. [7] postulated that the intestinal microbial community of cephalopods was composed of distinctive microbes which differed from those of other mollusk groups or marine fish. This microbiota was strongly associated with their phylogeny. The gills are essential organs for exchanging material with the environment. In this study, the AWCD of FYG was significantly higher than other samples after 72 h of incubation (p < 0.05). The mantle length and body mass of FY were significantly (p < 0.05) higher than those of the other types. Furthermore, the mantle length of the dwarf-form populations of S. oualaniensis was significantly (p < 0.01) positively correlated with the AWCD in the intestinal and gill bacterial community. This result indicates that the bacterial communities in the larger dwarf-form populations of S. oualaniensis utilize carbon sources more robustly.
Among the carbon sources in the Biolog EcoPlates, carbohydrates and amino acids were found in marine environment [34], which were the most abundant components of organic matter in marine organisms, suspended and sinking particles, as well as DOM [35]. The utilization of carbohydrates, amino acids, carboxylic acids, and polymers in gill bacterial communities of dwarf-form populations was higher than that in intestinal groups. These data suggest that the metabolic potential of the gill microbial community of dwarf-form populations is higher than that of the intestinal microbial community. However, compared with the carbon source utilization rate of culturable bacteria in water environment in South China Sea, the carbon source utilization rates of gill bacterial community of dwarf-form S. oualaniensis is were lower than that of water [18].
According to the Correlation and Redundancy analysis, AWCD had a significant (p < 0.01) positive correlation with the relative abundance of the BD1-7 clade. Moreover, the utilization of six types of carbon sources, including polymers, carboxylic acids, amino acids, carbohydrates, amines, and others, positively correlated with the abundance and distribution of the BD1-7 clade. Cho and Giovannoni [27] considered that BD1-7 clade was a dominant bacteria in marine environments crucial for the health of these holobionts [28,29]. Since BD1-7 clade is significantly related to microbial community activities and the utilization rate of various carbon sources, how to obtain its cultivable microbial resource would be the focus of subsequent strain utilization and function research.
5. Conclusions
This study demonstrated the microbial community characteristics of the intestine and gills of dwarf-form populations of S. oualaniensis. Mycoplasma, belonging to Tenericutes, was the most dominant group of bacteria in the intestinal samples of FN, FY, and MY and showed the second-highest relative abundance in MN samples of S. oualaniensis. The microbial community structure in the squid gills differed from that of the intestinal flora. BD1-7 clade was the dominant bacteria and metabolic function indicator of the gill microbial community. The bacterial communities in the larger dwarf-form populations of S. oualaniensis had a stronger utilization of carbon sources. This study provides insight into the microbial community structure and metabolic characteristics of the intestinal and gill tissues of dwarf-form populations of S. oualaniensis.
Author Contributions
Conceptualization, Y.C.; methodology, Y.C. and G.W.; formal analysis, X.H. and H.S.; investigation, P.Z. and J.L.; data curation, X.H. and W.X.; writing—original draft preparation, X.H.; writing—review and editing, X.H. and Y.X.; Supervision, Y.C.; funding acquisition, Y.C. and Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Agriculture and Rural Affairs, P. R. China (NFZX2021); the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2020TD54); the earmarked fund for CARS-48; the Major Projects of Basic and Applied Basic Research Programs in Guangdong Province (2019B030302004); and the Central Public-Interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2021SD08).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (SCSFRI-CAFS, No. nhdf2022-07).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We want to thank Mingke Biotechnology Co., Ltd. (Hangzhou, China) for sequencing analysis.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, P.; Yan, L.; Yang, B.Z.; Tan, Y.G.; Zhang, X.F.; Chen, S.; Li, J. Population structure of purpleback flying squid (Sthenoteuthis oualaniensis) in Nansha area in spring. South China Fish. Sci. 2015, 11, 11–19. [Google Scholar] [CrossRef]
- Jiang, Y.E.; Zhang, P.; Lin, Z.J.; Qiu, Y.S.; Fang, Z.Q.; Chen, Z.Z. Statolith morphology of purpleback flying squid (Sthenoeuthis oualaniensis) in the offshore South China Sea. South China Fish. Sci. 2015, 11, 27–37. [Google Scholar] [CrossRef]
- Jiang, Y.E.; Chen, Z.Z.; Lin, Z.J.; Qiu, Y.S.; Zhang, P.; Fang, Z.Q. Comparison of fishery biology between medium-form and dwarf-form of Sthenoeuthis oualaniensis in South China Sea. J. Fish. China 2019, 43, 454–466. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Kong, X.L.; Yang, Y.T.; Zhan, F.P.; Zhang, P.; Jiang, Y.E.; Chen, Z.Z. Feeding habits of dwarf-form Sthenoteuthis oualaniensis population in the South China Sea. Mar. Fish. 2018, 40, 395–403. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.H.; Zhang, P.; Du, F.Y.; Qiu, Y.S. A study on morphological variations and discrimination of medium and dwarf forms of purple flying squid Sthenoteuthis oualaniensis in the southern South China Sea. J. Trop. Oceanogr. 2016, 35, 82–88. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, L.C.; Xiao, C.Y.; Chen, X.J.; Lin, D.M.; Zhu, J.L. Characterizing Fecundity of Dwarf form of Female Purple Flying Squid (Sthenoteuthis oualaniensis) in the South China Sea. Prog. Fish. Sci. 2020, 41, 140–148. [Google Scholar] [CrossRef]
- Kang, W.; Kim, P.S.; Tak, E.J.; Sung, H.; Shin, N.R.; Hyun, D.W.; Whon, T.W.; Kim, H.S.; Lee, J.Y.; Yun, J.H.; et al. Host phylogeny, habitat, and diet are main drivers of the cephalopod and mollusk gut microbiome. Anim. Microbiom. 2022, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Taylor, M.W.; Behnam, F.; Lücker, S.; Rattei, T.; Whalan, S.; Horn, M.; Wagner, M. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ. Microbiol. 2010, 12, 2070–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.X.; Wu, S.S.; Zeng, Z.Y.; Fu, Z.W. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.F.; Huang, J.H.; Wang, Y.; Zhang, J.S. Characterization of bacterial community in intestinal and rearing water of Penaeus monodon differing growth performances in outdoor and indoor ponds. Aquac. Res. 2020, 51, 4279–4289. [Google Scholar] [CrossRef]
- Wang, R.G.; Chen, X.J. World Oceanic Economic Ommastrephidae Resources and Their Fisheries; Ocean Press: Beijing, China, 2005; pp. 284–296. [Google Scholar]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS ONE 2012, 7, e48289. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genom. Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.P.; Legge, R.L. Dynamics in the bacterial community-level physiological profiles and hydrological characteristics of constructed wetland mesocosms during start-up. Ecol. Eng. 2011, 37, 666–677. [Google Scholar] [CrossRef]
- Zhuang, K.; Hu, X.J.; Cao, Y.C.; Xu, Y.N.; Zhang, J.S.; Wen, G.L. Bacterial community structure and its utilization characteristics of carbon sources in water of South China Sea under different low-nutrient culture conditions. Microbiol. China 2020, 47, 2697–2710. [Google Scholar] [CrossRef]
- Hu, X.J.; Wen, G.L.; Cao, Y.C.; Gong, Y.X.; Li, Z.J.; He, Z.L.; Yang, Y.F. Metabolic and phylogenetic profiles of microbial communities from a mariculture base on the Chinese Guangdong coast. Fish. Sci. 2017, 83, 465–477. [Google Scholar] [CrossRef]
- Hines, I.S.; Ferguson, C.S.; Bushman, T.J.; Gatlin, D.M.; Jensen, R.V.; Smith, S.A.; Kuhn, D.D.; Stevens, A.M. Impact of a yeast-based dietary supplement on the intestinal microbiome of rainbow trout, Oncorhynchus mykiss. Aquac. Res. 2021, 52, 1594–1604. [Google Scholar] [CrossRef]
- Ransom, B.L. Intestinal Microbial Community Composition of Six Actinopterygii Fish. Species in the Southeastern United States; University of Georgia: Athens, GA, USA, 2008. [Google Scholar]
- Kirchhoff, H.; Rosengarten, R. Isolation of a motile Mycoplasma from fish. J. Gen. Microbiol. 1984, 130, 2439–2445. [Google Scholar] [CrossRef] [Green Version]
- Huyben, D.; Sun, L.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Dietary live yeast and increased water temperature influence the gut microbiota of rainbow trout. J. Appl. Microbiol. 2018, 124, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Mora-Sánchez, B.; Pérez-Sánchez, T.; Balcázar, J.L. Phylogenetic analysis of intestinal microbiota reveals novel Mycoplasma phylotypes in salmonid species. Microb. Pathog. 2020, 145, 104210. [Google Scholar] [CrossRef]
- Bano, N.; deRae Smith, A.; Bennett, W.; Vasquez, L.; Hollibaugh, J.T. Dominance of mycoplasma in the guts of the Long-Jawed mudsucker, Gillichthys mirabilis, from five California salt marshes. Environ. Microbiol. 2007, 9, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.X.; He, A.Y.; Lin, Y.F.; Huang, X.D.; Liu, L.; Zhou, L. Comparative Analysis of Microbial Communities Associated with the Gill, Gut, and Habitat of Two Filter-Feeding Fish. Aquacult. Rep. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Cho, J.C.; Giovannoni, S.J. Cultivation and growth characteristics of a diverse group of oligotrophic marine Gammaproteobacteria. Appl. Environ. Microbiol. 2004, 70, 432–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.P.; Yu, K.F.; Liao, Z.H.; Chen, B.; Deng, C.Q.; Yu, J.Y.; Yao, Q.C.; Qin, Z.J.; Liang, J.Y. Seasonal fluctuations in symbiotic bacteria and their role in environmental adaptation of the scleractinian coral Acropora pruinosa in high-latitude coral reef area of the South China Sea. Sci. Total Environ. 2021, 792, 148438. [Google Scholar] [CrossRef] [PubMed]
- van de Water, J.A.J.M.; Voolstra, C.R.; Rottier, C.; Cocito, S.; Peirano, A.; Allemand, D.; Ferrier-Pagès, C. Seasonal stability in the microbiomes of temperate gorgonians and the red coral Corallium rubrum across the Mediterranean Sea. Microb. Ecol. 2018, 75, 274–288. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Tao, X.Y.; Yang, Y.H.; Sun, P.; Jin, M.; Zhou, Q.C.; Jiao, L.F. Effects of dietary montmorillonite supplementation on the growth performance, antioxidant capacity, intestinal barrier and microbiota composition in Marsupenaeus japonicus. Aquaculture 2022, 557, 738330. [Google Scholar] [CrossRef]
- Mohammadian, T.; Alishahi, M.; Tabandeh, M.R.; Ghorbanpoor, M.; Gharibi, D. Effect of Lactobacillus plantarum and Lactobacillus delbrueckii subsp. bulgaricus on growth performance, gut microbial flora and digestive enzymes activities in Tor grypus (Karaman, 1971). Iran. J. Fish. Sci. 2017, 16, 296–317. [Google Scholar]
- Su, H.C.; Hu, X.J.; Xu, W.J.; Xu, Y.; Wen, G.L.; Cao, Y.C. Diversity, abundances and distribution of antibiotic resistance genes and virulence factors in the South China Sea revealed by metagenomic sequencing. Sci. Total Environ. 2022, 814, 152803. [Google Scholar] [CrossRef]
- Paerl, H.W.; Dyble, J.; Twomey, L.; Pinckney, J.L.; Nelson, J.; Kerkhof, L. Characterizing man-made and natural modifications of microbial diversity and activity in coastal ecosystems. Antonie Leeuwenhoek 2002, 81, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L.; Meon, B.; Ducklow, H.W.; Carlson, C.A.; Hansell, D.A.; Steward, G.F. Glucose fluxes and concentrations of dissolved combined neutral sugars (polysaccharides) in the Ross Sea and Polar Front Zone, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 4179–4197. [Google Scholar] [CrossRef]
- He, B.; Dai, M.; Huang, W.; Liu, Q.; Chen, H.; Xu, L. Sources and accumulation of organic carbon in the Pearl River Estuary surface sediment as indicated by elemental, stable carbon isotopic, and carbohydrate compositions. Biogeosciences 2010, 7, 3343–3362. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).