Introduction
Benzamides correspond to a carbonic acid amide of the benzoic acid. Amide has the general formula RCONH2, in such compounds the carbon atom being attached to oxygen and also a hydroxyl group. Generally, amides are divided into distinct subclasses according to the number of nitrogen substituents. Primary amide results through NH2 replacement of the carboxylic hydroxyl group, and usually it has a higher boiling point than secondary and tertiary amides. The secondary and tertiary amides result when one or both hydrogens are replaced by other groups in primary amides [
1,
2,
3]
From the group of benzamides, two active substances have been extensively in use in psychiatry and other related medical fields, namely Sulpiride and Amisulpiride. Remoxipride is another benzamide that was removed from the market due to life threatening side effects in 1993 [
4,
5,
6]. Depending on the compound, benzamides have antidepressant activity, anticonvulsant activity, anti-inflammatory activity, analgesic properties, serotonin (5-HT) activity, antitumor activity, and antimicrobial activity [
7,
8].
The purpose of this study was to identify new molecules with a higher degree of selectivity on receptors implicated in neurotransmission that is directly involved in the normal psychological functions, but with a lower rate of side effects.
Materials and Methods
A sample of 80 white NMRI mice having reached maturity, weighing 23±2g, divided in 4 groups, was used in this study. They were treated for 21 days as follows.
Group 1 – I5C: 9,82mg/bw (1/20 x LD50) intraperitoneal injection
Group 2 – I14C: 10,7mg/bw (1/20 x LD50) intraperitoneal injection
Group 3 – II5C: 7,83mg/bw (1/20 x LD50) intraperitoneal injection
Group 4 – Control: distilled water in similar injection volume.
Mice were kept in an environment with constant temperature and relative humidity (22-24
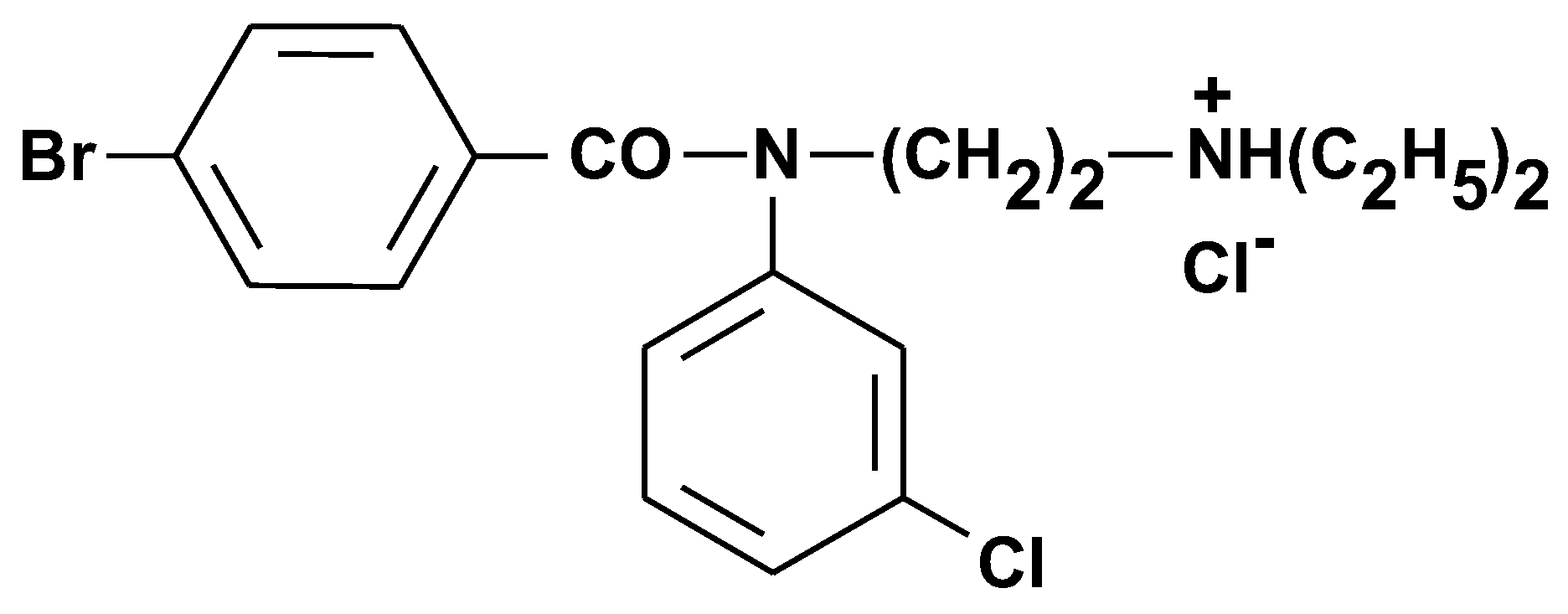
oC, 45-60%), while having free access to food and water. The chemical structures of the tested compounds are shown below [
2]:
Figure 1.
Chemical structure of compound I5C.
Figure 1.
Chemical structure of compound I5C.
Figure 2.
Chemical structure of compound I14C.
Figure 2.
Chemical structure of compound I14C.
Figure 3.
Chemical structure of compound II5C.
Figure 3.
Chemical structure of compound II5C.
Testing investigation–evasion capacity [
7].
Each mouse was placed at the center of a circular platform 20cm in diameter with 2cm high margins suspended at a 50cm height from the horizontal surface of a table. The number of times the mouse popped its head over the margins of the platform in a one minute time frame was recorded. This test was carried out on days 3, 7, 14, 21 of the experiment.
Testing motor activity [
7].
Each mouse was placed in an Autotrackactometer for 1 minute and three parameters were determined: the distance traveled by the mouse (m), the speed of traveling (m/s), and the time spent in the central area of observation (s). This test was carried out on days 4, 9, 15, 22 of the experiment.
All experimental procedures were carried out in accordance with the Directive 2010/63/UE of 22nd September 2010, regarding the protection of animals used for experimental and other scientific purposes. All experimental procedures were approved by the Ethical Committee of the Faculty of Pharmacy Bucharest.
Statistical calculation used the software GraphPad Prism 5. Statistical comparison between groups used the Student t test (for normal distribution) for two groups. Normality of response distribution in collectivity was tested with the D’Agostino & Pearson test. Figures were made using a Microsoft Office Excel 2013 computer program.
Results
The evasion–investigation test yielded the following results:
Table 1.
Dynamics of mouse crossings over the side of the platform.
Table 1.
Dynamics of mouse crossings over the side of the platform.
Figure 4.
Effect dynamics of 3 benzamides over the course of the experiment versus Control in the investigation-evasion test.
Figure 4.
Effect dynamics of 3 benzamides over the course of the experiment versus Control in the investigation-evasion test.
Compounds I5C and II5C proved to have a negative impact on the evasion–investigation capacity of mice, especially after long term exposure when results became statistically significant. The motor activity test yielded the following results:
Table 2.
Evolution of the average travelled distance (measured in meters) inside the actometer.
Table 2.
Evolution of the average travelled distance (measured in meters) inside the actometer.
Figure 5.
Travelled distance dynamics inside the actometer over the course of the experiment versus Control conditions
Figure 5.
Travelled distance dynamics inside the actometer over the course of the experiment versus Control conditions
Figure 6.
Speed dynamics inside the actometer over the course of the experiment versus Control conditions.
Figure 6.
Speed dynamics inside the actometer over the course of the experiment versus Control conditions.
Table 3.
Evolution of the average traveled speed (measured in meters/second) inside the actometer.
Table 3.
Evolution of the average traveled speed (measured in meters/second) inside the actometer.
Figure 7.
Dynamics of time spent in the center of the actometerover the course of the experiment versus Control conditions.
Figure 7.
Dynamics of time spent in the center of the actometerover the course of the experiment versus Control conditions.
Table 4.
Evolution of the average time (measured in seconds) spent in the center of the actometer.
Table 4.
Evolution of the average time (measured in seconds) spent in the center of the actometer.
Discussion
Benzamides are a well known class of pharmacological agents with multiple uses in managing a wide range of nervous and psychic disorders. Tiapride, for instance, is used as an antipsychotic drug because it inhibits the D2 dopaminergic receptors, but it also has an indirect analgesic effect by activating the 5HT1 and 5HT2 serotoninergic receptors [
1,
2,
3]. Sulpiride is another well-known example of an antipsychotic drug with a similar mechanism that is effective in the treatment of various forms of psychosis, but it also induces unpleasant side effects such as dizziness, headaches, tremor, dystonia, akathisia [
4,
5,
6].
The biological activities examined by several studies in organic scaffolds are therefore related to the pharmacological class of benzamides systems. Such biological activities are generally obtained through substitutions on benzamide either by the aliphatic, aromatic, or heteroaromatic path. In fact most substituted benzamides manifest bioactive properties, so they should be viewed as possible medicinal compounds.
In our study, compound II5C induced a statistically significant increase in motor activity (distance and speed) after 4 days of treatment (+71.34% and +73.14%) and after 22 days of treatment (+17.76% and +17.52%).
Compound I5C induced a statistically significant decrease in motor activity (distance and speed) after 4 days of treatment (-11.45% and -12.78%) and after 22 days of treatment (-11.82% and -12.31%).
After 9 days of treatment, compound II5C yielded a statistically significant decrease in the time spent in the central area of the actometer (-24.15%), but after 22 days of treatment, the same compound resulted in a statistically significant increase of the same parameter (+23.38%), possibly a sign of anxiety decrease.
Compound I5C yielded a decrease in the time spent in the central area of the actometer over the course of the experiment, but this was statistically significant only at the 4th day of the experiment.
Conclusions
The lack of effective treatment for many psychiatric diseases and the side effect complexity of many current drugs used for this purpose justify the need for medical research to find new alternatives [
9].
After performing two specific tests relevant for the normal functioning of the central nervous system, our findings revealed the following:
Compounds I5C and II5C decreased in a statistically significant manner the evasion–investigation capacity of mice (-45.16% and -45.62%), suggesting an intense central effect.
Compound I5C induced a statistically significant decrease in motor activity, suggesting a sedative type of antipsychotic profile.
Compound II5C induced a statistically significant increase in motor activity, suggesting a possible incisive type of neuroleptic profile.
Further studies are necessary in order to confirm these preliminary findings.