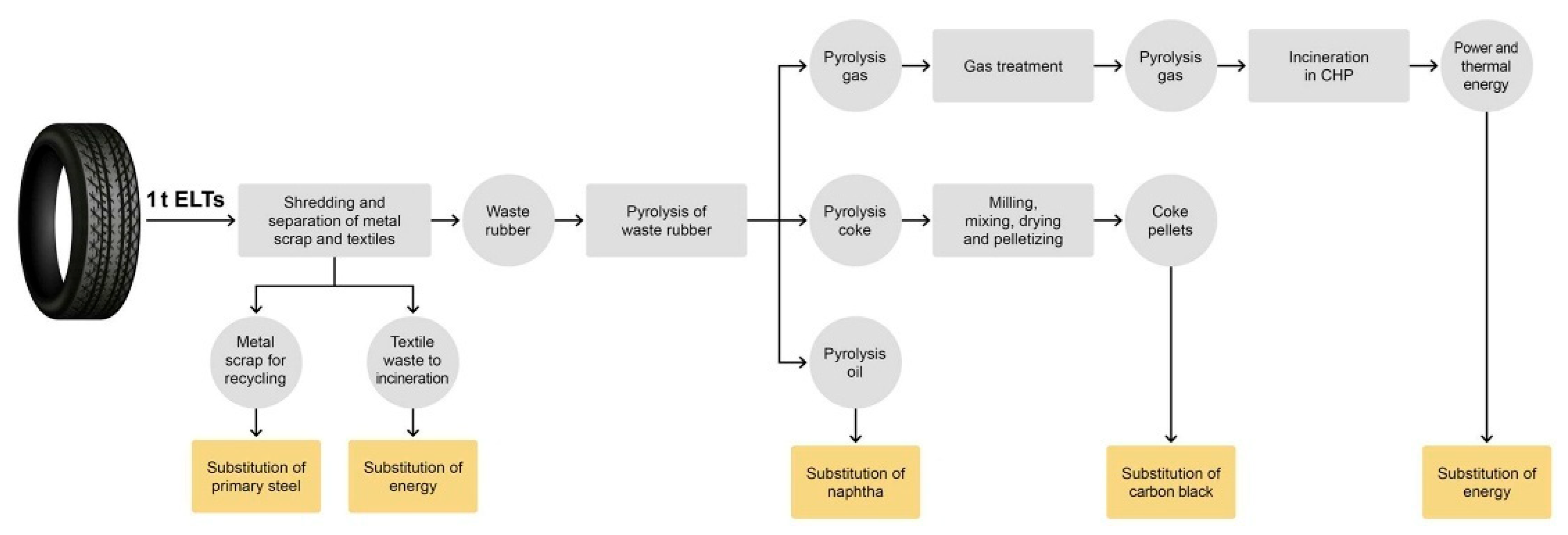
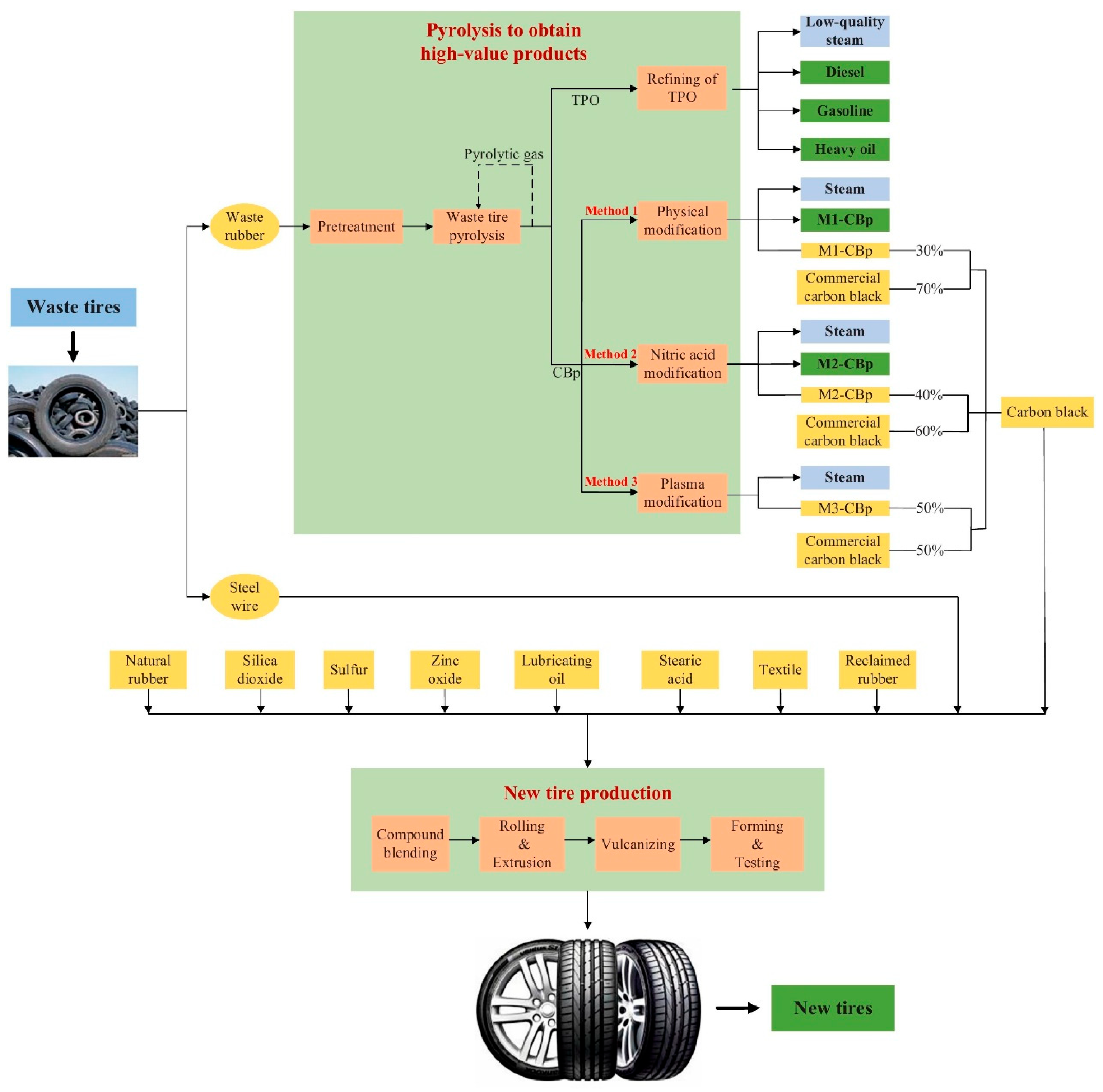
1. Introduction
In recent years, the rubber industry has been developing rapidly, along with the high-speed development of the global automobile industry. In order to meet the development needs of the automobile industry, the production and waste of tires have been rising year by year. According to recent estimates, the world produces approximately 2.3 to 3.0 billion waste tires (WTs) annually, generating around 25–30 million tons of solid waste per year [
1]. This number is expected to continue increasing at a rate of 3–5% annually in line with global vehicle growth. China’s tire production reached 1.187 billion units in 2024, with demand accounting for more than 40% of the global total [
2,
3].
In Europe (EU28+ Norway/Switzerland/Serbia/Turkey), the 2021 data indicate that ~96–97% of used tires were treated, with ~55–56% used for material recovery (granulation and partial mineral incorporation in cement) and ~40–42% for energy recovery (including co-processing in cement kilns, expressed as 75% energy and 25% material by convention) [
4]. These figures reflect the growth of extended producer responsibility (EPR) systems in most Member States.
In the United States (2023), ~79% of WTs enter recycling/reclamation markets; tire-derived fuel (TDF) accounts for ~33% and ground rubber for ~28%, with the remainder split among smaller material uses [
5].
In Japan (2023), the effective utilization rate has reached ~99.2%, dominated by industrial thermal uses (paper mills, chemical plants, cement kilns), with crumb rubber and pyrolysis being smaller but emerging outlets [
6].
These region-specific snapshots illustrate that portfolio mixes differ and that both material and energy recovery routes currently underpin WT management worldwide. A broader literature review reports ~90% recovery in Europe and the U.S. but emphasizes regional variability and data method harmonization needs [
7]. Globally, however, recovery patterns remain uneven. Europe maintains high recovery rates (~91% in 2018) with a balanced portfolio of material (~56%) and energy (~35%) recovery. The U.S. reported ~86% recovery in 2022, divided between ~38–42% material recycling and ~32–39% energy recovery. Japan reached ~92% utilization in 2021, dominated by thermal recovery (~64–73%), with smaller contributions from mechanical recycling (~14%). In contrast, China reported only ~47% recovery in 2019, of which reclaimed rubber accounted for ~63.8%, rubber powder ~16.6%, and pyrolysis ~13.8% of the recovered share [
1,
8,
9,
10]. These comparative data confirm that mechanical recycling and energy recovery remain the dominant global WT treatment technologies, while pyrolysis is emerging as a complementary but still minor method (<5% in Europe and the U.S., 10–15% in some Asian contexts).
The extremely low recycling rate highlights a critical gap in global WT management. Bridging this gap is essential not only for reducing environmental harm but also for achieving circular economy goals and carbon neutrality targets in the materials and mobility sectors.
Building on these regional statistics, the main WT treatment routes include retreading, mechanical recycling (e.g., crumb rubber, reclaimed rubber, granulation), thermochemical conversion (primarily pyrolysis, with limited gasification), and energy recovery such as cement kiln co-processing or dedicated incineration, while landfilling is increasingly restricted or banned.
Retreading, which involves reapplying new treads to waste tires, is an effective form of reuse with relatively low environmental impact. However, it is limited to certain tire types (e.g., commercial truck tires) and depends on strict quality control of the casing.
Mechanical recycling, particularly the production of crumb rubber, allows tires to be reused in road surfacing, playgrounds, and rubber-modified asphalt. Yet, such downcycling applications often represent low-value use of high-value materials and do not contribute meaningfully to carbon mitigation. Moreover, the market for crumb rubber remains volatile due to performance concerns and potential environmental leaching [
11].
In contrast, incineration recovers energy by direct combustion but emits approximately 2.8 t of CO
2 per ton of tire burned, along with harmful compounds such as sulfur dioxide (SO
2), nitrogen oxides (NO
x), and dioxins [
12]. Landfilling, while still practiced in some regions, occupies large areas and creates persistent environmental risks due to the non-biodegradable nature of rubber and potential leaching of heavy metals. Crucially, both incineration and landfilling fail to recover valuable tire constituents such as carbon black, steel wire, and liquid hydrocarbons–representing significant losses in material circularity [
13,
14].
Given these limitations, pyrolysis has emerged as a promising thermochemical conversion pathway, offering both high recovery efficiency and lower environmental impact. The process involves heating WTs in an oxygen-deficient or inert atmosphere—typically between 250 °C and 900 °C–resulting in multiple outputs: tire pyrolysis oil (TPO), pyrolysis gas (TPG), recovered carbon black (rCB). Unlike incineration, pyrolysis avoids direct oxidation and significantly reduces process-related CO
2 emissions. Compared with retreading, it is less constrained by casing quality; compared with mechanical recycling, it achieves higher valorization by recovering all tire constituents rather than downcycling into low-value uses; and compared with gasification, it faces fewer challenges in gas purification and integration. The pyrolysis process is a promising method of processing, as well as energy and fuel recovery in the field of used tire recycling, and it can fulfill the three principles of solid waste recycling: reduction, resource recovery, and reduction in pollutant emissions. It enables the comprehensive recovery of resources contained in WTs, thereby aligning more closely with circular economy principles and long-term sustainability objectives [
15,
16,
17].
While pyrolysis is widely recognized for its potential in resource recovery, its carbon reduction performance remains debated. Three key challenges—uncertain emission mechanisms, technical inefficiencies, and missing policy support—hinder a clear assessment of its decarbonization role.
First, although pyrolysis yields potentially valuable products—such as TPO, rCB, and TPG—that could replace fossil-based fuels and materials, current studies vary widely in how they define system boundaries, allocate environmental burdens, and model substitution effects. As a result, estimates of pyrolysis’s climate benefits are highly inconsistent across the literature. This lack of methodological coherence makes it difficult to assess whether pyrolysis can be considered a genuinely low-carbon solution and under what conditions it may outperform other WT management pathways.
Second, product quality issues and high energy use reduce the carbon benefits of pyrolysis. High-sulfur TPO requires further treatment, increasing emissions [
18], while electricity use may contribute to 60–80% of total emissions [
19], especially in carbon-intensive grids.
Third, the absence of recognized carbon offset protocols for pyrolysis limits access to climate finance. Unlike renewable energy, products like TPO and rCB lack standard crediting methods under systems like the EU ETS (European Union Emissions Trading System) or VCS (Verified Carbon Standard), weakening economic incentives for scaling up.
To address these gaps, this review aims to clarify whether pyrolysis can fulfill its dual role in waste valorization and carbon mitigation. It focuses on three core questions:
What carbon reduction mechanisms underlie the pyrolysis of WTs, and how are they enabled by its major products—TPO, rCB, and TPG?
How do existing LCA-based studies evaluate and compare the carbon performance of pyrolysis systems, and what methodological challenges remain?
What are the key factors required to transition pyrolysis from laboratory validation to scalable, standardized, and policy-integrated industrial deployment?
By integrating region-specific statistics with mechanism-level analysis and comparative LCA evidence, this paper aims to provide decision-useful insights for industry and policy on the credible, scalable decarbonization role of WT pyrolysis within a diversified WT management portfolio.
2. WT Pyrolysis Technology
To understand the emission reduction mechanisms of WT pyrolysis, it is essential to first examine the pyrolysis process itself. Pyrolysis is a thermochemical treatment carried out under oxygen-deficient conditions, in which rubber polymers are broken down into a mixture of gaseous, liquid, and solid fractions. This chapter provides an overview of both conventional and modified pyrolysis technologies, with emphasis on their operational characteristics, product profiles, and relevance to carbon mitigation.
Conventional pyrolysis of WTs typically operates at temperatures between 400 °C and 700 °C, decomposing tire materials into three major product streams: TPO, TPG, and rCB. TPO is a complex liquid composed of aliphatic and aromatic hydrocarbons, usually accounting for 40–50% of the input mass, and has a calorific value comparable to diesel. TPG, composed mainly of light hydrocarbons such as CH4, CO, and H2, constitutes approximately 10–15% and is often used to supply heat back to the system. The solid residue, rCB, represents 30–40% of the output and consists primarily of carbon and inorganic fillers. Beyond their yields, these fractions also have diverse applications: TPO can be refined into lighter and heavier fractions suitable for fuel blending, hydrogen production, or chemical feedstocks; rCB can be utilized in rubber compounding, pigments, or adsorbents, particularly after upgrading treatments to reduce ash and improve surface chemistry; and TPG can serve as an internal fuel to make the process self-sufficient.
All three pyrolysis fractions (TPO, TPG, and rCB) are valuable outputs with diverse applications. However, their quality-related limitations hinder large-scale or high-value utilization without further treatment. TPO suffers from high sulfur content, polycyclic aromatic hydrocarbons, and poor stability, requiring upgrading by hydrotreatment, distillation, or blending before use in engines or refineries [
20,
21]. rCB contains significant ash and heavy metals and has insufficient surface activity, which restrict its reinforcement performance compared with virgin carbon black; upgrading methods such as acid washing, activation, or chemical modification are necessary to improve its applicability [
22,
23]. TPG, while energy-rich, often contains H
2S and other acidic gases, necessitating cleaning and desulfurization prior to external utilization [
24,
25]. Without such improvements, these products risk transferring pollutants or delivering inferior performance, thereby weakening the environmental benefits of pyrolysis [
26].
Nevertheless, pyrolysis itself remains an energy-intensive process, especially in large-scale electrically heated reactors. External energy demand can account for a significant share of total emissions if not mitigated. Two main strategies are commonly proposed: (i) circulating TPG to achieve partial or full energy self-sufficiency, which can cut external fuel or electricity inputs substantially, and (ii) developing improved reactor designs, such as microwave-assisted, solar-assisted, or fluidized bed systems, which offer higher thermal efficiency and reduce heat losses [
27]. These innovations are critical to ensuring that pyrolysis remains environmentally advantageous compared with competing WT treatment routes.
To address the limitations of conventional pyrolysis, various modified technologies have emerged that enhance both energy efficiency and product usability.
Figure 1 summarizes the main technological routes of WT pyrolysis, including conventional, catalytic, microwave-assisted, and vacuum processes. These advancements are directly linked to the carbon reduction mechanisms elaborated in subsequent chapters. Modified systems aim to reduce external energy inputs and improve the quality of output products, thereby increasing the overall emission reduction potential. For instance, the reuse of TPG for internal heating significantly reduces dependence on grid electricity or natural gas, lowering Scope 2 emissions. More advanced designs, such as microwave-assisted and solar-heated reactors, have demonstrated even greater reductions in external energy demand [
28]. In parallel, the integration of catalysts, such as zeolites or metal oxides, during the pyrolysis process promotes cleaner cracking pathways, resulting in lighter, lower-sulfur oil fractions [
29]. Similarly, the post-treatment of rCB through acid washing, activation, and blending techniques can significantly increase its compatibility with rubber formulations, thus increasing its substitution ratio for virgin carbon black [
30].
Table 1 summarizes the typical differences between conventional and modified pyrolysis systems in terms of process parameters, product composition, and emission reduction potential. As shown, modified systems tend to operate at lower temperatures, produce higher-quality outputs, and achieve greater carbon savings—making them increasingly relevant in the context of circular economy and decarbonization targets.
This technological progression lays the groundwork for understanding how pyrolysis enables indirect emission reductions through both energy self-sufficiency and material substitution. These mechanisms are further explored in the following chapter.
WT technology is a final disposal method with the advantages of environmental friendliness, high resource recycling rate, low raw material quality requirements, large utilization volume, and high product value. This is considered to be the future direction of WT resource utilization [
34]. Conventional systems establish a basic pathway, while modified technologies enhance product quality and energy efficiency. Yet, persistent challenges—such as the high sulfur content of TPO, the ash and heavy metal impurities in rCB, the impurities in TPG, and the high external energy demand of the process—continue to constrain its large-scale deployment. Addressing these issues through integrated upgrading, catalytic improvements, and optimized reactor designs is essential to consolidate pyrolysis as a cornerstone technology for sustainable and circular management of end-of-life tires.
3. Carbon Reduction Mechanisms
To assess the decarbonization potential of waste tire pyrolysis, it is essential to understand the mechanisms through which it contributes to greenhouse gas (GHG) mitigation. The current literature identifies two major pathways: material substitution and energy self-sufficiency. This chapter conceptually classifies these pathways and explains the underlying mechanisms by which different pyrolysis-derived products contribute to emission reductions.
Although the energy content of TPG theoretically allows for its broader application, such as in power generation or fuel substitution, practical challenges—including its complex chemical composition and sulfur content and safety concerns during compression and transport—have largely confined its use to internal circulation within pyrolysis facilities [
25,
35]. Therefore, in most LCA models, emission reduction from TPG is framed as an avoided energy input rather than a product-based substitution.
3.1. Overview of Material Substitution Pathways
One of the most widely recognized carbon mitigation pathways in WT pyrolysis is the substitution of fossil-based materials with pyrolysis-derived products. This pathway operates on the principle that the environmental burden of manufacturing conventional fuels and materials—such as diesel and virgin carbon black (vCB)—can be avoided if equivalent functionality is provided by TPO and rCB. From a life cycle perspective, such substitution reduces upstream emissions linked to fossil resource extraction, chemical processing, and thermal transformation.
3.1.1. TPO as a Diesel Substitute
TPO is one of the principal products obtained from the thermal decomposition of WTs and typically constitutes 40–50 wt% of the pyrolysis output. Its high heating value—ranging from 44.20 to 45.09 MJ/kg—is nearly equivalent to that of commercial diesel fuel (≈45 MJ/kg), making it a viable alternative in energy-intensive applications such as industrial boilers, cement kilns, and stationary diesel engines The energy substitution pathway enabled by TPO is widely regarded as a cornerstone of carbon mitigation in pyrolysis systems.
From a feedstock perspective, the carbon advantage of TPO lies in its origin from waste materials. Unlike fossil diesel, which requires the extraction and refinement of approximately 3.8 t of crude oil per ton and generates 0.43 t of CO
2e in upstream processes [
36], TPO is derived from used tires—an end-of-life product containing both biogenic carbon (from natural rubber) and industrial carbon (from synthetic rubber). During pyrolysis, 60–70% of the tire’s volatile content is thermally decomposed under oxygen-limited conditions into liquid hydrocarbons. Approximately 88% of the C/H/O elements are retained in the TPO, with only 12–15% lost as CO
2 due to energy consumption in the process. The resulting oil generally contains 85–88 wt% carbon, 10–12 wt% hydrogen, and 0.5–2 wt% oxygen, indicating a composition favorable for fuel applications [
37,
38,
39,
40].
TPO is a chemically complex mixture, primarily composed of aromatic hydrocarbons (such as benzene, toluene, ethylbenzene, and xylene), along with aliphatic chains and minor sulfur- and nitrogen-containing compounds. This composition provides TPO combustion characteristics similar to those of diesel fuel, though with some operational differences. For example, its relatively low cetane number (CN ≈ 35, compared to CN > 50 for diesel) results in a longer ignition delay but can reduce peak combustion temperatures, which in turn lowers NO
x emissions by 8–12%. Due to its cyclic aromatic content, particulate emissions are also reduced by an estimated 15–20% relative to diesel, offering co-benefits in local air pollution control [
41].
Beyond direct fuel substitution, TPO can also be refined into value-added products with further emission reduction potential. Vacuum distillation separates TPO into light and heavy fractions. The light fraction (C
5–C
10), with volatility and octane ratings comparable to gasoline, can displace petroleum-based fuels and reduce emissions [
42]. The heavy fraction, rich in aromatics and resins, is suitable for steam reforming, yielding 110–130 g H
2/kg TPO with a carbon intensity of 1.5 kg CO
2e/kg H
2, about 55% lower than hydrogen from natural gas reforming [
43]. Another noteworthy compound is limonene (5–8%), a renewable terpene that serves as a green solvent [
44].
In summary, TPO serves as both a direct and indirect substitute for fossil fuels, contributing significantly to the carbon mitigation capacity of pyrolysis systems. Its versatility, energy density, and compatibility with industrial combustion processes make it a strategic asset in the transition to low-carbon and circular waste management models.
3.1.2. rCB as a Material Substitute
rCB, a major solid product obtained from the pyrolysis of WTs, exhibits a ahigh carbon content (typically 80–90%), a porous morphology, and a heterogeneous microstructure. Studies have shown that rCB undergoes structural evolution from amorphous to partially graphitized carbon as the pyrolysis temperature increases from 400 to 600 °C, with enhanced crystallinity, strong C-C and S-S bonds, and reduced volatile matter, indicating its potential as a sustainable substitute for vCB in industrial applications [
45].
Unlike vCB, which is traditionally produced through the energy-intensive furnace black process using fossil-based hydrocarbons, rCB is derived from the intrinsic carbon content of tires without additional fossil fuel input. The production of 1 ton of vCB emits approximately 3.2 tCO
2, while rCB production emits significantly less, ranging from 0.8 to 1.5 t CO
2 per ton, depending on the pyrolysis conditions and energy sources used [
46,
47]. This substantial difference in carbon footprint highlights the potential of rCB in mitigating GHG emissions during the tire production life cycle.
In terms of material performance, numerous studies have demonstrated that rCB can partially replace vCB in rubber formulations. For instance, Zhang et al. (2024) confirmed that rCB contains residual zinc sulfide and silica, which can independently catalyze the vulcanization of brominated butyl rubber (BIIR), achieving comparable curing behavior and mechanical strength to rubber filled with N660 carbon black and zinc oxide [
48]. Similarly, Bogdahn et al. (2025) reported that although rCB-based elastomers exhibited slightly lower mechanical properties than those filled with standard vCB, increasing the rCB loading partially compensated for performance loss and contributed to sustainability goals [
49].
Moreover, the quality of rCB can be significantly improved via post-treatment techniques such as chemical activation, acid demineralization, and plasma modification. These treatments enhance surface area, introduce oxygen-containing functional groups, and reduce ash and heavy metal content, thereby improving dispersion and filler–polymer interaction in rubber matrices [
23,
50,
51]. When incorporated into natural rubber composites, properly modified rCB demonstrated mechanical performance approaching that of vCB, especially under high-loading scenarios [
52].
Based on an industrial-scale study by Dziejarski et al. (2024) [
53], modified rCB (M-CBp) activated at 900 °C with KOH can directly replace ≥80% of vCB in rubber products. Given that vCB production emits 3.2 t CO
2e per ton while M-CBp requires only 0.5–0.7 t CO
2e, each ton of rCB substituted avoids 2.5–2.7 t of CO
2e. Moreover, the off-gas from the pyrolysis process provides on-site energy, cutting an additional 0.8–1.1 t of CO
2e per ton of finished product.
In summary, rCB offers a promising low-carbon alternative to vCB, especially when appropriately upgraded. Its integration into rubber products not only reduces dependence on fossil-based carbon black but also transforms WTs into value-added resources, thereby playing a crucial role in achieving low-carbon and circular manufacturing systems.
3.2. Energy Self-Sufficiency and Process Optimization
The pyrolysis gas generated from WT decomposition exhibits favorable fuel properties, making it a promising alternative energy source for internal energy self-supply within pyrolysis systems. The gas typically contains a high proportion of combustible compounds such as hydrogen (H
2), methane (CH
4), ethylene (C
2H
4), propane (C
3H
8), and other light hydrocarbons, along with small amounts of CO, CO
2, and sulfur-containing gases. The lower heating value (LHV) of this pyrolytic gas generally ranges between 30 and 40 MJ/m
3, comparable to natural gas and significantly higher than that of syngas derived from biomass or municipal solid waste [
54].
Due to this high energy content, pyrolytic gas is widely regarded as a viable energy carrier capable of directly supplying the thermal energy required for the pyrolysis process itself. This internal recycling of pyrolytic gas reduces, or in many cases entirely replaces, the need for external fossil fuel input. Studies indicate that in continuous or semi-continuous pyrolysis systems, the energy recovered from gas combustion can cover up to 95–100% of the reactor’s thermal demand [
55]. As such, WT pyrolysis can be considered a near energy-autonomous process, especially when combined with heat recovery and insulation optimization.
Replacing external energy sources with pyrolytic gas significantly contributes to GHG reduction. According to some studies, using TPG instead of coal or diesel oil can effectively reduce CO
2 emissions, depending on system efficiency and gas purification steps [
55,
56]. Furthermore, the direct use of in situ-generated gas avoids upstream emissions related to fuel extraction, refining, and transportation.
Several experimental and industrial studies have demonstrated the feasibility of using pyrolytic gas as a primary energy source in WT treatment systems. For instance, Campuzano et al. (2020) reported that a twin-auger continuous pyrolysis reactor operating at 475 °C could maintain stable operation using its own gas as the heating source, while also producing high-quality TPO and rCB [
57]. Similarly, Hoang et al. (2024) emphasized that pyrolytic gas, when properly cleaned, not only supplies sufficient thermal energy for pyrolysis but can also be used for power generation or industrial heating in cement, lime, or steel plants [
58]. Some pilot-scale systems have even integrated gas turbines or boilers for co-generation (heat and power), pushing the system toward full energy circularity.
The reuse of pyrolytic gas significantly enhances the energy efficiency and environmental performance of WT pyrolysis. It transforms the process into a self-sustaining, low-carbon system and reduces reliance on external energy inputs, thus aligning with broader goals of clean energy transition and circular economy implementation.
3.3. Summary of Emission Reduction Mechanisms
WT pyrolysis offers a dual pathway for greenhouse gas mitigation: material substitution and energy self-sufficiency. As demonstrated in this chapter, TPO and rCB can partially replace fossil-based diesel and virgin carbon black, yielding considerable upstream emission savings. Simultaneously, the reuse of TPG as an internal heat source significantly lowers the system’s external energy demand, further contributing to emission reduction.
However, the extent of these benefits depends heavily on product quality, upgrading technologies, system configurations, and regional energy contexts. Variability in sulfur content, ash fraction, and calorific value can influence substitution rates and downstream compatibility. Moreover, most of the current emission reduction estimates rely on attributional LCA with simplified boundaries, which may not fully capture rebound effects or cross-sector interactions.
Overall, while pyrolysis is not a zero-emission technology, it presents a credible and scalable pathway for decarbonizing waste management and material production—especially when combined with advanced upgrading techniques and integrated into circular economy frameworks. The following chapter will examine how LCA has been applied to quantify these emission impacts and guide technology optimization.
4. Quantitative Carbon Reduction Strategies Based on LCA
4.1. Role and Methodological Framework of LCA
Life cycle assessment (LCA) has emerged as the most widely adopted and scientifically recognized tool for quantifying the environmental and climate impacts of WT management pathways. As defined by the ISO 14040 [
59] and 14044 standards [
60], LCA comprises four main stages: (1) goal and scope definition, (2) inventory analysis, (3) impact assessment, and (4) interpretation. Within the context of WT pyrolysis, LCA enables a holistic evaluation of upstream and downstream processes—including raw material acquisition, energy inputs, emissions, product substitution, and end-of-life scenarios—under a consistent and standardized accounting framework.
In recent years, LCA has played a crucial role in comparing pyrolysis against other WT recovery methods such as incineration, mechanical grinding, retreading, and landfilling. Unlike attributional LCA models that focus solely on direct emissions, more advanced studies have incorporated consequential LCA approaches to capture indirect system-wide effects, including the displacement of fossil-based fuels and materials by pyrolysis-derived products [
61,
62]. These “avoided emissions” are particularly relevant in pyrolysis systems.
Tsangas et al. (2024) [
63] emphasized that the integration of substitution effects and circularity indicators is critical for assessing the true environmental value of pyrolysis in a circular economy framework. Similarly, Nunes et al. (2022) [
64] highlighted that the most climate-effective tire recovery scenarios are those that prioritize high substitution efficiency, product upgrading, and system integration. As such, the ability of LCA to model cross-boundary emission interactions is particularly valuable for evaluating pyrolysis systems that offer both material recovery and energy self-sufficiency.
Despite these strengths, several methodological challenges remain. There are significant differences in modeling assumptions among published studies—particularly with respect to system boundary definitions, functional unit selection, allocation methods, and the inclusion of background datasets. For instance, many LCA models limit their scope to a gate-to-gate boundary, thereby neglecting downstream product utilization phases. This omission can lead to a serious underestimation of pyrolysis’ climate benefits, especially those derived from material substitution and energy self-sufficiency. Additionally, reliance on static attributional models may fail to reflect the dynamic nature of evolving technologies, regional energy mixes, and policy interventions.
A further consideration is the lack of standardization in methodological transparency. Not all LCA studies clearly define their modeling assumptions, nor do they consistently apply sensitivity analysis or uncertainty assessment. This undermines the comparability and reproducibility of findings across different studies and regions.
In conclusion, LCA serves as the analytical backbone for assessing the carbon mitigation potential of pyrolysis-based tire recycling. Its value lies in its ability to incorporate both direct emissions and indirectly avoided burdens into a unified evaluation framework. However, future studies must strive for greater methodological rigor, transparency, and integration of dynamic variables. The following sections will delve into how specific factors—including system boundary settings, carbon reduction pathways, and comparative results—affect the interpretation of LCA findings in the pyrolysis literature.
Although this review does not adopt a formal systematic review protocol such as PRISMA, the selection of the included studies followed consistent and representative criteria. We focused on peer-reviewed journal articles published between 2015 and 2024, written in English, and publicly disclosing core carbon footprint metrics, such as GHG emissions per ton of WTs processed. Studies were selected based on their relevance to pyrolysis systems, the clarity of their methodological reporting, and the inclusion of comparative LCA data. While we acknowledge that this approach may not fully meet systematic review standards, it nevertheless ensures that the findings presented are grounded in widely cited, high-quality literature, reflecting the main contours of current research. Future reviews may benefit from more structured selection protocols to further enhance transparency and reproducibility.
4.2. System Boundary Definition and Functional Units
In LCA modeling of WT pyrolysis, the definition of system boundaries and the selection of functional units are critical methodological choices that directly affect the comparability and interpretation of results. These elements determine not only which processes are included in the assessment but also how the environmental burdens and benefits are normalized and communicated.
4.2.1. System Boundaries
Three typical types of system boundaries are observed in the literature:
Narrow (gate-to-gate) boundaries focus solely on the pyrolysis process itself, excluding upstream material sourcing and downstream product use. These models are useful for analyzing process energy efficiency but cannot account for substitution effects or avoided burdens.
Intermediate (cradle-to-gate) boundaries include the upstream impacts of tire collection and transportation, along with pyrolysis operations, but terminate at the factory gate without modeling product utilization.
Broad (cradle-to-grave) boundaries extend further to include downstream product applications and disposal, thereby capturing the full life cycle benefits of substituting fossil-derived fuels and materials.
As illustrated in
Figure 2, pyrolysis-derived products—including TPO, rCB, and TPG—enable the substitution of fossil diesel, vCB, and conventional fuels, respectively. These downstream applications represent the primary source of avoided emissions in tire pyrolysis systems. However, such substitution effects can only be captured when the LCA model adopts a broad system boundary. Therefore, the inclusion or exclusion of product utilization phases directly determines the credibility and completeness of the decarbonization assessment.
To better interpret system boundary choices, it is important to distinguish between attributional and consequential LCA frameworks. Attributional LCA (aLCA) assigns average environmental burdens to a specific process or product and often aligns with narrow or intermediate system boundaries. In contrast, consequential LCA (cLCA) models broader system changes triggered by decision-making, such as the market effects of replacing fossil fuels or virgin materials. cLCA studies often employ cradle-to-grave boundaries and are able to reflect substitution benefits—thus offering a more comprehensive picture of carbon mitigation.
This point is further supported by recent studies. Broad system boundaries are essential for evaluating the full carbon mitigation role of pyrolysis. For instance, models that exclude downstream utilization phases will miss the majority of the emission savings—such as those derived from using TPO in place of diesel or rCB in rubber manufacturing. Briones-Hidrovo et al. (2025) [
61] demonstrated that over 70% of tire pyrolysis’ positive effect on the climate arises from avoided emissions via substitution pathways. Their consequential LCA framework revealed that when these effects are omitted—or when system boundaries are restricted to the gate-to-gate level—the true decarbonization value of pyrolysis is significantly underestimated.
Methodologically, these differences in system boundaries explain why reported GHG emissions vary so widely across studies. Studies using aLCA with limited boundaries tend to only account for process energy demand and direct emissions, while omitting downstream benefits. Conversely, cLCA studies adopting broader boundaries incorporate market-driven consequences—such as the avoided impacts of using pyrolysis-derived products—thus producing more favorable carbon reduction estimates.
As shown in
Figure 3, adopting a cradle-to-grave perspective not only captures these downstream benefits but also reflects the broader circularity of pyrolysis systems. The diagram illustrates a closed-loop configuration in which pyrolysis products are reintegrated into new tire production, reinforcing the importance of product valorization and systemic integration. This highlights the need for expanded, cross-boundary LCA approaches to fully reflect the climate value of pyrolysis within circular economy strategies.
4.2.2. Functional Units
Equally important is the choice of functional unit, which defines the basis for impact quantification. Common units used in tire recycling LCA include
Per ton of WTs processed;
Per unit of useful output (e.g., 1 MJ of energy, 1 kg of product);
Per functional item (e.g., one passenger car tire, one tire lifetime).
The functional unit must align with the study’s objectives and enable fair comparisons across treatment options. For example, using “per ton of WTs” enables direct benchmarking across pyrolysis, incineration, and mechanical recycling systems. In contrast, energy-based units (e.g., per MJ) may favor energy recovery routes, while product-based units better highlight the material recovery potential of rCB or TPO. Some recent studies have adopted dual-functional units to address this bias, reporting both per-ton and per-function results for completeness.
4.2.3. Implications
Overall, system boundaries and functional units are not merely technical settings—they shape the narrative of pyrolysis’s environmental profile. Models employing attributional LCA and narrow system boundaries tend to underreport the climate benefits of pyrolysis, focusing solely on process emissions. In contrast, consequential LCA frameworks with cradle-to-grave boundaries provide a more accurate and comprehensive assessment by accounting for avoided burdens, market dynamics, and cross-sectoral substitutions.
The next section will explore the specific carbon reduction mechanisms reflected in the LCA literature, including the substitution of fuels, materials, and process energy.
4.3. Key Carbon Reduction Pathways in Pyrolysis-Based LCA
In conventional LCA modeling across diverse sectors, greenhouse gas (GHG) mitigation is typically achieved through mechanisms such as improved process efficiency, fuel switching, material substitution, energy recovery, and waste minimization.
4.3.1. Fuel Substitution: TPO Replacing Diesel
TPO can substitute diesel fuel in industrial burners, engines, or co-processing applications like cement kilns. Given its high calorific value (≈44 MJ/kg), comparable to commercial diesel, TPO presents a viable fossil fuel alternative. Multiple LCA studies [
65,
66,
67] estimate that replacing 1 kg of diesel with TPO can save approximately 2.7–3.2 kg of CO
2e, though this figure decreases if pre-treatment (e.g., hydrotreatment or desulfurization) is required.
On a more conservative basis, LCA models often adopt an emission savings factor of 0.5–0.7 kg CO
2e/kg TPO, reflecting partial substitution and regional fuel mix variability. These values remain significant when scaled across system outputs, particularly in configurations prioritizing TPO valorization [
68].
4.3.2. Material Substitution: rCB Replacing Virgin Carbon Black
rCB, derived from the solid residue of tire pyrolysis, can partially or fully replace vCB, which is traditionally produced through fossil fuel-intensive, high-temperature processes. According to Briones-Hidrovo et al. (2025) [
61], the production of 1 ton of vCB typically emits 2.4–3.0 t of CO
2 equivalent, depending on the feedstock and process efficiency. In contrast, rCB production via pyrolysis results in 0.8–1.5 t of CO
2e per ton, mainly due to lower energy consumption and avoided extraction of petroleum-based raw materials. This results in a net emission reduction of up to 72% when rCB is used as a substitute for vCB in tire or rubber manufacturing applications.
When rCB is used in tire or rubber manufacturing—particularly in secondary applications like industrial tires, flooring, or automotive parts—it can avoid up to 2.0–2.2 t of CO
2e per ton of product, assuming functional equivalence and sufficient dispersion quality. Some studies [
23] report lower substitution ratios (50–70%) due to ash content or limited reinforcement properties, but modified or post-treated rCB (M-CBp) has shown improved mechanical compatibility.
4.3.3. Energy Self-Sufficiency: TPG Replacing Natural Gas or Grid Electricity
The gaseous fraction from pyrolysis—commonly referred to as TPG—has an LHV between 30 and 40 MJ/m
3 and can fully meet the reactor’s thermal demand when combusted onsite. Replacing external fossil energy (typically natural gas or coal-fired electricity) with TPG can reduce emissions by approximately 0.4–0.8 t of CO
2e per ton of tires processed [
69].
LCA results consistently show that systems maximizing product utilization and closing material–energy loops outperform conventional recovery methods in terms of GHG reduction. However, the actual benefits depend on product quality, market integration, and boundary assumptions. As such, the next section will synthesize published LCA results to highlight these variations and identify key influencing factors.
4.4. Comparative LCA Results and Influencing Factors
The carbon reduction performance of WT pyrolysis varies across studies due to methodological choices and technological configurations. To enable like-for-like interpretation, this section integrates two complementary lenses: (i) a methodological comparison across LCAs and (ii) an intra-pyrolysis pathway comparison.
4.4.1. Methodological Comparison Across LCA Studies
Despite a growing number of LCAs on WT pyrolysis, their reported carbon footprints vary significantly across studies. These differences arise not only from geographical and technological variations, but also from methodological choices—such as boundary definitions, functional units, data sources, and assumptions about product substitution and upgrading. This section compares representative LCA studies and highlights key factors that drive discrepancies in emission estimates.
The table below summarizes four recent LCA studies that assess pyrolysis-based tire recycling under different scenarios. For comparability, all values are normalized to a functional unit of 1 ton of WTs processed, with boundary types and modeling features indicated.
These comparative results underscore the critical influence of methodological assumptions on LCA outcomes for WT pyrolysis.
First, system boundary choices play a decisive role in shaping reported carbon footprints. Studies adopting cradle-to-grave frameworks (e.g., Aryan et al. [
65] and Wu et al. [
47]) tend to capture substantial emission reductions by accounting for downstream substitution benefits from TPO and rCB. In contrast, gate-to-gate or cradle-to-gate models (e.g., Briones-Hidrovo et al. [
61]; Tsangas et al. [
63]) often underestimate mitigation potential by excluding product end-use and avoided burdens.
This is particularly evident for TPO, where reported substitution credits range from 2.7 to 3.2 kg CO2e/kg diesel when full well-to-wheel (WTW) emissions of diesel are credited, to only 0.5–0.7 kg CO2e/kg TPO under a well-to-tank (WTT) approach that excludes combustion. For rCB, variation is likewise significant: virgin carbon black (vCB) footprints range from ≈2.4 to 3.4 kg CO2e/kg depending on the dataset, while industrial-scale rCB typically falls between ≈0.8 and 1.5 kg CO2e/kg, yielding net savings of ≈1.7–2.4 kg CO2e/kg rCB. These differences stem not only from boundary scope but also from assumptions about product quality, post-treatment, and functional substitution ratios.
Second, the degree of substitution modeling—both in terms of presence and realism—significantly affects outcomes. Wu et al. [
47] explicitly modeled M3-CBp as a functional equivalent to vCB, incorporating both physicochemical upgrade processes and market re-entry assumptions, which resulted in a 55% reduction relative to conventional routes. In contrast, Briones-Hidrovo et al. excluded substitution entirely, reflecting a more conservative but less comprehensive estimate.
Third, energy source selection exerts a non-negligible influence, particularly in electricity-intensive or thermally demanding processes. Studies integrating renewable electricity (e.g., Briones-Hidrovo [
61]) or internal TPG combustion (e.g., Wu et al. [
47]) report significantly better climate profiles than those relying solely on grid- or fossil-based power. This suggests that decarbonizing the pyrolysis process itself is as important as valorizing its outputs.
Taken together, these findings highlight that while pyrolysis consistently shows potential for carbon mitigation, its quantified benefits are highly contingent on system design, modeling rigor, and the quality of substitution pathways. By harmonizing conventions—crediting fuels on a WTT basis and rCB on a cradle-to-gate basis—
Table 2 provides a more consistent basis for cross-study comparison, while still acknowledging the sensitivity of results to methodological assumptions.
4.4.2. Intra-Pyrolysis Pathway Comparison
In this subsection, different pyrolysis implementation pathways are systematically compared in order to highlight their relative contributions to circular economy goals and carbon mitigation.
Table 3 summarizes the main options, including baseline rCB recovery, advanced upgrading processes, coupled circulation strategies, and energy valorization via TPO and TPG. By presenting these pathways side by side, the table allows for a structured evaluation of their environmental benefits and trade-offs.
First, rCB substitution remains the dominant driver of carbon savings, with baseline scenarios already achieving net reductions of ~700 kg CO2e per ton of tires processed. However, the actual benefit is highly sensitive to product quality and substitution assumptions. Upgrading methods, such as demineralization or plasma modification, can improve material compatibility with tire compounding, but at the expense of additional environmental burdens, particularly in toxicity-related categories. Coupled circulation pathways, where modified rCB is reintegrated with reclaimed rubber into new tire production, represent a stronger alignment with circular economy principles and yield significantly higher eco-efficiency scores.
In parallel, TPO and TPG utilization illustrate the dual role of pyrolysis as both material and energy recovery technology. While TPO can substitute diesel or refinery feedstocks, its direct contribution to climate mitigation is constrained by sulfur and PAH contents that necessitate hydrotreatment. TPG, in contrast, reliably supplies internal energy demand (up to 70% of process heat) and thus reduces dependency on external fossil energy, though prospects for external grid injection remain limited due to strict quality standards.
Overall, intra-pyrolysis comparisons underscore the idea that pyrolysis should not be regarded as a homogeneous process but as a portfolio of pathways with distinct trade-offs. Recognizing these differences is vital for policymakers and industry stakeholders to identify which technological configurations can credibly deliver carbon mitigation in practice.
4.4.3. Conclusion and Implications
This comparative analysis demonstrates that the climate performance of WT pyrolysis is shaped by both methodological assumptions and technological choices. Variability in reported carbon footprints underscores the necessity of harmonized and transparent LCA practices. In particular, four factors consistently drive outcome differences: (i) the system boundary and the treatment of avoided emissions, (ii) the assumed substitution rates and the extent of product upgrading, (iii) the process energy source and regional electricity mix, and (iv) the quality and application scope of recovered products, especially rCB.
From a strategic perspective, pyrolysis occupies a middle ground between purely energy-oriented recovery and high-function mechanical recycling. Its unique advantage lies in combining material substitution with energy self-sufficiency, which makes it more robust under diverse regional contexts. To fully realize these benefits, future LCAs should move toward cradle-to-grave system boundaries, incorporate realistic market-driven substitution ratios, and explicitly account for product quality differentials. Scenario-based modeling and open data sharing will be essential to improve comparability across studies and to inform evidence-based policy.
Overall, the evidence positions pyrolysis not as a universal best option but as a cornerstone technology in an integrated WT management portfolio. Its scaling should be pursued in tandem with retreading and selected mechanical recycling, thereby maximizing both carbon mitigation and circular economy gains. The next chapter will therefore shift from environmental metrics to the policy, regulatory, and market frameworks that can enable the scale-up of low-carbon pyrolysis within global circular economy transitions.
5. Future Perspectives
Building on the established carbon reduction potential of WT pyrolysis, the path forward requires transitioning from theoretical and pilot-scale studies to scalable, standardized, and policy-integrated industrial applications. This calls for cross-disciplinary innovation and coordinated policy support to unlock the full climate and resource value of pyrolysis systems.
One of the key priorities lies in enhancing process efficiency and product quality. While current pyrolysis operations demonstrate environmental advantages, variability in feedstock composition and process control continues to yield inconsistent product quality—especially in TPO and rCB. Future research should prioritize modular, adaptive pyrolysis systems capable of handling heterogeneous waste streams while ensuring standardized outputs. Product upgrading technologies such as hydrodesulfurization, chemical activation, and advanced filtration systems will be crucial to meet industrial specifications, reduce downstream emissions, and improve product marketability.
At the same time, accurate environmental evaluation frameworks must evolve. Current LCAs often suffer from outdated emission factors, oversimplified boundary definitions, or regional mismatches. Future efforts should develop dynamic, geographically explicit LCA models that reflect real-world logistics, power mixes, and local regulatory contexts. In parallel, coupling LCA with techno-economic assessment (TEA) and social life cycle assessment (S-LCA) can help decision-makers evaluate the environmental, financial, and societal co-benefits of pyrolysis investments. As climate policy tightens, the credible quantification of avoided emissions will become essential for securing carbon financing or participating in voluntary carbon markets.
Beyond technical and methodological improvements, institutional and policy mechanisms will play a pivotal role in driving adoption. Currently, pyrolysis remains underrepresented in climate action plans and circular economy strategies. Governments should consider formally recognizing pyrolysis-derived products (e.g., rCB, TPO) within green taxonomy frameworks and carbon offset registries. Subsidy mechanisms, green bonds, and emissions trading schemes could provide powerful financial incentives for facilities that demonstrate verifiable emission savings. Moreover, establishing technical standards for pyrolysis outputs and life cycle accounting will help align industry practices, improve investor confidence, and foster market transparency.
In the broader context of sustainable development, tire pyrolysis should not be viewed merely as an end-of-life treatment, but rather as a node in a circular, decarbonized material economy. Integrated systems that combine pyrolysis with upstream reuse and downstream material reintegration—such as using rCB in new tires or TPO in regional transport fuel blends—can maximize both resource recovery and emission mitigation. To realize this vision, closer collaboration is needed between researchers, industry actors, policymakers, and financial institutions.
The future of WT pyrolysis lies in its ability to scale sustainably, demonstrate clear carbon benefits, and embed itself within national and global sustainability agendas. As technological innovation continues and policy support strengthens, pyrolysis has the potential to become not only a viable waste solution but also a significant contributor to climate action, industrial decarbonization, and circular economy transformation.
6. Conclusions
This review has examined the carbon reduction potential of WT pyrolysis through the lens of LCA. It focused on the three main products(rCB, TPO, and TPG) and assessed how they can reduce GHG emissions through material substitution and energy self-sufficiency.
The novelty of this review lies in its integration of mechanism-level analysis with comparative LCA evidence. Unlike earlier surveys that primarily cataloged pyrolysis technologies, this study explicitly examined why reported emission reduction factors vary so widely across the literature. Differences in system boundaries (well-to-wheel vs. well-to-tank), product upgrading assumptions, and substitution ratios explain much of the inconsistency in TPO and rCB credits. By harmonizing accounting conventions—crediting fuels on a WTT basis and rCB on a cradle-to-gate basis—this review provides a more consistent basis for comparing studies and quantifying realistic mitigation effects.
Furthermore, the intra-pyrolysis comparison demonstrates that product quality upgrading (e.g., desulfurization of TPO, activation of rCB) and energy integration (e.g., TPG reuse, microwave-assisted heating) are essential to maximize emission savings. In contrast to incineration, which recovers energy but forfeits materials, and mechanical recycling, which suffers from downcycling and limited carbon benefits, pyrolysis enables comprehensive recovery of all major tire constituents. This positions it as a cornerstone technology in a diversified WT management portfolio.
Nevertheless, challenges remain. High energy intensity, variable product quality, and the absence of standardized carbon crediting protocols limit the current scalability of pyrolysis. Addressing these barriers will require coordinated advances in reactor design, upgrading technologies, and policy recognition.