Abstract
Large-scale energy storage using sodium ion batteries (SIBs) as a hub for the conversion of renewable energy has become a topic of great importance. However, the application of SIBs is hindered by low energy density arising from inferior capacity and operation voltage. In this regard, vanadium-based phosphate polyanions with multiple valence changes (III–V), high redox potential, abundant resources, spacious frame structure, and remarkable thermal stability are promising avenues to address this dilemma. In this review, following the principle of electronic structure and function relationship, we summarize the recent progress in phosphates, pyrophosphates, fluorophosphates, and mixed polyanions of vanadium-centered polyanionic materials for SIBs. This review may provide comprehensive understanding and guidelines to further construct high performance, low-cost sodium-ion batteries.
1. Introduction
In recent years, as the price of lithium resources has rapidly grown, sodium ion batteries (SIBs) have become more competitive in the field of large-scale electrochemical energy storage (EES) [1,2]. Sodium is abundant in the Earth’s crust and in sea water, and is thus more available than lithium (element concentrations in the upper continental crust: Li = 22 ppm, Na = 25,670 ppm) [3]. As shown in Figure 1a, the working principle of SIBs is similar to that of lithium-ion batteries (LIBs) [4]. However, SIBs have been plagued by low theoretical capacity and sluggish insertion/extraction kinetics, owing to the larger ionic radius and greater atomic mass of Na+ [5]. Considering that large-scale EES demands higher power density to conduct frequent peak regulation, SIBs have encountered challenges with respect to their practical application [6]. Cathode materials are also an essential aspect of the electrochemical performance and reliability of SIBs. Their inherent crystal structure determines essential attributes such as electron transfer number, redox potential, and the charge-transfer kinetics.
There are four primary kinds of cathode materials for SIBs: Prussian blue, transition metal oxides, organic compounds, and polyanionic compounds. The comparison of these four types of compounds is displayed in Figure 1b. Generally, Prussian blue (AxM[Fe(CN)6]y·zH2O) has an open framework structure and good structural stability [7,8]. However, the crystal water in the structure results in large amounts of Fe(CN)6 vacancies, largely restricting the material’s electrochemical properties. Transition metal oxides possess a high specific capacity and a moderate working voltage, but are also limited by the structural changes and phase transitions that occur during electrochemical processes and by inferior air stability [9]. Organic compounds have also been considered as potential cathodes for SIBs owing to their structural diversity, micro-regulatory properties, and resource sustainability, but their widespread application has been limited by their low intrinsic electronic conductivity and low operating voltage [10]. Compared with these other candidates, polyanionic compounds possess a comprehensive manifestation of relatively high operating voltage, specific energy (vs. Na+/Na), operation reliability, and safety [11,12,13].
Among polyanionic compounds, vanadium (V)-based polyanionic compounds have tended to receive more attention in recent years due to their many merits [14]. On one hand, V has a higher abundance in the upper continental crust as compared to Co, Ni, Cu, and Cr, making it more available (Figure 1c) [3]. On the other hand, vanadium possesses a valence electron configuration of 3d34s2, which allows it to experience a multivalent electrochemical reaction from V3+ to V5+. Additionally, many V-based polyanionic compounds exhibit a high operating voltage of over 3.4 V (vs. Na+/Na) owing to the inductive effect of polyanionic groups and V redox (see Table 1). A schematic diagram of inductive effects is presented in Figure 1d [15]. When the M–O bond is more covalent, the antibonding orbitals will be pushed up, resulting in larger energy splitting between antibonding and bonding orbitals, as well as lower redox potential. As shown in Figure 1e, with a similar theoretical capacity of ~97 mAh g−1, a higher operating voltage remarkably increases the theoretical specific energy (vs. Na+/Na). When another atom X with stronger electronegativity is introduced to form M–O–X bonds, the inductive effect begins to take effect since the covalency between M–O will be lowered, leading to higher voltage [16]. Hence, the redox voltage can be effectively improved by introducing different polyanionic groups in cathode materials. In addition, the unique open framework often endows V-based polyanionic compounds with outstanding cycle stability and rate performance [4].
To date, several reviews of polyanionic compounds have been conducted with respect to their classification, crystal structures, and common electrochemical performance [4,14,17,18,19]. Nevertheless, to the best of our knowledge, there has not been a unique summary and interpretation of the high voltage properties of V-based polyanionic compounds, although this type of material is an essential candidate for SIBs. For a simple sample, and to have a comprehensive comparison of typical cathode materials for SIBs, we included their voltages and specific energies (vs. Na+/Na) in Figure 1f [4,7,8]. As can be seen, V-based polyanionic compounds have a moderate specific energy (vs. Na+/Na) but a relatively high voltage; thus, a systematic interpretation is necessary to build better a understanding of the causes, effects, and strategies for further enhancing V-based electrodes.
In this review, we will concentrate on high-voltage V-based polyanionic compounds (≥3.4 V) and present a comprehensive interpretation in terms of their structure, charge transfer kinetics, Na+ storage mechanisms, and electrochemical performance. In addition, modification approaches, major defects, challenges, and perspectives on the application of V-based electrode materials for SIBs will be also presented.
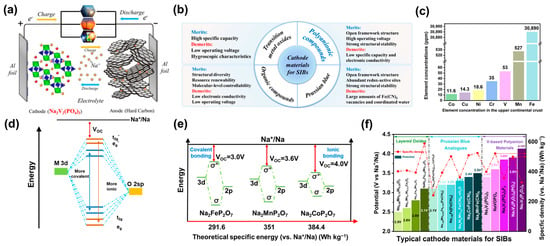
Figure 1.
(a) Working principle of room-temperature SIBs [20]. (b) Comparison of various cathode materials for SIBs [20]. (c) Element concentrations of Co, Cu, Ni, Cr, V, Mn, and Fe (in ppm) in the upper continental crust [3]. (d) Schematic diagram of the influence of the M-O bond on the orbital energy level and operating potential (Voc). (e) Schematic illustration showing the relationship of Voc and theoretical specific energy (vs. Na+/Na). (f) Potential and specific energies (vs. Na+/Na) of typical cathode materials for SIBs.

Table 1.
A comparison of V-based polyanionic compounds as cathode materials for SIBs.
Table 1.
A comparison of V-based polyanionic compounds as cathode materials for SIBs.
| Materials | Structure | Redox (V) | Redox Couple | Theoretical Capacity (mAh g−1) | Theoretical Specific Energy (vs. Na+/Na) (Wh kg−1) | Electrochemical Activity |
|---|---|---|---|---|---|---|
| Na3V2(PO4)3 [21,22] | Rhombohedral | 3.4 | V4+/V3+ | 117.6 | 400 | 117 mAh g−1 at 1 C 82 mAh g−1 at 100 C |
| Na3V3(PO4)4 [23] | Monoclinic (layered structure) | 3.9 | V4+/V3+ | 44.5 | 174 | ~40 mAh g−1 at 0.6 C |
| Na3V(PO4)2 [24] | Monoclinic (layered structure) | 3.5 | V4+/V3+ | 90 | 315 | ~90 mAh g−1 at 0.2 C |
| VOPO4 [25] | Tetragonal (layered structure) | 3.4 | V5+/V4+ | 165.5 | 563 | 150 mAh g−1 at 0.05 C |
| NaVOPO4 [26] | Monoclinic | 3.6 | V5+/V4+ | 145 | 522 | 101 mAh g−1 at 5 mA g−1 |
| NaVOPO4 [27] | Orthorhombic | 3.3 | V5+/V4+ | 145 | 479 | 115 mAh g−1 at 0.1 C |
| NaVOPO4 [28] | Triclinic | 3.5 | V5+/V4+ | 145 | 508 | 144 mAh g−1 at 0.05 C |
| Na4VO(PO4)2 [29,30] | Orthorhombic | 3.5 | V5+/V4+ | 78 | 273 | 41.3 mAh g−1 at 10 C |
| NaVPO4F [31] | Tetragonal (NASICON) | 3.7 | V4+/V3+ | 142.5 | 527 | 120.9 mAh g−1 at 0.05 C 70.1 mAh g−1 at 0.5 C |
| NaVPO4F [32,33,34] | Monoclinic (NASICON) | 3.4 | V4+/V3+ | 142.5 | 484.5 | 135 mAh g−1 at 0.2 C 86.5 mAh g−1 at 100 C |
| Na5V(PO4)2F2 [35] | Trigonal/Orthorhombic | 3.4/3.5 | V4+/V3+ | 68 | 231.2/238 | 61 mAh g−1 at 0.1C |
| Na3V2(PO4)2F3 [36,37] | Tetragonal (NASICON) | 3.9 | V4+/V3+ | 128.3 | 500 | 125 mAh g−1 at 0.2 C |
| Na3V2(PO4)2O2F [38,39] | Tetragonal | 3.8 | V5+/V4+ | 130 | 494 | 127.4 mAh g−1 at 0.2C |
| Na3V2(PO4)2O1.6F1.4 [40,41] | Tetragonal | 3.8 | V5+/V4+ | 129.7 | 492.9 | 134 mAh g−1 at 0.1 C |
| NaVP2O7 [42] | Monoclinic | 3.9 | V4+/V3+ | 108.1 | 421 | 104 mAh g−1 at 0.1 C |
| Na7V3(P2O7)4 [43,44] | Monoclinic | 4.13 | V4+/V3+ | 79.6 | 329 | 67.2 mAh g−1 at 8 C |
| Na2VOP2O7 [45] | Tetragonal | 3.8 | V5+/V4+ | 93.4 | 355 | 80 mAh g−1 at 0.05 C |
| Na7V4(P2O7)4(PO4) [46,47] | Tetragonal | 3.85 | V4+/V3+ | 92.7 | 357 | 92 mAh g−1 at 0.05 C 70.2 mAh g−1 at 10 C |
| Na2(VO)2(HPO4)2(C2O4) [48] | Monoclinic | 4.0 | V5+/V4+ | 116.6 | 466.4 | 105 mA h g−1 at 0.1 C |
2. Phosphate
V-based phosphates have been widely investigated owing to their high operating potential, and outstanding thermal and structural stability. The inductive effect of the (PO4)3− polyanion endows them with high potential, while the strong P–O bond ensures a stable structure during the charge–discharge process. To date, numerous compounds including Na3V2(PO4)3, Na3V3(PO4)4, VOPO4, NaVOPO4, and Na4VO(PO4)2 have been confirmed to have high operation potential.
2.1. Na3V2(PO4)3 (3.4 V vs. Na+/Na, the Same Hereinafter)
The well-known Na3V2(PO4)3 (NVP) has the rhombohedral NASICON structure with the space group Rc [49,50]. Figure 2a shows its 3D structure; the isolated VO6 octahedra and PO4 tetrahedra are connected to form the framework [V2(PO4)3]3− along the c direction. The unit cell of Na3V2(PO4)3 is constructed of six formula units and two kinds of Na+ sites with different coordination environments, which are referred to as the Na1 site and Na2 site. The structure can provide spacious diffusion paths to ensure sodium ions’ intercalation/deintercalation in the structure [50]. Meanwhile, two different types of Na+ occupy two oxidation state channels in the lattice, namely the Na1 site and the Na2 site. The skeleton structure is prone to be stable during the extraction of two Na+ ions because of the tightly bonded covalent influence of (PO4)3. Figure 2b displays the galvanostatic cycling profile within the voltage range of 2.3–3.9 V for a Na3V2(PO4)3/C electrode [51]. With a flat plateau at 3.4 V vs. Na+/Na, two Na+ ions can be released from the host material to form NaV2(PO4)3, resulting in a high theoretical capacity of 117.6 mAh g−1 [20]. During the charge/discharge process, a highly reversible bi-phase reaction based on V3+/V4+ has been observed [52]. When charge from Na3V2(PO4)3 to form NaV2(PO4)3, calculations using density functional theory (DFT) have indicated that Na+ prefers to diffuse through the inside Na layers, such that Na+ ions located at the Na1 site tend to remain while the rest of the Na+ ions at the Na2 site can be extracted [53,54].
To optimize the performance of high-voltage NVP, several types of modification strategies have been tested to address the poor intrinsic conductivity of Na3V2(PO4)3, including carbon coating, nano crystallization, and ionic doping. Carbon coating has been widely used to promote the electronic conductivity of electrode materials. Generally, construction of a carbon layer and conductive agents can enhance electronic conductivity, and thus the electrochemical performance of electrode materials [52,55,56]. Additionally, heteroatom doping (N, S, P, B, etc.) can also cause carbon layers by defects [57,58,59,60]. Nano crystallization of the cathode materials can increase the specific surface area and decrease ions’ diffusion paths [61,62,63]. Although carbon coating and nano crystallization are efficient approaches to improve electrochemical performance, they cannot change the inherent characteristics of a material. Hence, tests of anionic doping (Ti4+, Fe3+, Li+, Mn2+, Cr2+) and anionic ions (F−, Cl−) have been conducted to specifically modify the deep electrons’ structure. To enhance the voltage, Zakharkin et al. selected Mn2+ to partially replace V3+ and successfully synthesized Na3+xMnxV2−x (PO4)3 (0 ≤ x ≤ 1) samples [57]. An additional high-voltage plateau (~3.9 V) was found in all Mn-substituted samples and Na3+xMnxV2−x(PO4)3 (x = 0.4) exhibited 8–10% additional specific energy (vs. Na+/Na) in comparison to bare Na3V2(PO4)3 (Figure 2c). Further phase transformations in the voltage window of 2.5–3.8 V were also investigated, and it was found that several single- and two-phase actions exist during Na+ (de)intercalation (Figure 2d), thus leaving more opportunities for novel composition. For anionic ions, Chen et al. synthesized F-doped Na3V2(PO4)3/C composites [58]. The modified Na3V2(PO4)2.93F0.07/C presented a higher electronic conductivity and better cycle performance (86% retention after 1000 cycles at 200 mA g−1).
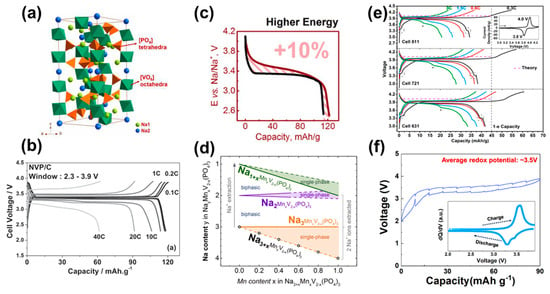
Figure 2.
(a) Crystal structure of Na3V2(PO4)3 [50]. (b) Charge/discharge curves of Na3V2(PO4)3/C at various current rates in the voltage range 2.3–3.9 V [51]. (c) Improvement of the specific energy (vs. Na+/Na) for Mn-doped Na3V2(PO4)3. (d) Schematic diagram of the Na+ deintercalation mechanism for Na3+xMnxV2−x(PO4)3 (0 ≤ x ≤ 1) during Na+ extraction [57]. (e) Charge/discharge curves of Na3V3(PO4)4 at different current densities between 3.0–4.3 V. Inset is the relative CV profile at 0.05 mV s−1 [23]. (f) The quasi-open-circuit potential profile of Na3V(PO4)2 tested at C/20 and calculated average voltage. Inset is the dQ/dV profiles of Na3V(PO4)2 [24].
2.2. Na3V3(PO4)4 (3.9 V)
Recently, a novel 3.9 V layered Na3V3(PO4)4 was explored as a potential cathode material for SIBs. It is isostructural with Na3Fe3(PO4)4 (ICSD No. 95532) with space group C2/c [23]. As shown in Figure 2e, due to the inductive effect, nearly one Na+ ion can be extracted from the structure of Na3V3(PO4)4 with a high voltage plateau at 3.9 V, which is the highest V3+/V4+ couple among V-based phosphates. From the perspective of thermodynamics, DFT calculations have also confirmed the high theoretical potential of 4.03 V when the first Na atom is removed from the original unit cell. However, it exhibited a low discharge capacity of 34 mAh g−1 and further exploration is still necessary.
2.3. Na3V(PO4)2 (3.5 V)
Another ratio in this derivative is the peculiar Na3V(PO4)2, which is identical to the structure of Na3Fe(PO4)2 in the monoclinic (C2/c) space group [24,64]. It has a layered structure, consisting of VO6 octahedra and PO4 tetrahedra. As shown in Figure 2f, a flat voltage plateau at ∼3.5 V (vs. Na+/Na) can be observed in quasi-open-circuit potential and dQ/dV profiles. During Na (de)intercalation, it experiences a biphasic reaction between Na3V(PO4)2 and Na2V(PO4)2 with only one Na able to be exacted form the structure, leading to a theoretical capacity of 90 mAh g−1, even when charged to 4.2 V [65,66]. To address this drawback, Liu et al. attempted to extend the upper cut-off voltage to 4.3 V [67]. 51V solid-state NMR was employed to confirm that the high-voltage plateau can be attributed to V4+/V5+ reactions. Therefore, Na3V(PO4)2 has the potential to deliver two electrons through V3+/V4+/V5+ reactions with a higher theoretical specific energy (vs. Na+/Na) of 657 Wh kg−1. However, the poor reversibility of V4+/V5+ severely influences the cycle performance, calling for more work to tackle this this dilemma.
2.4. VOPO4 (3.4 V)
Besides Na–V orthophosphates with different element ratios, vanadyl orthophosphates are enriching the high-voltage family. Prior to its application to SIBs in the last decade, VOPO4 had already been recognized as a potential cathode material in LIBs owing to its high operating voltage and specific capacity. There are several types of crystal structures for VOPO4 in terms of layered α1, α2, δ, ω, and γ, as well as three-dimensional β and ε phases, and all of them are composed of independent PO4 tetrahedra sharing vertices with VO6 octahedra, but they differ with respect to the orientation of the vanadyl bond [68,69]. He et al. first prepared layered VOPO4 via chemical delithiation of the tetragonal α1-LiVOPO4, delivering a reversible capacity of 110 mAh g−1 at 0.05 C with an obvious potential plateau at 3.4~3.5 V (Figure 3a) [25]. Additionally, introducing highly conductive reduced graphene oxide (rGO) can significantly increase the specific capacity of α1-VOPO4 to 150 mAh g−1, amounting to 0.9 Na+ per formula. Yu et al. synthesized tetragonal VOPO4 to achieve a high-rate capability of 70 mAh g−1 at 5 C, as well as 136 mAh g−1 at 0.1 C [70]. Further, a Na2Ti3O7/VOPO4 full cell was tested by Yu’s group [71]. The full cell presented a high operating voltage (~2.9 V) and reversible capacity (114 mAh g−1 at 0.1C and 74 mAh g−1 at 2 C) with a high specific energy (vs. Na+/Na) of 220 Wh kg−1. Very recently, Zhang et al. found that VOPO4•2H2O can fulfill consecutive V5+/V4+/V3+ redox reactions [72]. Abundant phases bring more possibilities but also troubles for synthesis, thus preparing the targeted phase without side products is a major challenge for VOPO4.
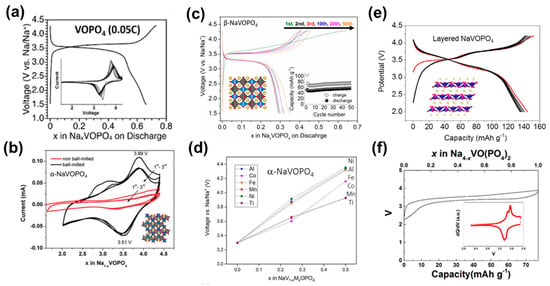
Figure 3.
(a) Typical charge–discharge curves and CV (inset) between 1.5–4.3 V of the layered VOPO4 electrodes [25]. (b) CV profiles (scan rate at 0.1 mV s−1) and crystal structure of α-NaVOPO4 [73]. (c) Charge–discharge curves of β-VOPO4 at C/20 (8 mA g−1) between 4.3 and 1.5 V. Insets show the crystal structure and cycling capacities [74]. (d) How the type and concentration of dopants on the V site influence the cell voltage (vs. Na+/Na) in the α-NaVOPO4 polymorphs (M = Al3+, Co2+, Fe3+, Mn4+, Ni2+, or Ti4+) [75]. (e) Lattice structure and galvanostatic charge–discharge profiles of the layered NaVOPO4 electrode at a current rate of 0.05 C (1 C = 145 mA g−1) [28]. (f) Charge/discharge curve of Na4VO(PO4)2. Inset is dQ/dV [29].
2.5. NaVOPO4 (3.3 V/3.5 V/3.6 V Due to Phase Difference)
Na-deficient VOPO4 needs to be pre-sodiated to become a Na-rich cathode for Na-ion battery applications, causing it to be quite costly, time consuming, and complex; however, NaVOPO4 containing reasonable amounts of Na has been studied as well. Similar to VOPO4, NaVOPO4 also has abundant phase states, such as monoclinic lattice α (space group: P21/c), tetragonal lattice α1 (space group: P4/nmm), orthorhombic lattice β (space group: Pnma), and layered structured NaVOPO4. α-NaVOPO4 was first explored as a cathode material for SIBs by Goodenough et al. [73]. As shown in Figure 3b, with an average potential of 3.6 V (vs. Na+/Na), it exhibits a reversible capacity of 90 mAh g−1 at 1/15 C. As shown in Figure 3c, β-NaVOPO4 possesses a moderate potential plateau at 3.3 V and a high theoretical capacity of 145 mAh g−1 based on a V5+/V4+ redox couple [27,74]. However, its actual capacity was greatly affected by the invasion of protons into the structure, leading to large irreversible capacity. α1-NaVOPO4 was built up of VOPO4 sheets stacked along the c axis to show a typical layered structure. Na+ ions are adequately accommodated in the space between two VOPO4 planes because of the large interlayer spacing. On the basis of molecular dynamics simulations, it has been found to have the highest Na+ mobility among the α, β, and α1 polymorphs [75]. DFT+U was also employed to investigate the influence of cell voltage on the NaVOPO4 polymorphs when doping on the vanadium site. As shown in Figure 3d, the promotion of cell voltage is tend to occur with cation doping (Al3+, Fe3+, Mn4+, Co2+, Ni2+, and Ti4+) at the vanadium site. Fang et al. prepared another layered NaVOPO4 with a triclinic lattice (Figure 3e) [28]. It attains a high voltage of ~3.5 V (vs. Na+/Na) and a high discharge capacity of 144 mAh g−1 at 0.05 C. Besides these crystals, an amorphous NaVOPO4 was reported to exhibit high reversible capacity (110 mAh g−1 at 0.05 C) at ∼3.5 V (vs. Na+/Na), as well as a capacity retention of 96% after 2000 cycles, showing an outstanding cyclability [76]. Additionally, some derivatives of NaVOPO4 have also been shown to be feasible hosts for Na ions. For instance, based on a multi-electron transfer with V3+/V5+ redox reactions, KVOPO4 could exhibit an extremely high specific capacity of 235 mAh g−1 at 2.56 V, which can be attributed to its unique polyhedral framework [77].
2.6. Na4VO(PO4)2 (3.5 V)
Na4VO(PO4)2, indexed into a space group of orthorhombic Pbca, is the Na sufficient member of V-based oxygenous phosphates [30]. As can be seen from the charge/discharge profile and the dQ/dV presented in Figure 3f, one Na ion in Na4VO(PO4)2 can be reversibly (de)intercalated from the structure with an average voltage of ∼3.5 V (vs. Na+/Na) and a theoretical capacity of 78 mAh g−1 [29]. Based on the V4+/V5+ redox reaction, the reaction mechanism turns out to be a one-phase reaction. Additionally, Deriouche et al. also examined the electrochemical performance of the Na4VO(PO4)2 compound, and found that is has high ionic conductivity and stability up to 700 °C [78]. Recently, DFT calculations and molecular dynamics simulations were employed to further study the electronic structure and influence of cation doping for Na4VO(PO4)2 [30]. The results indicated a diffusion coefficient of DNa = 5.1*10−11 cm2 s−1 at 300 K and a Na ion activation energy of 0.49 eV. Additionally, the cell voltage is also predicted to increase by cation doping at the vanadium site. Nevertheless, similar to the prediction of NaVOPO4, the practical performance of cation doping should be confirmed by experiment.
In short, V-based phosphates have excellent durability and fast kinetics, which can be attributed to their open channel structure and compositional stability. However, the operation voltage is still limited due to the inherent nature of PO4, limiting the feasibility of its introduction into other groups with strong inductive effect.
3. Fluorophosphate and Vanadyl Fluorophosphate
Fluorine substitution for phosphate groups is possible to promote the operating voltage because of the stronger inductive effect of F−. To date, several F-containing V-based polyanionic compounds, including NaVPO4F, Na5V(PO4)2F2, Na3V2(PO4)2F3, and Na3V2O2x(PO4)2F3−2x have been investigated as high voltage cathodes for SIBs.
3.1. NaVPO4F (3.7 V)
Compared with the other members of V-based polyanionic compounds, NaVPO4F has a higher theoretical specific capacity of 143 mAh g−1 (compare 117 mAh g−1 for Na3V2(PO4)3 and 128 mAh g−1 for Na3V2(PO4)2F3), making it a promising choice for SIBs. Currently, research shows that NaVPO4F can exist in two phases: a tetragonal symmetric structure with space group I4/mmm, and a monoclinic structure with space group C2/c [79]. The tetragonal phase is isostructural with Na3Al2(PO4)2F3 while the monoclinic phase presents an analogous structure to NaAlPO4F (ICSD no. 040522) (Figure 4a) [80,81]. Even with exactly the same chemical composition, the sodium storage properties of the two compounds vary greatly. The tetragonal NaVPO4F possesses a theoretical capacity of 143 mAh g−1 based on a V4+/V3+ redox reaction at 3.7 V. The monoclinic NaVPO4F shows a relatively low working plateau of 3.4 V with a single-phase reaction (Figure 4b) [32,34]. Further reports found that the tetragonal NaVPO4F tends to experience an irreversible transition to the monoclinic phase when the sintering temperature reaches up to ~750 °C, due to lower formation energy (i.e., more stable nature) of monoclinic NaVPO4F as compared to the tetragonal phase (Figure 4a) [79,82]. Additionally, after surface coating and structure modification, the monoclinic phase seems to have capacity and rate merits compared to the other phase [83,84].
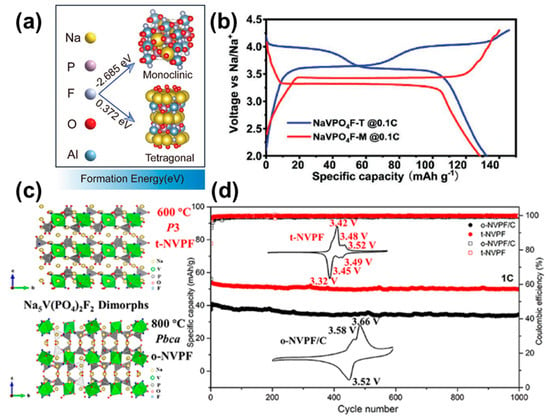
Figure 4.
(a) The formation energy calculated by DFT methods and (b) galvanostatic charge/discharge curves at 0.1C of NaVPO4F-M and NaVPO4F-T [79]. (c) The crystal structure and (d) cyclability, as well as CV curves, of o-Na5V(PO4)2F2/C and t-Na5V(PO4)2F2 [35].
In short, the tetragonal phase is more suitable as a high-energy-density cathode, mainly due to strong ionic bonding, while the monoclinic crystal is feasible for high power applications due to the intrinsically faster charge transfer kinetics.
3.2. Na5V(PO4)2F2 (3.4 V/3.5 V)
Layered Na5V(PO4)2F2 has recently been investigated as a potential cathode material for SIBs, owing to its high voltage and good cycling stability. Interestingly, as shown in Figure 4c, it has two types of structures depending on the calcination temperatures. Under high temperatures, reaching 800 °C, the obtained phase turns out to be identical with the orthorhombic Na5Fe(PO4)2F2 (PDF 87-1031) with a space group P3, which is referred as o-Na5V(PO4)2F2. The other phase, t-Na5V(PO4)2F2, is isostructural to the trigonal Na5Cr(PO4)2F2 (PDF 77-0506), indexed into space group Pbca, and can be formed at a relatively low temperature of 600 °C [35]. Due to the different sodium coordinative environments in the two phases, Na+ ions in t-Na5V(PO4)2F2 are more likely to migrate along the c axis, while they prefer to migrate along the ac plane in o-Na5V(PO4)2F2. The material presents an operating voltage of 3.4 V for trigonal-type and 3.5 V for orthorhombic-type, based on the V3+/V4+ redox couple (Figure 4d). During the charging process, a biphasic process followed by a monophasic process was confirmed by ex situ XRD and DFT calculations for both phases. However, only one Na ion can be reversibly extracted/inserted, resulting in a poor specific capacity of ~62 mAh g−1 at 0.1C (1C = 136 mA g−1). Further reports have indicated that higher sodium ion activation barriers and possible structural decomposition of the intermediate phase make it difficult to remove two sodium ions from Na5V(PO4)2F2 [85].
3.3. Na3V2(PO4)2F3 (3.9 V)
Among V-based fluorophosphate, Na3V2(PO4)2F3 displays the highest working voltage (~3.9 V vs. Na+/Na) due to the strong ionicity of the F–V bond, and high specific energy (vs. Na+/Na).
Na3V2(PO4)2F3 has a tetragonal symmetry structure with a space group of P42/mnm, and is composed of [V2O8F3] bi-octahedral units and [PO4] tetrahedral units connected by shared O atoms (Figure 5a) [86]. This arrangement generates channels along the a and b directions with sodium located in the tunnel sites, providing apparent pathways for diffusion of Na ions [87,88]. Na3V2(PO4)2F3 delivers a theoretical capacity of 128 mAh g−1 with two potential plateaus at 3.7 V and 4.2 V, based on a two-step redox reaction of V3+/V4+ (Figure 5b) [86]. Two different environments for Na ions resulted in respective Na (de)insertion, which generated a difference of 0.4–0.5 V between the two voltage plateaus. Although the high working voltage (about 3.9 V), large theoretical capacity, and fast ionic mobility enables Na3V2(PO4)2F3 to be a promising cathode material for SIBs, the poor intrinsic electronic conductivity often leads to high inner resistance, low coulombic efficiency, and limited rate performance, which seriously affects its applications.
Many approaches have been employed to enhance the electronic conductivity of Na3V2(PO4)2F3, such as heteroatom-doping [89], carbon-coating [90], morphology design [91,92], etc. In order to explore the possibility of modifying the values of the redox couples, anionic substitution of oxygen for fluorine has also been conducted. Hence, several vanadyl fluorophosphates including Na3V2(PO4)2O2F and Na3V2(PO4)2O1.6F1.4 have been further investigated.
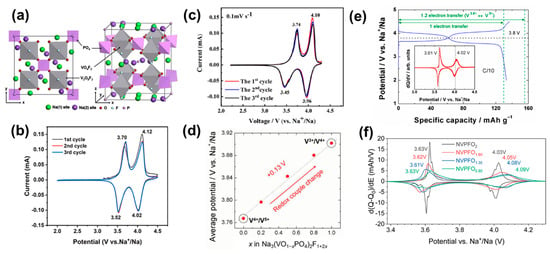
Figure 5.
(a) The crystal structure of Na3V2(PO4)2F3 [86]. (b) CV curves of Na3V2(PO4)2F3 for the first three cycles at 0.1 mV s−1 [93]. (c) CV curves of Na3V2(PO4)2O2F at a scan rate of 0.5 mV s−1 [94]. (d) Tends in average voltages of the Nay(VO1−xPO4)2F1+2x compound as x increases. [95]. (e) Charge/discharge profile at a C/10 rate for the Na3V2(PO4)2O1.6F1.4 cathode and the corresponding dQ/dV curve inset [40]. (f) The dQ/dV curves for Na3V3+2−yV4+y(PO4)2F3−yOy electrode materials corresponding to the 5th cycle performed at C/20 [96].
3.4. Na3V2O2x(PO4)2F3−2x (0 < x ≤ 1)
Na3V2(PO4)2F3 is actually the special member for x = 0 of a series of isostructural Na3V2O2x(PO4)2F3−2x (0 ≤ x ≤ 1) compounds, the latter of which has three-dimensional O8F3−2xO2x bi-octahedral units linked together by phosphate groups via oxygen atoms, with the highly mobile Na ions present at the tunnel sites [97]. In spite of their similar crystal framework, the redox mechanism and phase reactions significantly deviate with the composition due to variations in covalent and ionic bonding, as we stated in the introduction section. Na3V2O2x(PO4)2F3−2x (0 < x < 1) exhibits a slightly lower operating voltage than Na3V2(PO4)2F3, because of the weaker electronegativity of O than of F [98]. Fortunately, they present a high theoretical capacity due to the extra Na+ insertion/extraction below 4.5 V, since partial substitution of O for F can reduce the Na+–Na+ repulsion, thus allowing more Na to be accommodated. Here, two typical compounds in the series, Na3V2(PO4)2O2F and Na3V2(PO4)2O1.6F1.4, are discussed in detail.
3.4.1. Na3V2(PO4)2O2F (3.8 V)
When x = 1, the formula turns out to be Na3V2(PO4)2O2F. It has a tetragonal symmetry within the I4/mnm space group [99]. Its crystal structure is constructed by layers of alternating [PO4] tetrahedra and [VO5F] octahedra. The layers in the structure are loosely interconnected over the F atoms along the c direction, forming a 3D open framework [100]. Two types of Na+ sites were found: Na1 sites coordinated by one F atom and six O atoms, and Na2 sites coordinated six O atoms. As shown in Figure 5c, the reversible extraction of two Na+ ions at voltages of ~3.6 and ~4.0 V (vs. Na+/Na) in Na3V2(PO4)2O2F resulted in a theoretical capacity of 130 mA h g−1 and a theoretical specific energy (vs. Na+/Na) of 486 Wh kg−1 [94]. As for the Na+ storage mechanism, different hypotheses have been proposed based on characterizations. Based on an in situ XRD test, one typical statement indicates that the Na3V2(PO4)2O2F experiences two completely reversible bi-phasic transitions from Na3V2(PO4)2O2F to Na2V2(PO4)2O2F, and then to NaV2(PO4)2O2F, as well as a low volumetric change during the charging and discharging processes [101]. Based on time-resolved in situ SXRD, another statement indicates a complex asymmetrical reaction during the Na+ insertion/extraction process [102].
Similar to Na3V2(PO4)2F3, the sluggish Na+ diffusion kinetics and poor electronic conductivity of Na3V2(PO4)2O2F cause a rapid fading of capacity during Na+ insertion/extraction and low specific energy (vs. Na+/Na). Therefore, multiple surfaces, morphology, and crystal structure modification strategies have been developed to address the problems [38,103,104,105]. Investigation on the family of Na3(VO1−xPO4)2F1+2x (0 ≤ x ≤ 1) has been conducted to evaluate their performance. As shown in the Figure 5d, when x = 0 (or 1), the reaction between the charge/discharge process is only based on the V3+/V4+ (or V4+/V5+) redox couple [95]. Owing to the strong inductive effect of F− in fluorine-rich samples, the V3+/V4+ redox potential of the latter (∼3.9 V) was higher by ∼0.13 V as compared to the V4+/V5+ redox potential of the former (∼3.77 V). The linear change in voltage may help us achieve a balance between voltage and capacity for higher specific energies (vs. Na+/Na) via changing the x.
3.4.2. Na3V2(PO4)2O1.6F1.4 (3.8 V)
When x = 0.8, the formula turns out to be Na3V2(PO4)2O1.6F1.4. It is isostructural to both Na3V2(PO4)2O2F and Na3V2(PO4)2F3 with a space group of P42/mnm [41]. Benefiting from the open framework, Na3V2(PO4)2O1.6F1.4 possesses a theoretical capacity of 129.7 mAh g−1 based on one electron transfer with two potential plateaus at 3.61 and 4.02 V (vs. Na+/Na). However, it can actually exhibit a higher reversible capacity of 134 mAh g−1 due to more electron transfer (Figure 5e) [40]. During the charge/discharge process, Na3V2(PO4)2O1.6F1.4 experiences a typical two-step solid-solution reaction while both V4+/V5+ and V3+/V4+ redox couples are active. Moreover, solid-state nuclear magnetic resonance and electron paramagnetic resonance have been employed to give a comprehensive understanding of the Na+ extraction/intercalation process [106]. The results indicated that V3+ mostly reacts in the high-potential charge region while V4+ continually translates to V5+ during the whole charge process. The influence of O content has also been investigated, and it was found that Na3V2(PO4)2O1.6F1.4 has broad and less defined peaks (Figure 5f) because of the solid solution reaction [96]. Meanwhile, the results indicated that the average redox potential of the second pseudo-plateau tends to rise with of the increase in fluorine content, which can be attributed to the more ionic V–F bond in comparison to the V=O one. Additionally, a small volume change (2.9%; 0.88 ≤ x ≤ 3) upon cycling was observed, which guarantees a remarkable cycling stability and rate performance. For instance, a nanostructured Na3V2(PO4)2O1.6F1.4 exhibited, via microwave-assisted solvothermal procedure, excellent rate capability (67.2 mAh g−1 under 30 C) and long-term cycle performance (61 mAh g−1 after 1000 cycles at 10 C) when tested as a cathode for Na-ion coin cells [107].
Compared with Na3V2(PO4)3, V-based fluorophosphates present an elevated specific capacity and an improved potential, which can be attributed to the lighter molecular mass and stronger inductive effect of F−. However, fluorine loss during heat treatment is a challenge, as fluorine is unstable in V-based fluorophosphates, and thus calls for proper preparation methods to precisely regulate material composition.
4. Pyrophosphates
Compared with phosphates and fluorophosphates, V-based pyrophosphates possess abundant crystal chemistry, robust structure, and superior Na+ mobility, manifesting potential in application. To date, NaVP2O7, Na7V3(P2O7)4, and Na2(VO)P2O7 are typical representatives.
4.1. NaVP2O7 (3.9 V)
NaVP2O7 presents a relatively high operating voltage of 3.9 V and a theoretical capacity of 108.4 mAh g−1 [108,109]. As shown in Figure 6a, it has two types of crystal structures: α- and β-phase. α-NaVP2O7 is isostructural with the monoclinic NaMoP2O7, delivering a poor reversible capacity of 40 mAh g−1 [42]. β-NaVP2O7 isotypic to the monoclinic KAlP2O7, and could be operated at 3.9 V vs. Na/Na+ (Figure 6b) with a reversible capacity of 104 mAh g−1. The different structures of the two phases leads to lower migration barrier in the β-phase and thus disparate electrochemical performances. The diffusion of Na in α-NaVP2O7 was slower by several orders of magnitude than that of β-NaVP2O7, making β-NaVP2O7 a more suitable cathode material than the α-phase. β-NaVP2O7 also exhibited good high-rate performance (77 mAh g−1 at 50 C) and low stable galvanostatic cycling with only 0.5% of volume change. Similar to Na2(VO)P2O7, further research on NaVP2O7 is still limited.
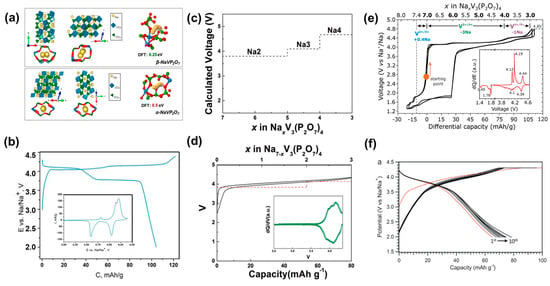
Figure 6.
(a) The crystal structure, shortest Na+ migration pathways, and migration barriers of β-NaVP2O7 and α-NaVP2O7. (b) Galvanostatic charge–discharge curves for β-NaVP2O7 collected at 10 mA g−1 current density between 2.0 and 4.4 V vs. Na+/Na. Inset is the CV measurement for β-NaVP2O7 performed within 2.0–4.5 V [42]. (c) Calculation results of average redox potential for NaxV3(P2O7). (d) Charge/discharge curves for Na7V3(P2O7)4. Inset is the dQ/dV profile of Na7V3(P2O7)4 [43]. (e) First two cycles of Na7V3(P2O7)4 in the extended range of 1.3–4.8 V [44]. (f) Galvanostatic characterization of Na2(VO)P2O7 cathode [45].
4.2. Na7V3(P2O7)4 (4.13 V)
Owing to the strong inductive effects of P2O74−, Na7V3(P2O7)4 possesses the highest redox potential to date among reported vanadium-based cathode materials for SIBs. It is isotypic to the disordered form of Na7Fe3(P2O7)4 (ICSD:86437), which can be indexed into a monoclinic space group of C2/c [110]. Previous studies have shown that Na7V3(P2O7)4 exhibits a reversibility of ~80 mAh g−1 at an average voltage of 4.13 V (vs. Na+/Na) based on the V3+/V4+ redox couple (Figure 6c,d) [43]. Additionally, the electrode also presented a good rate capability with 75% initial capacity retention after 600 cycles at 1 C, which can be attributed to the open framework and low volume change (~1%) of the material during charge/discharge possess. Recently, detailed Na+ extraction/insertion processes of Na7V3(P2O7)4 have been investigated. As shown in Figure 6e, the charge capacity can be increased up to 106 mA h g−1 with extraction of about 4 Na+ ions via activation of the V4+/V5+ redox couple at higher voltages [44]. However, this Na+ insertion/extraction reaction at high voltage is not fully reversible. In a wider voltage window of 1.3–4.8 V vs. Na+/Na, operando X-ray diffraction was employed to determine the theoretical selective order of Na+ extraction/insertion, demonstrating that the capacity of polyanionic materials may be promoted by the activation of additional redox couples for metal ions.
4.3. Na2(VO)P2O7 (3.8 V)
Introducing vanadyl into pyrophosphates may be another promising approach. Dating back to 1998, tetragonal Na2(VO)P2O7 has been reported to have an excellent ionic conductivity of 3.05 × 10−5 S cm−1, rendering it a potential candidate for cathode applications [111]. However, it has only been explored as a potential cathode material for SIBs in the last decade. Slabs of [VP2O8]∞ are piled up along the c direction, intercepted by slabs of Na atoms, and thus a layered framework with Na atoms located along the tunnels is constructed. Moreover, the structure lets Na+ preferentially diffuse between the layers, which can be considered a 1D conductor for Na diffusion. As can be seen in the charge/discharge curves displayed in Figure 6f, the Na2(VO)P2O7 can deliver a reversible capacity of ~80 mAh g−1 at 3.8 V based on a V5+/V4+ redox reaction, while its theoretical capacity is 93.4 mAh g−1 [45]. Different from Na7V3(P2O7)4, the reaction of Na2(VO)P2O7 is mainly based on the V5+/V4+ redox couple rather than activating it for higher voltage.
Generally, pyrophosphates possess a relatively high operating voltage but a low specific capacity because of the strong inductive effect and large molecular mass of P2O74−. Additionally, low theoretical specific capacity and poor conductivity mainly limited their development. Therefore, partially (rather than completely) replacing PO4 with P2O74− and other polyanions to generate mixed polyanions may be an effectively way to handle this dilemma.
5. Mixed Polyanions
As mentioned above, by combining various polyanionic units, the concept of “mixed polyanionic compounds” has been proposed. This kind of combination can not only result in multiple electron redox activity and abundant structural diversity, but also regulate the redox voltage via different degrees of inductive effect. Among V-based mixed-polyanionic compounds, Na7V4(P2O7)4(PO4) and Na2(VO)2(HPO4)2(C2O4) are two typical representatives.
5.1. Na7V4(P2O7)4(PO4) (3.85 V)
Na7V4(P2O7)4(PO4) is indexed to a tetragonal structure (P-421c) in which a 3D open framework and well-defined ionic channels can serve for Na (de)insertion. Lim et al. reported that Na7V4(P2O7)4(PO4) can exhibit an initial capacity of 91.0 mAh g−1 with a retention of ~78% over 1000 cycles [112]. The CV curve and charge/discharge profiles of Na7V4(P2O7)4(PO4) are displayed in Figure 7a,b [46]. It exhibits a single-valued voltage plateau at 3.88 V vs. Na+/Na based on the V3+/V4+ redox reaction. Moreover, Deng et al. found the intermediate phase of Na5V3.5+4(P2O7)4(PO4) during the sodium de/intercalation of a Na7V4(P2O7)4(PO4) nanorod [113]. As a result, the potential plateau can be subdivided into 3.87 V (V3+/V3.5+) and 3.89 V (V3.5+/V4+) (Figure 7c). Methods of carbon coating and structure have also been employed to improve the performance of Na7V4(P2O7)4(PO4) [47,114,115,116]. Recently, an interesting study indicated that Na7V4(P2O7)4PO4 can be used for both cathode and anode materials owing to the operating voltage of 3.85 V and 0.94 V, respectively [46]. They assemble a symmetric full battery which exhibits an initial capacity of 81.9 mAh g−1 at 2.84 V. Although the compound possesses a high operating voltage and well-defined plateau, a relatively low capacity due to the heavy P2O7 group and the complexity still restricts its wide application.
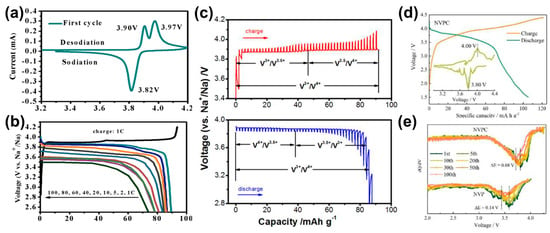
Figure 7.
(a) CV curve at a scan rate of 0.1 mV s−1 in 3.3–4.2 V and (b) the charge/discharge curves at various rates of the Na7V4(P2O7)4(PO4)/C-GA cathode [46]. (c) The GITT charge and discharge curves of a Na7V4(P2O7)4(PO4) nanorod [115]. (d) Galvanostatic charge/discharge curves and the corresponding dQ/dV patterns of Na2(VO)2(HPO4)2(C2O4). (e) The dQ/dV plots of different cycles of Na2(VO)2(HPO4)2(C2O4) and Na3V2(PO4)3 at 0.2 C [48].
5.2. Na2(VO)2(HPO4)2C2O4 (4.0 V)
Generally, the V5+/V4+ redox reaction suffers from poor reversibility in other polyanionic compounds, leading to rapid capacity degradation. A solution is to employ the coupling of (C2O4)2− and (HPO4)2− to result in a smaller forbidden band gap and lower energy barrier 2D Na+ ion migration paths, as well as to stabilize reversible high-valent redox of V4+/V5+. Very recently, Li et al. reported a V-based mixed-polyanion sodium oxalate-phosphate compound, formulated Na2(VO)2(HPO4)2(C2O4) [48]. As shown in Figure 7d, it exhibited a high redox potential of 4.0/3.8 V and a considerable reversible capacity of 105.4 mAh g−1 based on the V4+/V5+ redox couple. The high redox potential is confirmed by the dQ/dV plots (inset), which can be attributed to the accumulated inductive effect of mixed anions. As shown in Figure 7e, the corresponding dQ/dV curves of different cycles indicated the low voltage decay of only 0.08 V in the Na2(VO)2(HPO4)2(C2O4) electrode. Hence, this showed the decent cycling stability with an initial capacity retention of 74.7% after 100 cycles at 0.2 C and 61.2% after 1000 cycles at 5 C.
The concept of mixed polyanions brings us new ideas regarding cathode materials. However, the complexity of the components limits the preparation methods and influences the phase purity; thus, to determine their electrochemical performance, more work is necessary to decipher this puzzle.
6. Summary and Perspectives
In this review, the structure, Na+ storage mechanisms, charge transfer kinetics, and electrochemical performance of members among V-based polyanionic phosphates, fluorophosphates, vanadyl fluorophosphates, pyrophosphates, and mixed polyanions were systematically introduced and interpreted. A comprehensive comparison of their potential, specific capacity, and specific energy (vs. Na+/Na) is displayed in Figure 8.
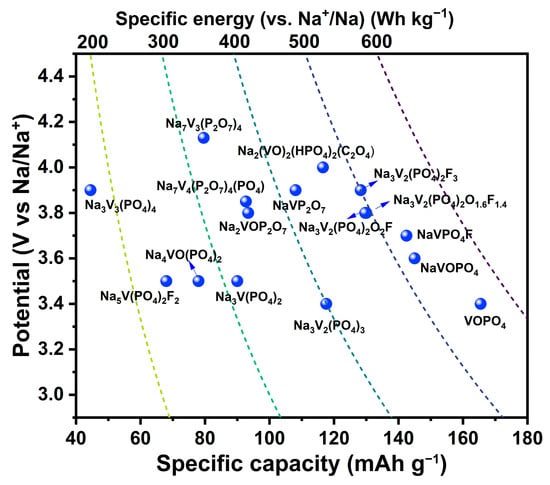
Figure 8.
Comparisons among V-based polyanionic compounds for SIBs.
V-based phosphates have been widely investigated because of their high operating potential, and outstanding structural and thermal stability. The inductive effect of the (PO4)3− polyanion endows them with high potential while the strong P–O bond ensures stable structure during charge–discharge process. However, they are short on efficient methods to further promote the working voltage, and the stronger inductive effect of F atoms made them a substitution alternative to further promote operating voltage. V-based fluorophosphates present a relatively higher operating voltage and specific capacity but also suffer from fluorine loss during heat treatment. Pyrophosphates possess rich crystal chemistry, stable structure, and superior Na+ mobility, manifesting potential in application. However, the high molecular mass of P2O74− leads to low theoretical specific capacity, although mixed polyanions seem to be a promising idea. Therefore, a balance of voltage and capacity is likely to be achieved using mixed polyanions, but the complexity of the components limits the synthetic methods and their electrochemical performance. In short, V-based phosphates are the most promising cathode material for massive applications, while fluorophosphates have the potential to achieve higher operating voltage and specific energy (vs. Na+/Na).
Although encouraging progress has been achieved in V-based polyanionic materials for SIBs, obstacles still remain that must be addressed. A comprehensive conclusion will help us to better understand the future of high-voltage V-based polyanionic materials:
- Improvement in the operating voltage. Although V-based polyanionic compounds present relatively high operating voltages, more work is still urgently needed to further enhance the voltage of Vn+1/Vn+ (n = 2,3,4) redox couples as well as specific energies (vs. Na+/Na). There are three primary strategies to improve the operating voltage: utilization of inductive effect, activation of the V5+/V4+ redox couple, and substitution of functional elements. On one hand, owing to the inductive effect, the stronger electronegativity of X and a more ionic M–O bond will always result in a higher working voltage for the Mn+1/Mn+ redox couple. For the V3+/V4+ redox couple, different groups exhibit diverse operating voltages, following the order of P2O7 > PO4F ≈ (PO4)m(P2O7) > (PO4). On the other hand, it is possible for V-based compounds are to exhibit a multi-electron reaction (involving V3+/V2+, V4+/V3+, and V5+/V4+ redox couples). V4+/V3+ is the most widespread redox couple with a moderate voltage, while an efficient way to achieve a much higher voltage is to activate the V5+/V4+ redox couple at a higher voltage. Lastly, partially substituting vanadium with other high-voltage redox elements is also regarded as an efficient approach to promote voltage. Mn2+ and Co2+ may be ideal options and further investigation is necessary.
- Developing new V-based polyanionic compounds. To achieve higher specific energy (vs. Na+/Na), partially substituting heavy polyanionic groups (PO43−, P2O74−) with other light groups (CO32−, BO33−, etc.) is a viable option. The oxalate–phosphate compound Na2(VO)2(HPO4)2(C2O4) mentioned above is a referential instance. Additionally, considering the higher electronegativity for SO42−, V-based sulfates may also an available way to achieve high-voltage material. Vanadium-based phosphate polyanionic compounds are competitive candidates for application in SIBs because of their high voltage, high power density, and cycle stability.
- Although high-voltage V-based polyanionic compounds seem to be competitive candidate for SIBs, there is still a long way to go before their wide application (Figure 9). For instance, Li3V2(PO4)3 is a well-studied cathode material in Li-ion batteries but has not been commercialized for large-scale EES; however, the analysis of it may help us figure out the obstacles towards commercialization. Li3V2(PO4)3 provides a high capacity of 156.9 mAh g−1 within the voltage range of 3.0–4.8 V. However, its instability under high pressure, which may be caused by lithium–vanadium antisite mixing, necessitates researchers to limit the upper cut-off voltage to 4.3 V, resulting in uncompetitive specific energy (380 Wh kg−1 for Li3V2(PO4)3, ~496 Wh kg−1 for LiFePO4, ~430 Wh kg−1 for LiMn2O4). In short, the performance gap with rival products makes it difficult to commercialize. Even if V-based compounds have comparative advantages over other transition metal compounds, challenges still remain before their mass production. First, while vanadium resources are abundant in the upper continental crust, the price of vanadium is higher when compared to Fe, etc. Additionally, the recycling of vanadium and vanadium dissolution are also urgent problems before commercialization. Lastly, the pollution and toxicity of vanadium have also been noteworthy issues. Research indicates that vanadium enrichment will become an ecological problem around vanadium-production areas. Meanwhile, vanadium is moderately toxic, and excess vanadium can cause certain damage to the body’s organs and tissues. In general, if we can improve the production process of vanadium resources to reduce pollutants, and recycle V-based batteries at the end of their life, then vanadium-based cathode materials will usher in a promising future and maintain exuberant vitality.
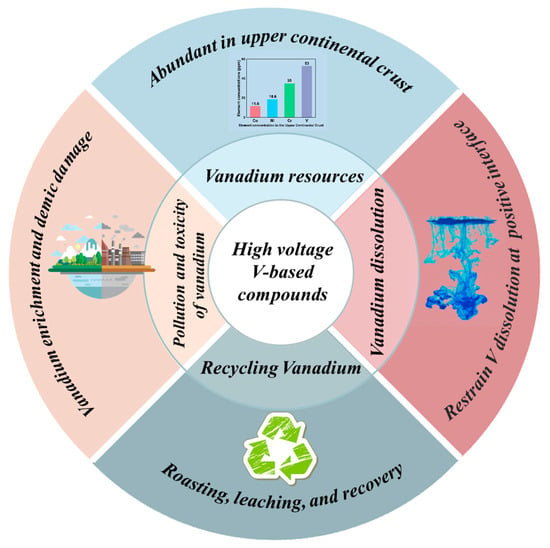 Figure 9. Perspective on the application of high-voltage V-based compounds [117].
Figure 9. Perspective on the application of high-voltage V-based compounds [117].
Author Contributions
Conceptualization, X.P. and Z.C.; investigation, H.W. and Y.C.; data curation, H.W., T.W. and L.C.; writing—original draft preparation, H.W. and X.P.; writing—review and editing, H.W., X.P. and Z.C.; supervision, X.P. and Z.C.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21875171 and U22A20438) and the Key R&D Plan of Hubei Province (2020BAA030).
Data Availability Statement
No data support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, Y.-S.; Lu, Y. 2019 Nobel Prize for the Li-Ion Batteries and New Opportunities and Challenges in Na-Ion Batteries. ACS Energy Lett. 2019, 4, 2689–2690. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-based polyanionic compounds as cathode materials for sodium-ion batteries: Toward high-energy and high-power applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can Hybrid Na–Air Batteries Outperform Nonaqueous Na–O2 Batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Zhao, D.; Yang, H.; Ai, X.; Cao, S.; Chen, Z.; Cao, Y. Recent Progress in Rechargeable Sodium-Ion Batteries: Toward High-Power Applications. Small 2019, 15, e1805427. [Google Scholar]
- Song, J.; Wang, L.; Lu, Y.; Liu, J.; Guo, B.; Xiao, P.; Lee, J.; Yang, X.; Henkelman, G.; Goodenough, J. Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Wei, C.; Hu, L.; Qian, J.; Cao, Y.; Ai, X.; Wang, J.; Yang, H. Highly Crystallized Na2CoFe(CN)6 with Suppressed Lattice Defects as Superior Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5393–5399. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.; Zhou, H. Adverse effects of interlayer-gliding in layered transition-metal oxides on electrochemical sodium-ion storage. Energy Environ. Sci. 2019, 12, 825–840. [Google Scholar] [CrossRef]
- Yin, X.; Sarkar, S.; Shi, S.; Huang, Q.A.; Zhao, H.; Yan, L.; Zhao, Y.; Zhang, J. Recent Progress in Advanced Organic Electrode Materials for Sodium-Ion Batteries: Synthesis, Mechanisms, Challenges and Perspectives. Adv. Funct. Mater. 2020, 30, 908445. [Google Scholar]
- Yuan, T.; Wang, Y.; Zhang, J.; Pu, X.; Ai, X.; Chen, Z.; Yang, H.; Cao, Y. 3D graphene decorated Na4Fe3(PO4)2(P2O7) microspheres as low-cost and high-performance cathode materials for sodium-ion batteries. Nano Energy 2019, 56, 160–168. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Yuan, T.; Cao, S.; Liu, S.; Xu, L.; Yang, H.; Ai, X.; Chen, Z.; Cao, Y. Na4Fe3(PO4)2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Storage Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Z.; Zhang, H.; Dong, C.; Ding, Y.; Cao, Y.; Chen, Z. A Green and Scalable Synthesis of Na3Fe2(PO4)P2O7/rGO Cathode for High-Rate and Long-Life Sodium-Ion Batteries. Small Methods 2021, 5, 2100372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Zhang, W.; Mao, M.; Wei, Z.; Wang, L.; Cui, C.; Zhu, Y.; Ma, J. Research progress on vanadium-based cathode materials for sodium ion batteries. J. Mater. Chem. A 2018, 6, 8815–8838. [Google Scholar] [CrossRef]
- Barpanda, P.; Lander, L.; Nishimura, S.-i.; Yamada, A. Polyanionic Insertion Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703055. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 8, 1701785. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, Z.; Xiao, L.; Ai, X.; Cao, Y.; Yang, H. Recent Progress in Iron-Based Electrode Materials for Grid-Scale Sodium-Ion Batteries. Small 2018, 14, 1703116. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.F.; Liu, P.; Jiao, L. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef]
- Pan, W.; Guan, W.; Jiang, Y. Research Advances in Polyanion-Type Cathodes for Sodium-Ion Batteries. Acta Phys. -Chim. Sin. 2020, 36, 1905017. [Google Scholar]
- Zheng, Q.; Yi, H.; Li, X.; Zhang, H. Progress and prospect for NASICON-type Na3V2(PO4)3 for electrochemical energy storage. J. Energy Chem. 2018, 27, 1597–1617. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Song, Y.; Li, Q.; Liu, Y.; Chen, J.; Xing, X. Understanding the superior sodium-ion storage in a novel Na3.5Mn0.5V1.5(PO4)3 cathode. Energy Storage Mater. 2019, 23, 25–34. [Google Scholar] [CrossRef]
- Zhu, C.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. High Power-High Energy Sodium Battery Based on Threefold Interpenetrating Network. Adv. Mater. 2016, 28, 2409–2416. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Sheng, T.; Zheng, S.; Zhong, G.; Zheng, G.; Liang, Z.; Ortiz, G.F.; Zhao, W.; Mi, J.; et al. Novel 3.9 V Layered Na3V3(PO4)4 Cathode Material for Sodium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3603–3606. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, G.; Kim, H.; Park, Y.-U.; Kang, K. Na3V(PO4)2: A New Layered-Type Cathode Material with High Water Stability and Power Capability for Na-Ion Batteries. Chem. Mater. 2018, 30, 3683–3689. [Google Scholar] [CrossRef]
- He, G.; Kan, W.H.; Manthiram, A. A 3.4 V Layered VOPO4 Cathode for Na-Ion Batteries. Chem. Mater. 2016, 28, 682–688. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R. Improved Electrochemical Performance of NaVOPO4 Positive Electrodes at Elevated Temperature in an Ionic Liquid Electrolyte. J. Electrochem. Soc. 2015, 162, A2093–A2098. [Google Scholar] [CrossRef]
- Ni, Y.; He, G. Stable cycling of β-VOPO4/NaVOPO4 cathodes for sodium-ion batteries. Electrochim. Acta 2018, 292, 47–54. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Xiao, L.; Rong, Y.; Liu, Y.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H.; Xie, J.; et al. A Fully Sodiated NaVOPO4 with Layered Structure for High-Voltage and Long-Lifespan Sodium-Ion Batteries. Chem 2018, 4, 1167–1180. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, S. High Power Cathode Material Na4VO(PO4)2 with Open Framework for Na Ion Batteries. Chem. Mater. 2017, 29, 3363–3366. [Google Scholar] [CrossRef]
- Aparicio, P.A.; de Leeuw, N.H. Electronic structure, ion diffusion and cation doping in the Na4VO(PO4)2 compound as a cathode material for Na-ion batteries. Phys. Chem. Chem. Phys. 2020, 22, 6653–6659. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Wang, K.; Song, S.-D.; Han, X.; Cheng, B.-W. Graphene modified sodium vanadium fluorophosphate as a high voltage cathode material for sodium ion batteries. Electrochim. Acta 2015, 160, 330–336. [Google Scholar] [CrossRef]
- Ling, M.; Li, F.; Yi, H.; Li, X.; Hou, G.; Zheng, Q.; Zhang, H. Superior Na-storage performance of molten-state-blending-synthesized monoclinic NaVPO4F nanoplates for Na-ion batteries. J. Mater. Chem. A 2018, 6, 24201–24209. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, S.; Zou, F.; Luo, L.; Chen, Y.; Chen, S.; Zhuo, H.; Zeng, X. Nano-NaVPO4F enwrapped in reduced graphene oxide as a cathode material for long-cycle and high-rate sodium-ion batteries. J. Alloys Compd. 2019, 811, 151828. [Google Scholar] [CrossRef]
- Chen, C.; Li, T.; Tian, H.; Zou, Y.; Sun, J. Building highly stable and industrial NaVPO4F/C as bipolar electrodes for high-rate symmetric rechargeable sodium-ion full batteries. J. Mater. Chem. A 2019, 7, 18451–18457. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, X.; Liu, R.; Ortiz, G.F.; Zhong, G.; Xiang, Y.; Chen, S.; Mi, J.; Wu, S.; Yang, Y. New Dimorphs of Na5V(PO4)2F2 as an Ultrastable Cathode Material for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 1181–1189. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.; Wang, H.; Zhou, T.; Xie, H.; Ren, Y. Synthesis and electrochemical performances of Na3V2(PO4)2F3/C composites as cathode materials for sodium ion batteries. RSC Adv. 2019, 9, 30628–30636. [Google Scholar] [CrossRef]
- Yi, H.; Ling, M.; Xu, W.; Li, X.; Zheng, Q.; Zhang, H. VSC-doping and VSU-doping of Na3V2-xTix(PO4)2F3 compounds for sodium ion battery cathodes: Analysis of electrochemical performance and kinetic properties. Nano Energy 2018, 47, 340–352. [Google Scholar] [CrossRef]
- Guo, J.Z.; Wang, P.F.; Wu, X.L.; Zhang, X.H.; Yan, Q.; Chen, H.; Zhang, J.P.; Guo, Y.G. High-Energy/Power and Low-Temperature Cathode for Sodium-Ion Batteries: In Situ XRD Study and Superior Full-Cell Performance. Adv. Mater. 2017, 29, 1701968. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Tao, L.; Tian, Z.; Zhou, S.; Zhao, N.; Wong, C.-P. Investigation of Na3V2(PO4)2O2F as a sodium ion battery cathode material: Influences of morphology and voltage window. Nano Energy 2019, 60, 510–519. [Google Scholar] [CrossRef]
- Park, Y.U.; Seo, D.H.; Kwon, H.S.; Kim, B.; Kim, J.; Kim, H.; Kim, I.; Yoo, H.I.; Kang, K. A new high-energy cathode for a Na-ion battery with ultrahigh stability. J. Am. Chem. Soc. 2013, 135, 13870–13878. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Yang, B.; Liu, L.; Li, J.; Yan, X.; He, D. A Dual Carbon-Based Potassium Dual Ion Battery with Robust Comprehensive Performance. Small 2018, 14, 1801836. [Google Scholar] [CrossRef]
- Drozhzhin, O.A.; Tertov, I.V.; Alekseeva, A.M.; Aksyonov, D.A.; Stevenson, K.J.; Abakumov, A.M.; Antipov, E.V. β-NaVP2O7 as a Superior Electrode Material for Na-Ion Batteries. Chem. Mater. 2019, 31, 7463–7469. [Google Scholar] [CrossRef]
- Kim, J.; Park, I.; Kim, H.; Park, K.-Y.; Park, Y.-U.; Kang, K. Tailoring a New 4V-Class Cathode Material for Na-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502147. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Chotard, J.-N.; Fauth, F.; Masquelier, C. Na7V3(P2O7)4 as a high voltage electrode material for Na-ion batteries: Crystal structure and mechanism of Na+ extraction/insertion by operando X-ray diffraction. J. Mater. Chem. A 2020, 8, 21110–21121. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Avdeev, M.; Yamada, A. t-Na2(VO)P2O7: A 3.8 V Pyrophosphate Insertion Material for Sodium-Ion Batteries. ChemElectroChem 2014, 1, 1488–1491. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, J.; Li, Z.; Liu, X.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y.; Ma, Z. Using Na7V4(P2O7)4(PO4) with superior Na storage performance as bipolar electrodes to build a novel high-energy-density symmetric sodium-ion full battery. J. Power Sources 2020, 451, 227734. [Google Scholar] [CrossRef]
- Fang, W.; An, Z.; Xu, J.; Zhao, H.; Zhang, J. Superior performance of Na7V4(P2O7)4PO4 in sodium ion batteries. RSC Adv. 2018, 8, 21224–21228. [Google Scholar] [CrossRef]
- Li, H.; Guan, C.; Xu, M.; Guo, J.; Yuan, K.; Cheng, K.; Xie, Y.; Zhang, L.; Zheng, J.; Lai, Y.; et al. Organic/inorganic anions coupling enabled reversible high-valent redox in vanadium-based polyanionic compound. Energy Storage Mater. 2022, 47, 526–533. [Google Scholar] [CrossRef]
- Bui, K.M.; Dinh, V.A.; Okada, S.; Ohno, T. Na-ion diffusion in a NASICON-type solid electrolyte: A density functional study. Phys. Chem. Chem. Phys. 2016, 18, 27226–27231. [Google Scholar] [CrossRef]
- Zhang, X.; Rui, X.; Chen, D.; Tan, H.; Yang, D.; Huang, S.; Yu, Y. Na3V2(PO4)3: An advanced cathode for sodium-ion batteries. Nanoscale 2019, 11, 2556–2576. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The First Report on Excellent Cycling Stability and Superior Rate Capability of Na3V2(PO4)3 for Sodium Ion Batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Jian, Z.; Han, W.; Lu, X.; Yang, H.; Hu, Y.-S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chen, D.; et al. Superior Electrochemical Performance and Storage Mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2013, 3, 156–160. [Google Scholar] [CrossRef]
- Jian, Z.; Yuan, C.; Han, W.; Lu, X.; Gu, L.; Xi, X.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, D.; et al. Atomic Structure and Kinetics of NASICON NaxV2(PO4)3 Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 4265–4272. [Google Scholar] [CrossRef]
- Bui, K.M.; Dinh, V.A.; Okada, S.; Ohno, T. Hybrid functional study of the NASICON-type Na3V2(PO4)3: Crystal and electronic structures, and polaron-Na vacancy complex diffusion. Phys. Chem. Chem. Phys. 2015, 17, 30433–30439. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, L.; Pan, H.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, L. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Guo, Z.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Double-Nanocarbon Synergistically Modified Na3V2(PO4)3: An Advanced Cathode for High-Rate and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15341–15351. [Google Scholar] [CrossRef]
- Zakharkin, M.V.; Drozhzhin, O.A.; Ryazantsev, S.V.; Chernyshov, D.; Kirsanova, M.A.; Mikheev, I.V.; Pazhetnov, E.M.; Antipov, E.V.; Stevenson, K.J. Electrochemical properties and evolution of the phase transformation behavior in the NASICON-type Na3+xMnxV2-x(PO4)3 (0≤x≤1) cathodes for Na-ion batteries. J. Power Sources 2020, 470, 228231. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Sun, X.; Zhang, B.; He, S.; Li, L.; Wang, C. Preventing structural degradation from Na3V2(PO4)3 to V2(PO4)3: F-doped Na3V2(PO4)3/C cathode composite with stable lifetime for sodium ion batteries. J. Power Sources 2018, 378, 423–432. [Google Scholar] [CrossRef]
- Nie, P.; Zhu, Y.; Shen, L.; Pang, G.; Xu, G.; Dong, S.; Dou, H.; Zhang, X. From biomolecule to Na3V2(PO4)3/nitrogen-decorated carbon hybrids: Highly reversible cathodes for sodium-ion batteries. J. Mater. Chem. A 2014, 2, 18606–18612. [Google Scholar] [CrossRef]
- Guo, J.Z.; Wu, X.L.; Wan, F.; Wang, J.; Zhang, X.H.; Wang, R.S. A Superior Na3V2(PO4)3-Based Nanocomposite Enhanced by Both N-Doped Coating Carbon and Graphene as the Cathode for Sodium-Ion Batteries. Chemistry 2015, 21, 17371–17378. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Li, D.; Cheng, X.; Liu, F.; Yu, Y. Highly Reversible Na Storage in Na3V2(PO4)3 by Optimizing Nanostructure and Rational Surface Engineering. Adv. Energy Mater. 2018, 8, 1800068. [Google Scholar] [CrossRef]
- Duan, W.; Zhu, Z.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F.; Chen, J. Na3V2(PO4)3@C core–shell nanocomposites for rechargeable sodium-ion batteries. J. Mater. Chem. A 2014, 2, 8668–8675. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, B.; Zhang, S.; Gao, X.; Deng, C. Superior sodium intercalation of honeycomb-structured hierarchical porous Na3V2(PO4)3/C microballs prepared by a facile one-pot synthesis. J. Mater. Chem. A 2015, 3, 7732–7740. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; David, R.; Chotard, J.N.; Recham, N.; Masquelier, C. A High Voltage Cathode Material for Sodium Batteries: Na3V(PO4)2. Inorg. Chem. 2018, 57, 8760–8768. [Google Scholar] [CrossRef]
- Hao, X.; Xie, H.; Zhang, X.; Wang, R.; Yu, S.; Liu, Y.; Yu, Y.; Xu, Y.; Sun, K. Electrochemical properties and sodium ion diffusion in Na3V(PO4)2. J. Solid State Chem. 2020, 281, 121047. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Na3V(PO4)2 cathode material for Na ion batteries: Defects, dopants and Na diffusion. Solid State Ion. 2019, 336, 75–79. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Z.; Xiang, Y.; Zhao, W.; Liu, H.; Chen, Y.; An, K.; Yang, Y. Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries. Molecules 2020, 25, 1000. [Google Scholar] [CrossRef]
- Boudin, S.; Guesdon, A.; Leclaire, A.; Borel, M.-M. Review on vanadium phosphates with mono and divalent metallic cations: Syntheses, structural relationships and classification, properties. J Int. J. Inorg. Mater. 2000, 2, 561–579. [Google Scholar] [CrossRef]
- Dupré, N.; Wallez, G.; Gaubicher, J.; Quarton, M. Phase transition induced by lithium insertion in αI- and αII-VOPO4. J. Solid State Chem. 2004, 177, 2896–2902. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, L.; Chen, D.; Yu, G. Intercalation Pseudocapacitance in Ultrathin VOPO4 Nanosheets: Toward High-Rate Alkali-Ion-Based Electrochemical Energy Storage. Nano Lett. 2016, 16, 742–747. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Zhu, Y.; Chen, D.; Zhang, X.; Yu, G. An advanced high-energy sodium ion full battery based on nanostructured Na2Ti3O7/VOPO4 layered materials. Energy Environ. Sci. 2016, 9, 3399–3405. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, Y.; Avdeev, M.; Kan, W.H.; He, G. Dual-ion intercalation to enable high-capacity VOPO4 cathodes for Na-ion batteries. Electrochim. Acta 2021, 365, 137376. [Google Scholar] [CrossRef]
- Song, J.; Xu, M.; Wang, L.; Goodenough, J.B. Exploration of NaVOPO4 as a cathode for a Na-ion battery. Chem. Commun. 2013, 49, 5280–5282. [Google Scholar] [CrossRef]
- He, G.; Huq, A.; Kan, W.H.; Manthiram, A. β-NaVOPO4 Obtained by a Low-Temperature Synthesis Process: A New 3.3 V Cathode for Sodium-Ion Batteries. Chem. Mater. 2016, 28, 1503–1512. [Google Scholar] [CrossRef]
- Aparicio, P.A.; Dawson, J.A.; Islam, M.S.; de Leeuw, N.H. Computational Study of NaVOPO4 Polymorphs as Cathode Materials for Na-Ion Batteries: Diffusion, Electronic Properties, and Cation-Doping Behavior. J. Phys. Chem. C 2018, 122, 25829–25836. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Amorphous NaVOPO4 as a High-Rate and Ultrastable Cathode Material for Sodium-Ion Batteries. CCS Chem. 2021, 3, 2428–2436. [Google Scholar] [CrossRef]
- Ding, J.; Lin, Y.C.; Liu, J.; Rana, J.; Zhang, H.; Zhou, H.; Chu, I.H.; Wiaderek, K.M.; Omenya, F.; Chernova, N.A.; et al. KVOPO4: A New High Capacity Multielectron Na-Ion Battery Cathode. Adv. Energy Mater. 2018, 8, 1800221. [Google Scholar] [CrossRef]
- Deriouche, W.; Anger, E.; Freire, M.; Maignan, A.; Amdouni, N.; Pralong, V. A vanadium oxy-phosphate Na4VO(PO4)2 as cathode material for Na ion batteries. Solid State Sci. 2017, 72, 124–129. [Google Scholar] [CrossRef]
- Ling, M.; Jiang, Q.; Li, T.; Wang, C.; Lv, Z.; Zhang, H.; Zheng, Q.; Li, X. The Mystery from Tetragonal NaVPO4F to Monoclinic NaVPO4F: Crystal Presentation, Phase Conversion, and Na-Storage Kinetics. Adv. Energy Mater. 2021, 11, 2100627. [Google Scholar] [CrossRef]
- Ling, M.; Lv, Z.; Li, F.; Zhao, J.; Zhang, H.; Hou, G.; Zheng, Q.; Li, X. Revisiting of Tetragonal NaVPO4F: A High Energy Density Cathode for Sodium-Ion Batteries. ACS Appl Mater Interfaces 2020, 12, 30510–30519. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Y.; Li, Y.; Cao, K.; Wang, X.; Jiao, L. Electrospun NaVPO4F/C Nanofibers as Self-Standing Cathode Material for Ultralong Cycle Life Na-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700087. [Google Scholar] [CrossRef]
- Zhao, J.; He, J.; Ding, X.; Zhou, J.; Ma Yo Wu, S.; Huang, R. A novel sol–gel synthesis route to NaVPO4F as cathode material for hybrid lithium ion batteries. J. Power Sources 2010, 195, 6854–6859. [Google Scholar] [CrossRef]
- Zhao, C.-D.; Guo, J.-Z.; Gu, Z.-Y.; Zhao, X.-X.; Li, W.-H.; Yang, X.; Liang, H.-J.; Wu, X.-L. Robust three-dimensional carbon conductive network in a NaVPO4F cathode used for superior high-rate and ultralong-lifespan sodium-ion full batteries. J. Mater. Chem. A 2020, 8, 17454–17462. [Google Scholar] [CrossRef]
- Kumar, V.K.; Ghosh, S.; Biswas, S.; Martha, S.K. Pitch-Derived Soft-Carbon-Wrapped NaVPO4F Composite as a Potential Cathode Material for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4059–4069. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, F.; Lü, T.-Y.; Wu, S.; Zhu, Z. Probing the Limiting Mechanism of Sodium-Ion Extraction in the Na5V(PO4)2F2 Cathode. J. Phys. Chem. C 2021, 125, 14583–14589. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Wu, Z.; Yang, Y.; Zhou, Z.; Li, F.; Chen, Q.; Banks, C.E. Exploration of ion migration mechanism and diffusion capability for Na3V2(PO4)2F3 cathode utilized in rechargeable sodium-ion batteries. J. Power Sources 2014, 256, 258–263. [Google Scholar] [CrossRef]
- Nguyen, L.H.B.; Sanz Camacho, P.; Broux, T.; Olchowka, J.; Masquelier, C.; Croguennec, L.; Carlier, D. Density Functional Theory-Assisted 31P and 23Na Magic-Angle Spinning Nuclear Magnetic Resonance Study of the Na3V2(PO4)2F3-Na3V2(PO4)2FO2 Solid Solution: Unraveling Its Local and Electronic Structures. Chem. Mater. 2019, 31, 9759–9768. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, Y.; Shao, H.; Yang, Y. Understanding the electrochemical mechanism of high sodium selective material Na3V2(PO4)2F3 in Li+/Na+ dual-ion batteries. Electrochim. Acta 2018, 292, 234–246. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Cui, Z.; Qi, S.; Peng, Q.; Sun, W.; Lv, L.-P.; Xu, Y.; Wang, Y.; Chen, S. In-situ structural evolution analysis of Zr-doped Na3V2(PO4)2F3 coated by N-doped carbon layer as high-performance cathode for sodium-ion batteries. J. Energy Chem. 2022, 65, 514–523. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Guo, J.-Z.; Sun, Z.-H.; Zhao, X.-X.; Li, W.-H.; Yang, X.; Liang, H.-J.; Zhao, C.-D.; Wu, X.-L. Carbon-coating-increased working voltage and energy density towards an advanced Na3V2(PO4)2F3@C cathode in sodium-ion batteries. Sci. Bull. 2020, 65, 702–710. [Google Scholar] [CrossRef]
- Qi, Y.; Mu, L.; Zhao, J.; Hu, Y.-S.; Liu, H.; Dai, S. pH-regulative synthesis of Na3(VPO4)2F3 nanoflowers and their improved Na cycling stability. J. Mater. Chem. A 2016, 4, 7178–7184. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Chang, R.; Wang, C.; He, S.; Ding, X. Unraveling the mechanism of optimal concentration for Fe substitution in Na3V2(PO4)2F3/C for Sodium-Ion batteries. Energy Storage Mater. 2021, 37, 325–335. [Google Scholar] [CrossRef]
- Liang, K.; Wang, S.; Zhao, H.; Huang, X.; Ren, Y.; He, Z.; Mao, J.; Zheng, J. A facile strategy for developing uniform hierarchical Na3V2(PO4)2F3@carbonized polyacrylonitrile multi-clustered hollow microspheres for high-energy-density sodium-ion batteries. Chem. Eng. J. 2022, 428, 131780. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, E.; Zhang, J.; Quan, J.; Wang, H.; Sun, Z.; Jiang, Y. Fabrication of a Sandwiched Core Carbon Sphere@Na3V2(PO4)2O2F@N-Doped Carbon Cathode for Superior Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 3952–3961. [Google Scholar] [CrossRef]
- Park, Y.-U.; Seo, D.-H.; Kim, H.; Kim, J.; Lee, S.; Kim, B.; Kang, K. A Family of High-Performance Cathode Materials for Na-ion Batteries, Na3(VO1−xPO4)2F1+2x(0 ≤ x ≤ 1): Combined First-Principles and Experimental Study. Adv. Funct. Mater. 2014, 24, 4603–4614. [Google Scholar] [CrossRef]
- Fang, R.; Olchowka, J.; Pablos, C.; Bianchini Nuernberg, R.; Croguennec, L.; Cassaignon, S. Impact of the F- for O2- Substitution in Na3V2(PO4)2F3-yOy on Their Transport Properties and Electrochemical Performance. ACS Appl. Energy Mater. 2022, 5, 1065–1075. [Google Scholar] [CrossRef]
- Olchowka, J.; Nguyen, L.H.B.; Broux, T.; Sanz Camacho, P.; Petit, E.; Fauth, F.; Carlier, D.; Masquelier, C.; Croguennec, L. Aluminum substitution for vanadium in the Na3V2(PO4)2F3 and Na3V2(PO4)2FO2 type materials. Chem. Commun. (Camb) 2019, 55, 11719–11722. [Google Scholar] [CrossRef]
- Nguyen, L.H.B.; Broux, T.; Camacho, P.S.; Denux, D.; Bourgeois, L.; Belin, S.; Iadecola, A.; Fauth, F.; Carlier, D.; Olchowka, J.; et al. Stability in water and electrochemical properties of the Na3V2(PO4)2F3-Na3(VO)2(PO4)2F solid solution. Energy Storage Mater. 2019, 20, 324–334. [Google Scholar] [CrossRef]
- Nguyen, L.H.B.; Olchowka, J.; Belin, S.; Sanz Camacho, P.; Duttine, M.; Iadecola, A.; Fauth, F.; Carlier, D.; Masquelier, C.; Croguennec, L. Monitoring the Crystal Structure and the Electrochemical Properties of Na3(VO)2(PO4)2F through Fe3+ Substitution. ACS Appl. Mater. Interfaces 2019, 11, 38808–38818. [Google Scholar] [CrossRef]
- Serras, P.; Palomares, V.; Goñi, A.; Gil de Muro, I.; Kubiak, P.; Lezama, L.; Rojo, T. High voltage cathode materials for Na-ion batteries of general formula Na3V2O2x(PO4)2F3−2x. J. Mater. Chem. 2012, 22, 22301–22308. [Google Scholar] [CrossRef]
- Serras, P.; Palomares, V.; Goñi, A.; Kubiak, P.; Rojo, T. Electrochemical performance of mixed valence Na3V2O2x(PO4)2F3−2x/C as cathode for sodium-ion batteries. J. Power Sources 2013, 241, 56–60. [Google Scholar] [CrossRef]
- Sharma, N.; Serras, P.; Palomares, V.; Brand, H.E.A.; Alonso, J.; Kubiak, P.; Fdez-Gubieda, M.L.; Rojo, T. Sodium Distribution and Reaction Mechanisms of a Na3V2O2(PO4)2F Electrode during Use in a Sodium-Ion Battery. Chem. Mater. 2014, 26, 3391–3402. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, R.; He, B.; Gong, Y.; Wang, H. Large-Area, Uniform, Aligned Arrays of Na3(VO)2(PO4)2F on Carbon Nanofiber for Quasi-Solid-State Sodium-Ion Hybrid Capacitors. Small 2019, 15, 1902466. [Google Scholar] [CrossRef] [PubMed]
- Olchowka, J.; Fang, R.; Bianchini Nuernberg, R.; Pablos, C.; Carlier, D.; Cassaignon, S.; Croguennec, L. Particle nanosizing and coating with an ionic liquid: Two routes to improve the transport properties of Na3V2(PO4)2FO2. Nanoscale 2022, 14, 8663–8676. [Google Scholar] [CrossRef]
- Palomares, V.; Iturrondobeitia, A.; Sanchez-Fontecoba, P.; Goonetilleke, D.; Sharma, N.; Lezama, L.; Rojo, T. Iron-Doped Sodium–Vanadium Fluorophosphates: Na3V2–yO2–yFey(PO4)2F1+y (y < 0.3). Inorg. Chem. 2019, 59, 854–862. [Google Scholar]
- Li, C.; Shen, M.; Lou, X.; Hu, B. Unraveling the Redox Couples of VIII/VIV Mixed-Valent Na3V2(PO4)2O1.6F1.4 Cathode by Parallel-Mode EPR and In Situ/Ex Situ NMR. J. Phys. Chem. C 2018, 122, 27224–27232. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Hu, B.; Lou, X.; Zhang, X.; Tong, W.; Hu, B. High-energy nanostructured Na3V2(PO4)2O1.6F1.4 cathodes for sodium-ion batteries and a new insight into their redox chemistry. J. Mater. Chem. A 2018, 6, 8340–8348. [Google Scholar] [CrossRef]
- Kee, Y.; Dimov, N.; Staikov, A.; Barpanda, P.; Lu, Y.-C.; Minami, K.; Okada, S. Insight into the limited electrochemical activity of NaVP2O7. RSC Adv. 2015, 5, 64991–64996. [Google Scholar] [CrossRef]
- Vellaisamy, M.; Reddy, M.V.; Chowdari, B.V.R.; Kalaiselvi, N. Exploration of AVP2O7/C (A = Li, Li0.5Na0.5, and Na) for High-Rate Sodium-Ion Battery Applications. J. Phys. Chem. C 2018, 122, 24609–24618. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Zhao, B. First exploration of ultrafine Na7V3(P2O7)4 as a high-potential cathode material for sodium-ion battery. Energy Storage Mater. 2016, 4, 71–78. [Google Scholar] [CrossRef]
- Daidouh, A.; Veiga, M.; Pico, C. New polymorphs of A2VP2O8 (A=Na, Rb): Structure determination and ionic conductivity. Solid State Ion. 1998, 106, 103–112. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, H.; Chung, J.; Lee, J.H.; Kim, B.G.; Choi, J.J.; Chung, K.Y.; Cho, W.; Kim, S.J.; Goddard, W.A., 3rd; et al. Role of intermediate phase for stable cycling of Na7V4(P2O7)4PO4 in sodium ion battery. Proc. Natl. Acad. Sci. USA 2014, 111, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, S.; Wu, Y. Hydrothermal-assisted synthesis of the Na7V4(P2O7)4(PO4)/C nanorod and its fast sodium intercalation chemistry in aqueous rechargeable sodium batteries. Nanoscale 2015, 7, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, C.; Meng, Y. Bicontinuous hierarchical Na7V4(P2O7)4(PO4)/C nanorod-graphene composite with enhanced fast sodium and lithium ions intercalation chemistry. J. Mater. Chem. A 2014, 2, 20538–20544. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S. 1D nanostructured Na7V4(P2O7)4(PO4) as high-potential and superior-performance cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 9111–9117. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Zhang, S.; Deng, C. Towards high potential and ultra long-life cathodes for sodium ion batteries: Freestanding 3D hybrid foams of Na7V4(P2O7)4(PO4) and Na7V3(P2O7)4@biomass-derived porous carbon. J. Mater. Chem. A 2016, 4, 5719–5729. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Ding, Y.; Ding, M.; Cao, Y.; Chen, Z. Will Vanadium-Based Electrode Materials Become the Future Choice for Metal-Ion Batteries? ChemSusChem 2022, 15, e202200479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).