The Advantages of EPR Spectroscopy in Exploring Diamagnetic Metal Ion Binding and Transfer Mechanisms in Biological Systems
Abstract
1. Introduction
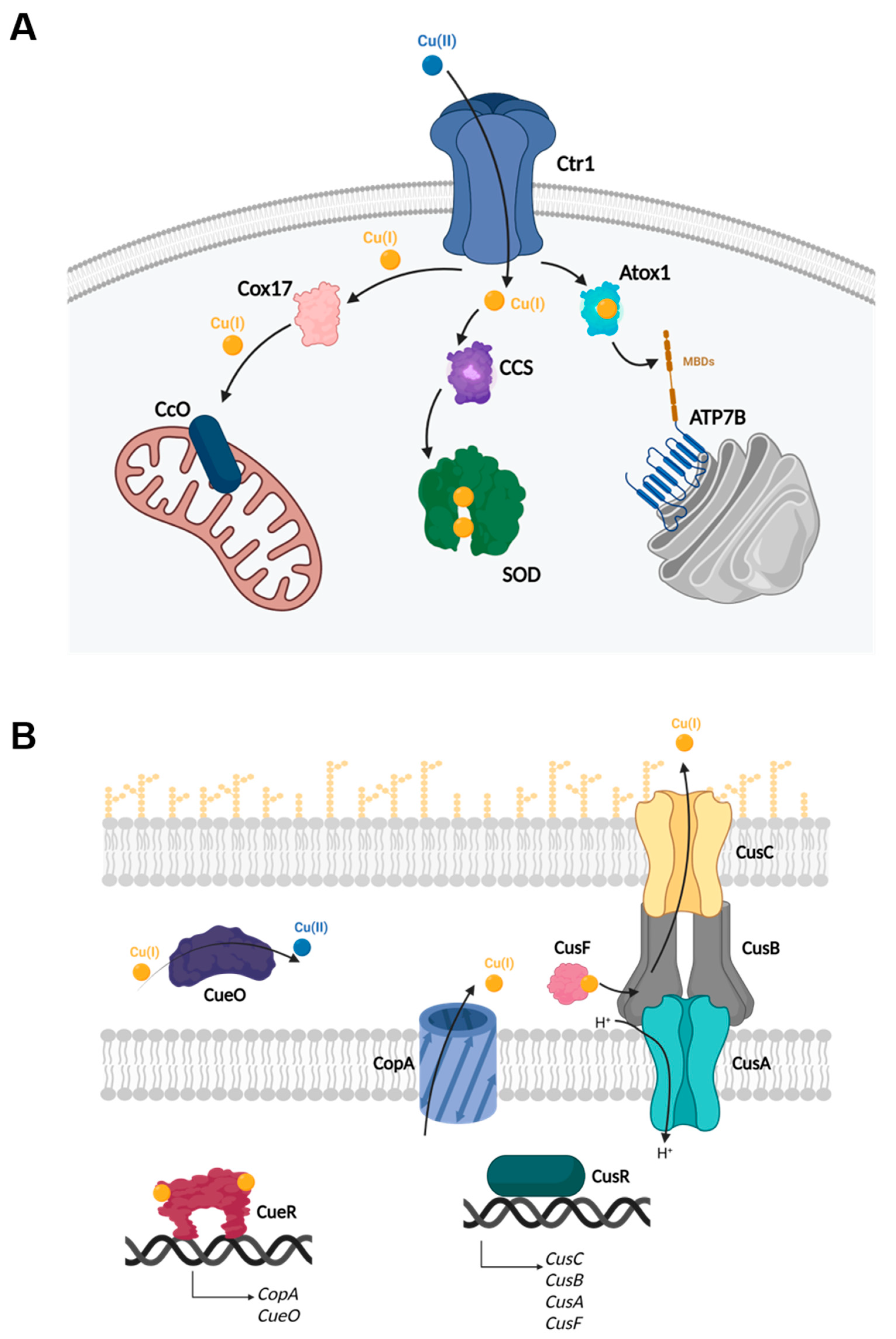
1.1. Copper Homeostasis
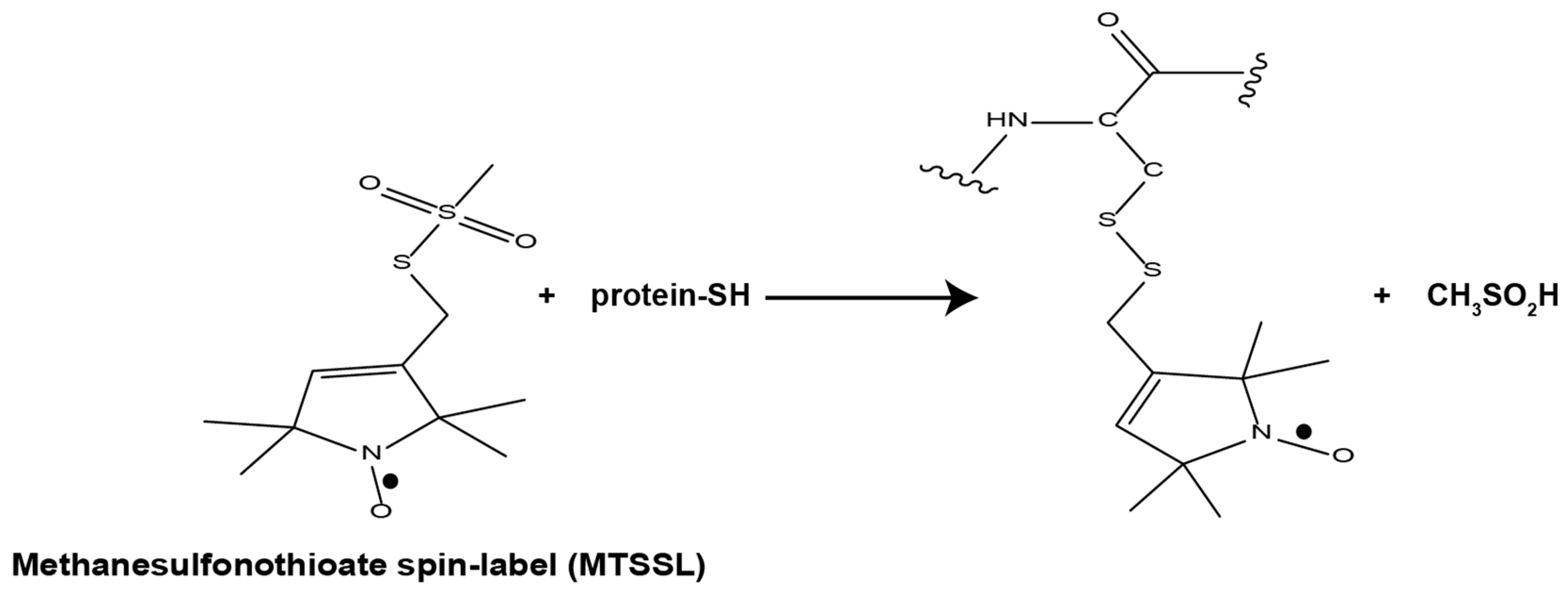
1.2. EPR Spectroscopy
2. Case Studies
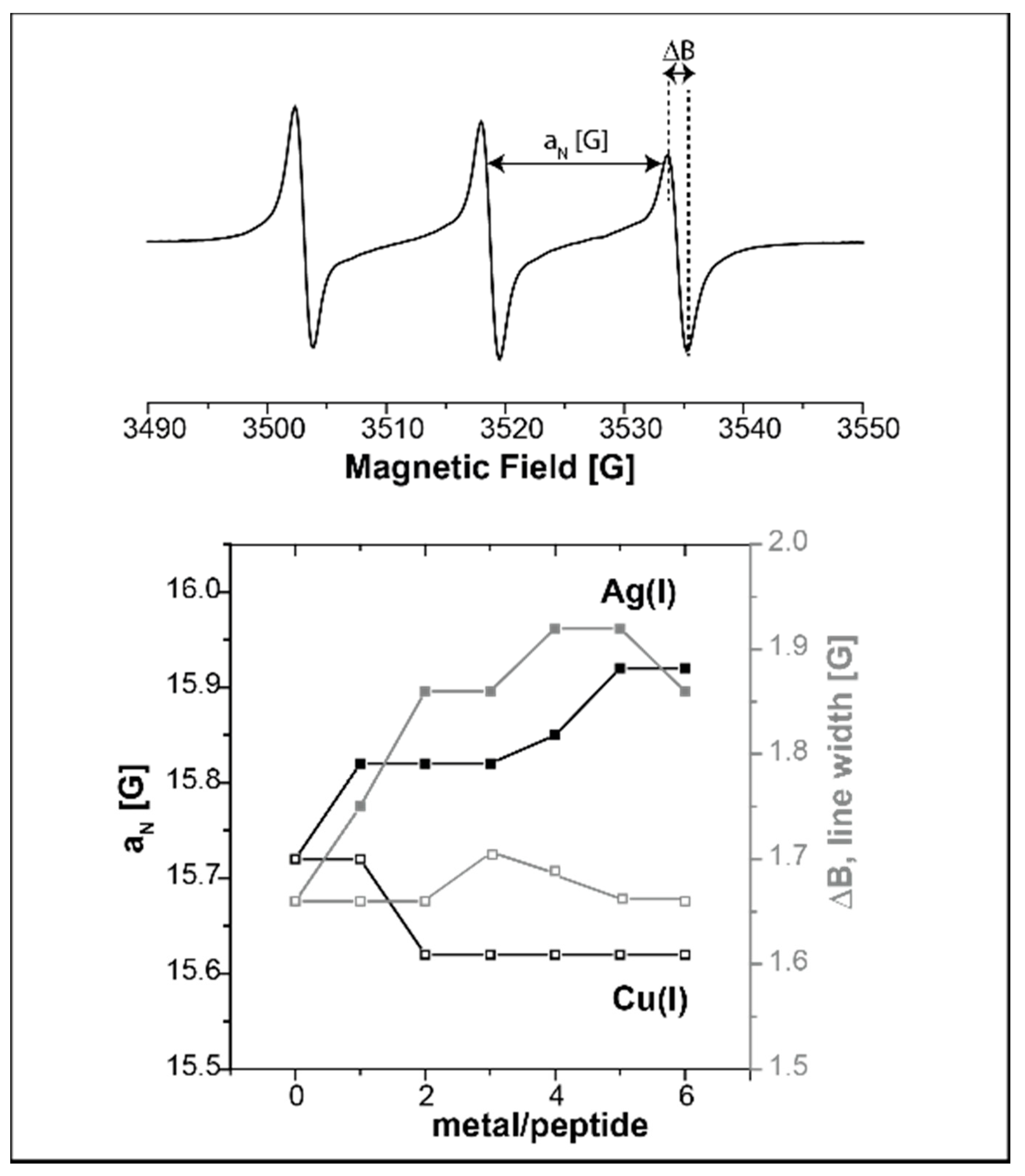
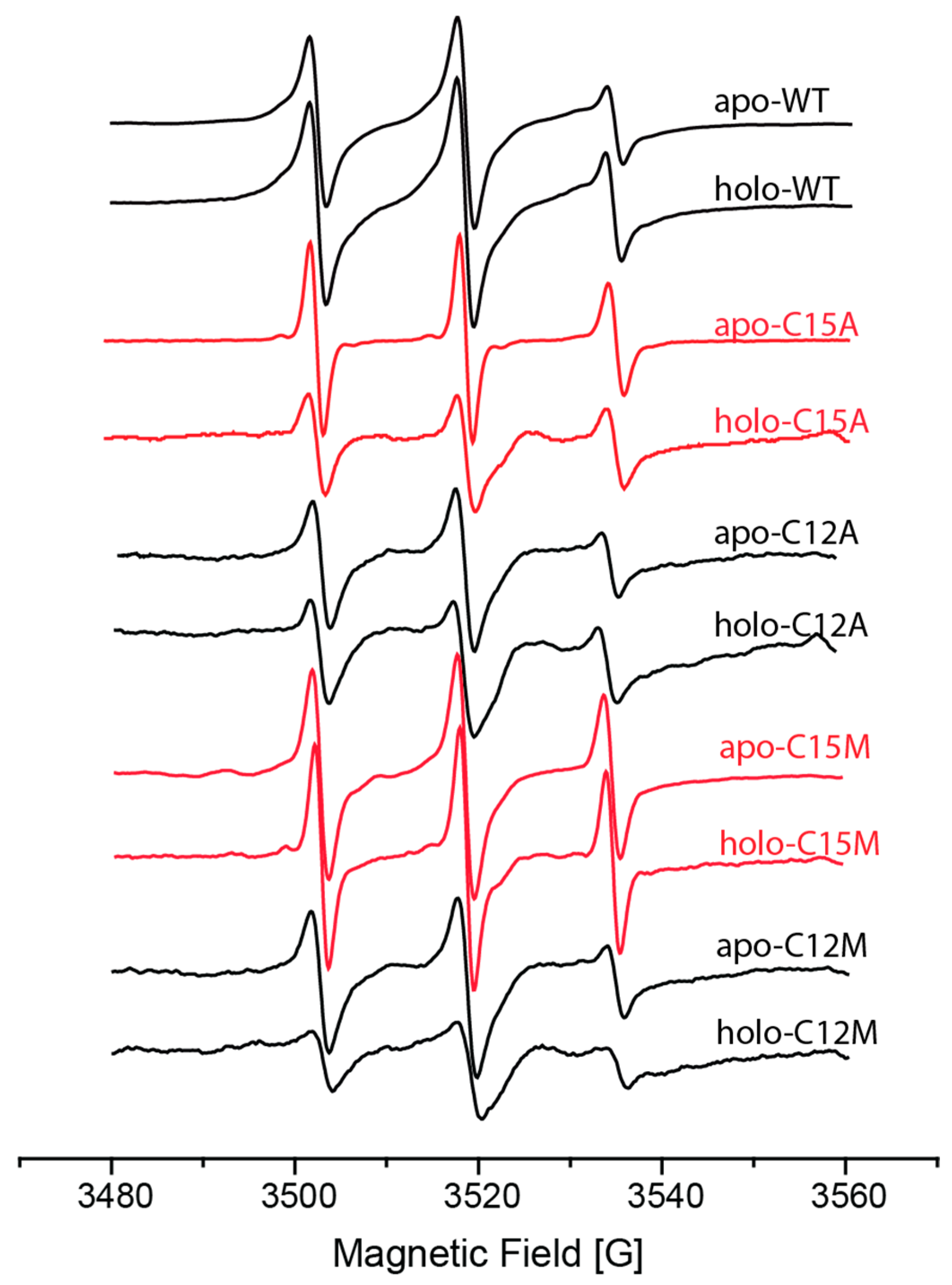
2.1. Following Conformational Changes in Biomolecules upon Metal Binding
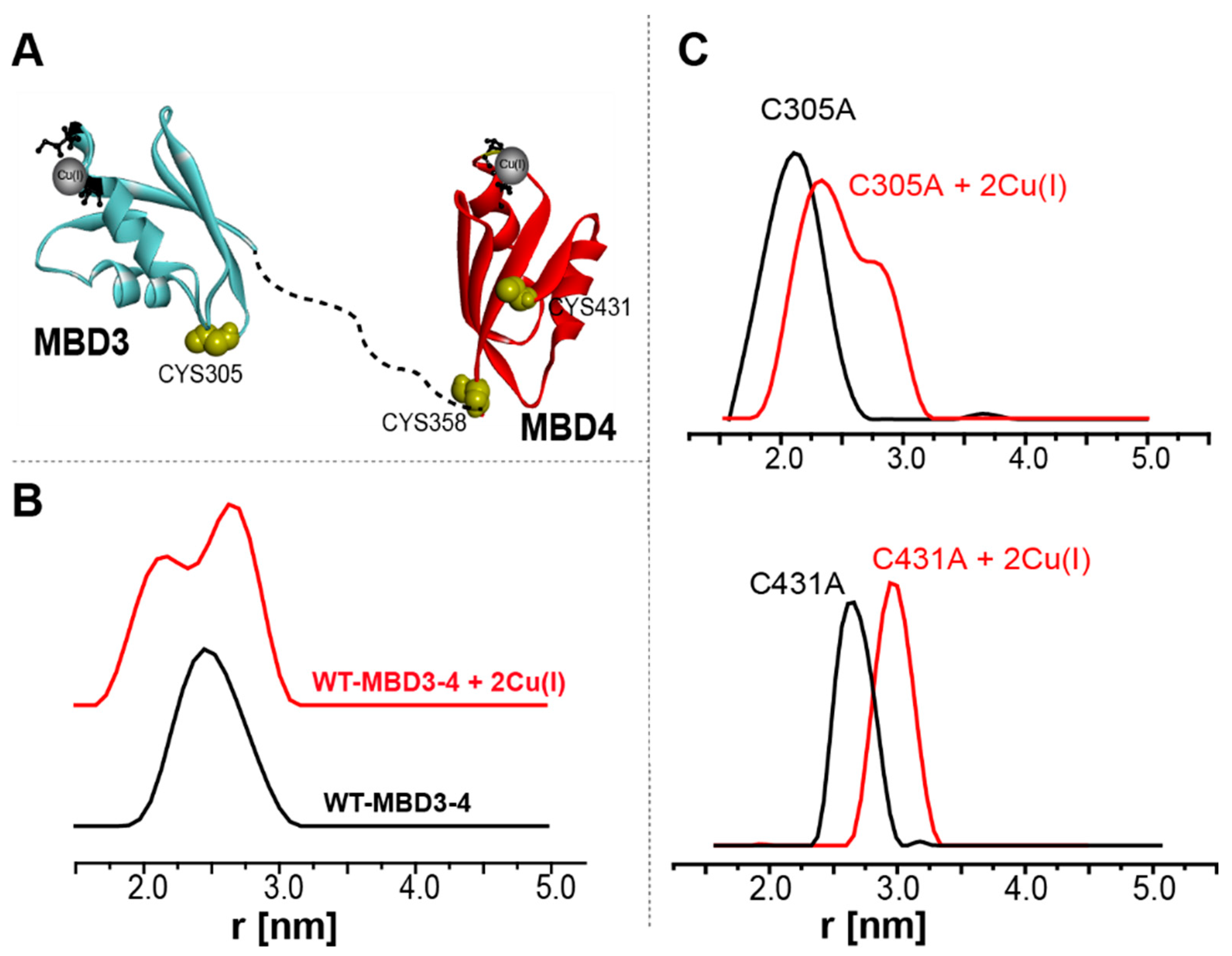
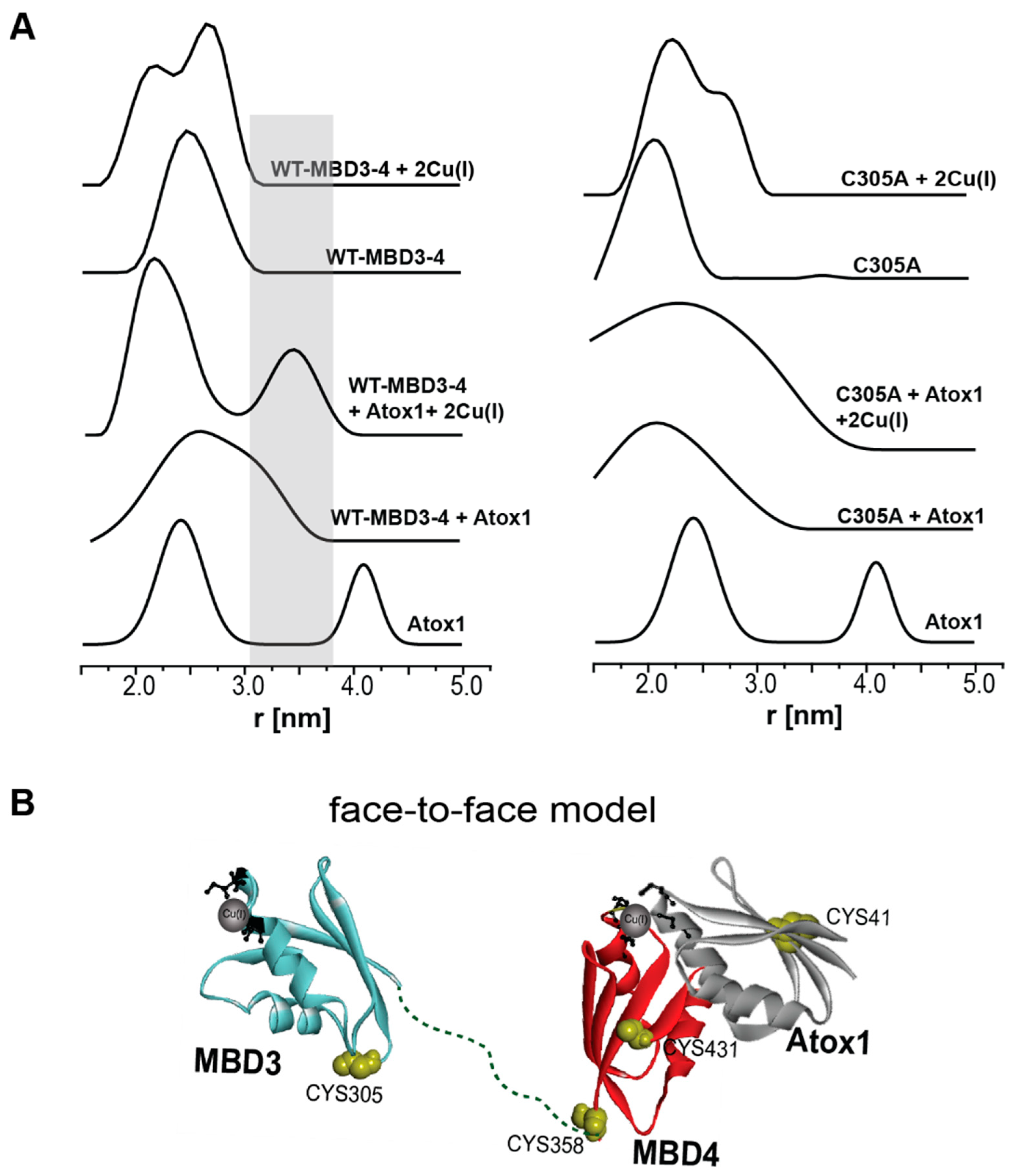
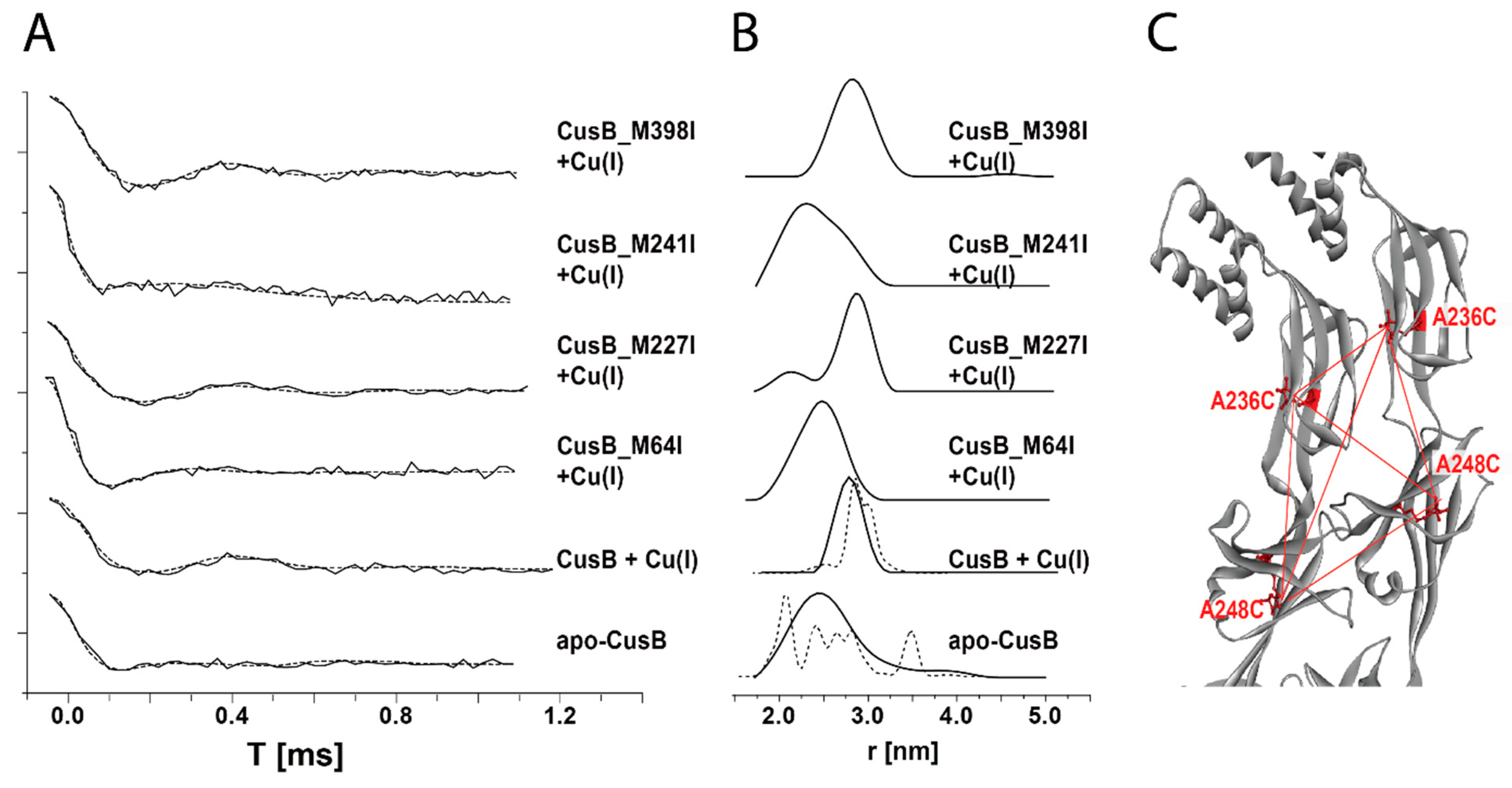
2.2. Following Conformational Changes in Biomolecules upon Metal Transfer
2.3. Identifying Metal Coordination Sites
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kosman, D.J. The teleos of metallo-reduction and metallo-oxidation in eukaryotic iron and copper trafficking. Metallomics 2018, 10, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper Homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, J.; Esser, A.; Kanzler, S.; Braunschweig, T.; Kintsler, S.; Spillner, J.; Schroder, T.; Kalverkamp, S.; Balakirski, G.; Gerhards, B.; et al. The effects of zinc- and copper-containing welding fumes on murine, rat and human precision-cut lung slices. J. Trace Elem. Med. Biol. 2018, 49, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.; Gitlin, J.D. Copper and Iron Disorders of the Brain. Annu. Rev. Neurosci. 2007, 30, 317–337. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. Adv. Microb. Physiol. 2017, 70, 193–260. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Dollwet, H.; Sorenson, J.R.J. Historic uses of copper compunds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Donnelly, P.S.; Xiao, Z.; Wedd, A.G. Copper and Alzheimer’s disease. Curr. Opin. Chem. Biol. 2007, 11, 128–133. [Google Scholar] [CrossRef]
- Innocenti, M.; Salvietti, E.; Guidotti, M.; Casini, A.; Bellandi, S.; Foresti, M.L.; Gabbiani, C.; Pozzi, A.; Zatta, P.; Messori, L. Trace copper(II) or zinc(II) ions drastically modify the aggregation behavior of amyloid-beta1-42: An AFM study. J. Alzheimers Dis. 2010, 19, 1323–1329. [Google Scholar] [CrossRef]
- Hofmann, L.; Hirsch, M.; Ruthstein, S. Advances in Understanding of the Copper Homeostasis in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 2050. [Google Scholar] [CrossRef]
- Magistrato, A.; Pavlin, M.; Qasem, Z.; Ruthstein, S. Copper trafficking in eukaryotic systems: Current knowledge from experimental and computational efforts. Curr. Opin. Struct. Biol. 2019, 58, 26–33. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S. Copper trafficking in biology: An NMR approach. HFSP J. 2009, 3, 165–175. [Google Scholar] [CrossRef]
- Shenberger, Y.; Gottlieb, H.E.; Ruthstein, S. EPR, NMR, and CD spectroscopy provide inputs on the coordination of Cu(I) and Ag(I) to a disordered methionine segment. J. Biol. Inorg. Chem. 2014, 20, 719–727. [Google Scholar] [CrossRef]
- Gui, Z.; Green, A.R.; Kasrai, M.; Bancroft, G.M.; Stillman, M.J. Sulfur K-Edge EXAFS Studies of Cadmium-, Zinc-, Copper-, and Silver-Rabbit Liver Metallothioneins. Inorg. Chem. 1996, 35, 6520–6529. [Google Scholar] [CrossRef]
- McDonald, A.; Pushie, M.J.; Millhauser, G.L.; George, G.N. New insights into metal interactions with the prion protein: EXAFS analysis and structure calculations of copper binding to a single octarepeat from the prion protein. J. Phys. Chem. B 2013, 117, 13822–13841. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, D.; Pike, K.J.; Persson, I.; Hanna, J.V.; Dupree, R.; Forsling, W.; Antzutkin, O.N. Solid-state NMR and EXAFS spectroscopic characterization of polycrystalline copper(I) O,O′-dialkyldithiophosphate cluster compounds: Formation of copper(I) O,O′-diisobutyldithiophosphate compounds on the surface of synthetic chalcocite. Chemistry 2006, 12, 5282–5292. [Google Scholar] [CrossRef]
- Sameach, H.; Ghosh, S.; Gevorkyan-Airapetov, L.; Saxena, S.; Ruthstein, S. EPR Spectroscopy Detects Various Active State Conformations of the Transcriptional Regulator CueR. Angew. Chem. Int. Ed. Engl. 2018, 58, 3053–3056. [Google Scholar] [CrossRef]
- Tottey, S.; Harvie, D.R.; Robinson, N.J. Understanding how cells allocate metals using metal sensors and metallochaperone. Acc. Chem. Res. 2005, 38, 775–783. [Google Scholar] [CrossRef]
- Eisses, J.F.; Kaplan, J.H. Molecular Characterization of hCTR1, the Human Copper Uptake Protein. J. Biol. Chem. 2002, 277, 29162–29171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pena, M.M.O.; Nose, Y.; Thiele, D.J. Biochemical Characterization of the Human Copper Transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Robinson, N.J.; Winge, D.R. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Rosenzweig, A.C. Copper delivery by metallochaperone proteins. Acc. Chem. Res. 2001, 34, 119–128. [Google Scholar] [CrossRef]
- Arnesano, F.; Banci, L.; Bertini, I.; Huffman, D.L.; O’Halloran, T.V. Solution strcuture of the Cu(I) and Apo forms of the yeast metallochaperone, Atx1. Biochemistry 2001, 40, 1528–1539. [Google Scholar] [CrossRef]
- Wernimont, A.M.; Huffman, D.L.; Lamb, A.L.; O’Halloran, T.V.; Rosenzweig, A.C. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proetin. Nat. Struct. Biol. 2000, 7, 766–771. [Google Scholar] [PubMed]
- Nevitt, T.; Ohrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochem. Biophys. Acta 2012, 1823, 1580–1593. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.K.; Abomoelak, B.; Hoye, E.A.; Steinberg, H.; Talaat, A.M. CtpV: A putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010, 77, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Long, F.; Yu, E.W. The Cus efflux system removes toxic ions via a methionine shuttle. Protein Sci. 2011, 20, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Chacón, K.N.; Mealman, T.D.; McEvoy, M.M.; Blackburn, M.E. Tracking metal ions through a Cu/Ag efflux pump assigns the functional roles of the periplasmic proteins. Proc. Nat. Acad. Sci. USA 2014, 111, 15373–15378. [Google Scholar] [CrossRef]
- Roberts, S.A.; Weichsel, A.; Grass, G.; Thakali, K.; Hazzard, J.T.; Tollin, G.; Rensing, C.; Montfort, W.R. Crystal structure and electron transfer kinetics of CueO, a multicopper oxicdase required for copper homeostasis in Escherichia coli. Proc. Nat. Acad. Sci. USA 2002, 99, 2766–2771. [Google Scholar] [CrossRef]
- Rowland, J.L.; Niederweis, M. Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload. Tuberculosis 2012, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Wladron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef]
- Hitomi, Y.; Outten, C.E.; O’Halloran, T.V. Extreme Zinc-binding thermodynamics of the metal sensor/regulator protein, ZntR. J. Am. Chem. Soc. 2001, 123, 8614–8615. [Google Scholar] [CrossRef]
- Outten, C.E.; Outten, F.W.; O’Halloran, T.V. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 1999, 274, 37517–37524. [Google Scholar] [CrossRef] [PubMed]
- Changela, A.; Chen, K.; Holschen, J.; Outten, C.E.; O’Halloran, T.V.; Mondragon, A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 2003, 301, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Outten, C.E.; Hale, J.A.; O’Halloran, T.V. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal Mer homologue, CueR. J. Biol. Chem. 2000, 275, 31024–31029. [Google Scholar] [CrossRef]
- Chen, K.; Yuldasheva, J.E.; Penner-Hahn, J.E.; O’Halloran, T.V. An Atypical Linear Cu(I)−S2 Center Constitutes the High-Affinity Metal-Sensing Site in the CueR Metalloregulatory Protein. J. Am. Chem. Soc. 2003, 125, 12088–12089. [Google Scholar] [CrossRef]
- Stoyanov, J.V.; Hobman, J.L.; Brown, N.L. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol. Microbiol. 2001, 39, 502–512. [Google Scholar] [CrossRef]
- Schultz, L.W.; Chivers, P.T.; Raines, R.T. The CXXC motif: Crystal structure of an active-site variant of Escherichia coli thioredoxin. Acta Cryst. D Biol Cryst. 1999, 55, 1533–1538. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Strausak, D.; Greenough, M.; Brooks, H.; Petris, M.; Smith, S.; Mercer, J.F.; Camakaris, J. Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J. Biol. Chem. 1999, 274, 22008–22012. [Google Scholar] [CrossRef]
- Pushie, M.J.; Shaw, K.; Franz, K.J.; Shearer, J.; Haas, K.L. Model Peptide Studies Reveal a Mixed Histidine-Methionine Cu(I) Binding Site at the N-Terminus of Human Copper Transporter 1. Inorg Chem. 2015, 54, 8544–8551. [Google Scholar] [CrossRef]
- Xue, Y.; Davis, A.V.; Balakrishnan, G.; Stasser, J.P.; Staehlin, B.M.; Focia, P.; Spiro, T.G.; Penner-Hahn, J.E.; O’Halloran, T.V. Cu(I) recognition via cation-pi and methionine interactions in CusF. Nat. Chem. Biol. 2008, 4, 107–109. [Google Scholar] [CrossRef]
- Rubino, J.T.; Riggs-Gelasco, P.; Franz, K.J. Methionine motifs of copper transport proteins provide general and flexible thioether-only binding sites for Cu(I) and Ag(I). J. Biol. Inorg. Chem. 2010, 15, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Banham, J.E.; Baker, C.M.; Ceola, S.; Day, I.J.; Grant, G.H.; Groenen, E.J.J.; Rodgers, C.T.; Jeschke, G.; Timmel, C.R. Distance measurements in the borderline region of applicability of CW EPR and DEER: A model study on a homologous series of spin-labelled peptides. J. Magn. Reason. 2008, 191, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Banham, J.E.; Jeschke, G.; Timmel, C.R. Evidence from EPR that nitroxide spin labels attached to human hemoglobin alter their conformation upon freezing. Mol. Phys. 2007, 105, 2041–2047. [Google Scholar] [CrossRef]
- Bennati, M.; Robblee, J.H.; Mugnaini, V.; Stubbe, J.; Freed, J.H.; Borbat, P. EPR distance measurements support a model for long-range radical initiation in E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2005, 127, 15014–15015. [Google Scholar] [CrossRef] [PubMed]
- Borbat, P.P.; Surendhran, K.; Bortolus, M.; Zou, P.; Freed, J.H.; Mchaourab, H.S. Conformational motion of the ABC transporter MsbA induced by ATP hydrolysis. PLoS Biol. 2007, 5, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Hemminga, M.A.; Berliner, L.J. ESR Spectroscopy in Membrane Biophysics; Springer Science+Business Media, LLC: New York, NY, USA, 2007; Volume 27. [Google Scholar]
- Hilger, D.; Jung, H.; Padan, E.; Wegener, C.; Vogel, K.P.; Steinhoff, H.J.; Jeschke, G. Assessing oligomerization of membrane proteins by four-pulse DEER: pH-dependent dimerization of NhaA Na+/H+ antiporter of E. coli. Biophys. J. 2005, 89, 1328–1338. [Google Scholar] [CrossRef]
- Jeschke, G.; Polyhach, Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2007, 9, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Raitsimring, A.M.; Gunanathan, C.; Potapov, A.; Efremenko, I.; Martin, J.M.L.; Milstein, D.; Goldfarb, D. Gd3+ complexes as potential spin labels for high field pulsed EPR distance measurements. J. Am. Chem. Soc. 2007, 129, 14138–14139. [Google Scholar] [CrossRef]
- Sicoli, G.; Mathis, G.; Delalande, O.; Boulard, Y.; Gasparutto, D.; Garnbarelli, S. Double electron-electron resonance (DEER): A convenient method to probe DNA conformational changes. Angew. Chem. Int. Ed. 2008, 47, 735–737. [Google Scholar] [CrossRef]
- Xu, Q.; Ellena, J.F.; Kim, M.; Cafiso, D.S. Substrate-Dependent unfolding of the energy coupling motif of a membrane transport protein determined by double electron-electron resonance. Biochemistry 2006, 45, 10847–10854. [Google Scholar] [CrossRef][Green Version]
- Sahu, I.D.; Kroncke, B.M.; Zhang, R.; Dunagan, M.M.; Smith, H.J.; Craig, A.; McCarrick, R.M.; Sanders, C.R.; Lorigan, G.A. Structural Investigation of the Transmembrane Domain of KCNE1 in Proteoliposomes. Biochemistry 2014, 53, 6391–6401. [Google Scholar] [CrossRef] [PubMed]
- Atherton, N.M. Principles of Electron. Spin Resonance; Ellis Horwood PTR Prentice Hall: Hemel Hempstead, UK, 1993. [Google Scholar]
- Weil, J.A.; Bolton, J.R. Electron. Paramagnetic Resonance; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schweiger, A.; Jeschke, G. Principles of Electron. Paramgnetic Resonance; University Press: Oxford, UK, 2001. [Google Scholar]
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656. [Google Scholar] [CrossRef]
- Columbus, L.; Hubbell, W.L. A new spin on protein dynamics. Trends Biochem. Sci. 2002, 27, 288–295. [Google Scholar] [CrossRef]
- Hubbell, W.L.; Mchaourab, H.S.; Altenbach, C.; Lietzow, M.A. Watching proteins move using site-directed spin labeling. Structure 1996, 4, 779–783. [Google Scholar] [CrossRef]
- Jeschke, G. Conformational dynamics and distribution of nitroxide spin labels. Prog. Nuc. Magn. Reson. Spec. 2013, 72, 42–60. [Google Scholar] [CrossRef]
- Shah, A.; Roux, A.; Starck, M.; Mosely, J.A.; Stevens, M.; Norman, D.G.; Hunter, R.I.; El Mkami, H.; Smith, G.M.; Parker, D.; et al. A Gadolinium Spin Label with Both a Narrow Central Transition and Short Tether for Use in Double Electron Electron Resonance Distance Measurements. Inorg. Chem. 2019, 58, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, A.; Yang, Y.; Gong, Y.-J.; Tan, X.; Feintuch, A.; Carmieli, R.; Bahrenberg, T.; Liu, Y.; Su, X.-C.; Goldfarb, D. DEER distance measurements on trityl/trityl and Gd(iii)/trityl labelled proteins. Phys. Chem. Chem. Phys. 2019, 21, 10217–10227. [Google Scholar] [CrossRef] [PubMed]
- Fleck, N.; Hett, T.; Brode, J.; Meyer, A.; Richert, S.; Schiemann, O. C-C Cross-Coupling Reactions of Trityl Radicals: Spin Density Delocalization, Exchange Coupling, and a Spin Label. J. Org. Chem. 2019, 84, 3293–3303. [Google Scholar] [CrossRef]
- Wu, Z.; Feintuch, A.; Collauto, A.; Adams, L.A.; Aurelio, L.; Graham, B.; Otting, G.; Goldfarb, D. Selective distance measurements using triple spin labeling with Gd3+, Mn2+, and a nitroxide. J. Phys. Chem. Lett. 2017, 8, 5277–5282. [Google Scholar] [CrossRef]
- Jassoy, J.J.; Berndhauser, A.; Duthie, F.; Kuhn, S.P.; Hagelueken, G.; Schiemann, O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules In Vitro and within Cells. Angew. Chem. Int. Ed. Engl. 2017, 56, 177–181. [Google Scholar] [CrossRef]
- Goldfarb, D. Gd3+ spin labeling for distance measurements by pulse EPR spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 9685–9699. [Google Scholar] [CrossRef] [PubMed]
- Abdullin, D.; Schiemann, O. Localization of metal ions in biomolecules by means of pulsed dipolar EPR spectroscopy. Dalton Trans. 2021, 50, 808–815. [Google Scholar] [CrossRef]
- Gorcester, J.; Millhauser, G.L.; Freed, J.H. In Modern Pulsed and Continuous Wave Electron Spin Resonance; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-Time free measurement of dipole-dipole interactions between electron spins. J. Magn. Res. 2000, 142, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Tsvetkov, Y.D. Double electron-electron resonance in electron spin echo: Conformations of spin-labeled poly-4-vinilpyridine in glassy solutions. App. Magn. Reson. 1997, 12, 495–504. [Google Scholar] [CrossRef]
- Joseph, B.; Morkhov, V.M.; Yulikov, M.; Jeschke, G.; Bordignon, E. Conformational cycle of the vitamin B12ABC importer in liposomes detected by Double Electron-Electron Resonance (DEER). J. Biol. Chem. 2014, 289, 3176–3185. [Google Scholar] [CrossRef]
- Sahu, I.D.; McCarrick, R.M.; Troxel, K.R.; Zhang, R.; Smith, H.J.; Dunagan, M.M.; Swartz, M.S.; Rajan, P.V.; Kroncke, B.M.; Sanders, C.R.; et al. DEER EPR measurements for membrane protein structures via bifunctional spin labels for lipodisp nanoparticles. Biochemistry 2013, 52, 6627–6632. [Google Scholar] [CrossRef][Green Version]
- Schiemann, O.; Heubach, C.A.; Abdullin, D.; Ackermann, K.; Azarkh, M.; Bagryanskaya, E.G.; Drescher, M.; Endeward, B.; Freed, J.H.; Galazzo, L.; et al. Benchmark Test and Guidelines for DEER/PELDOR Experiments on Nitroxide-Labeled Biomolecules. J. Am. Chem. Soc. 2021, 143, 17875–17890. [Google Scholar] [CrossRef]
- Saxena, S.; Freed, J.H. Double quantum two-dimensional Fourier transform electron spin resonance: Distance measurements. Chem. Phys. Lett. 1996, 251, 102–110. [Google Scholar] [CrossRef]
- Saxena, S.; Freed, J.H. Theory of double quantum two-dimensional electron spin resonance with application to distance measurements. J. Chem. Phys. 1997, 107, 1317–1340. [Google Scholar] [CrossRef]
- Becker, J.S.; Saxena, S. Double quantum coherence electron spin resonance on coupled Cu(II)-Cu(II) electron spins. Chem. Phys. Lett. 2005, 414, 248–252. [Google Scholar] [CrossRef]
- Akhmetzyanov, D.; Ching, H.Y.; Denysenkov, V.; Demay-Drouhard, P.; Bertrand, H.C.; Tabares, L.C.; Policar, C.; Prisner, T.F.; Un, S. RIDME spectroscopy on high-spin Mn(2+) centers. Phys. Chem. Chem. Phys. 2016, 18, 30857–30866. [Google Scholar] [CrossRef]
- Wort, J.L.; Arya, S.; Ackermann, K.; Stewart, A.J.; Bode, B.E. Pulse Dipolar EPR Reveals Double-Histidine Motif Cu(II)-NTA Spin-Labeling Robustness against Competitor Ions. J. Phys. Chem. Lett. 2021, 12, 2815–2819. [Google Scholar] [CrossRef] [PubMed]
- Peariso, K.; Huffman, D.L.; Penner-Hahn, J.E.; O’Halloran, T.V. The PcoC copper resistance protein coordinates Cu(I) via novel S-methionine interactions. J. Am. Chem. Soc. 2003, 125, 342–343. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Del Conte, R.; Gonnelli, L. Understanding copper trafficking in bacteria: Interaction between the copper transport protein CopZ and the N-terminal domain of the copper ATPase CopA from Bacillus subtilis. Biochemistry 2003, 42, 1939–1949. [Google Scholar] [CrossRef]
- Arnesano, F.; Banci, L.; Bertini, I.; Mangani, S.; Thompsett, A.R. A redox switch in CopC: An intriguing copper trafficking protein that binds copper(I) and copper(II) at different sites. Proc. Natl. Acad. Sci. USA 2003, 100, 3814–3819. [Google Scholar] [CrossRef]
- Meir, A.; Abdelhai, A.; Moskovitz, Y.; Ruthstein, S. EPR spectroscopy targets conformational and topological changes in the E. coli membrane fusion CusB dimer upon Cu(I) binding. Biophys. J. 2017, 112, 2494–2502. [Google Scholar] [CrossRef][Green Version]
- Su, C.-C.; Long, F.; Zimmermann, M.T.; Rajashankar, K.R.; Jernigan, R.L.; Edward, W.Y. Crystal Structure of the CusBA heavy-metal efflux complex of Escherichia coli. Nature 2011, 470, 558–563. [Google Scholar] [CrossRef]
- Su, C.-C.; Yang, F.; Long, F.; Reyon, D.; Routh, M.D.; Kuo, D.W.; Mokhtari, A.K.; Ornam, J.D.V.; Rabe, K.L.; Hoy, J.A.; et al. Crystal structure of the membrane fusion protein CusB from Escherichia Coli. J. Mol. Biol. 2009, 393, 342–355. [Google Scholar] [CrossRef]
- Jeschke, G. MMM: A toolbox for integrative structure modeling. Protein Sci. 2018, 27, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. MMM: Integrative ensemble modeling and ensemble analysis. Protein Sci. 2021, 30, 125–135. [Google Scholar] [CrossRef]
- Krivov, G.G.; Shapovalov, M.V.; Dunbrack, R.L.J. Improved prediction of protein side-chain conformations with SCWRL4. Proteins 2009, 77, 778–795. [Google Scholar] [CrossRef]
- Qasem, Z.; Pavlin, M.; Ritacco, I.; Gevorkyan-Airapetov, L.; Magistrato, A.; Ruthstein, S. The pivotal role of MBD4-ATP7B in the human Cu(I) excretion path as revealed by EPR experiments and all-atom simulations. Metallomics 2019, 11, 1288–1297. [Google Scholar] [CrossRef]
- Scushan, M.; Bhattacherjee, A.; Ben-Tal, N.; Lutsenko, S. A structural model for the copper ATPase ATP7B to facilitate analysis of Wilson disease causing mutations and studies of tranport mechanism. Metallomics 2012, 4, 669–678. [Google Scholar] [CrossRef]
- Yu, C.H.; Yang, N.; Bothe, J.; Tonelli, M.; Nokherin, S.; Dolgova, N.V.; Braiterman, L.; Lutsenko, S.; Dmitriev, O.Y. The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics. J. Biol. Chem. 2017, 292, 18169–18177. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Rosenzweig, A.C.; Yatsunyk, L.A. Metal binding domains 3 and 4 of the Wilson disease protein: Solution Structure and interaction with the copper(I) chaperone HAH1. Biochemistry 2008, 47, 7423–7429. [Google Scholar] [CrossRef]
- Holt, B.T.O.; Merz, K.M. Insights into Cu(I) exchange in HAH1 using quantum mechanical and molecular simulations. Biochemistry 2007, 46, 8816–8826. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.K.; Rosenzweig, A.C. Crystal structures of cisplatin bound to human copper chaperone. J. Am. Chem. Soc. 2009, 131, 14196–14197. [Google Scholar] [CrossRef] [PubMed]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-Dimensional structure of the human copper transporter hCTR1. Proc. Nat. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Kahra, D.; Kovermann, M.; Wittung-Stafshede, P. The C-Terminus of Human Copper Importer Ctr1 Acts as a Binding Site and Transfers Copper to Atox1. Biophys. J. 2016, 110, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.R.; Yarmiayev, V.; Moskovitz, Y.; Ruthstein, S. Probing the structural flexibility of the human copper metallochaperone Atox1 dimer and its interaction with the CTR1 c-terminal domain. J. Phys. Chem. B 2014, 118, 5832–5842. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Massagni, C.; Migliardi, M.; Rosato, A. An NMR Study of the Interaction of the N-terminal Cytoplasmic Tail of the Wilson Disease Protein with Copper(I)-HAH1. J. Biol. Chem. 2009, 284, 9354–9360. [Google Scholar] [CrossRef]
- Levy, A.R.; Nissim, M.; Mendelman, N.; Chill, J.; Ruthstein, S. Ctr1 Intracellular Loop Is Involved in the Copper Transfer Mechanism to the Atox1 Metallochaperone. J. Phys. Chem. B 2016, 120, 12334–12345. [Google Scholar] [CrossRef]
- Perkal, O.; Qasem, Z.; Turgeman, M.; Schwartz, R.; Gevorkyan-Airapetov, L.; Pavlin, M.; Magistrato, A.; Major, D.T.; Ruthstein, S. Cu(I) Controls Conformational States in Human Atox1 Metallochaperone: An EPR and Multiscale Simulation Study. J. Phys. Chem. B 2020, 124, 4399–4411. [Google Scholar] [CrossRef]
- Zaccak, M.; Qasem, Z.; Gevorkyan-Airapetov, L.; Ruthstein, S. An EPR Study on the Interaction between the Cu(I) Metal Binding Domains of ATP7B and the Atox1 Metallochaperone. Int. J. Mol. Sci. 2020, 21, 5536. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.; Walke, G.; Schwerdtfeger, F.; Gevorkyan Airapetov, L.; Ruthstein, S. Exploring the role of the various methionine residues in the Escherichia coli CusB adapter protein. PLoS ONE 2019, 14, e0219337. [Google Scholar] [CrossRef]
- Von Hagens, T.; Polyhach, Y.; Sajid, M.; Godt, A.; Jeschke, G. Suppression of Ghost Distances in Multi-Spin Double Electron-Electron Resonance. Phys. Chem. Chem. Phys. 2013, 15, 5854–5866. [Google Scholar] [CrossRef]
- Ji, M.; Tan, L.; Jen-Jacobson, L.; Saxena, S. Insights into copper coordination in the EcoRI-DNA complex by ESR spectroscopy. Mol. Phys. 2014, 112, 3173–3182. [Google Scholar] [CrossRef]
- Hunsicker-Wang, L.; Vogt, M.; Derose, V.J. EPR methods to study specific metal-ion binding sites in RNA. Methods Enzym. 2009, 468, 335–367. [Google Scholar] [CrossRef]
- Gamble Jarvi, A.; Bogetti, X.; Singewald, K.; Ghosh, S.; Saxena, S. Going the dHis-tance: Site-Directed Cu(2+) Labeling of Proteins and Nucleic Acids. Acc. Chem. Res. 2021, 54, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Wort, J.L.; Ackermann, K.; Giannoulis, A.; Stewart, A.J.; Norman, D.G.; Bode, B.E. Sub-Micromolar Pulse Dipolar EPR Spectroscopy Reveals Increasing Cu(II) -labelling of Double-Histidine Motifs with Lower Temperature. Angew. Chem. Int. Ed. Engl. 2019, 58, 11681–11685. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.; Lepechkin-Zilbermintz, V.; Kahremany, S.; Schwerdtfeger, F.; Gevorkyan-Airapetov, L.; Munder, A.; Viskind, O.; Gruzman, A.; Ruthstein, S. Inhibiting the copper efflux system in microbes as a novel approach for developing antibiotics. PLoS ONE 2019, 14, e0227070. [Google Scholar] [CrossRef] [PubMed]
- Walke, G.R.; Meron, S.; Shenberger, Y.; Gevorkyan-Airapetov, L.; Ruthstein, S. Cellular Uptake of the ATSM-Cu(II) Complex under Hypoxic Conditions. ChemistryOpen 2021, 10, 486–492. [Google Scholar] [CrossRef]
- Walke, G.R.; Ruthstein, S. Does the ATSM-Cu(II) Biomarker Integrate into the Human Cellular Copper Cycle? ACS Omega 2019, 4, 12278–12285. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meron, S.; Shenberger, Y.; Ruthstein, S. The Advantages of EPR Spectroscopy in Exploring Diamagnetic Metal Ion Binding and Transfer Mechanisms in Biological Systems. Magnetochemistry 2022, 8, 3. https://doi.org/10.3390/magnetochemistry8010003
Meron S, Shenberger Y, Ruthstein S. The Advantages of EPR Spectroscopy in Exploring Diamagnetic Metal Ion Binding and Transfer Mechanisms in Biological Systems. Magnetochemistry. 2022; 8(1):3. https://doi.org/10.3390/magnetochemistry8010003
Chicago/Turabian StyleMeron, Shelly, Yulia Shenberger, and Sharon Ruthstein. 2022. "The Advantages of EPR Spectroscopy in Exploring Diamagnetic Metal Ion Binding and Transfer Mechanisms in Biological Systems" Magnetochemistry 8, no. 1: 3. https://doi.org/10.3390/magnetochemistry8010003
APA StyleMeron, S., Shenberger, Y., & Ruthstein, S. (2022). The Advantages of EPR Spectroscopy in Exploring Diamagnetic Metal Ion Binding and Transfer Mechanisms in Biological Systems. Magnetochemistry, 8(1), 3. https://doi.org/10.3390/magnetochemistry8010003





