Abstract
Petunia (Petunia × hybrida Hort. Vilm.-Andr.) is a well-suited plant for sustainable landscape issues in borderline areas with irrigation with saline water. Salicylic acid (SA) as a modulator performs an imperative function in modulating plant salt tolerance. However, there are a few reports on the effect of SA on petunia plants irrigated with saline water. During the 2022/2023 season, a factorial pot experiment in a randomized complete block design was carried out in Riyadh, Saudi Arabia, to assess the effect of SA concentration (0, 500, 1000, 2000 mgL−1) on petunia plant growth, flowering, ion content, chlorophyll level, and proline concentration under irrigation with salty water (230, 1500, 3000 mgL−1). Saline water up to 3000 mgL−1 dramatically reduced plant growth, chlorophyll, ions, and flowering attributes, while the contrary was observed in proline and sodium concentrations as compared to the control treatments (irrigation with tap water). Foliar spraying with 1000 mgL−1 SA considerably boosted plant growth and flowering as well as chlorophyll, proline, and ion content compared to untreated plants under such salinity levels. Alternatively, the application of 1000 mgL−1 to normal or salinized water significantly decreased the Na content in non-treated plants under such a salinity level. Accordingly, using 1000 mgL−1 of SA under salt stress conditions could be a useful technique to lessen the mutilation induced by the use of salinized water in the era of climate change.
1. Introduction
Water availability represents a serious problem in most arid and semi-arid climates worldwide [1]. Policymakers encourage the usage of marginal water resources for irrigation in dry regions like the Kingdom of Saudi Arabia (KSA) in order to avoid freshwater resource scarcity [2,3]. However, excessive dissolved salt concentrations in marginal water are often harmful to plant growth and development [4,5].
Salinity induces a series of morpho-physiological responses and molecular modifications that prevent plant development and flowering behavior [5,6,7,8,9]. Salinity injury in plants is primarily caused by osmotic stress, cytotoxicity, and nutritional conflict [5,6,7,8,9]. Salinity reduces a plant’s ability to absorb enough water to meet its evaporative demands, causing growth and flowering decline and lessened metabolic pathways [8,10,11,12]. Excessive accumulation of sodium (Na) and chloride (Cl) deactivates numerous metabolic pathways by evoking the over-production of reactive oxygen species (ROS) [3,7,8,13]. In addition, the accumulation of Na and Cl induces marginal chlorosis and leaf necrosis by impeding crucial ion uptake and transport activities [14,15]. The impact of salt can be seen in a variety of biochemical activities, including photosynthesis, nucleic acid production, enzyme activation, and ion transport [16]. Additionally, many development and blooming characteristics are affected by salt [17,18]. In this concern, Fornes et al. [19] and Elhindi et al. [18] found that irrigation with saline water significantly lessens flowering aptitude and flower quality as well as reducing the flowering date.
To mitigate the dramatic impacts of salinity on plant establishment, several control strategies have been developed and implemented, including the use of various natural or synthetic compounds such as ions, biostimulants, and plant growth substances [7,10,18,20,21]. Using phytohormones for enhancing growth and development under abiotic stress is highly recommended and provides new ideas for irrigation with saline water [11,22].
Salicylic acid (SA) as an endogenous plant phytohormone/plant growth substance, plays an important role in several plant biochemical pathways under normal or stressful conditions and modulates a plant’s ability to withstand stress [11,23,24,25,26]. The influence of SA on plant development differs based on its concentration, the plant species, and ecological circumstances [11,22,27]. Exogenous SA regulates a variety of plant metabolic pathways, i.e., germination, nutrient uptake, photosynthetic rates, chlorophyll assimilation, and organic solute accumulation [8,11,12,24,25,26]. Several studies show that SA boosts plant growth and induces stress tolerance by enhancing antioxidant capacity and reducing ROS accumulation [8,28,29]. Flowering is another important parameter that is directly regulated by SA in several plants [30,31].
The use of ornamental and flowering plants (OFP) in gardens, roadways, landscaping, and cut flowers is critical in the horticultural industry [32,33]. The annual ornamental petunia (Petunia × hybrida Hort. Vilm. -Andr.) plant has a lengthy flowering time, it easily grows, and it comes in a variety of forms and sizes [34]. It is widely planted in sustainable urban landscapes in marginal sites because of its high salt tolerance. It is also primarily utilized as an annual bedding and container plant in dry and semi-arid climates [35]. However, there have been few studies on the effect of SA on Petunia plants under salinity, and whether or not SA can improve Petunia salt tolerance is still unknown.
Accordingly, the aim of this investigation was to assess the impact of SA on petunia plant growth, flowering, ion content, chlorophyll level, and proline concentration in irrigation with saline water. We hypothesized that using SA would mitigate the negative effects of salty water on Petunia plant growth and flowering. Furthermore, the occasion to examine the potential usage of saline water in Petunia plants is anticipated to open novel possibilities for the expansion of OFP business in KSA.
2. Materials and Methods
2.1. Experimental Setup
From 15 November 2022 to 1 May 2023, a completely randomized block pot design was carried out at King Saud University, Riyadh, KSA (longitude: 46°43′ E, latitude: 24°55′ N, and above mean sea level: 625 m), with a temperature of 24/20 °C, relative humidity of 60–70%, and photoperiod of 11 h, to investigate the impact of saline water (230 ‘tap water control’, 1500, and 3000 mgL−1 sodium chloride ‘NaCl’), spraying of SA concentration (0, 500, 1000, and 2000 mgL−1), and their combinations on Petunia plant growth, flowering, chlorophyll index, proline concentration, and nutrient content.
Petunia seeds (Petunia hybrida) cv “Madness White” were seeded into peat moss-filled plug trays on 15 November 2022. At emergence, uniform seedlings (5–8 leaves) were transplanted into plastic pots (30 cm in diameter) containing 5.5 kg of experimental garden soil, in greenhouse environments with usual day and night temperatures of 30 °C and 19 °C, and natural light (800 mmol m−2 s−1) with an 11/13 h day/night photoperiod. The garden soil characteristics are indicated in Table 1

Table 1.
Physical and chemical analysis of garden soil.
The plants were irrigated with tap water for 15 days until plant establishment, and then with saline water (NaCl). The plants were watered three times a week−1, utilizing tap water (control, 230 mgL−1), 1500, or 3000 mgL−1 NaCl, to keep the soil moisture at 80% of field capacity. The plants received SA with Tween 20 (0.05%) as a surfactant via monthly spraying from 15 January to 15 April. All plants received NPK chemical fertilization (Sangral NPK 20:20:20, SQM Europe, NV, Antwerpen, Belgium) at the rate of 2 g pot−1. Fertilization was added four times every 30 days through the growing period (from 15 January to 15 April).
2.2. Data Recorded
On 1 May, nine plants from each treatment were dispensed for deliberation of morphological, flowering, and biochemical attributes.
2.3. Vegetative and Flowering Attributes
Stem length (SL, cm), stem diameter (SD, mm), and leaf number plant−1 (LNP), as well as shoot fresh weights (SFW, g) and dry weights (SDW, g) were determined. Also, leaf area plant−1 (LA, cm2) was assessed with leaf area meter LI-3000 COR (Walz Co., Temecula, CA, USA). The flower number plant−1 (FN) and flower diameter (FD, mm), in addition to flower fresh weights (FFW, g) and dry weights (FDW, g) were correspondingly documented.
2.4. Physiological and Chemical Analysis
The chlorophyll content was quantified as SPAD units using a Minolta (chlorophyll meter) SPAD 502 Plus chlorophyll meter (Konica Minolta, Tokyo, Japan). According to Yadava [36], 9 measurements were taken from the 5th upper leaf and averaged to yield a single record per leaf.
Proline level was estimated in fresh leaf tissues, as designated by Bates et al. [37], with a ninhydrin reagent. In a boiling water bath, the sulfosalicylic acid extract was mixed with acid ninhydrin for one hour. Toluene was used to capture the chromophore, and the optical density was read at 520 nm. Proline level (g g−1 fresh weight ‘FW’) was calculated using the proline standard curve.
Ion content (nitrogen ‘N’, phosphorous ‘P’, potassium ‘K’, calcium ‘Ca’, magnesium ‘Mg’, iron ‘Fe’, zinc ‘Zn’, copper ‘Cu’, and manganese ‘Mn’) was determined within acid extraction of shoot dry weight following the Association of Official Analytical Chemists (A.O.A.C.) protocol [38]. The micro-Kjeldahl method was used to estimate N content. The procedure of Cooper [39] was followed for the assessment of P. Alternatively, K, Ca, Mg, Fe, Zn, Cu, and Mn were appraised using iCAPTM 7000 Plus Series ICP-OES (Thermo ScientificTM, Waltham, MA, USA) following Bettinelli et al. [40] protocols.
2.5. Statistical Analysis
The data homogeneity was realized earlier before the analysis of variance (ANOVA). The Costatc Software, version number 6.303 statistical package (CoHort software, 2006; Birmingham, UK) was used for data analysis. Differences between treatments were distinguished using a two-way ANOVA to assess the effect of SA in alleviating salinity damage. When significant (p ≤ 0.05), a comparison of means (Tukey test) was instigated. The existing data are mean with standard error (SE).
3. Results
3.1. Growth Attributes
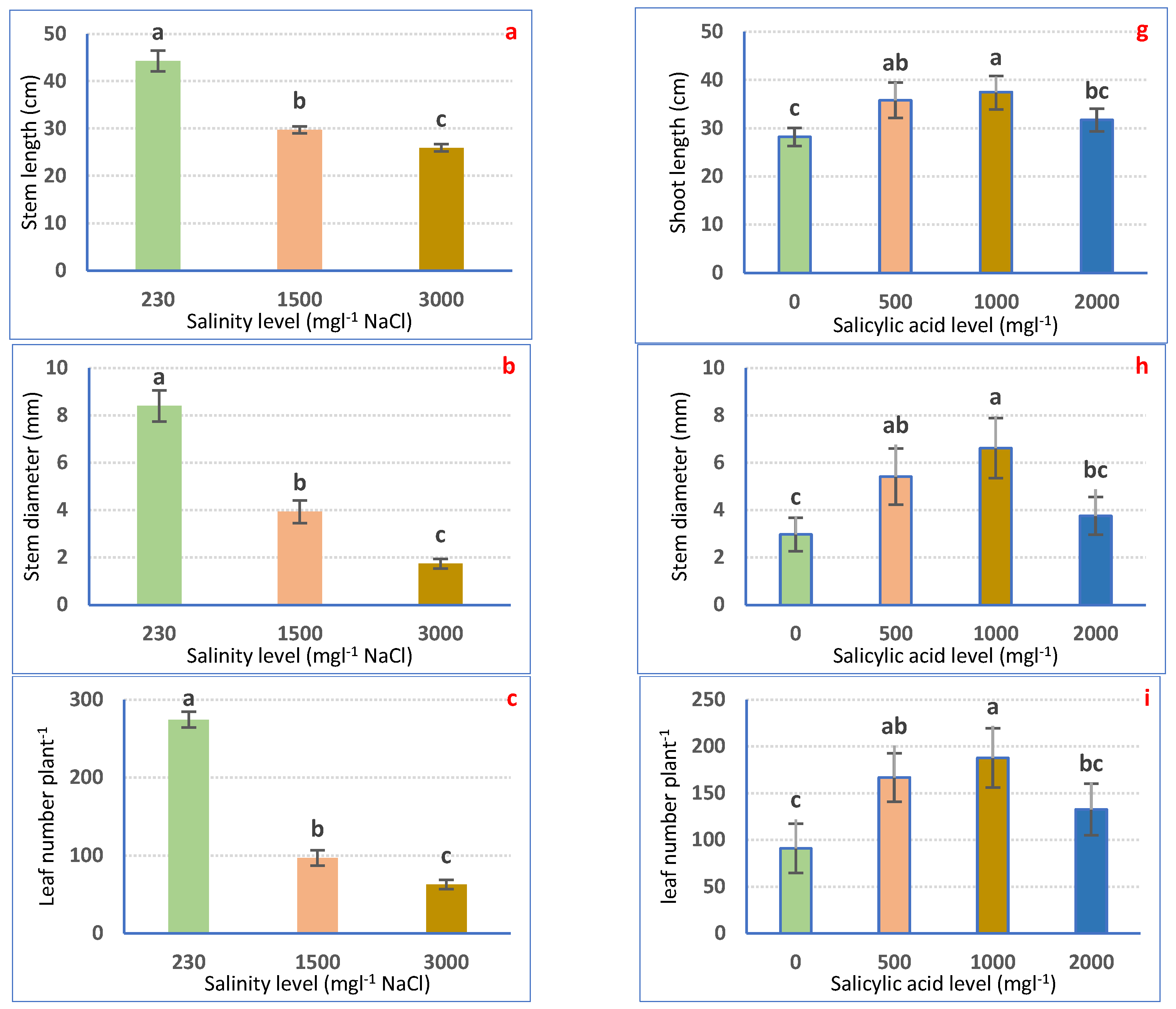
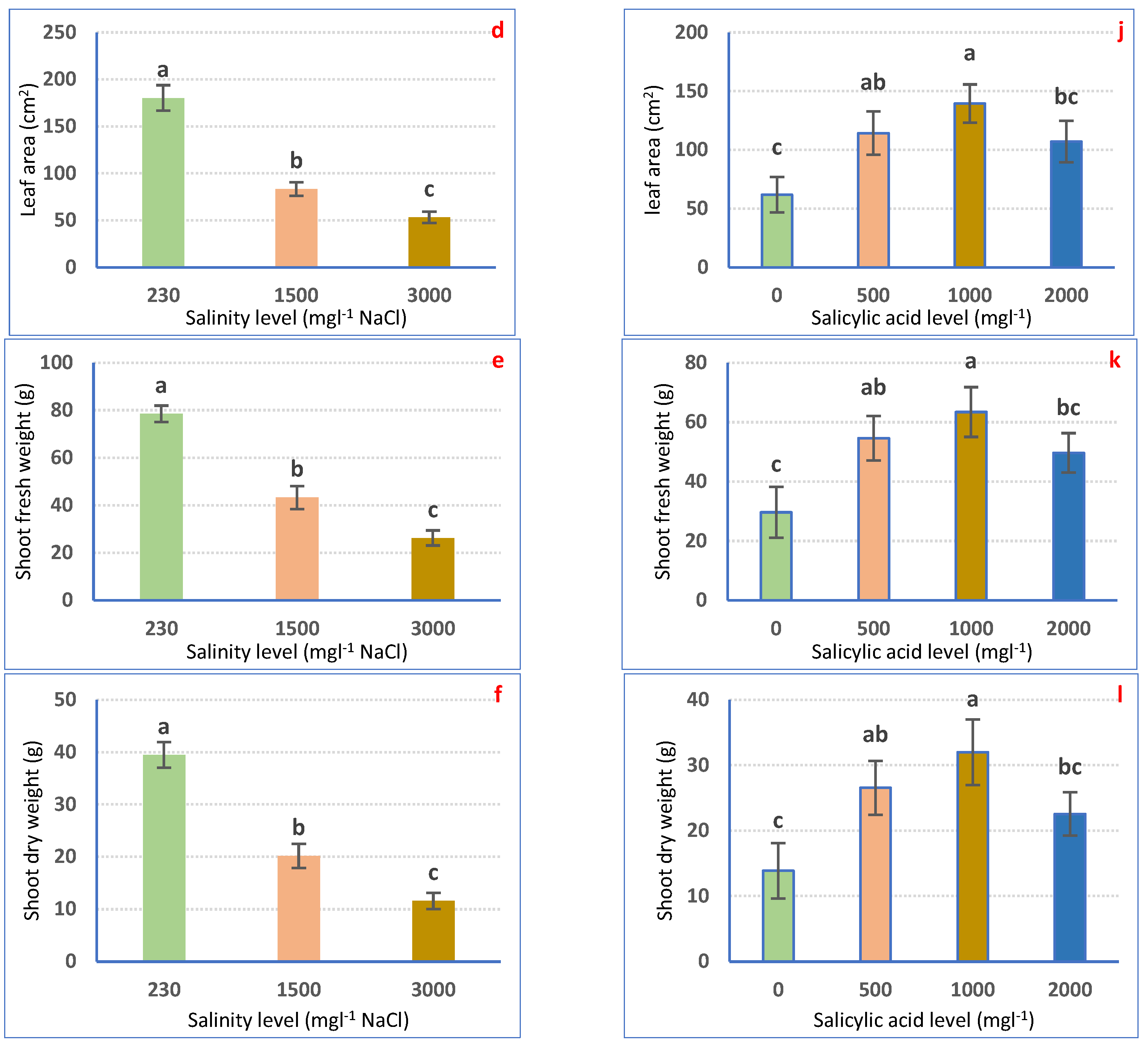
Irrigation with saline water significantly (p ≤ 0.05) decreased petunia plant growth in a dose-dependent means (Figure 1a–f). Nonetheless, all examined traits considerably declined to a superior range for 3000 mgL−1 to the 1500 mgL−1 NaCl treatment as compared with irrigation with tap water. Irrigation with 1500 mgL−1 NaCl decreased SL (32.39%), SD (53.15%), LNP (64.80%), LA (53.81%), SFW (44.93%), and SDW (48.96%). Using 3000 mgL−1 NaCl significantly lessened SL (41.45%), SD (79.31%), LNP (77.14%), LA (70.40%), SFW (66.57%), and SDW (70.68%) relative to the non-salinized control plants (Figure 1a–f).
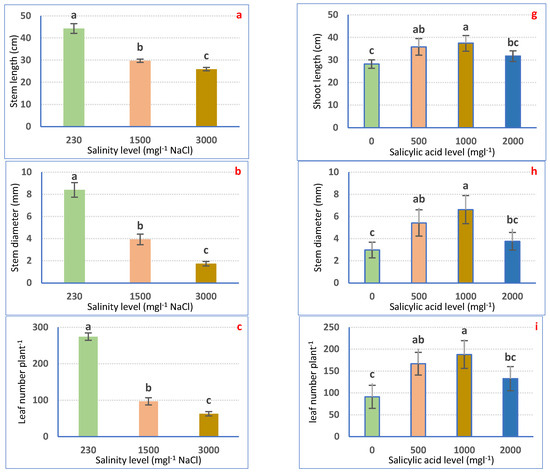
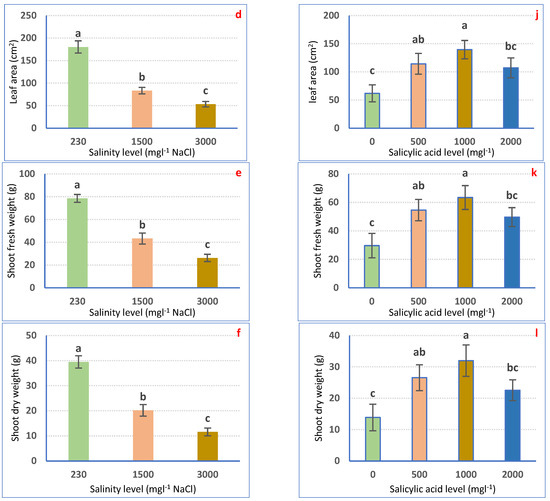
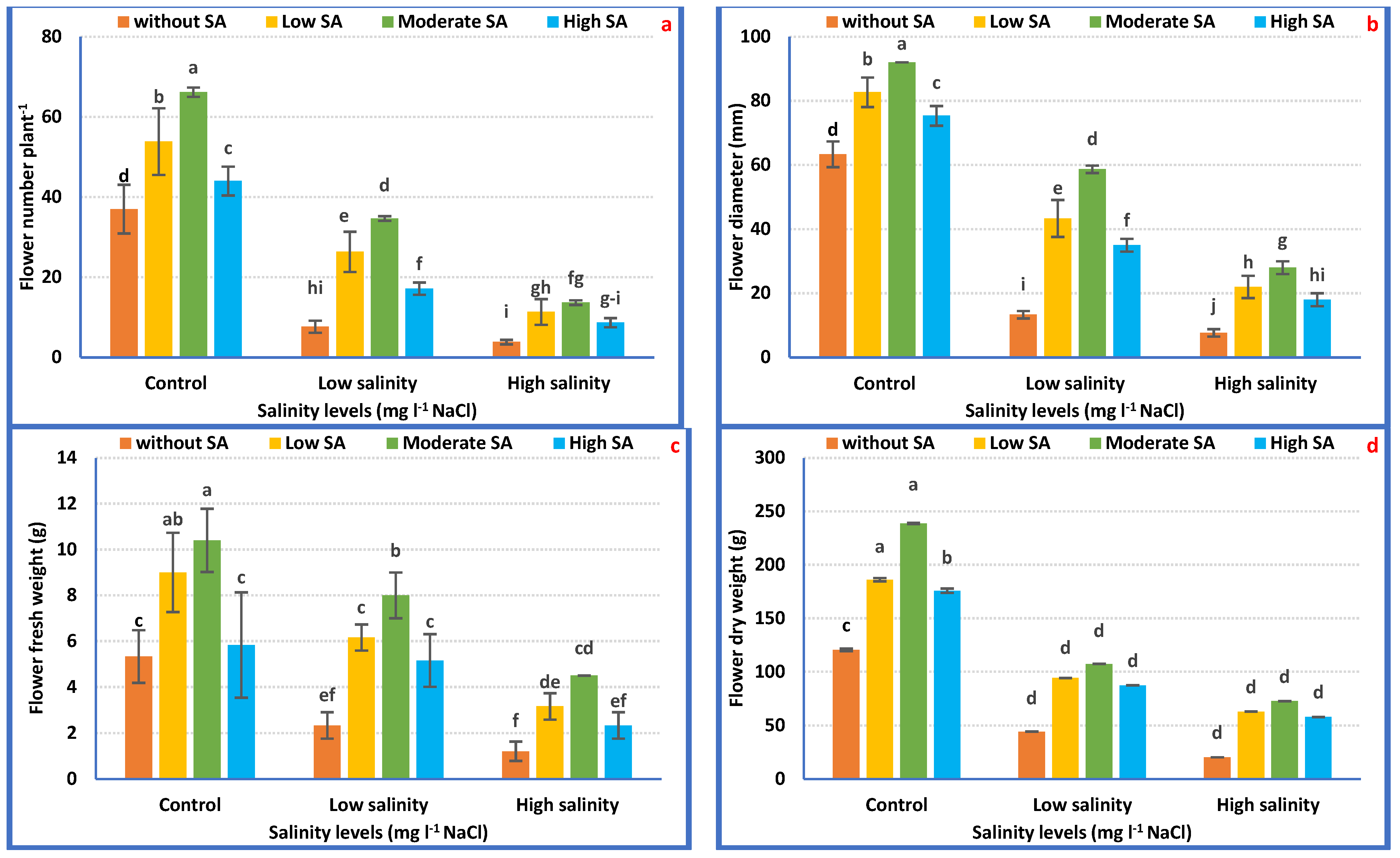
Figure 1.
Vegetative growth attributes of Petunia plants as affected by irrigation with saline water (a–f) and salicylic acid (g–l). Mean values ± standard error in a column for each characteristic with dissimilar letters are significantly different (Tukey test at p ≤ 0.05).
SA spraying expressively (p ≤ 0.05) boosted the growth features of petunia plants as compared with non-treated plants. The highest values of growth attributes were achieved after 1000 mgL−1 SA foliar spraying, followed by 500 mgL−1 SA and 2000 mgL−1 SA. SA at 1000 mgL−1 expressively increased SL, SD, LNP, LA, SFW, and SDW by 33.04, 121.70, 106.71, 126.1, 113.89, and 130.54%, correspondingly, relative to 0 mgL−1 SA-sprayed plants (Figure 1g–l).
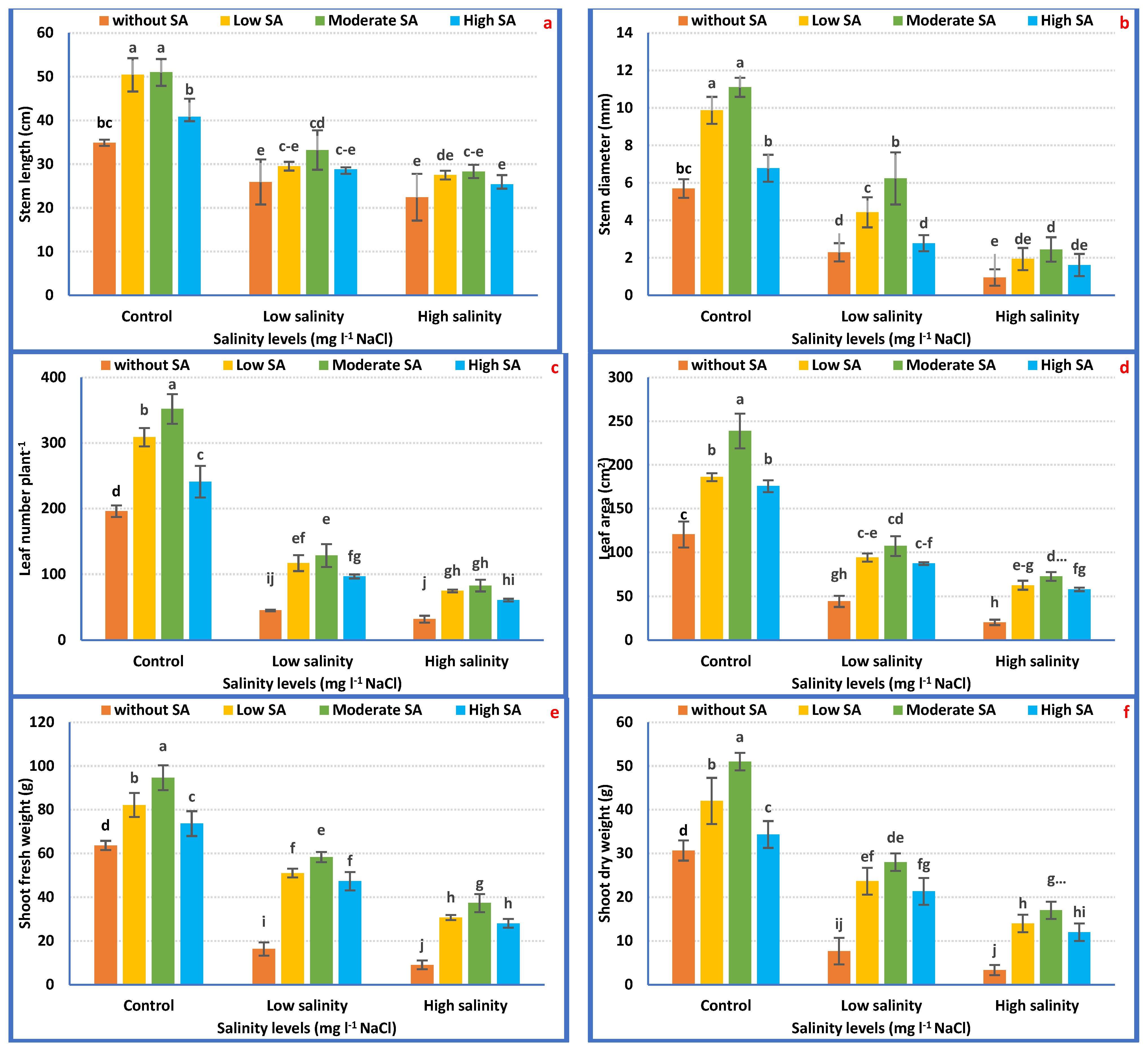
The application of 1000 mgL−1 SA had the capacity to reverse the harmful effect of salinity on petunia plants, chiefly those grown under 3000 mgL−1 NaCl salt stress. Accordingly, it is feasibly declared that SA application might reduce the negative impressions of salt stress (1500 and 3000 mgL−1 NaCl), which enhanced all the growth attributes of Petunia plants in saline conditions. SA at 1000 mgL−1 achieved the maximum severe salt tolerance (3000 mgL−1) and improved plant growth features (Figure 2a–f).
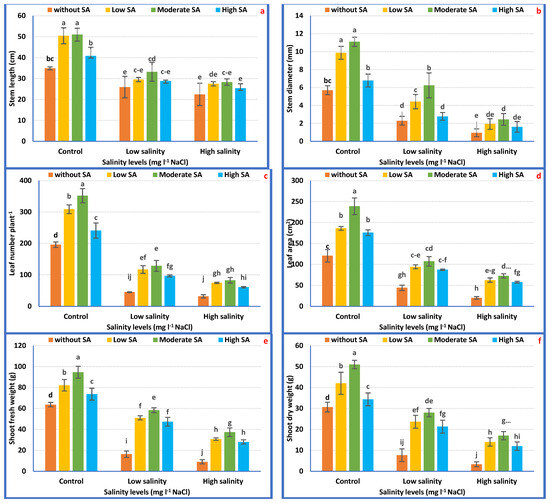
Figure 2.
Vegetative growth attributes of Petunia plants as affected by the interaction between irrigation with saline water and salicylic acid (a–f). Mean values ± standard error in a column for each characteristic with dissimilar letters are significantly different (Tukey test at p ≤ 0.05).
3.2. Flowering Attributes
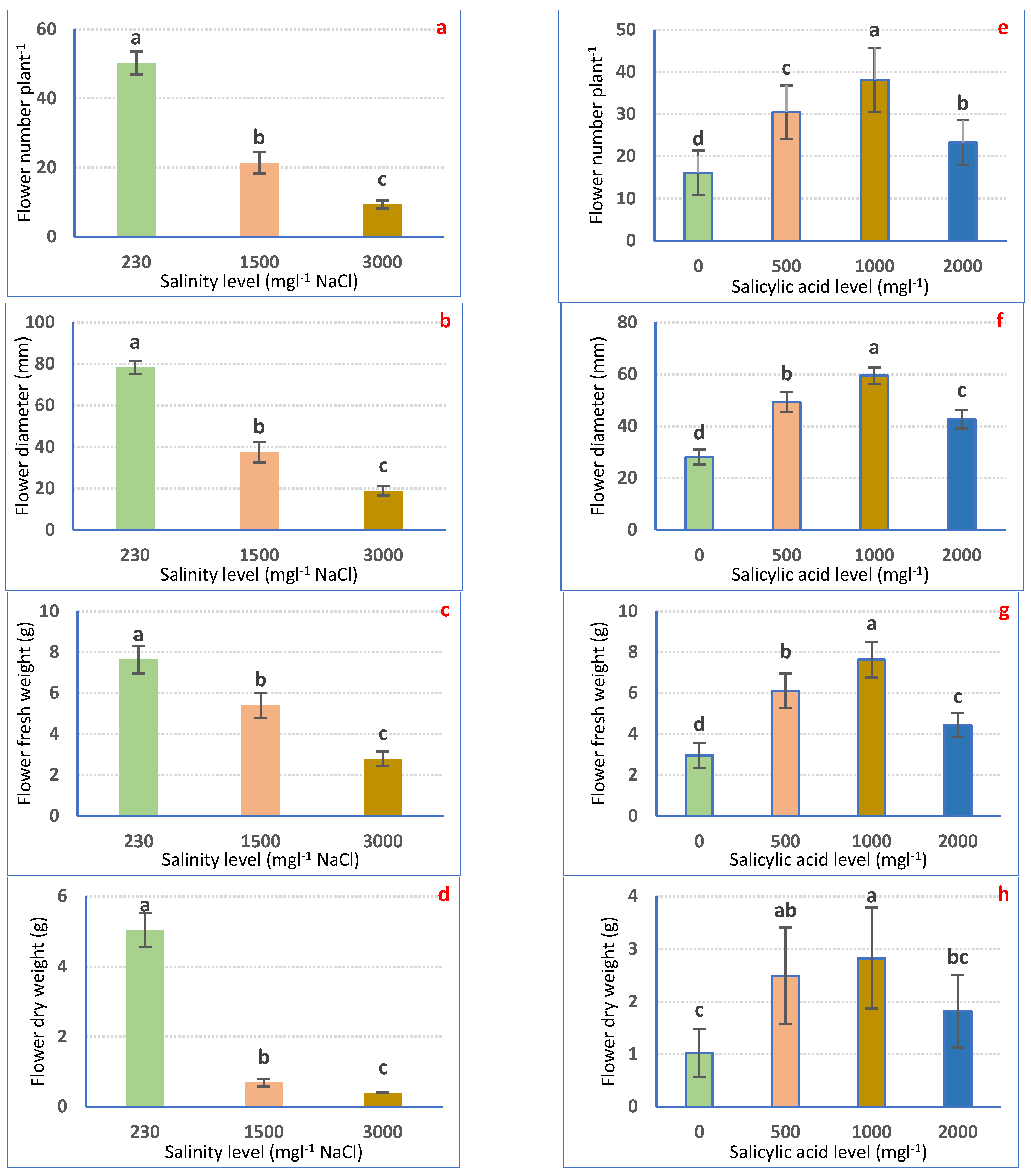
Salt stress considerably (p ≤ 0.05) declined flowering features relative to irrigation with tap water. The lowest records were attained in plants irrigated with 3000 mgL−1 NaCl which reduced FN, FD, FFW, and FDW by 81.34, 75.85, 63.35, and 92.14%, correspondingly, compared with the control plants (Figure 3a–d).
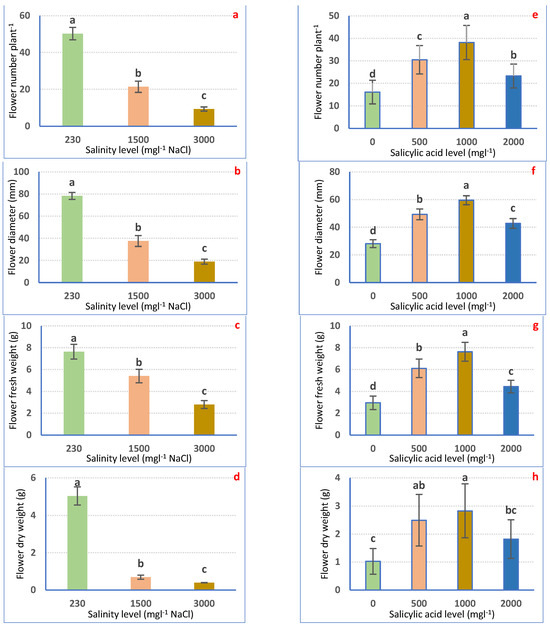
Figure 3.
Flowering attributes of Petunia plants as affected by irrigation with saline water (a–d) and salicylic acid (e–h). Mean values ± standard error in a column for each characteristic with dissimilar letters are significantly different (Tukey test at p ≤ 0.05).
Instead, SA treatment increased flowering features. Foliar application with SA at 1000 mgL−1 provides the highest FN (136.13%), FD (111.84%), FFW (157.78%), and FDW (175.46%) weights as compared with the non-treated plants (Figure 3e–h).
As for the interaction effects, the maximum FN, FD, FFW, and FDW were achieved with irrigation with tap water plus 1000 mgL−1 SA relative to the rest of the treatments. Under 3000 mgL−1 NaCl, the application of 1000 mgL−1 SA improved FN, FD, FFW, and FDW by 256.37, 265.24, 270.06, and 616.25%, correspondingly, compared with plants irrigated with 3000 mgL−1 NaCl alone (Figure 4a–d).
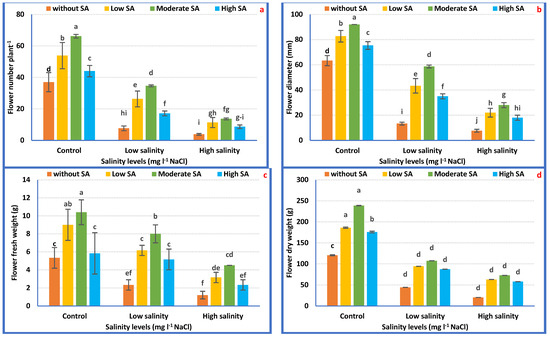
Figure 4.
Flowering attributes of Petunia plants as affected by the interaction between irrigation with saline water and salicylic acid (a–d). Mean values ± standard error in a column for each characteristic with dissimilar letters are significantly different (Tukey test at p ≤ 0.05).
3.3. Chlorophyll
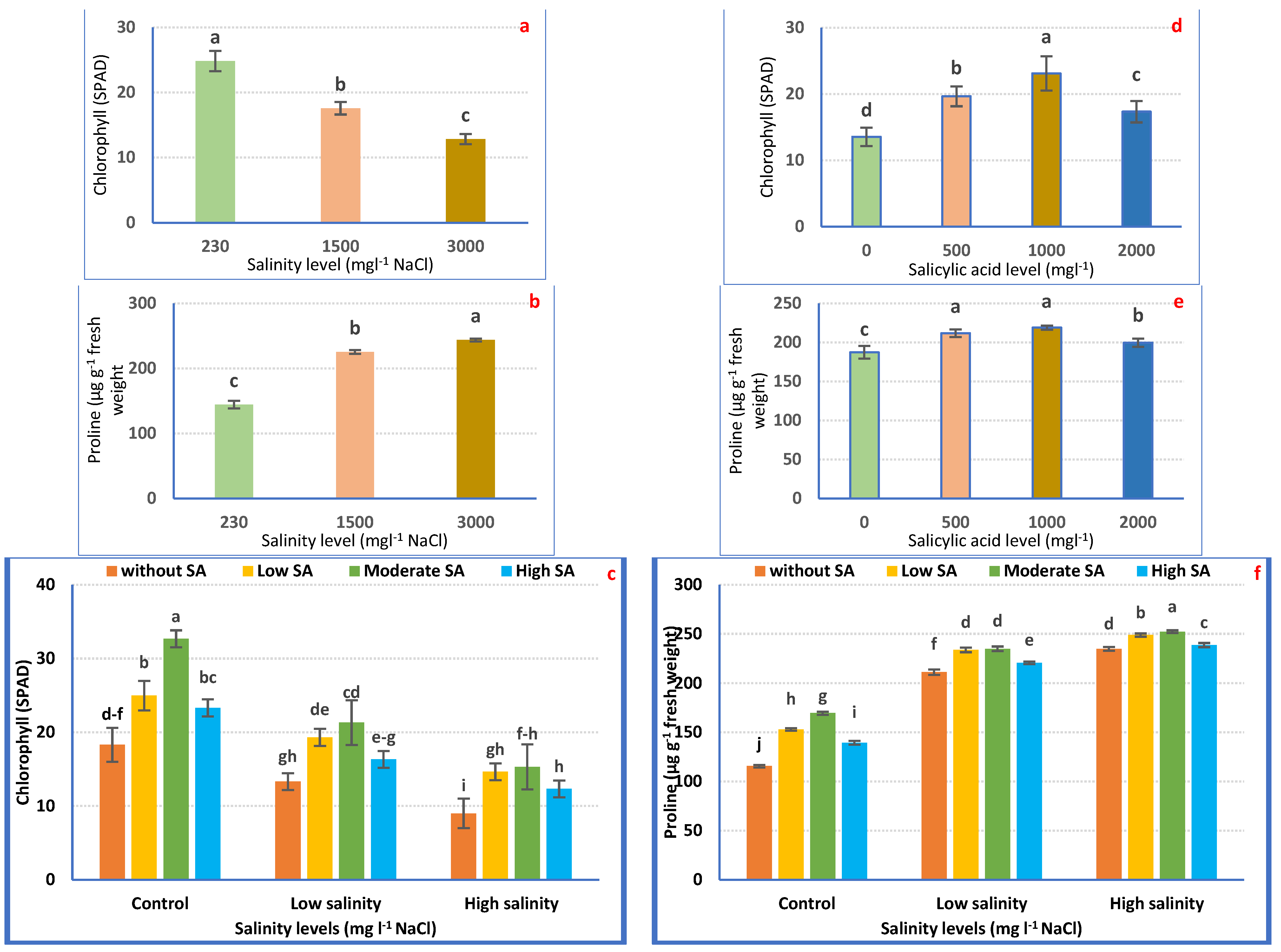
Chlorophyll levels were significantly (p ≤ 0.05) affected by irrigation with saline water and/or SA foliar application (Figure 5a). The supreme chlorophyll level (SPAD) was attained from petunia plants irrigated with tap water. Chlorophyll concentration in petunia leaves significantly decreased with increasing salinity stress (Figure 5a). The lowest values (12.83 SPAD) were achieved in plants irrigated with 3000 mgL−1 NaCl compared with plants irrigated with tap water.
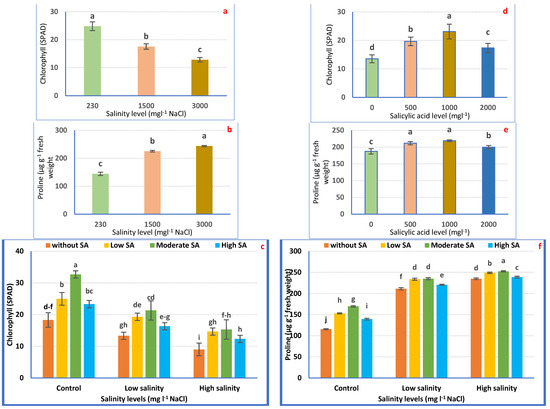
Figure 5.
Chlorophyll (SPAD) and proline (µg/g FW) concentration of Petunia plants as affected by irrigation with saline water (a,b) and salicylic acid (d,e) and their combination (c,f). Mean values ± standard error in a column for each characteristic with dissimilar letters are significantly different (Tukey test at p ≤ 0.05).
The same table proves that SA spraying hastened the production of chlorophyll relative to non-treated ones (0 mgL−1 SA). The maximum chlorophyll index was noted in plants sprayed with 1000 mgL−1, followed by 500 mgL−1 and 2000 mgL−1, compared to non-treated plants (Figure 5d).
Regarding the combination treatments, it was noted that SA spraying, notably 1000 mgL−1, causes hyper-accumulation of chlorophyll compared with non-treated plants in such salinity levels. Within severe salinity, the supplementation of SA attenuated the destructive impacts of salinity on chlorophyll content (Figure 5c).
3.4. Proline
Petunia plants irrigated with saline water evoked the hyperaccumulation of proline (Figure 5b). The greatest concentration (68.65%) was recorded in severe saline water relative to irrigation with tap water (Figure 5b).
Spraying petunia plants with SA significantly increased proline concentration relative to non-treated plants. The highest concentration was observed in plants treated with 1000 mgL−1 (Figure 5e).
The same table proves that the interaction treatments evoked an over-accumulation of proline, compared with untreated plants. The ultimate concentration (106.29%) was obtained due to irrigation with 3000 mgL−1 with a foliar application of 1000 mgL−1 SA (Figure 5f).
3.5. Mineral Composition
Nutrient contents in petunia plant shoots were suggestively (p ≤ 0.05) influenced by salinity, SA, and their interactions (Table 2 and Table 3). Irrigation with saline water gradually reduced all examined nutrients excluding Na, which is elevated with salinity levels. The lowermost N (54.11%), P (48.35%), K (70.63%), Ca (45.12%), Mg (85.06%), Mn (39.33%), Cu (50.32%), Fe (72.01%), and Zn (52.69%) were documented as a result of irrigation with 3000 mgL−1 NaCl water compared to plants irrigated with tap water. Alternatively, 3000 mgL−1 NaCl expressively (p ≤ 0.05) raised the Na content by 152.96% relative to non-salinized treatment.

Table 2.
Macronutrient concentration (mg/total dry weight) of Petunia plants as affected by irrigation with saline water and salicylic acid and their combination.

Table 3.
Micro-nutrient concentration (mg/total dry weight) of Petunia plants as affected by irrigation with saline water and salicylic acid and their combination.
Alternatively, SA levels in foliar application raised all the nutrient contents studied, but decreased Na concentration in petunia shoots relative to non-treated plants. The supreme effective SA level for boosting N, P, K, Ca, Mg, Mn, Cu, Fe, and Zn, as well as reducing Na, was 1000 mgL−1, as compared with unsprayed plants (Table 2 and Table 3).
SA supplementation in salt-affected plants conspicuously invalidates the drastic injuries to nutrient levels. The prime effective level was 1000 mgL−1 SA, which boosted N (63.40%), P (4.43%), K (61.88%), Ca (164.63%), Mg (179.27%), Mn (83.23%), Cu (269.44%), Fe (769.23%), and Zn (81.03%), while decreasing Na (27.43%), compared to plants irrigated with 3000 mgL−1 NaCl (Table 2 and Table 3).
4. Discussion
One of the biggest issues in the agriculture sector is salt stress which reduces plant growth as indicated previously and in the present study [5,12,25,41]. Salt stress limits plant growth by interfering with various physiological processes and molecular alterations, i.e., photosynthesis, ionic imbalance, and increasing ROS buildup [7,8,11]. This decline in plant growth might also result from the blocking of vascular tissues and the disruption of water uptake, nutrient uptake, and assimilation capacity [11,42]. Additionally, salt stress defeats plant photosynthetic effectiveness, withdraws ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), and diminishes the ATP assimilation required for plant establishment [43]. Furthermore, salinity causes a significant drop in plant water status, and/or reduces the cyclin-dependent kinase (CDK) activity that reduces cell division and elongation, and ultimately reduces plant growth [44,45]. Exogenous spraying of SA is one of the successful treatments being developed by researchers to minimize the negative consequences of salt [11,12,22]. SA is a unique plant growth substance and essential antioxidant that has the capacity to moderate the drastic effects of salinity [8,26]. In this concern, Stevens et al. [46] stated a comparable increase in tomato plant growth under 200 mM NaCl irrigation via the amendment of 0.1 mM SA by the root medium. Data reported here show that SA promotes petunia plant growth in either non-saline or saline circumstances. In fact, NaCl-affected plants treated with SA exhibited plant growth relative to that of those grown without SA treatment. The exogenous application of SA under stress conditions is anticipated to regulate stomatal movement during stress, which lowers transpiration and water loss, maintains turgor, and regulates plant establishment [47]. Moreover, SA-enhanced physiological pathways, i.e., enhanced carbon assimilates, improved secondary metabolites assimilation, preserved plant water status, and improved plant growth under normal or salinized conditions [25,48]. Additionally, SA enhanced mitosis and cell extension of apical meristems [49], along with sustained RWC and photosynthetic capacity [25,50]. Finally, SA reinforced the antioxidant defense systems required to mitigate oxidative injuries [31].
As recorded in the current study, flowering features distinctly declined due to irrigation with saline water, which was established before [4,18,51]. So, plants irrigated with saline water may have diminished flowering intensity, delayed flowering, and a reduced flowering date [18,19]. Consistent with Ahmad et al. [52], irrigation with saline water hinders the vascular system and ultimately limits water uptake. The flowering reduction under salinity could result from the deterioration of plant photosynthesis and the devastation of chloroplasts [53]. Furthermore, it hampers photochemical pathways, diminishes the Calvin cycle enzyme activities [54], and modifies plant hormones [55]. As indicated earlier and in the current study, it can be said that SA application induces early flowering of several plants [30,31] due to its role in overcoming the demand for photoperiod [56]. Improving flowering attributes with SA application within non-salinized and salinized treatments could be attributable to maintaining water homeostasis and developed antioxidant activity as well as water use efficiency [31,57]. Moreover, the spraying of SA might be a novel implement for molecular biologists to explicate the genes and mechanisms that control flowering, since this process is not entirely implicit and needs more investigation [26].
Current outcomes revealed that irrigation with saline water considerably decreased the chlorophyll index relative to irrigation with tap water. A decline in chlorophyll has been stated previously in several plants [8,11,12,58]. The deterioration of chlorophyll caused by saline water might be related to boosted chlorophyllase activity [59,60], decreased assimilation of the key chlorophyll pigment complexes encoded by the cab gene family [61], and/or a drop in chlorophyll biosynthesis intermediation concentrations [62]. The reduced SPAD index under salinity was previously anticipated due to the generation of ROS, which accelerates the deterioration of the thylakoid membranes, chlorophyll devastation, and modifications in chlorophyll protein complexes, that ultimately mutilate chlorophyll [63,64]. Conversely, earlier investigations displayed a reduction in chlorophyll levels resulting from the decline in the assimilation of 5-aminolinolic acid, an essential intermediate of protochlorophyllide, which transforms into chlorophyll in light conditions [65]. Furthermore, salt stress persuades a decline in chlorophyll assimilation of intermediation and reductions in the expression of ChlD, Chl H, and Chl I-1 gene encoding subunits of Mg-chelatase [66,67]. Current results revealed that the spraying of SA boosted chlorophyll accumulation in salt-affected plants. Plants treated with SA had higher levels of chlorophyll than control or salt-affected plants [8,11,58,68,69]. Variations in chlorophyll with SA spraying are advocated to be concentration dependent and species specific [11,12]. These outcomes supported the findings of Fariduddin et al. [70], who discovered that foliar spraying of SA up to 30% mitigated the inhibition of chlorophyll production caused by 120 mM NaCl in the growing medium. SA mitigates the harmful effect of salinity on chlorophyll by decreasing ROS, elevating the antioxidant systems that accelerate cell division and elongation, and possibly inhibiting chlorophyll oxidase enzymes, subsequently preventing chlorophyll breakdown and enhancing photosynthesis [71,72]. The stimulatory effect of SA on chlorophyll content might be attributable to its role in increment-triggered chlorophyll biosynthesis [73], and conjointly due to the phenomenon of antioxidant scavenging to provide protection to chloroplast and chlorophyll against degradation [74].
Recent outcomes showed that the application of saline water with or without SA supplementation considerably boosted proline accretion in petunia plans. Enhancement in the accumulation of proline in saline- and/or SA-treated plants [8,11,58,75] indicates the role of SA in synthesizing proline to maintain a favorable solute potential. Proline is well known for its ability to maintain cell turgor and stabilize cell membranes by reducing membrane leakage [76]. Proline is known to work as an energy sink, to regulate redox potential and mitigate salt stress, and act as an antioxidant to quench singlet oxygen species by decreasing oxygenase and carboxylase activities of Rubisco and protecting plants [77,78]. Proline accumulation under the experimental condition results from changes in the activities of proline-synthesizing and -degrading enzymes and the up- and down-regulation of genes responsible for proline biosynthesis [77]. Recently, proline metabolism under SA application has received less attention and needs further study. The increment in proline under SA may be due to rising nitrogen in the plant which is taken into account as the main ingredient of proline [69]. Besides the upregulation of genes involving proline synthesis, Nazar et al. [79] showed that increases in proline could be observed in mustard under SA treatment through an increase in γ-glutamyl kinase and a decrease in proline oxidase activity.
The results of the current research show that raised salinity up to 3000 mgL−1 reduced ion content. Salinity typically induces ionic imbalance through drops in N, P, K, Mg, Ca, Mn, Cu, and Zn, accompanied by the extra accumulation of Na, which was established formerly [3,11,18,41]. A reduction in ion uptake and/or transport in addition to ionic poisonousness may be to blame for the dramatic effects of salinity on plant nutritional status [58,80]. Extreme levels of saline ions in plant tissues typically inhibit N uptake, resulting in a decrease in N concentration [11,81], as well as ion uptake, such as K and Ca [11,58,82]. Within salinity, the decline of N or P could be caused by the antagonism between either chloride and nitrate [83] or phosphate [84], correspondingly. The inhibition of K uptake is primarily caused by the physical and chemical similarities between K and Na that encouraged the competition on main binding sites [85]. Furthermore, there is an antagonism between either Ca and Mg with Na which disturbs membrane functions and induces a deterioration of membrane integrity and selectivity [86]. Plant ion uptake typically degenerated within saline environments owing to a substantial drop in transpiration rates as well as diminished membrane permeability and decreased root-absorbing capacity [87]. The decline of root development under salinity might be one of the reasons behind the decrease in plants’ ion concentration [88]. Numerous crucial physiological processes in salt-affected plants are influenced by SA, including an increase in nutrient absorption and a decrease in Na concentrations [58,89]. Similarly, Roshdy et al. [90] have described that the application of SA up to 90 ppm lessens the deleterious impact of salinity on N, P, and K content and declines the Na concentration of strawberry leaves under 40 mM NaCl. It was stated that the exogenous application of SA at 0, 1.0, 1.5, and 2.0 mM declined the Na uptake and was effective in improving ion uptake in salt-affected cotton seedlings [58,91]. Likewise, SA at 1.0 mmol/l with NaCl expressively enhanced essential ion buildup and reduced Na and Cl content relative to NaCl treatment only [92]. SA is able to modify essential ion uptake, thus improving ion concentration under normal or stress conditions [11,58,93].
5. Conclusions
SA might be taken into consideration as a prospective growth substance to enhance the growth of petunia plants under salinity conditions. The current findings displayed that elevated salinity decreased petunia plant development and flowering characteristics, which was partially restored with foliar SA administration. Additionally, data showed that a threshold rate of 1000 mgL−1 SA was necessary to reduce salt stress by raising ion homeostasis, increasing the concentration of chlorophyll index and decreasing sodium. However, additional investigation is required to entirely recognize the best concentrations and conditions for SA usage to attain supreme benefits under saline water application.
Author Contributions
Conceptualization, K.M.E., F.A.A. and M.A.A.-Y.; methodology, K.M.E. and M.A.A.-Y.; software, K.M.E.; validation, K.M.E., F.A.A. and M.A.A.-Y.; formal analysis, K.M.E., F.A.A. and M.A.A.-Y.; investigation, K.M.E. and M.A.A.-Y.; resources, K.M.E., F.A.A. and M.A.A.-Y.; data curation, K.M.E.; writing—original draft preparation, K.M.E., F.A.A. and M.A.A.-Y.; writing—review and editing, K.M.E. and F.A.A.; visualization, K.M.E.; supervision, F.A.A.; project administration, F.A.A.; funding acquisition, K.M.E., F.A.A. and M.A.A.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Researchers Supporting Project number (RSPD2023R952) King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to The Researchers Supporting Project number (RSPD2023R952) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernández García, I.; Lecina, S.; Ruiz-Sánchez, M.C.; Vera, J.; Conejero, W.; Conesa, M.R.; Domínguez, A.; Pardo, J.J.; Léllis, B.C.; Montesinos, P. Trends and challenges in irrigation scheduling in the semi-arid area of Spain. Water 2020, 12, 785. [Google Scholar] [CrossRef]
- Murtaza, G.; Zia-ur-Rehman, M.; Rashid, I.; Qadir, M. Use of Poor-Quality Water for Agricultural Production. In Research Developments in Saline Agriculture; Dagar, J.C., Yadav, R.K., Sharma, P.C., Eds.; Springer: Singapore, 2019; pp. 769–783. ISBN 9789811358326. [Google Scholar]
- Elhindi, K.M.; Al-Mana, F.A.; Algahtani, A.M.; Alotaibi, M.A. Effect of irrigation with saline magnetized water and different soil amendments on growth and flower production of Calendula officinalis L. plants. Saudi J. Bio. Sci. 2020, 27, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Anny Mrudhula, K.; Venkata Subbaiah, G.; Sambaiah, A.; Sunil Kumar, M. Performance of flower and medicinal plants with saline irrigation water through drip system. Pharma Innov. J. 2021, 10, 1514–1519. Available online: https://www.thepharmajournal.com/archives/2021/vol10issue8/PartU/10-7-439-247.pdf (accessed on 23 July 2021).
- Banon, D.; Lorente, B.; Ortunõ, M.F.; Bañon, S.; Sanchez-Blanco, M.J.; Alarcon, J. Effects of saline irrigation on the physiology and ornamental quality of Euphorbia Ascot Rainbow and its relationship with salinity indexes based on the bulk electrical conductivity. Sci. Hort. 2022, 305, 111406. [Google Scholar] [CrossRef]
- El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-kenawy, M.A.; Abd El-Khalek, A. Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L.) to brassinolide spraying. Horticulturae 2022, 8, 568. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Mahdavian, K. Effect of salicylic acid and calcium chloride on lipid peroxidation and scavenging capacity of radical of red bean (Phaseolus calcaratus L.) under salt stress. Int. J. Hortic. Sci. Technol. 2022, 9, 55–72. [Google Scholar]
- Sousa, V.F.O.; Santos, A.S.; Sales, W.S.; Silva, A.J.; Gomes, F.A.L.; Dias, T.J.; Gonçalves-Neto, A.C.; Faraz, A.; Santos, J.P.O.; Santos, G.L.; et al. Exogenous application of salicylic acid induces salinity tolerance in eggplant seedlings. Braz. J. Biol. 2024, 84, e257739. [Google Scholar] [CrossRef]
- Farouk, S.; Arafa, S.A. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span. J. Agric. Res. 2018, 16, e0802. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of salt stress and foliar application of salicylic acid on morphological, biochemical, anatomical, and productivity characteristics of cowpea (Vigna unguiculata L.) plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef]
- Ashraf, K.; Siddiqi, E.H.; Bhatti, K.H.; Iqbal, I.; Nasir, M.; Hassan, A.; Aslam, K.; Mehmood, S. Role of salicylic acid in the alleviation of salt stress on pea cultivars using growth, biochemical and physiological attributes. GU J. Phytosci. 2023, 3, 94–101. [Google Scholar] [CrossRef]
- Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of antioxidants and alkaloids in Catharanthus roseus Suspension cultures using silver nanoparticles with expression of CrMPK3 and STR genes. Plants 2021, 10, 2202. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, C.; Romano, D.; Flowers, J.T. The response of ornamental plants to saline irrigation water. In Irrigation-Water Management, Pollution and Alternative Strategies; Garcia-Garizabal, I., Abrahao, R., Eds.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0421-6. [Google Scholar]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Álvarez, S.; Gómez-Bellot, M.J.; Castillo, M.; Bañón, S.; Sánchez-Blanco, M.J. Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environ. Exp. Bot. 2012, 78, 138–145. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Almana, F.A.; Al-Yafrsi, M.A. Role of humic acid on inducing salt tolerance of Ivy Geranium (Pelargonium peltatum L.) plants. Horticulturae 2023, 9, 1012. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Carrion, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hort. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil. Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging roles of salicylic acid in plant saline stress tolerance. Int. J. Mol. Sci. 2023, 24, 3388. [Google Scholar] [CrossRef]
- Hassoon, A.S.; Abduljabbar, I.A. Review on the role of salicylic acid in plants. In Sustainable Crop Production; Hasanuzzaman, M., Ed.; Intech: London, UK, 2019; pp. 1–6. [Google Scholar]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Ali, Z.H.; Ali, F.H. Influence of salicylic acid on the physiological properties of two Petunia species. AIP Conf. Proc. 2022, 2398, 040032. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Khattab, S.; Yap, Y.K.; El Sherif, F. Salicylic acid foliar spray enhanced Silybum marianum growth and yield, as well as its chemical constituents and chalcone synthase gene activity. Horticulturae 2022, 8, 556. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M.; Arvin, M.J. Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination. Crop Pasture Sci. 2019, 70, 36–43. [Google Scholar] [CrossRef]
- Costa, A.A.; Paiva, E.P.; Torres, S.B.; Souza Neta, M.L.; Pereira, K.T.O.; Leite, M.S.; SÁ, F.V.S.; Benedito, C.P. Osmoprotection in Salvia hispanica L. seeds under water stress attenuators. Braz. J. Biol. 2022, 82, e233547. [Google Scholar] [CrossRef]
- Martin-Mex, R.; Villanueva-Couob, E.; Herrera-Campos, T.; Larque-Saavedra, A. Positive effect of salicylates on the flowering of African violet. Sci. Hort. 2005, 103, 499–502. [Google Scholar] [CrossRef]
- Safari, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2022, 19, 969–984. [Google Scholar] [CrossRef]
- Keles, B.; Ertürk, Y. Advantages of microorganism containing biological fertilizers and evaluation of their use in ornamental plants. Int. J. Agric. For. Life Sci. 2021, 5, 189–197. Available online: https://dergipark.org.tr/en/pub/ijafls/issue/66205/983444 (accessed on 28 December 2021).
- Rocha, C.S.; Rocha, D.C.; Kochi, L.Y.; Carneiro, D.N.M.; Dos Reis, M.V.; Gomes, M.P. Phytoremediation by ornamental plants: A beautiful and ecological alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef]
- Khalighi, A. Floriculture—Ornamental Plant Production of Iran; Golshan Press: Tehran, Iran, 1997. [Google Scholar]
- Baily, L.H.; Baily, E.Z. Petunia. In Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada; Macmillan Publishing: New York, NY, USA, 1976; pp. 850–851. [Google Scholar]
- Yadava, U.L. A rapid and nondestructive method to determine chlorophyll in intact leaves. HortScience 1986, 21, 1449–1450. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Cooper, T.G. The Tools of Biochemistry; John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- Bettinelli, M.; Baffi, C.; Beone, G.M.; Spezia, S. Soil and sediment analysis by spectroscopic techniques Part I: Determination of Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn. At. Spectrosc. 2000, 21, 50–59. [Google Scholar]
- Amarin, R.; Kafawin, O.; Ayad, J.; Al-Zyoud, F.; Ghidan, A. Effect of saline water irrigation and growing media on growth, physiological and mineral parameters of clove pink Dianthus caryophyllus. Jor. J. Agric. Sci. 2020, 16, 55–62. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 858. [Google Scholar]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Hasan, M.A.; Al-Taweel, S.K.; Alamrani, H.A.; Al-Naqeeb, M.A.; Al-Baldawwi, M.H.K.; Hamza, J.H. Anatomical and physiological traits of broad bean (Vicia faba L.) seedling affected by salicylic acid and salt stress. Indian. J. Agric. Res. 2018, 52, 368–373. [Google Scholar] [CrossRef]
- Habibi, G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biológica Szeged. 2012, 56, 57–63. [Google Scholar]
- Sakhabutdinova, A.R.; Fatkutdinova, D.R.; Bezrukova, M.V.; Shakirova, F.M. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 2003, 21, 314–319. [Google Scholar]
- Mathur, N.; Vyas, A. Physiological effect of some bioregulators on vegetative growth, yield and chemical constituents of pearl millet (Pennisetum typhoides (Burm) Stapf. and Hubb). Int. J. Agric. Res. 2007, 2, 238–245. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Tanveer, M.; Matloob, A.; Hussain, H.A. Aspirin priming circumvents the salinity-induced effects on wheat emergence and seedling growth by regulating starch metabolism and antioxidant enzyme activities. Acta Physiol. Plant. 2018, 40, 68. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.A.; Qasim, M.; Ahmad, R. Growth, yield and quality of Rosa hybrida L. as influenced by NaCl salinity. J. Orn. Hort. Pl. 2013, 3, 143–153. Available online: https://jornamental.rasht.iau.ir/article_513390_8543f77e6319e13c7dee80836060c0e7.pdf (accessed on 23 July 2021).
- Lyengar, E.R.; Reddy, M.P. Photosynthesis in highly salt tolerant plants. In Handbook of Photosynthesis; Pesserkali, M., Ed.; Marshal Dekar: Baten Rose, LA, USA, 1996; pp. 897–909. [Google Scholar]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Current Sci. 2004, 86, 408–421. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Cleland, C.F.; Tanaka, O. Effect of day length on the ability of salicylic acid to induce flowering in the longday plant Lemna gibba G3 and the short-day plant Lemna paucicostata. Plant Physiol. 1979, 64, 421–424. [Google Scholar] [CrossRef]
- Okuma, E.; Nozawa, R.; Murata, Y.; Miura, K. Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis. Plant Signal Behav. 2014, 9, e28085. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zhang, T.; Deng, Y.; Yan, J.; Wang, L. Salicylic acid improves the salt tolerance capacity of Saponaria officinalis by modulating its photosynthetic rate, osmoprotectants, antioxidant levels, and ion homeostasis. Agronomy 2022, 12, 1443. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk. J. Biol. 2009, 32, 79–83. [Google Scholar]
- Freire, J.L.O.; Cavalcante, L.F.; Nascimento, R.; Rebequi, A.M. Teores de clorofila e composição mineral foliar do maracujazeiro irrigado com águas salinas e biofertilizante. Rev. De Ciências Agrárias 2013, 36, 57–70. [Google Scholar]
- Allakhverdiev, I.; Hayashi, H.; Nishiyama, Y.; Ivanov, A.G.; Aliev, J.A.; Klimov, V.V.; Murata, N.; Carpentier, R. Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J. Plant Physiol. 2003, 160, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 2017, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mustafiz, A.; Singla-Pareek, S.L.; Srivastava, P.S.; Sopory, S.K.; Srivastava, S.E.; Sopory, S.K. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal Behav. 2012, 7, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Alamri, S.; Al-Khaishany, M.; Khan, M.; Al-Amri, A.; Ali, H.; Alaraidh, I.; Alsahli, A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.A.; Farghaly, F.A.; Hamada, A.M.; Noreen, S.; Siddiq, A.; Hussain, K.; Ahmad, S.; Hasanuzzaman, M. Foliar application of salicylic acid with salinity stress on physiological and biochemical attributes of sunflower (Helianthus annuus L.) crop. Acta Sci. Pol. Hortorum Cultus 2013, 16, 57–74. [Google Scholar]
- Farouk, S.; Al-Ghamdi, A.A.M. Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis, sustaining ion homeostasis, and enhancing osmotic adjustment capacity. Arab. J. Geosci. 2021, 14, 430. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A.; El-Gamal, S.M.A. Potential role of biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic acid: Biosynthesis, metabolism and physiological role in plants. In Salicylic Acid: A Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–14. [Google Scholar]
- El-Tayeb, M.A. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Reg. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Kang, G.; Li, G.; Guo, T. Molecular mechanism of salicylic acid-induced abiotic stress tolerance in higher plants. Acta Physiol. Plant. 2014, 36, 2287–2297. [Google Scholar] [CrossRef]
- Farahat, M.M.; Ibrahim, S.M.M.; Lobna, T.S.; El-Quesni, E.M.F. Response of vegetative growth and some chemical constituents of Cupressus sempervirn L. to foliar application of ascorbic acid and zinc at Nubaria. World J. Agric. Sci. 2007, 3, 282–288. [Google Scholar]
- Aldesuquy, H.; Ghanem, H. Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. J. Hortic. 2015, 2, 139. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A. Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol. Biochem. 2021, 159, 347–362. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants—Focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzi, A. Response of Ornithogalum saundersiae Bak. to salinity stress. Acta Sci. Pol. Hortorum Cultus 2016, 15, 123–134. [Google Scholar]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil. 2012, 352, 353–362. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stresses, 2nd ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Rakszegi, M.; Darkó, E.; Lovegrove, A.; Molnár, I.; Láng, L.; Bedő, Z.; Molnár-Láng, M.; Shewry, P. Drought stress affects the protein and dietary fiber content of wholemeal wheat flour in wheat/Aegilops addition lines. PLoS ONE 2019, 14, e0211892. [Google Scholar] [CrossRef]
- Roshdy, A.E.D.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The effect of salicylic acid on the performances of salt stressed strawberry plants, enzymes activity, and salt tolerance index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel papaverine metal complexes with potential anticancer activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).