The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Micheliamaudiae ‘Rubicunda’ Flower at Five Development Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Flowers Collection and Parameters Measurement
2.3. Determination of C, N, and P Contents
2.4. Determination of NSC Content
2.5. Endogenous Hormone Measurement
2.6. Statistical Analysis
3. Results
3.1. Flower Developmentand Morphological Features
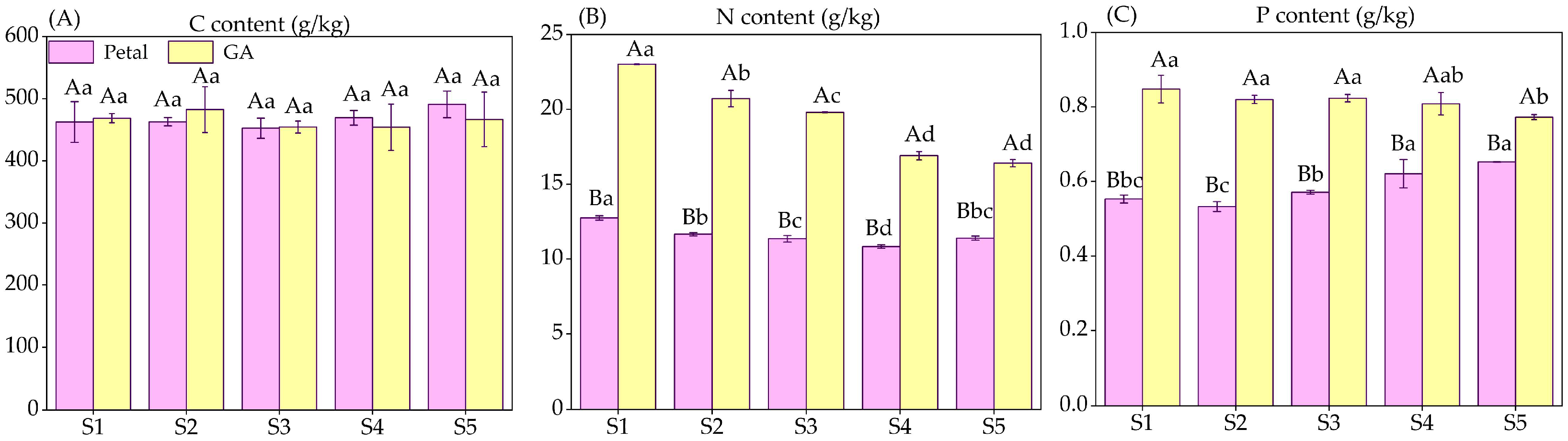
3.2. C, N, and Pcontents
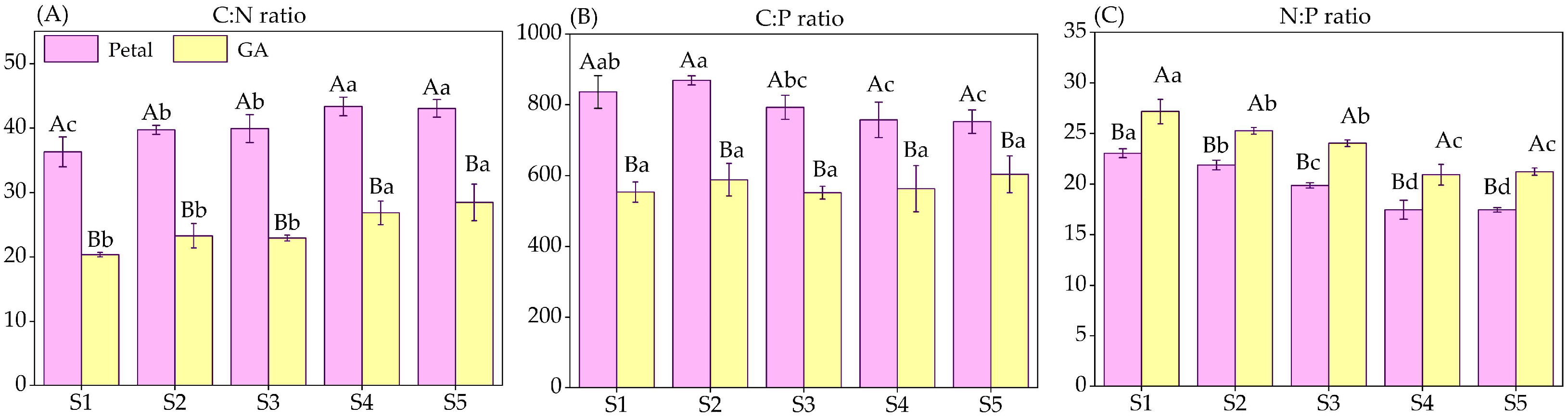
3.3. C/N/P Stoichiometry
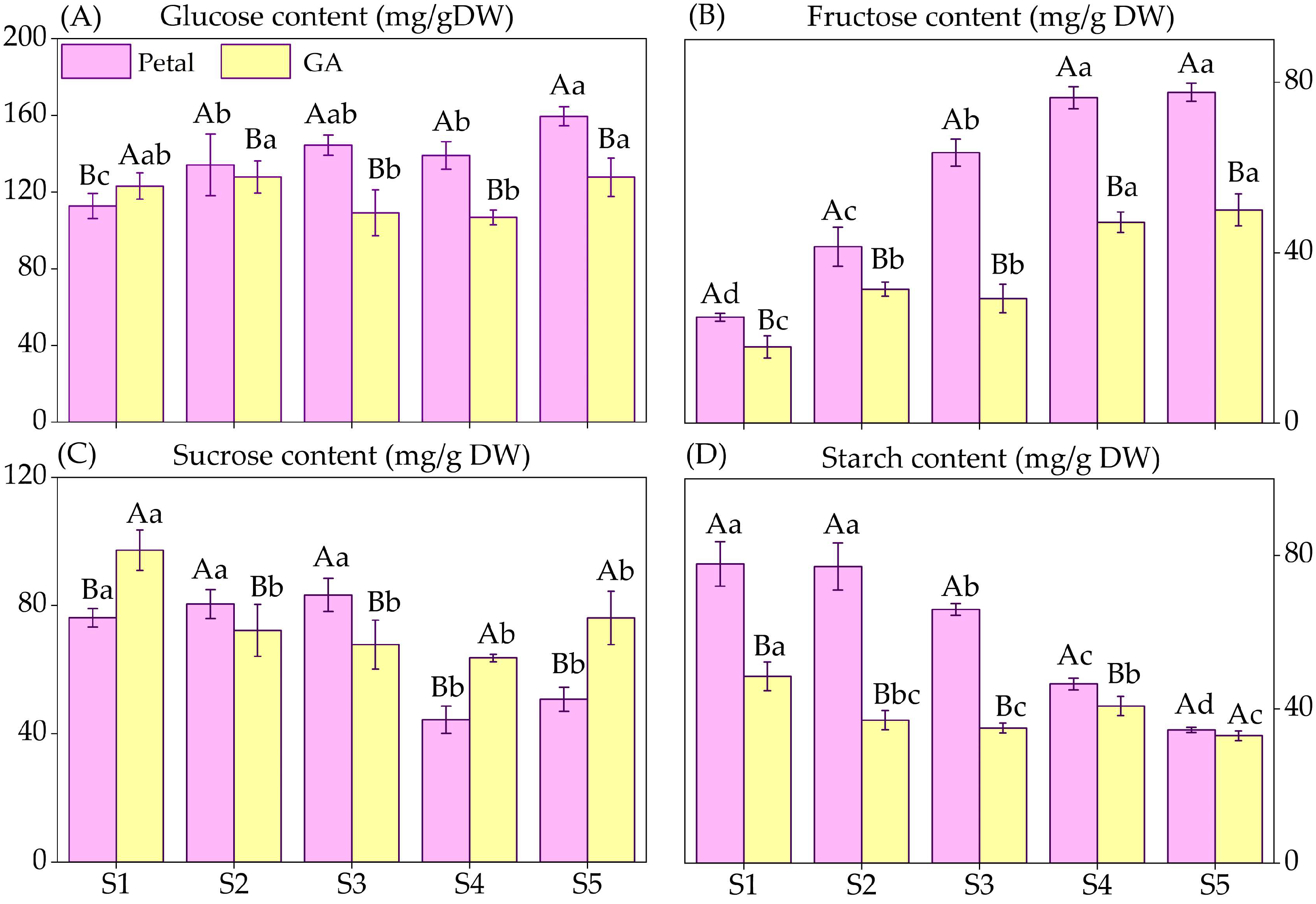
3.4. Non-Structural Carbohydrates (NSC) Contents
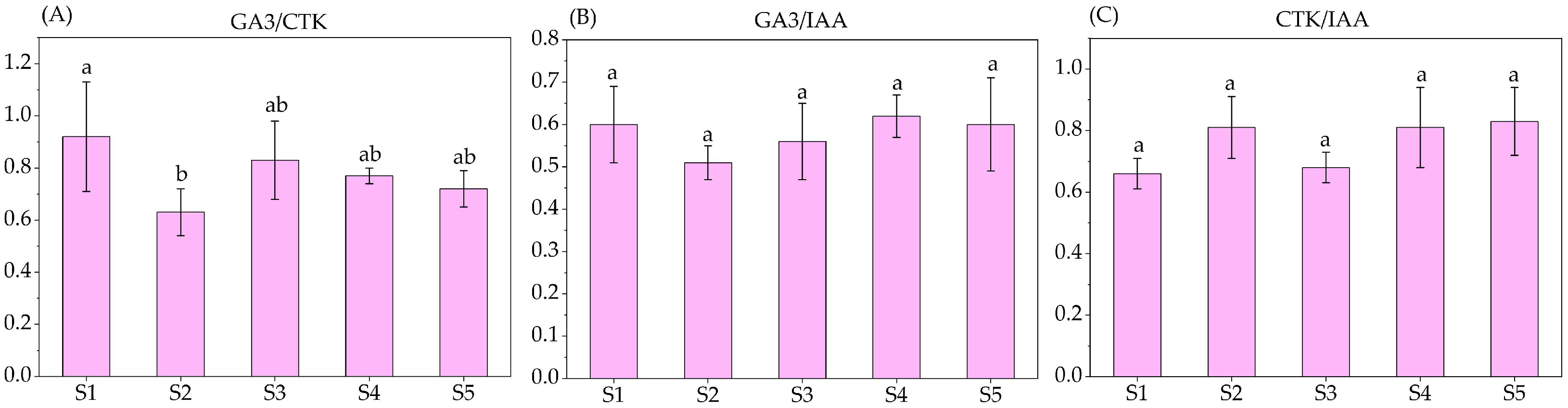
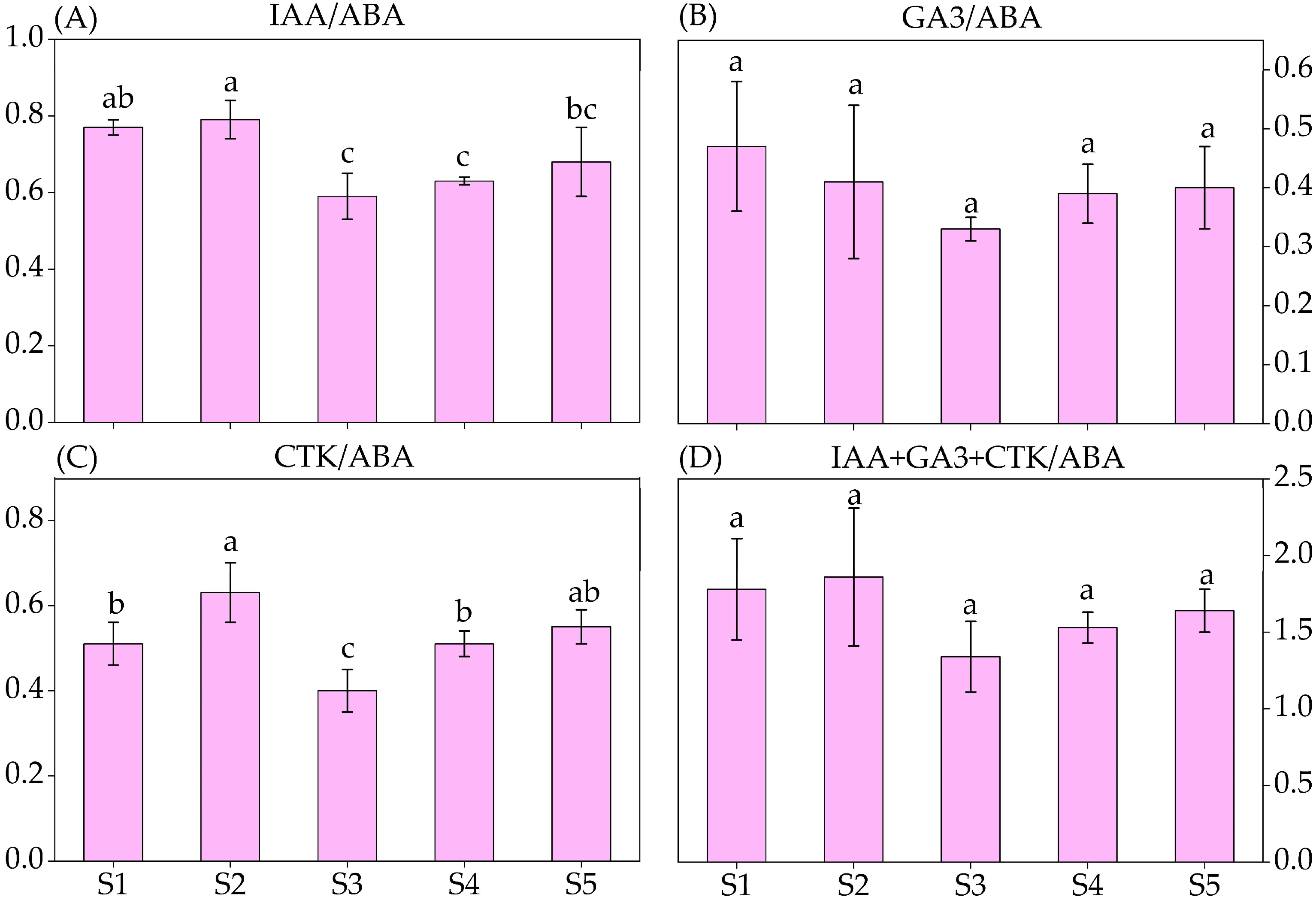
3.5. Endogenous Hormones Content and Their Specific Values
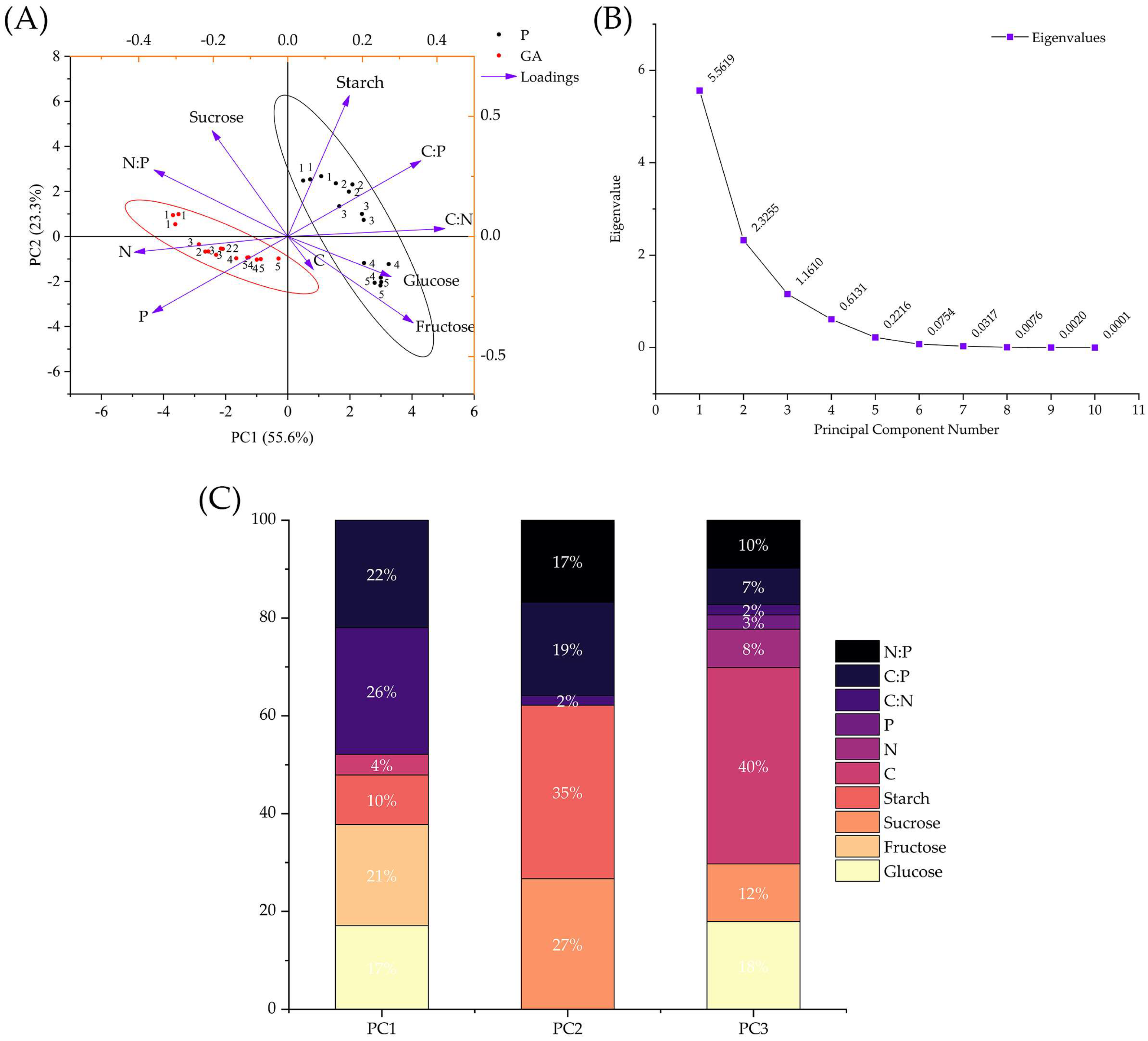
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinakin, D.J.; Kumar, V.; Suri, S.; Sharma, R.; Kaushal, M. Nutraceutical potential of tree flowers: A comprehensive review on biochemical profile, health benefits, and utilization. Food Res. Int. 2020, 127, 108724. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S. Flower development: From bud to bloom. In Proceedings of the ISHS Acta Horticulturae 669: VIII International Symposium on Postharvest Physiology of Ornamental Plants, Doorwerth, The Netherlands, 1 February 2005; ISHS: Korbeek-Lo, Belgium, 2005; Volume 669, p. 12. [Google Scholar] [CrossRef]
- Sood, S.; Vyas, D.; Nagar, P.K. Physiological and biochemical studies during flower development in two rose species. Sci. Hortic. 2006, 108, 390–396. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Kamdee, C. Flower opening and closure: An update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Agren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Hu, J.; Ding, Z.; Liang, Q.; Zhang, Y.; Wang, H. Mineral and metabolic profiles in tea leaves and flowers during flower development. Plant Physiol. Biochem. 2016, 106, 316–326. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Mensuali-Sodi, A.; Serra, G. Effects of promoters and inhibitors of ethylene and ABA on flower senescence of Hibiscus rosa-sinensis L. J. Plant Growth Regul. 2011, 30, 175–184. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of phenological, primary and secondary metabolites changes during flower developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhang, W.; Njie, A.; Pan, X. The changes in C/N, carbohydrate, and amino acid content in leaves during female flower bud differentiation of Juglans sigillata. Acta Physiol. Plant. 2022, 44, 19. [Google Scholar] [CrossRef]
- Ren, H.Y.; Qian, W.Z.; Yi, L.; Ye, Y.L.; Gu, T.; Gao, S.; Cao, G.X. Nutrient composition and antioxidant activity of Cercis chinensis flower in response to different development stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Y.; Xue, Y.; Wang, X.; Huang, Z.; Xue, J.; Zhang, X. Non-structural carbohydrates coordinate tree peony flowering both as energy substrates and as sugar signaling triggers, with the bracts playing an essential role. Plant Physiol. Biochem. 2021, 159, 80–88. [Google Scholar] [CrossRef]
- Han, H.; He, H.; Wu, Z.; Cong, Y.; Zong, S.; He, J.; Fu, Y.; Liu, K.; Sun, H.; Li, Y.; et al. Non-structural carbohydrate storage strategy explains the spatial distribution of treeline species. Plants 2020, 9, 384. [Google Scholar] [CrossRef]
- Cho, L.H.; Pasriga, R.; Yoon, J.; Jeon, J.S.; An, G. Roles of sugars in controlling flowering time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, Y.J.; Nguyen, B.V.; Park, Y.E.; Sathasivam, R.; Kim, J.K.; Park, S.U. Profiles of secondary metabolites (phenolic acids, carotenoids, anthocyanins, and galantamine) and primary metabolites (carbohydrates, amino acids, and organic acids) during flower development in Lycoris radiata. Biomolecules 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.M.; Loh, C.S.; Ong, B.L. Regulation of flower development in Dendrobium crumenatum by changes in carbohydrate contents, water status and cell wall metabolism. Sci. Hortic. 2008, 119, 59–66. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Önder, D.; Erbaş, S.; Mutlucan, M. Flower color and carbohydrate metabolism changes during the floral development of Rosa damascena. S. Afr. J. Bot. 2023, 156, 234–243. [Google Scholar] [CrossRef]
- Roderick, B.; John, E.; Julian, H.; Allan, W. Flower opening in asiatic lily is a rapid process controlled by dark-light cycling. Ann. Bot. 2000, 86, 1169–1174. [Google Scholar] [CrossRef][Green Version]
- Vergauwen, R.; Ende, W.V.D.; Van Laere, A. The role of fructan in flowering of Campanula rapunculoides. J. Exp. Bot. 2000, 51, 1261–1266. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, S.E.; Casal, B. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res.Int. 2019, 123, 771–778. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Hormonal changes during flower development in floral tissues of Lilium. Planta 2012, 236, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, L.; Yu, H.; Yang, M. Endogenous hormones and biochemical changes during flower development and florescence in the buds and leaves of Lycium ruthenicum Murr. Forests 2022, 13, 763. [Google Scholar] [CrossRef]
- Callaghan, C.B.; Png, S.K. Twenty-six additional new combinations in the Magnolia (Magnoliaceae) of China and Vietnam. PhytoKeys 2020, 146, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Yang, L.; Cui, T.; Zhang, S.; Qi, J. Relationship between diurnal changes of Micheliamaudiae var. rubicunda photosynthetic rate and environmental factors. J. Southwest For. Univ. 2017, 37, 22–27. [Google Scholar]
- Lang, X.A.; Yang, L.L.; Zhang, S.Z.; Li, L.F.; Zhang, S.Z. Morphological variation and reproductive application of Micheliamaudiae var. rubicunda. Subtrop. Plant Sci. 2019, 48, 50–55. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van denBoom, T. The BBCH system to coding the phenological growth stages of plants, history and publications. J. Für Kult. 2009, 61, 41–52. [Google Scholar]
- Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological growth stages of Abelmoschus manihot: Codification and description according to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Zhi, X.; Zou, J.; Ma, N.; Liu, T.; Hu, Y.; Yao, Y.; Wang, H.R.; Gao, S. Responses of the growth and nutrient stoichiometry in Ricinus communis seedlings on four soil types. J. Elem. 2022, 27, 223–238. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Wang, Z.; Chen, Y.; Wen, Z.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Ni, M.; Yu, F. A study on petal morphological and physiological characteristics of Styrax japonicus during the flowering period. Agronomy 2021, 11, 1498. [Google Scholar] [CrossRef]
- Chumber, M.; Jhanji, S. Morpho-physiological and biochemical characterization of Chrysanthemum varieties for early flowering under heat stress. S. Afr. J. Bot. 2022, 146, 603–613. [Google Scholar] [CrossRef]
- Polito, J.I.H.S. Pistillate and staminate flower development in dioecious Pistacia vera (Anacardiaceae). Amer. J. Bot. 1996, 83, 759–766. [Google Scholar] [CrossRef]
- Ramírez, F.; Fischer, G.; Davenport, T.L.; Pinzón, J.C.A.; Ulrichs, C. Cape gooseberry (Physalis Peruviana L.) phenology according to the BBCH phenological scale. Sci. Hortic. 2013, 162, 39–42. [Google Scholar] [CrossRef]
- Ulger, S.; Sonmez, S.; Karkacier, M.; Ertoy, N.; Akdesir, O.; Aksu, M. Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant GrowthRegul. 2004, 42, 89–95. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006, 168, E103–E122. [Google Scholar] [CrossRef]
- Handa, H.; Itani, K.; Sato, H. Structural features and expression analysis of a linear mitochondrial plasmid in rapeseed (Brassica napus L.). Mol. Genet. Genom. 2002, 267, 797–805. [Google Scholar] [CrossRef]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiou, C.Y.; Wang, H.L.; Krishnamurthy, R.; Venkatagiri, S.; Tan, J.; Yeh, K.W. Carbohydrate mobilization and gene regulatory profile in the pseudobulb of Oncidium orchid during the flowering process. Planta 2008, 227, 1063–1077. [Google Scholar] [CrossRef]
- Msaada, K.; Hammami, M.; Marzouk, B.; Salem, N.; Limam, F. Variation in anthocyanin and essential oil composition and their antioxidant potentialities during flower development of Borage (Borago officinalis L.). Plant Biosyst. 2014, 148, 444–459. [Google Scholar] [CrossRef]
- Shalit, A.; Rozman, A.; Alvarez, J.P.; Bowman, J.L.; Eshed, Y.; Lifschitz, E. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 2009, 106, 8392–8397. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Yamane, H.; Gao-Takai, M.; Tao, R. Changes in plant hormone contents in Japanese apricot flower buds during prolonged chilling exposure. In Proceedings of the ISHS Acta Horticulturae 1208: II Asian Horticultural Congress, Chengdu, China, 30 August 2018; ISHS: Korbeek-Lo, Belgium, 2018; Volume 1208, pp. 251–256. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Y.; Deng, Q.; Zhang, H.; Pan, C.; Yang, Z. Endogenous phytohormones and the expression of flowering genes synergistically induce flowering in loquat. J. Integrat. Agric. 2020, 19, 2247–2256. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, C.; Yu, X.; Shen, Y. Biochemical Changes during fruit and seed development in Nanjing Linden (Tilia miqueiana M.). Forests 2023, 14, 969. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Yang, Y.; Wang, H.; Qian, W.; Hu, Y.; Gao, S.; Liao, H. The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Micheliamaudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae 2023, 9, 1198. https://doi.org/10.3390/horticulturae9111198
Yu T, Yang Y, Wang H, Qian W, Hu Y, Gao S, Liao H. The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Micheliamaudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae. 2023; 9(11):1198. https://doi.org/10.3390/horticulturae9111198
Chicago/Turabian StyleYu, Ting, Yao Yang, Hongrui Wang, Wenzhang Qian, Yunyi Hu, Shun Gao, and Hai Liao. 2023. "The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Micheliamaudiae ‘Rubicunda’ Flower at Five Development Stages" Horticulturae 9, no. 11: 1198. https://doi.org/10.3390/horticulturae9111198
APA StyleYu, T., Yang, Y., Wang, H., Qian, W., Hu, Y., Gao, S., & Liao, H. (2023). The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Micheliamaudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae, 9(11), 1198. https://doi.org/10.3390/horticulturae9111198





