Abstract
A method for stimulating the growth of spruce and strawberry in the early phases of development is proposed. A technology for obtaining plasma-activated water (PAW) with the help of a glow discharge plasma generator was developed. The method is proposed for increasing the shelf life of PAW by adding an aqueous colloid of polyvinylpyrralidone (PVP) polymer. It is shown that after treatment with a PAW + PVP mixture, the seeds have a higher percentage of germination, the plants develop faster in the early stages, and they are more viable. At the physicochemical level, after seed treatment with PAW + PVP, higher rates of metabolite outflow from seeds are observed. At the biological level, seed treatment with PAW + PVP leads to a slight decrease in the activity of antioxidant enzymes and a higher content of chlorophylls in the leaves, and a slightly higher assimilation rate is observed. In the leaves, there is higher content of the growth hormone indole-3-acetic acid (IAA), whereas the content of the growth-inhibiting hormone abscisic acid decreases. The use of a stimulating drug based on the composition of an aqueous solution activated by plasma and polyvinylpyrrolidone (PAW + PVP) polymer can be an effective means of a single pre-sowing treatment of spruce seeds in solving the problem of reforestation and strawberry during plant propagation.
1. Introduction
Climate change is accompanied by significant fluctuations in the maximum values of atmospheric pressure and temperatures, and in many areas, there is a shallowing of water bodies and prolonged droughts [1]. These negative changes are accompanied by a widespread reduction in the world’s forests and the green cover of the planet, which exacerbates the crisis state of the environment, with unpredictable consequences [2]. The cultivation of many plant species is difficult in conditions of natural anomalies; moisture deficiency is especially critical [3].
It is known that the greatest loss of seedlings of both woody and garden plants occurs at the initial stages of plant development [4]. To solve the problem, various agricultural practices are used, including those based on the use of chemicals, which often adversely affect the ecological situation [5]. Several decades ago, it was proposed to use aqueous solutions treated with plasma as a plant growth stimulator [6]. Plasma is an ionized gas, one of the four classical aggregate states of matter [7]. When plasma interacts with condensed matter, for example, water, the following is observed: Some of the plasma electrons and ions interact with water molecules and molecules of gases dissolved in water, transferring part of their energy to them [8,9]. As a result, more than 100 different chemical compounds are formed in the aqueous phase, some of which have significant biological activity and a significant lifetime [10]. Some of these products are called reactive oxygen species [11], whereas some are classified as reactive nitrogen species [12]. Reactive oxygen species at high concentrations can cause oxidative stress and at low concentrations have a significant stimulating effect on living systems [13]. Plasma-treated water containing a large number of active compounds is commonly referred to as plasma-activated water (PAW).
The main problem with PAW is its short shelf life. The problem of PAW storage is solved in different ways. One approach involves the suppression to PAW of compounds that stabilize hydrogen peroxide molecules. We suggest that polyvinylpyrrolidone (PVP), a water-soluble biocompatible polymer widely used in biological research and medical practice, can become such a compound [14]. PVP has the ability to bind hydrogen peroxide up to 50–60% by weight [15]. The mechanism of H2O2 binding with PVP is explained by the fact that in complexes with PVP, H2O2 molecules form associates of the chain type, in which each subsequent molecule is connected to the previous one by two equivalent hydrogen bonds and the addition of the second and subsequent molecules is energetically more favorable than the first [16]. Using quantum chemical calculations, binding energies were determined for PVP-H2O2 model systems [17]. The calculations made it possible to substantiate the structure of complexes formed by PVP and a chain of H2O2 molecules of optimal length [18]. As a result, confirmation of the fact that the polymer forms strong complexes with H2O2 was obtained, which can be used to develop biologically active complexes of prolonged action, especially in cases where H2O2 is formed directly in the active system [15].
At the same time, we are not aware of any work on the use of the PAW + PVP system in crop production. In this regard, the first purpose of this work was to create a technology for obtaining PAW using a portable glow discharge plasma generator and to develop a method for increasing the shelf life of PAW by adding PVP. Investigation of the effect of PAW + PVP colloid on the percentage of seed germination of difficult-to-cultivate plants such as spruce and strawberry, on the rate of development in the early stages, and on the viability of these plants was the second purpose of the work. Two types of plants were selected that are maximally different from each other evolutionarily (spruce belongs to Gymnospermae; strawberry belongs to Spermatophytae), with different types of seed coatings (spruce has seeds with a fossilized shell; strawberry has a thin net shell) and different economic importance (spruce—forests and ornamental horticulture; strawberry fruit—horticultural crop). Investigation of the physicochemical and biological mechanisms underlying the stimulatory effect of the PAW + PVP colloid was the third purpose of this work.
2. Materials and Methods
2.1. Portable PAW Reactor
To obtain plasma-activated water, the known hydrostatic experimental scheme (reactor geometry, overflow vessels, gas outlets, mixing system) [19,20,21] involves significant changes in the dimensions, the reactor part, modified principles of the ignition and maintenance of plasma, and control of the processing of aqueous solutions. The developed scheme makes it possible to carry out two methods of activating liquids—plasma–chemical and electrochemical. The design of the reactor is based on an electrochemical cell, which includes a vessel with an electrolyte in which two electrodes are immersed—active and passive. The active electrode (cathode) is made of tungsten and has the shape of a cylinder with a diameter of 0.6 mm and a length of up to 20 mm. The neutral electrode (anode) is made of pyrolytic graphite and has an area of 12 cm2. The reactor is powered by specially designed high-frequency generator operating at a frequency of 0.44 MHz. The operating voltage on the reactor electrodes is maintained in the range of 250 to 350 V. The peak power of the generator during ignition is 1500 W; the power consumption during operation is about 300 W. For the correct operation of the device, the current strength in the negative half-cycle (cathode polarity) must significantly exceed the current strength in the positive half-cycle (anodic polarity). The shape of the change in the current strength with time in the negative half-cycle and the positive half-cycle also differs significantly. The essence of this technique is that the current flowing through the reactor has a constant component and two regions are formed in the reactor.
Zone 1 (active electrode) is in the gas phase formed by water vapor. In this region, there is high-frequency glow discharge and plasma–chemical reactions take place. The electrodes of zone 1 are the cathode and the plasma–electrolyte interface. Zone 2 (neutral electrode) is located in the electrolyte part of the reactor. A pulsating current with a constant component flows through this region. The electrodes of zone 2 are the plasma–electrolyte interface and the anode. Since zone 1 and zone 2 are connected via the plasma–electrolyte interface, the composition and nature of the electrolyte obviously have a rather strong influence on the process. The system developed by us has a rather high frequency of 0.44 MHz and works effectively in a rather small range of electrolyte characteristics. In this case, the volume of the experimental reactor is about 0.2 L. The initial solution is fed into the reactor through an adjustable valve (leak) set to a flow of 50 cm3/min, which ensures sufficient activation of the solution. The resulting hydrogen is removed from the reactor through a special channel. In principle, any dissociating salt can be used to obtain an electrolyte. In preliminary experiments it was determined that K2SO4 80 mM, pH = 5.5, could be used effectively. A schematic view of the reactor assembly and a disassembled appearance of the reactor assembly are shown in Figure 1.
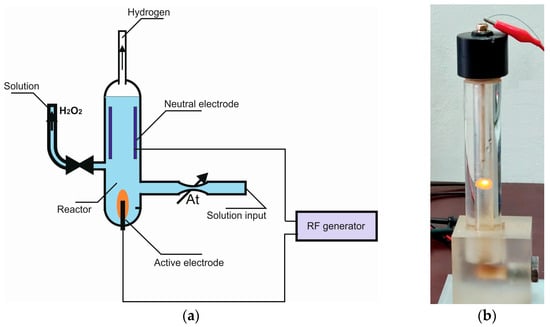
Figure 1.
Schematic view of the reactor unit (a) and the disassembled appearance of the reactor unit (b).
The current–voltage characteristic of the reactor discharge circuit is shown in Figure 2, which shows an oscillogram of the current (red curve) and voltage (yellow curve) in the tab. It can be seen from the figure that in the stage of raising the voltage to the plasma ignition threshold (section AB), the current increases according to a linear law. In the plasma combustion section (BCD), the voltage stabilizes and the discharge is close to a normal glow discharge. In the DF section, the voltage drop after plasma quenching follows a linear law.
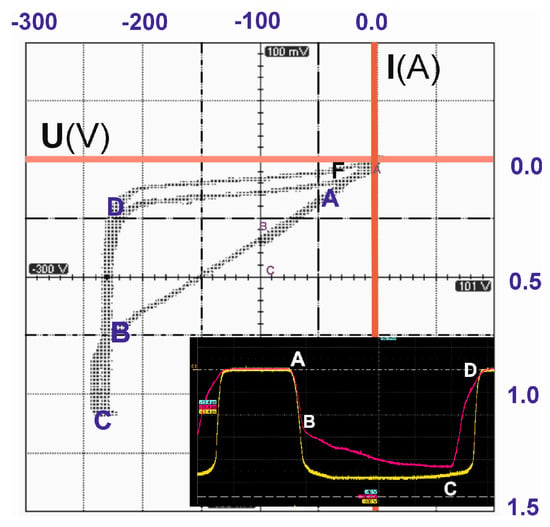
Figure 2.
The current–voltage characteristic of the reactor discharge circuit.
Let us consider the processes taking place in the electrochemical part of the reactor at the gas–liquid interface in its near-surface layer. In the cathodic polarity of the active electrode in the gas phase covering the electrode, the glow discharge burns in its characteristics close to an anomalous glow discharge. Electrons, as the main charge carriers in plasma and with an energy of about 5 eV, bombard the liquid surface at the gas–liquid interface. At the same time, they lose their energy at a small depth and, upon reaching thermal equilibrium with the liquid, enter a hydration reaction with the formation of a solvated state. In addition, taking into account that current flows through the electrolyte part of the reactor, which has a constant component in the cathode polarity of the plasma electrode, the process of electrolysis of the K2SO4 solution should proceed. As a result of the electrolysis, on the surface of the plasma electrode (gas–liquid interface), the H+ ions are recharged with the release of molecular hydrogen. It should be noted that the current through the glow discharge plasma flows not only in the cathode polarity of the active electrode but also in the anode polarity. In this case, the current pulse is smaller in magnitude and shorter in duration. Possibly, it is associated with the reverse ejection of solvated electrons into the plasma discharge zone upon the change in the polarity of the supply voltage together with a thin layer of liquid at the plasma–electrolyte interface.
2.2. Investigation of PAW Properties
Using the high-precision measuring station S470 SevenEx Cellence (Mettler Toledo, Columbus, OH, USA) with an InLab Expert Pro-ISM sensor electrode, we measured the pH and redox potential on the InLab Redox sensor and conductivity on the InLab731-ISM sensor. The measured solutions were mixed in laminar mode with a frequency of about 2–3 Hz. The measurement details are described earlier [22]. The concentration of molecular oxygen dissolved in PAW was determined using an AKPM-1-02 polarograph (BSS, Moscow, Russia). Atmospheric pressure was taken into account, and thermal compensation worked. The details of the measurements are described earlier [23]. The concentration of nitrate anions was determined with a Horiba LAQUAtwin B-741 nitrate meter. The instrument was calibrated using the method described earlier [24]. To determine the concentration of hydrogen peroxide in PAW, the method of enhanced chemiluminescence in the “luminol—4-iodophenol—horseradish peroxidase” system was used [25]. The details of the experimental setup and measurements are described earlier [26].
2.3. Preparation of PAW Complex and Polyvinylpyrrolidone
The aqueous colloid of polyvinylpyrlidone (Ashland, Moscow, Russia) 1% had a pH = 5.0 and an electrical conductivity of less than 0.1 mS/cm, contained about 270 μM of dissolved molecular oxygen and had less than 0.01 mM NO3− and H2O2. The aqueous colloid of polyvinylpyrlidone was mixed with PAW in a ratio of 1/10.
2.4. Growing Plants
Seeds of the common spruce, Picea abies, were used. The sample size of the seeds for one case was 100 pieces. There were 5 independent experiments. The sampling law was random. Pre-sowing treatment was carried out by immersing the spruce seeds in the test solution for 5 min, with further drying at room temperature. Evaluation of the effectiveness of the pre-sowing treatment of the seeds with solution was carried out by testing the state of the plant objects in the early phases of development after treatment. The testing methodology was divided into two stages. Stage 1: After being pre-treated and dried to a free-flowing state, the seeds were laid out for germination in Petri dishes of 25 pcs. on paper filters moistened with water, then transferred to a thermostat with a temperature of +20 °C, and on the 6th day the number of live germinated seeds was estimated. Seeds with a root length of more than 5 mm were considered to have germinated. Stage 2: Germinated seeds were transferred to transparent culture vessels made of PET plastic on depleted soil–sand once moistened with water, and the vessels were hermetically sealed and placed in a climate chamber. Per plant, the volume of sand was 5 mL, the volume of the humidifier was 2 mL, and the volume of air was 10 mL. Seedling cultivation conditions: illumination 1500 lux, light/dark alternation—12:12, temperature +20 °C. For different variants of pre-sowing treatment of spruce seeds, the process of seedling germination was recorded simultaneously at each stage of development. The experiment was performed for 4 treatment options: initial aqueous solution, aqueous PVP colloid (1%), PAW, and PAW + PVP colloid (1%). The state of the germinating seeds was recorded on the 6th, 9th, and 15th days. On the 6th day, the number of germinated seeds in Petri dishes was recorded. On the 9th day, the number of seedlings with free needles was taken into account, and on the 15th day, the number of formed seedlings was estimated.
Strawberry seeds of the garden variety Regina, Fragaria × ananassa, were used. The sample size of the seeds for one experiment was 100 pieces, and there were 5 independent experiments. The seeds were selected randomly. A pre-sowing treatment was carried out by immersing the strawberry seeds in a special strainer in the test solution for 5 min, followed by drying at room temperature. The seeds were laid out for germination on cellulose filters moistened with water and placed in a climate chamber with a temperature of +20 °C [27]. The state of the germinating seeds was recorded on the 8th, 10th, and 15th days.
2.5. Evaluation of the Release of Metabolites from Seeds during Soaking
For the experiments, spruce seeds were selected, pre-treated, and dried for two hours. Experimental samples (30 seeds, treated with PAW + PVP) were thoroughly washed for 10 min. The procedure was repeated in a similar way with control (untreated with PAW + PVP) seeds, and they were placed in quartz cuvettes with deionized water in a volume of 4.5 mL. Absorption spectra were recorded on a Cary 300 spectrophotometer (Varian, Belrose, NSW, Australia) (reference cell, deionized water). Fluorescence spectra were recorded on an FP-8300 spectrofluorimeter (Jasco, Tokyo, Japan) at room temperature. The metabolites released into the solution had a characteristic absorption spectrum in the UV region of the spectrum, with a clearly defined maximum at λ = 207 nm and an absorption shoulder of up to λ = 330 nm. At certain intervals before the next measurement, the liquid in the cuvette with the control and experimental samples was gently mixed, and then the optical density was measured at the absorption maximum or the fluorescence intensity at a wavelength of 330 nm and at an excitation wavelength of 280 nm. The spectral technique settings and procedures are described earlier [28,29].
2.6. Markers of Oxidative Stress
SOD activity was measured colorimetrically using nitro blue-tetrazolium as described in [30]. Peroxidase activity was assessed by guaiacol oxidation, which was monitored by a change in optical density at 470 nm [31]. Catalase activity was measured by hydrogen peroxide consumption via the method described above. Lipid peroxidation was assessed by measuring the content of malondialdehyde (MDA) as described previously [32].
2.7. Characterization of Plant Photosynthesis
To measure FChl and the intensity of carbon dioxide assimilation and transpiration in the plant leaves, a GFS-3000 gas analyzer integrated with a DUAL-PAM-100 was used (Waltz, Eichenring, Effeltrich, Germany). The measurements were carried out in a measuring cuvette on untouched leaves at room temperature and 65% humidity in a laminar CO2 flow 400 with a concentration of 200 ppm. The measurements were preceded by a 1 h incubation of the plants in the dark at room temperature to ensure complete relaxation of all photoinduced processes and 30 minute adaptation in a cuvette [33]. The concentration of chlorophyll a and chlorophyll b was measured spectrally according to the method described earlier [34].
2.8. Definition of Phytohormones
Extraction and purification of phytohormones indole-3-acetic acid (IAA) and abscisic acid (ABA) was carried out according to the protocol in [35]. Detection of IAA was performed according to the modified protocol in [36] and ABA according to the protocol in [37] using ELISA.
2.9. Statistical Analysis
Data in graphs are presented as means ± standard error of the mean. Data from at least three independent experiments (n ≥ 3) were used for averaging. To check the normality of the sample distribution, the Shapiro–Wilk test was used. To compare two independent samples subject to normal distribution, the Student’s t-test was used.
3. Results and Discussion
Table 1 shows the physicochemical properties of glow discharge plasma-activated aqueous solutions (PAW) and aqueous PVP colloids prepared on their basis (PAW + PVP). Plasma exposure to samples of aqueous solutions took place for 30 min, since during long times of treatment of aqueous solutions in the developed reactor (approximately 45–50 min), a decrease in the generation rate of a number of chemical products is observed. For example, the concentration of hydrogen peroxide in an aqueous solution ceases to increase significantly. The maximum values to which it was possible to bring the concentration of hydrogen peroxide was 7–10 mM.

Table 1.
Physicochemical characteristics of the aqueous solution before treatment with glow discharge plasma (control), after treatment (PAW), and immediately after the preparation of a colloidal solution with PVP polymer.
In the process of exposure to glow discharge plasma, the specific electrical conductivity of the aqueous solution increased by 2.5 times. After preparing a colloidal solution based on glow discharge plasma-treated water (PAW + PVP), the electrical conductivity decreased by about 10% relative to PAW. When exposed to an aqueous solution of plasma, alkalization occurred. After plasma treatment, the pH of PAW increased by more than 1 unit. In this case, when making a colloidal solution (addition of PVP), the pH values did not return to the neutral region. The concentration of molecular oxygen in all the cases studied did not change significantly and was at the level of standard indicators. It is known that in water and weak electrolytes, the redox potential correlates to the concentration of dissolved molecular oxygen. In this case, this was not observed. After plasma treatment, the redox potential of the aqueous solution increased by 75%. The preparation of a colloidal solution of the polymer somewhat decreased the value of the redox potential relative to PAW. In the initial aqueous solution, the concentration of nitrate anions was less than 0.01 mM. After plasma treatment of the aqueous solution, the detectable concentration of nitrate anions was about 15 mM. When a polymer (10% by volume) was added to PAW, dilution was observed and the concentration of the nitrate anion decreased by about 10%, which was expected [38]. In the initial aqueous solution, the concentration of hydrogen peroxide was less than 0.01 mM. This agrees with the literature data for water (4–7 nM) [39] and weak electrolytes (2–15 nM) [40]. After plasma treatment of the aqueous solution, about 5 mM of hydrogen peroxide was detected in it. When the polymer was added, a decrease in the concentration of hydrogen peroxide by about 10% was observed.
It is known that the concentration of hydrogen peroxide in aqueous solutions at room temperature and atmospheric pressure decreases with time [41]. It was assumed that the PVP polymer would bind and stabilize the hydrogen peroxide molecules. To test this assumption, PAW and PAW + PVP were stored for 10 days under the same conditions. In this case, the concentration of hydrogen peroxide was recorded. The data are presented in Figure 3. It is shown that for 10 days in an aqueous solution containing no polymer, the concentration of hydrogen peroxide decreased by about 2 times. When a polymer was added to PAW, a colloid was formed in which the concentration of hydrogen peroxide decreased by 10–15% in 10 days.
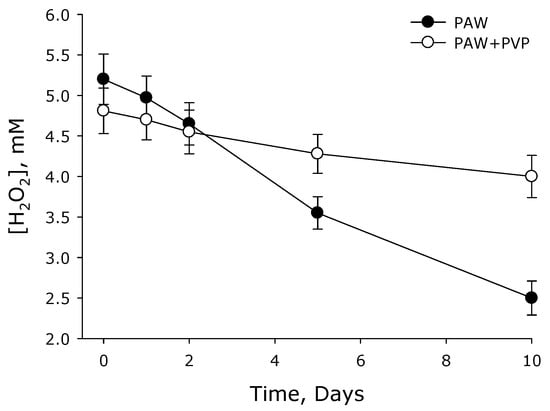
Figure 3.
Change in the concentration of hydrogen peroxide in plasma-activated water (PAW) and a colloidal solution of PAW with PVP polymer (PAW + PVP) during storage (n = 3).
Thus, PVP decreased the rate of decomposition of hydrogen peroxide in the aqueous solution treated with glow discharge plasma by 3–5 times; however, whether this will have any advantage when used in the agricultural industry remains unknown. To find out, the effect of plasma-activated water and a colloidal solution based on PAW was studied, and experiments were carried out with a pre-sowing treatment of seeds of common spruce (Picea abies) plants. It was shown that, after seed treatment with PAW + PVP colloid, the number of germinated seeds was significantly higher compared to the control. Plants germinated from such seeds had a significantly larger biomass and size (Figure 4).

Figure 4.
Representative photographs of spruce plants on the 15th day of growth. Plants grown from seeds pre-sowing treated with a control aqueous solution (on the left, denoted by the letter K) and PAW + PVP colloid (on the right, denoted by the letter O).
More detailed data on the cultivation of seedlings of common spruce (Picea abies) are shown in Table 2. It is shown that for the control by the 6th day of the experiment, approximately one seed out of five had germinated. Treatment with PVP colloid did not lead to a significant improvement in the situation. Seed treatment with PAW led to a significant increase in seed germination by the 6th day of the experiment. Approximately two out of three seeds had germinated. At the same time, seed treatment with PAW + PVP colloid led to a 20% increase in seed germination by the 6th day of the experiment relative to the PAW group.

Table 2.
Results of the influence of pre-sowing treatment of spruce seeds with control solution, PVP, PAW, and PAW + PVP, n = 5.
It is shown that approximately every fifth plant in the control had free needles by the 9th day of the experiment. Treatment with PVP colloid led to some improvement in the situation. The number of plants with free needles by the 9th day increased by 50% relative to the control. By the 9th day of the experiment, seed treatment with PAW led to about half of the plants having free needles. At the same time, seed treatment with PAW + PVP colloid led to an increase of 20% in the number of plants with free needles by the 9th day relative to the PAW group.
It is shown that, by the 15th day of the experiment for the control, the number of living plants was about 10%. Treatment with PVP colloid led to some improvement in the situation. By the 15th day of the experiment, seed treatment with PAW led to about two out of five plants remaining alive. At the same time, seed treatment with PAW + PVP colloid led to an increase in the number of living plants by 30% relative to the PAW group and by 350% relative to the control group.
Thus, it is shown that the pre-sowing treatment of spruce seeds with the PAW + PVP colloid was the most effective, but it is not clear what this effect is associated with, both at the level of biology and at the level of physical chemistry. In the following, we focused on studying the effects of the PAW + PVP colloid alone. Spruce seeds have the required dimensions, density, and solidity for studying germination processes at the physicochemical level. One of the processes by which one can judge the effect of a stimulating effect on seed germination is the speed of the release of metabolites from seeds into their cultivation medium [42]. The possibility of early release of metabolites during seed treatment was studied using the methods of spectrophotometry and spectrofluorimetry. The change in value of the optical density of the solution in which the seeds are soaked can be considered an indicator of the activation of seeds in the aquatic environment in the first few hours of the experiment.
Organic molecules often absorb ultraviolet radiation intensely. In the spectrum, the highest intensity was observed at 207 nm; it was this wavelength that was used in the experiments. Figure 5 shows the kinetic curves of the release of metabolites over time for the control and experimental samples. It follows from the data obtained that the process of increasing the optical density of the liquid surrounding the seeds after the contact of the seeds with water occurred quite quickly in the control group, with an initial velocity of 4 × 10−2 min−1. When using PAW + PVP, the initial speed was twice as high, of about 8 × 10−2 min−1. Subsequently, the release rate of metabolites decreased to 1 × 10−2 min−1 for the control and to 3 × 10−2 min−1 for the PAW + PVP group.
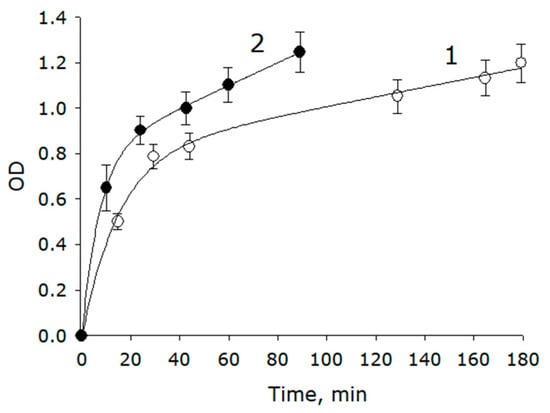
Figure 5.
Dynamics of changes in the optical density of the medium for cultivating seeds of spruce immediately after soaking at 207 nm. The yield of metabolites was recorded using the change in the optical density of the control samples (1) and PAW + PVP samples (2). Background absorbance values are subtracted (n = 5).
Figure 6 shows a 3D fluorescence map of the solution with released metabolites 90 min after the start of soaking. The map shows two areas of intense fluorescence. Usually, the area of a fluorescent map with a center of λex = 280 nm, λem = 330 nm is associated with proteins and peptides containing aromatic amino acid residues, as well as with the amino acids tryptophan, phenylalanine, and tyrosine [43]. The region below is associated with the fluorescence of a large number of secondary metabolites. More details about substances capable of fluorescence and their place on the 3D fluorescence map can be found in the review article in [44]. Obviously, proteins and their derivatives are a more homogeneous group in terms of chemical composition than secondary metabolites. For this reason, the dynamics of the release of metabolites from plant seeds was studied with the instrument settings λex = 280 nm, λem = 330. Figure 6 shows the dynamics of the release of metabolites of the control and samples soaked in PAW + PVP. It is shown that the rate of release of metabolites into the medium when using PAW + PVP was almost 50% higher compared to the control in the initial section of the graph and almost 25% higher at later times.
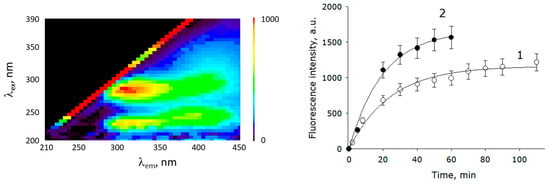
Figure 6.
The dynamics of the release of metabolites during spruce seed soaking, measured by fluorescence. On the left is a 3D fluorescence map of the solution with released metabolites 90 min after the start of soaking. The graph on the right shows the dynamics of changes in the fluorescence of the seed cultivation medium immediately after soaking (λex = 280 nm, λem = 330 nm). Control samples (1), PAW + PVP samples (2) (n = 5).
It is rather difficult to study the effects of plasma-activated water at the biological level on spruce seeds due to their density and solidity, as well as the rather large size of one seedling. In this regard, further studies were carried out on strawberries (Fragaria × nananassa) cv. Regina. The convenience of using strawberry seeds is explained by a number of factors. (1) Small seed size: This allows you to use a large number of seeds to determine the statistically significant sowing qualities of seeds—energy and germination. This is relevant in the case of using digital processing methods because it allows seedlings to be grown in a limited area to the stage of full opening of the cotyledons and the formation of the first true leaves (without significant overlap). An increase in the time of observation and further analysis allows one to more reliably judge the effects of stimulation or inhibition, if any. (2) A short amount of time is needed to determine the germination energy [21]. This fact allows, at the time of observation and analysis, for the phases of pecking, germination, opening of the cotyledons, and coloring of the leaf surface to be more clearly separated. In the case of a preliminary impact on seeds and further analysis of sowing qualities, we can more reliably judge the positive or negative impact on the further development of the plant object. (3) Average percentage of seed germination [45]: Strawberry seeds do not have a high germination rate. Seeds with a germination capacity of 30% or more are allowed for commercial sale. As in the case of the germination energy, this fact, together with a high sample size, allows us to draw more reliable conclusions if, after the impact on the seeds, an increase or decrease in the percentage of seed germination is observed.
As in the case of spruce seeds, strawberry seeds were pretreated according to the method described above. The method for checking the sowing qualities was divided into two stages. In the first stage, 25 strawberry seeds that were pretreated and dried to a free-flowing state were laid out in bowls for germination on moistened paper filters. The bowls were transferred to a thermostat with a temperature of 25 °C, and on the 8th day the number of germinated seeds was estimated. Seeds with open cotyledons or with pecked roots were considered to have germinated. The experiment compared seeds pretreated with a control aqueous solution and PAW + PVP (similar to the common spruce seeds). As a result of the pre-sowing treatment of the strawberry seeds, the final seed germination increased from 28% to 44% in comparison to the control.
At the second stage, seed germination was assessed in terms of the total area of leaf blades. Figure 7 shows representative photographs of strawberry plants on the 8th, 10th, and 15th days of growth. A total of 120 strawberry seeds that were pre-treated and dried to a free-flowing state were laid out randomly in containers for germination on moistened cotton pads. Starting from the 8th day, the total area of the leaf surface was measured. The measurement results are presented in Table 3. From the results obtained, it can be seen that by the 8th day, the leaf surface area in the pre-treated PAW + PVP was 1.4 times greater than the control. By the 10th day, the difference reached the maximum of 1.6 times, which is close to the difference in germination measured in the first stage. By future days, the difference between the control and the experiment was less significant. Taking into account the measurement error and the large number of seeds involved in the measurement, it was possible to reliably judge the positive effect of the PAW + PVP stimulating solution on the sowing qualities of the seeds.

Figure 7.
Representative photographs of strawberry plants on the 8th, 10th, and 15th days of growth. Plants grown from seeds treated with pre-sowing with control aqueous solution (top row) and PAW + PVP colloid (bottom row).

Table 3.
Leaf area of strawberry plants in mm2 measured at different stages of germination.
Thus, it is shown that the pre-sowing treatment of strawberry seeds with the PAW + PVP colloid was effective, but it is not clear which biological mechanism underlies the effect. It is possible that plants are slightly damaged when exposed to PAW + PVP, leading to the effect of hormesis [46], which is also observed in plants [47,48]. At hormesis, after 5–12 h, activation of antioxidant systems is usually observed [49]. To test this assumption, the effect of PAW + PVP treatment on the antioxidant status of strawberry plants was studied (Figure 8). It is shown that after treatment with PAW + PVP, a decrease in the activity of antioxidant enzymes in the leaves of strawberry plants was observed. Activity with SOD 8 days after treatment was decreased by 35% compared to the control. Peroxidase activity did not change significantly. The activity of the enzyme catalase decreased by more than 20% compared to control values. At the same time, the amount of malondialdehyde in the leaf tissues was increased by 15–20% relative to the control. Thus, it was found that PAW + PVP treatment did not cause the development of hormesis in plants.
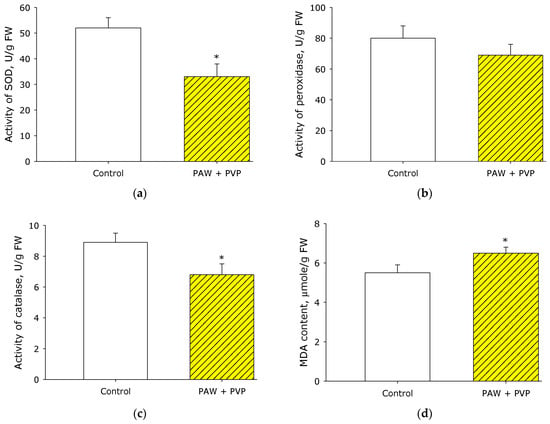
Figure 8.
Effect of PAW + PVP treatment on the antioxidant status of strawberry plants. Effect of PAW + PVP treatment on the activity of SOD (a), peroxidase (b), and catalase (c) enzymes. Effect of PAW + PVP treatment on malondialdehyde (MDA) content (d). The measurements were carried out on the leaves on the 8th day of the experiment. * Statistical differences relative to the control group (p < 0.05) (n = 3).
Faster plant development may be associated with more efficient assimilation processes [50]. More productive assimilation may be due to plants absorbing light more efficiently [51]. It is known that light absorption is predominantly carried out by plant pigments, or chlorophylls [52]. Figure 9 shows the effect of PAW + PVP treatment on the content of chlorophyll a and chlorophyll b in the leaves of strawberry plants. It is shown that when treated with PAW + PVP, the content of chlorophyll a in the leaves increased by almost 10% compared to the control. The content of chlorophyll b in comparison to the control increased by 20%. The effect of PAW + PVP processing on the assimilation rate was also measured. It is shown that the assimilation rate in the control was about 1.8 ± 0.2 µmol·m−2·s−1, whereas the assimilation rate after treatment with PAW + PVP reached 2.3 ± 0.2 µmol·m−2·s−1. In general, the data obtained can only partly explain the observed effects.
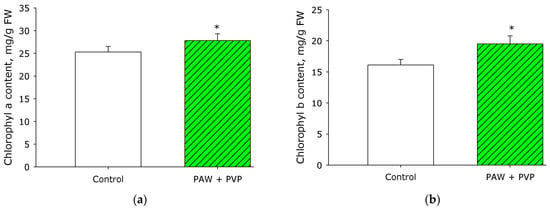
Figure 9.
Effect of PAW + PVP treatment on the content of chlorophyll a (a) and chlorophyll b (b) in the leaves of strawberry plants. The measurements were carried out in leaves on the 8th day of the experiment. * Statistical differences relative to the control group (p < 0.05) (n = 3).
Biologically active substances of an organic nature—hormones—are also responsible for the growth and development of plants. One of the plant hormones is indole-3-acetic acid (IAA) [53]. This is a chemical substance with high physiological activity that is formed in plants and affects growth processes (the so-called growth hormone) [54]. One of the most widely distributed is auxins [55]. This hormone can also be synthesized by microorganisms and fungi [56]. The effect of PAW + PVP treatment on the content of indole-3-acetic acid (IAA) in the leaves of strawberry plants was studied (Figure 10a). It was found that after treatment with PAW + PVP, there was an increase in the content of the hormone in leaf tissues by 30% compared to the control.
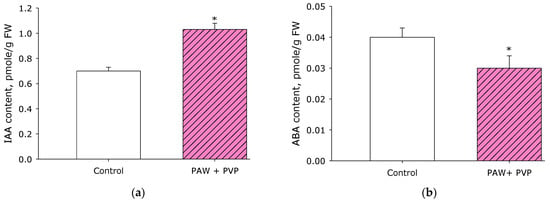
Figure 10.
Effect of PAW + PVP treatment on the content of indole-3-acetic acid (IAA) (a) and abscisic acid (ABA) (b) in strawberry plant leaves. The measurements were carried out in leaves on the 8th day of the experiment. * Statistical differences relative to the control group (p < 0.05) (n = 3).
Another important hormone affecting plant growth and development is abscisic acid (ABA) [57]. Abscisic acid is a plant hormone that inhibits growth and development. Its chemical nature classifies it as an isoprenoid [58]. It is known that ABA and IAA can cross-influence each other [59]. The effect of PAW + PVP treatment on the content of abscisic acid (ABA) in the leaves of strawberry plants was studied (Figure 10b). It was found that after treatment with PAW + PVP, there was a decrease in the content of the hormone in leaf tissues by 25% compared to the control. In general, the data obtained, coupled with data on the content of pigments and the rate of assimilation, suggest that the effect of PAW + PVP treatment at the biological level can also be realized through these mechanisms.
4. Conclusions
A portable compact reactor for the production of plasma-activated water was developed. The reactor volume is 0.2 L, and the power consumed during operation is 300 W. It is shown that after treatment, the seeds had a higher percentage of germination, the plants developed faster, and they were more viable. At the physicochemical level, after seed treatment with PAW + PVP, higher rates of metabolite outflow from seeds were observed. At the biological level, seed treatment with PAW + PVP led to a slight decrease in the activity of antioxidant enzymes and a higher content of chlorophylls in the leaves, and a slightly higher assimilation rate was observed. In the leaves, the content of the growth hormone indole-3-acetic acid (IAA) was higher, whereas the content of the growth-inhibiting hormone abscisic acid was decreased. Analysis of review articles devoted to pre-sowing seed treatment using PAW [60,61,62,63] allows us to assert that the reactor we developed is comparable in efficiency to both well-known scientific installations and commercial reactors. The advantage of our development is its compactness, small reactor volume, unpretentiousness to water quality, ability to use any dissociating salts, and low energy consumption. The use of a stimulating solution based on the composition of PAW + PVP can be an effective means of carrying out a single pre-sowing treatment for spruce seeds to solve the problem of reforestation and for strawberry seeds during plant propagation.
Author Contributions
Conceptualization, Y.K.D., E.N.O., A.S.D., A.Y.I. and V.S.; methodology, S.V.B. and A.V.L.; software, M.E.A.; validation, A.V.L. and S.V.B.; formal analysis, V.I.L.; investigation, A.B.E., V.I.L., L.M.A., A.V.S. (Alexander V. Simakin), E.M.K., D.A.Z., E.V.S., M.O.P., D.V.K. and R.V.P.; data curation, A.V.S. (Alexey V. Shkirin); writing—original draft preparation, Y.K.D. and S.V.B.; writing—review and editing, V.S.; funding acquisition, A.S.D. and A.Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Science and Higher Education of the Russian Federation for large scientific projects in priority areas of scientific and technological development (subsidy identifier 075-15-2020-774).
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical.
Acknowledgments
The authors are grateful to the Shared Use Center of the GPI RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castree, N.; Adams, W.M.; Barry, J.; Brockington, D.; Büscher, B.; Corbera, E.; Demeritt, D.; Duffy, R.; Felt, U.; Neves, K.; et al. Changing the Intellectual Climate. Nat. Clim. Chang. 2014, 4, 763–768. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate Change and the Need for Agricultural Adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar Applications of ZnO and SiO2 Nanoparticles Mitigate Water Deficit and Enhance Potato Yield and Quality Traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]
- Pozner, E.; Bar-On, P.; Livne-Luzon, S.; Moran, U.; Tsamir-Rimon, M.; Dener, E.; Schwartz, E.; Rotenberg, E.; Tatarinov, F.; Preisler, Y.; et al. A Hidden Mechanism of Forest Loss under Climate Change: The Role of Drought in Eliminating Forest Regeneration at the Edge of Its Distribution. For. Ecol. Manag. 2022, 506, 119966. [Google Scholar] [CrossRef]
- Istriningsih; Dewi, Y.A.; Yulianti, A.; Hanifah, V.W.; Jamal, E.; Dadang; Sarwani, M.; Mardiharini, M.; Anugrah, I.S.; Darwis, V.; et al. Farmers’ Knowledge and Practice Regarding Good Agricultural Practices (GAP) on Safe Pesticide Usage in Indonesia. Heliyon 2022, 8, e08708. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Bulanov, S.V. Electron Dynamics in the Field of Strong Plasma and Electromagnetic Waves: A Review. Phys. Wave Phenom. 2021, 29, 1–46. [Google Scholar] [CrossRef]
- Piskarev, I.M. Nitration of Phenol with Water Activated by Pulsed Hot Plasma Radiation. High Energy Chem. 2023, 57, 379–383. [Google Scholar] [CrossRef]
- Simakin, A.V.; Baymler, I.V.; Smirnova, V.V.; Astashev, M.E.; Voronov, V.V.; Dorokhov, A.S.; Gudkov, S.V. Comparison of the Intensities of Chemical and Physical Processes Occurring during Laser-Induced Breakdown of Colloidal Solutions of Tb Nanoparticles with Different Oxidation States. Dokl. Phys. 2022, 67, 465–471. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Apasheva, L.M.; Ovcharenko, E.N.; Lobanov, A.V. Water Activated by a Microwave Plasma Argon Jet as a Factor Stimulating the Germination of Plant Seeds. Russ. J. Phys. Chem. B 2022, 16, 84–89. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Usacheva, A.M.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Formation of Long-Lived Reactive Species of Blood Serum Proteins Induced by Low-Intensity Irradiation of Helium-Neon Laser and Their Involvement in the Generation of Reactive Oxygen Species. J. Photochem. Photobiol. B Biol. 2017, 176, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Zenkov, N.K.; Kozhin, P.M.; Vcherashnyaya, A.V.; Martinovich, G.G.; Kandalintseva, N.V.; Menshchikova, E.B. Features of redox regulation in tumor cells. Sib. Sci. Med. J. 2019, 39, 11–26. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The Role of Peroxiredoxin 6 in Neutralization of X-ray Mediated Oxidative Stress: Effects on Gene Expression, Preservation of Radiosensitive Tissues and Postradiation Survival of Animals. Free. Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Forysenkova, A.A.; Trofimchuk, E.S.; Gafurov, M.R.; Ahmed, A.I.; Davidova, G.A.; Antonova, O.S.; Barinov, S.M. Porous Matrixes Based on Polyvinylpyrrolidone Containing Calcium Phosphates for Medical Application. Russ. Chem. Bull. 2022, 71, 543–548. [Google Scholar] [CrossRef]
- Kirsh, Y.E. Poly-N-vinylpyrrolidone and Other Poly-N-vinylamides: Synthesis and Physico-Chemical Properties; Nauka: Moscow, Russia, 1998; p. 252. ISBN 5-02-004498-9. [Google Scholar]
- Chambers, L.I.; Yufit, D.S.; Fox, M.A.; Musa, O.M.; Steed, J.W. Structure and Hydration of Polyvinylpyrrolidone–Hydrogen Peroxide. Chem. Commun. 2022, 58, 80–83. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Mudretsova, S.N.; Sinko, G.V.; Komissarov, G.G.; Stoyanov, O.V.; Zaikov, G.E. On the nature of the anomal effect of stabilization of tetrapyrroles in complexes with poly-n-vinylpyrrolidone and hydrogen peroxide. Vestn. Kaz. Technol. Univ. 2014, 17, 20–22. [Google Scholar]
- Panarin, E.F.; Kalninsh, K.K.; Pestov, D.V. Complexation of Hydrogen Peroxide with Polyvinylpyrrolidone: Ab Initio Calculations. Eur. Polym. J. 2001, 37, 375–379. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 616. [Google Scholar] [CrossRef]
- Danileiko, Y.K.; Lukanin, V.I.; Altukhov, E.L.; Yakovlev, A.A.; Osmanov, E.G.; Kogan, E.A.; Shulutko, A.M.; Simakin, A.V.; Baimler, I.V.; Serov, D.A.; et al. Therapy of Pressure Sores via Activation of Regenerative Processed in Tissues by Low-Temperature Glow-Type Plasma Discharges of Glow Type. Opera Medica Physiol. 2022, 9, 15–30. [Google Scholar] [CrossRef]
- Danilejko, Y.K.; Belov, S.V.; Egorov, A.B.; Lukanin, V.I.; Sidorov, V.A.; Apasheva, L.M.; Dushkov, V.Y.; Budnik, M.I.; Belyakov, A.M.; Kulik, K.N.; et al. Increase of Productivity and Neutralization of Pathological Processes in Plants of Grain and Fruit Crops with the Help of Aqueous Solutions Activated by Plasma of High-Frequency Glow Discharge. Plants 2021, 10, 2161. [Google Scholar] [CrossRef]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and Inosine (Riboxin) Eliminate the Long-Lived Protein Radicals Induced X-ray Radiation. Dokl. Biochem. Biophys. 2007, 413, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Heat-Induced Formation of Nitrogen Oxides in Water. J. Biol. Phys. 2013, 39, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Chernikov, A.V.; Gudkov, S.V.; Masalimov, Z.K. Thermal Activation of the Reducing Properties of Seawater Anions. Biofizika 2003, 48, 1022–1029. [Google Scholar] [PubMed]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar]
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684. [Google Scholar] [CrossRef]
- Matveeva, T.A.; Baimler, I.V.; Artemiev, K.V.; Gorudko, I.V.; Sarimov, R.M. Laser Optical Breakdown Modified Physical Properties of Lysozyme in Aqueous Solution. Opera Medica Physiol. 2022, 4, 126–136. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Tikhonova, I.V.; Reut, V.E.; Pobedonostsev, R.V.; Astashev, M.E. Study of the Effects of Selenium Nanoparticles and Their Combination with Immunoglobulins on the Survival and Functional State of Polymorphonuclear Cells. Opera Medica Physiol. 2022, 4, 137–159. [Google Scholar] [CrossRef]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morphological and Physiological Responses of Two Chrysanthemum Cultivars Differing in Their Tolerance to Waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Hu, Y.; Zhao, P.; Xu, T.; Sun, J.; Gao, X. Proline, Sugars, and Antioxidant Enzymes Respond to Drought Stress in the Leaves of Strawberry Plants. Korean J. Hortic. Sci. Technol. 2015, 33, 625–632. [Google Scholar] [CrossRef]
- Grinberg, M.A.; Gudkov, S.V.; Balalaeva, I.V.; Gromova, E.; Sinitsyna, Y.; Sukhov, V.; Vodeneev, V. Effect of Chronic β-Radiation on Long-Distance Electrical Signals in Wheat and Their Role in Adaptation to Heat Stress. Environ. Exp. Bot. 2021, 184, 104378. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Popov, A.V.; Pobedonostsev, R.V.; Kazantseva, D.V.; Dorokhov, A.S.; Izmailov, A.Y.; Vyatchinov, A.A.; Orlovskaya, E.O.; Shaidulin, A.T.; et al. Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae 2023, 9, 846. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise Estimation of Chlorophyll a, b and Carotenoid Content by Deconvolution of the Absorption Spectrum and New Simultaneous Equations for Chl Determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lai, T.; Huang, Q.-W.; Yang, X.-M.; Shen, Q.-R. Effect of N Fertilizers on Root Growth and Endogenous Hormones in Strawberry. Pedosphere 2009, 19, 86–95. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.Z.; Mustafina, A.R.; Kof, E.M. Modified Solvent Partitioning Scheme Providing Increased Specificity and Rapidity of Immunoassay for Indole-3-Acetic Acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Current Trends in the Studies of Aqueous Solutions. Phys. Wave Phenom. 2022, 30, 129–134. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Influence of External Impacts on the Properties of Aqueous Solutions. Phys. Wave Phenom. 2021, 29, 89–93. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Ivanov, V.E.; Karp, O.E.; Chernikov, A.V.; Belosludtsev, K.N.; Bobylev, A.G.; Astashev, M.E.; Gapeyev, A.B.; Bruskov, V.I. Impact of Biologically Relevant Anions on Reactive Oxygen Species Formation in Water under the Action of Non-Ionizing Physical Agents. Biophysics 2014, 59, 700–707. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Karmanova, E.E.; Chernikov, A.V.; Usacheva, A.M.; Ivanov, V.E.; Emel’yanenko, V.I. Formation of Hydrated Electrons in Water under Thermal Electromagnetic Exposure. Phys. Wave Phenom. 2021, 29, 94–97. [Google Scholar] [CrossRef]
- Singh, S. A Review on Possible Elicitor Molecules of Cyanobacteria: Their Role in Improving Plant Growth and Providing Tolerance against Biotic or Abiotic Stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V.; et al. Investigation of Aggregation and Disaggregation of Self-Assembling Nano-Sized Clusters Consisting of Individual Iron Oxide Nanoparticles upon Interaction with HEWL Protein Molecules. Nanomaterials 2022, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-L. Detection and Characterization of Biological and Other Organic-Carbon Aerosol Particles in Atmosphere Using Fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Ashurov, M.K.; Ashurov, E.M.; Astashev, M.E.; Baimler, I.V.; Gudkov, S.V.; Konchekov, E.M.; Lednev, V.N.; Lukina, N.A.; Matveeva, T.A.; Markendudis, A.G.; et al. Development of an Environmentally Friendly Technology for the Treatment of Aqueous Solutions with High-Purity Plasma for the Cultivation of Cotton, Wheat and Strawberries. ChemEngineering 2022, 6, 91. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation Hormesis and Radioadaptive Response in Drosophila Melanogaster Flies with Different Genetic Backgrounds: The Role of Cellular Stress-Resistance Mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Volkova, P.Y.; Bondarenko, E.V.; Kazakova, E.A. Radiation Hormesis in Plants. Curr. Opin. Toxicol. 2022, 30, 100334. [Google Scholar] [CrossRef]
- Geras’kin, S.; Churyukin, R.; Volkova, P. Radiation Exposure of Barley Seeds Can Modify the Early Stages of Plants’ Development. J. Environ. Radioact. 2017, 177, 71–83. [Google Scholar] [CrossRef]
- Ushakov, I.; Vasin, M. The Drugs and Natural Antioxidants as the Components of Anti-Radiation Countermeasures during Cosmic Flights. Med. Radiol. Radiat. Saf. 2017, 62, 66–73. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and Application of Photoconversion Fluoropolymer Films for Greenhouses Located at High or Polar Latitudes. J. Photochem. Photobiol. B Biol. 2020, 213, 112056. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Proshkin, Y.A.; Godyaeva, M.M.; Vodeneev, V.; Sukhov, V.; Panchenko, V.; Chilingaryan, N.O. Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) While Cultivated in Vertical Farms. Agriculture 2022, 12, 1988. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae 2022, 8, 885. [Google Scholar] [CrossRef]
- Solano, C.; Artola, A.; Barrena, R.; Ballardo, C.; Sánchez, A. Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy 2023, 13, 2204. [Google Scholar] [CrossRef]
- Korobova, A.; Ivanov, R.; Timergalina, L.; Vysotskaya, L.; Nuzhnaya, T.; Akhiyarova, G.; Kusnetsov, V.; Veselov, D.; Kudoyarova, G. Effect of Low Light Stress on Distribution of Auxin (Indole-3-Acetic Acid) between Shoot and Roots and Development of Lateral Roots in Barley Plants. Biology 2023, 12, 787. [Google Scholar] [CrossRef]
- Bispo, R.L.B.; Ceccato-Antonini, S.R.; Takita, M.A.; Rosa-Magri, M.M. Exogenous Indole-3-Acetic Acid Production and Phosphate Solubilization by Chlorella vulgaris Beijerinck in Heterotrophic Conditions. Fermentation 2023, 9, 116. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants 2023, 12, 1023. [Google Scholar] [CrossRef]
- Pizzio, G.A. Abscisic Acid Perception and Signaling in Chenopodium Quinoa. Stresses 2022, 3, 22–32. [Google Scholar] [CrossRef]
- Ortiz-García, P.; González Ortega-Villaizán, A.; Onejeme, F.C.; Müller, M.; Pollmann, S. Do Opposites Attract? Auxin-Abscisic Acid Crosstalk: New Perspectives. Int. J. Mol. Sci. 2023, 24, 3090. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef]
- Meier, A.; Essumang, D.; Hummerick, M.; Johnson, C.; Kruger, M.; Massa, G.; Engeling, K. Reviewing Plasma Seed Treatments for Advancing Agriculture Applications on Earth and Into the Final Frontier. Gravitational Space Res. 2021, 9, 133–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).