Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Carpometric Determinations
2.3. Biochemical Analyses
2.3.1. Total Carotenoids Concentration
2.3.2. Total Phenolic Content
2.3.3. DPPH Assay
2.3.4. FRAP Assay
2.4. Determination of Nitrogen and Mineral Contents
Determination of Leaf Fe Content
2.5. Statistical Procedures
3. Results
3.1. Yield and Carpometric Traits
3.2. Biochemical Variables
3.3. Nutrient Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carrasco-Gil, S.; Rios, J.J.; Álvarez-Fernández, A.; Abadía, A.; García-Mina, J.M.; Abadía, J. Effects of individual and combined metal foliar fertilisers on iron- and manganese-deficient Solanum lycopersicum plants. Plant Soil 2016, 402, 27–45. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164. [Google Scholar] [PubMed]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Tong, S.; Vichinsky, E. Iron deficiency: Implications before anemia. Pediatrics Rev. 2021, 42, 11–20. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Distefano, M.; Steingass, C.B.; Leonardi, C.; Giuffrida, F.; Schweiggert, R.; Mauro, R.P. Effects of a plant-derived biostimulant application on quality and functional traits of greenhouse cherry tomato cultivars. Food Res. Int. 2022, 157, 111218. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Buturi, C.V.; Sabatino, L.; Mauro, R.P.; Navarro-León, E.; Blasco, B.; Leonardi, C.; Giuffrida, F. Iron Biofortification of Greenhouse Soilless Lettuce: An Effective Agronomic Tool to Improve the Dietary Mineral Intake. Agronomy 2022, 12, 1793. [Google Scholar] [CrossRef]
- Mauro, R.P.; Lo Monaco, A.; Lombardo, S.; Restuccia, A.; Mauromicale, G. Eradication of Orobanche/Phelipanche spp. seedbank by soil solarization and organic supplementation. Sci. Hortic. 2015, 193, 62–68. [Google Scholar] [CrossRef]
- Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy 2022, 12, 376. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Golubkina, N.A.; Pietrantonio, L.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Florin, I.; Caruso, G. Tomato yield, quality, mineral composition and antioxidants as affected by beneficial microorganisms under soil salinity induced by balanced nutrient solutions. Agriculture 2019, 9, 110. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; ISBN 9780123849052. [Google Scholar]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Li, G.; Kronzucker, H.J.; Shi, W. Root developmental adaptation to Fe toxicity: Mechanisms and management. Plant Signal. Behav. 2016, 11, e1117722. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and iron agronomic biofortification of Brassicaceae microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Kromann, P.; Valverde, F.; Alvarado, S.; Vélez, R.; Pisuña, J.; Potosí, B.; Taipe, A.; Caballero, D.; Cabezas, A.; Devaux, A. Can Andean potatoes be agronomically biofortified with iron and zinc fertilizers? Plant Soil 2017, 411, 121–138. [Google Scholar] [CrossRef]
- Mauro, R.P.; Agnello, M.; Distefano, M.; Sabatino, L.; Primo, A.S.B.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef]
- Giuffrida, F.; Agnello, M.; Mauro, R.P.; Ferrante, A.; Leonardi, C. Cultivation under salt stress conditions influences postharvest quality and glucosinolates content of fresh-cut cauliflower. Sci. Hortic. 2018, 236, 166–174. [Google Scholar] [CrossRef]
- Distefano, M.; Arena, E.; Mauro, R.P.; Brighina, S.; Leonardi, C.; Fallico, B.; Giuffrida, F. Effects of Genotype, Storage Temperature and Time on Quality and Compositional Traits of Cherry Tomato. Foods 2020, 9, 1729. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Chlorophyll and Carotenoid Determination. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Mendoza Cortez, J.W.; de Sordi, D.; Urrestarazu, M. Common Chicory Performance as Influenced by Iron Concentration in the Nutrient Solution. J. Plant Nutr. 2015, 38, 1489–1494. [Google Scholar] [CrossRef]
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Valverde-Miranda, D.; Díaz-Pérez, M.; Gómez-Galán, M.; Callejón-Ferre, Á.J. Total soluble solids and dry matter of cucumber as indicators of shelf life. Postharvest Biol. Technol. 2021, 180, 111603. [Google Scholar] [CrossRef]
- Brown, J.C.; Ambler, J.E. Iron-Stress Response in Tomato (Lycopersicon esculentum) 1. Sites of Fe Reduction, Absorption and Transport. Physiol. Plant. 1974, 31, 221–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Han, J.; Hu, X.; Li, X.; Zhao, H.; Bai, L.; Shi, Y.; Golam, J.A. Interactive Effects of Iron and Photoperiods on Tomato Plant Growth and Fruit Quality. J. Plant Growth Regul. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Das, U.; Rahman, M.M.; Roy, Z.R.; Rahman, M.M.; Kabir, A.H. Morpho-physiological retardations due to iron toxicity involve redox imbalance rather than photosynthetic damages in tomato. Plant Physiol. Biochem. 2020, 156, 55–63. [Google Scholar] [CrossRef]
- Olowolaju, E.D.; Okunlola, G.O.; Ayeotan, O.J. Growth, yield and uptake of some nutrients by tomato as affected by iron concentration. Int. J. Veg. Sci. 2021, 27, 378–387. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M. Lou Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef]

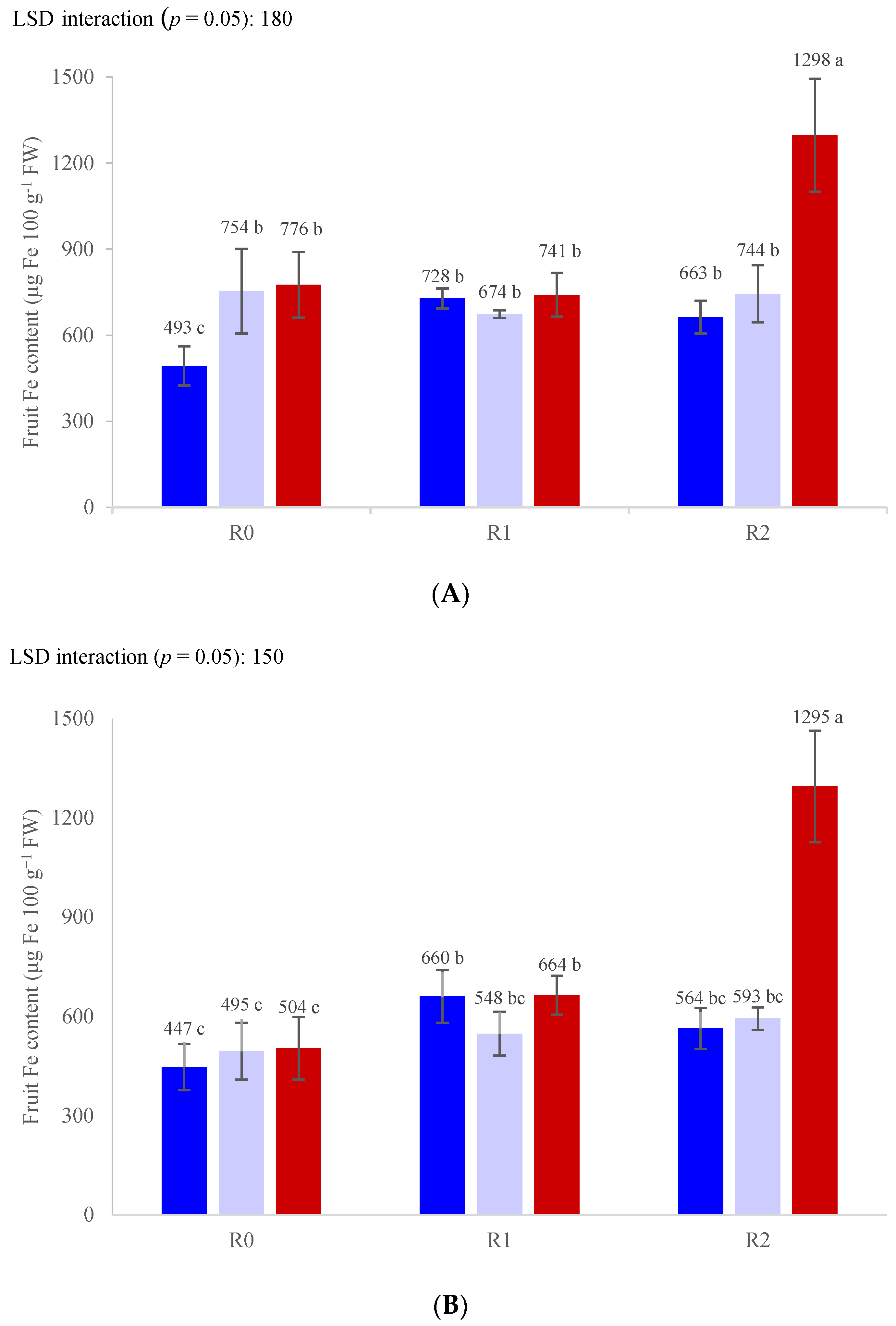
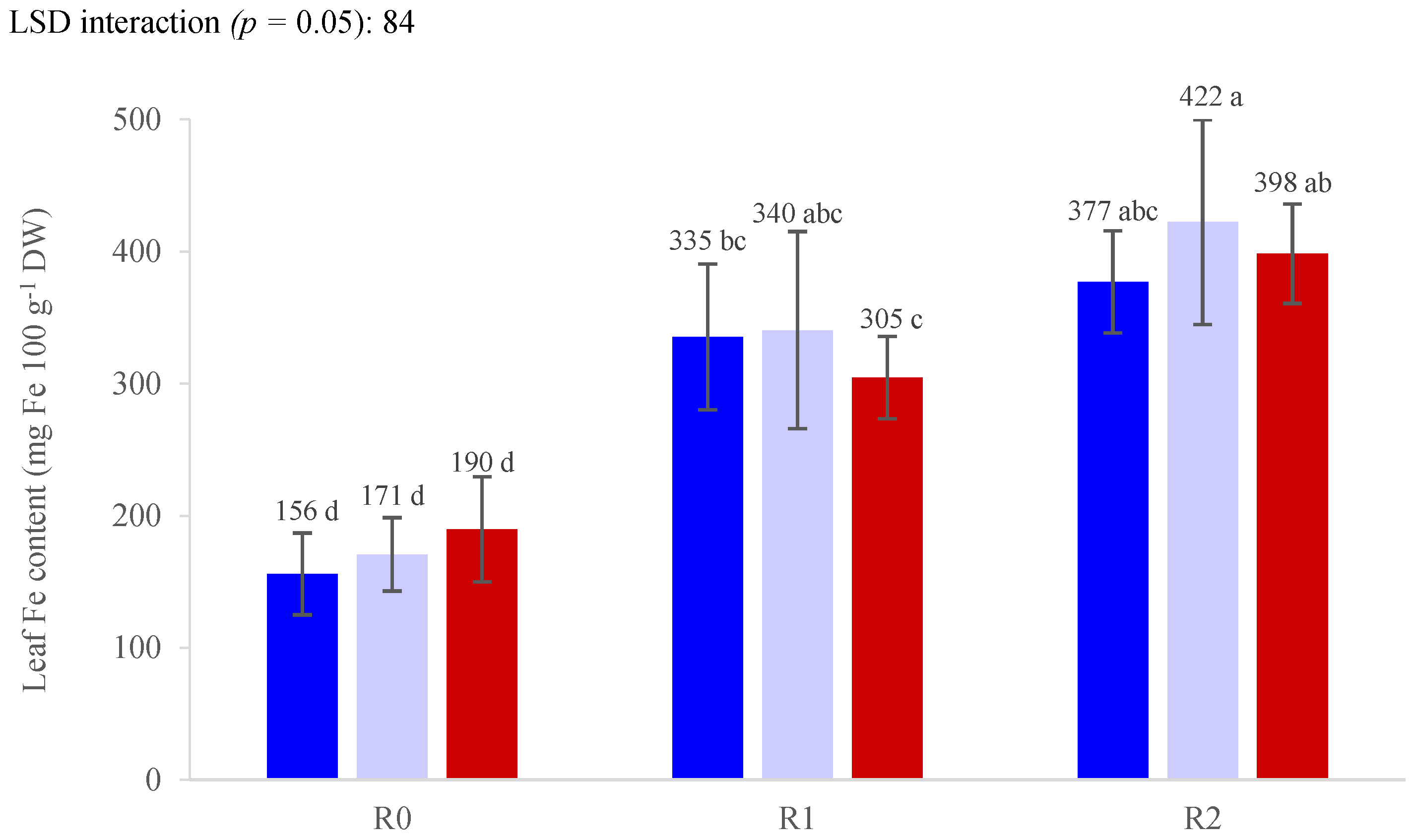
| Yield (g Plant−1) | Average Fruit Weight (g) | Dry Matter Content (%) | Soluble Solids (Brix°) | Titratable Acidity (g L−1) | Firmness (N) | |
|---|---|---|---|---|---|---|
| Fe Nutrient Solution | ||||||
| R0 | 1067 ± 41 a | 14.8 ± 1.0 a | 9.69 ± 0.6 b | 8.56 ± 0.4 b | 6.35 ± 0.5 b | 8.87 ± 0.7 b |
| R1 | 981 ± 86 b | 13.2 ± 1.1 b | 10.24 ± 0.5 ab | 9.22 ± 0.6 a | 6.78 ± 0.6 b | 9.37 ± 0.5 ab |
| R2 | 932 ± 65 b | 12.6 ± 1.1 b | 10.69 ± 0.9 a | 9.57 ± 0.9 a | 7.65 ± 0.9 a | 9.70 ± 0.5 a |
| F-test | * | *** | ** | ** | ** | * |
| Fe Leaf Spray | ||||||
| L0 | 958 ± 79 | 13.2 ± 1.2 | 10.35 ± 0.6 | 9.30 ± 0.6 | 7.01 ± 0.9 | 9.22 ± 0.7 |
| L250 | 999 ± 100 | 13.3 ± 1.8 | 10.32 ± 1.0 | 9.27 ± 1.0 | 7.10 ± 1.0 | 9.28 ± 0.7 |
| L500 | 1024 ± 72 | 14.1 ± 1.0 | 9.95 ± 0.6 | 8.78 ± 0.4 | 6.67 ± 0.7 | 9.45 ± 0.7 |
| F-test | NS | NS | NS | NS | NS | NS |
| Interaction | NS | NS | NS | NS | NS | NS |
| L * | a * | b * | (a */b *)2 | Chroma | |
|---|---|---|---|---|---|
| Fe Nutrient Solution | |||||
| R0 | 42.14 ± 0.8 a | 17.52 ± 1.2 | 24.71 ± 0.6 | 0.49 ± 0.1 | 30.30 ± 1.0 |
| R1 | 41.87 ± 0.9 ab | 16.89 ± 1.4 | 24.37 ± 0.6 | 0.50 ± 0.1 | 29.67 ± 0.8 |
| R2 | 41.30 ± 0.5 b | 17.43 ± 1.5 | 23.84 ± 0.9 | 0.54 ± 0.1 | 29.56 ± 1.2 |
| F-test | * | NS | NS | NS | NS |
| Fe Leaf Spray | |||||
| L0 | 42.01 ± 0.3 | 17.67 ± 1.1 | 24.46 ± 0.9 | 0.54 ± 0.1 | 30.01 ± 1.1 |
| L250 | 41.88 ± 0.2 | 17.24 ± 1.6 | 24.24 ± 0.3 | 0.50 ± 0.1 | 29.95 ± 0.8 |
| L500 | 41.43 ± 0.3 | 16.93 ± 1.5 | 24.22 ± 1.0 | 0.49 ± 0.1 | 29.57 ± 1.2 |
| F-test | NS | NS | NS | NS | NS |
| Interaction | NS | NS | NS | NS | NS |
| TPC | FRAP | DPPH | Total Carotenoids | |
|---|---|---|---|---|
| (GA μmol 100 g−1 FW) | (TE μmol 100 g−1 FW) | (TE μmol 100 g−1 FW) | (μg 100 g−1 FW) | |
| Fe Nutrient Solution | ||||
| R0 | 670 ± 58 | 247 ± 18 | 266 ± 17 | 1534 ± 174 |
| R1 | 665 ± 70 | 235 ± 19 | 251 ± 24 | 1686 ± 160 |
| R2 | 673 ± 36 | 232 ± 19 | 246 ± 24 | 1587 ± 109 |
| F-test | NS | NS | NS | NS |
| Fe Leaf Spray | ||||
| L0 | 670 ± 36 | 233 ± 16 | 256 ± 20 | 1576 ± 111 |
| L250 | 653 ± 61 | 243 ± 16 | 259 ± 24 | 1604 ± 189 |
| L500 | 684 ± 63 | 238 ± 25 | 248 ± 25 | 1628 ± 178 |
| F-test | NS | NS | NS | NS |
| Interaction | NS | NS | NS | NS |
| N | P | K | Mg | Ca | Na | |
|---|---|---|---|---|---|---|
| Fe Nutrient Solution | ||||||
| R0 | 181 ± 3.9 | 47.8 ± 3.9 | 272 ± 44 b | 16.0 ± 2.6 b | 5.2 ± 0.7 | 9.6 ± 1.8 b |
| R1 | 183 ± 5.8 | 46.6 ± 6.3 | 323 ± 52 a | 15.8 ± 2.2 b | 5.3 ± 0.5 | 12.4 ± 2.4 a |
| R2 | 191 ± 11.8 | 48.7 ± 6.6 | 342 ± 73 a | 18.8 ± 1.9 a | 4.9 ± 0.4 | 13.6 ± 4.4 a |
| F-test | NS | NS | * | ** | NS | * |
| Fe Leaf Spray | ||||||
| L0 | 186 ± 7.6 | 50.5 ± 6.3 | 326 ± 76 | 17.8 ± 2.3 a | 5.1 ± 0.5 | 13.1 ± 3.9 |
| L250 | 188 ± 11.8 | 47.2 ± 5.0 | 309 ± 71 | 15.1 ± 2.5 b | 4.9 ± 0.4 | 11.7 ± 3.6 |
| L500 | 181 ± 4.3 | 45.4 ± 4.5 | 301 ± 42 | 17.7 ± 2.2 a | 5.5 ± 0.6 | 10.8 ± 2.7 |
| F-test | NS | NS | NS | ** | NS | NS |
| Interaction | NS | NS | NS | NS | NS | NS |
| Zn | Mn | Cu | |
|---|---|---|---|
| Fe Nutrient Solution | |||
| R0 | 239 ± 13 b | 70.8 ± 12 | 120 ± 30 |
| R1 | 237 ± 12 b | 75.5 ± 10 | 130 ± 30 |
| R2 | 266 ± 31 a | 70.8 ± 6 | 128 ± 23 |
| F-test | ** | NS | NS |
| Fe Leaf Spray | |||
| L0 | 259 ± 27 a | 73.4 ± 9 | 133 ± 23 |
| L250 | 250 ± 25 a | 71.3 ± 9 | 129 ± 27 |
| L500 | 233 ± 8 b | 72.3 ± 12 | 117 ± 31 |
| F-test | ** | NS | NS |
| Interaction | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buturi, C.V.; Coelho, S.R.M.; Cannata, C.; Basile, F.; Giuffrida, F.; Leonardi, C.; Mauro, R.P. Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System. Horticulturae 2022, 8, 858. https://doi.org/10.3390/horticulturae8100858
Buturi CV, Coelho SRM, Cannata C, Basile F, Giuffrida F, Leonardi C, Mauro RP. Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System. Horticulturae. 2022; 8(10):858. https://doi.org/10.3390/horticulturae8100858
Chicago/Turabian StyleButuri, Camila Vanessa, Silvia Renata Machado Coelho, Claudio Cannata, Federico Basile, Francesco Giuffrida, Cherubino Leonardi, and Rosario Paolo Mauro. 2022. "Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System" Horticulturae 8, no. 10: 858. https://doi.org/10.3390/horticulturae8100858
APA StyleButuri, C. V., Coelho, S. R. M., Cannata, C., Basile, F., Giuffrida, F., Leonardi, C., & Mauro, R. P. (2022). Iron Biofortification of Greenhouse Cherry Tomatoes Grown in a Soilless System. Horticulturae, 8(10), 858. https://doi.org/10.3390/horticulturae8100858









