Genetic Variability Assessment of a Diploid Pre-Breeding Asparagus Population Developed Using the Tetraploid Landrace ‘Morado de Huétor’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. SSR Marker Analysis
2.3. Assessment of Morpho-Agronomic Traits
3. Results
3.1. Genetic Diversity Analysis
3.2. Phenotypic Evaluation
| Traits b | Mean Square a | ||
|---|---|---|---|
| Year df = 1 | Individuals df = 766 | Error df = 766 | |
| Spear number | 471.52 (<0.0001) | 27.71 (<0.0001) | 11.55 |
| Phenological stage | 27.79 (<0.0001) | 1.97 (<0.0001) | 1.37 |
| Stalk number | 18.23 (<0.0001) | 0.22 (<0.0001) | 0.11 |
| Branching height | 3.61 (<0.0001) | 0.10 (<0.0001) | 0.06 |
| Stalk thickness | 1.25 (0.0016) | 0.20 (<0.0001) | 0.06 |
| Trait | Population | Year | N | Mean ± SE | SD |
|---|---|---|---|---|---|
| Spring | |||||
| Spear number | Total | 2017 | 841 | 5.4 ± 0.13 | 3.81 |
| 2018 | 823 | 4.4 ± 0.17 | 4.80 | ||
| Selected | 2017 | 71 | 8.1 ± 0.60 | 5.06 | |
| 2018 | 71 | 8.3 ± 0.80 | 6.72 | ||
| Phenological stage | Total | 2017 | 791 | 2.3 ± 0.04 | 1.12 |
| 2018 | 793 | 2.0 ± 0.05 | 1.43 | ||
| Selected | 2017 | 65 | 3.0 ± 0.11 | 0.87 | |
| 2018 | 71 | 3.0 ± 0.11 | 0.94 | ||
| Autumn | |||||
| Stalk number | Total | 2016 | 846 | 1.7 ± 0.01 | 0.39 |
| 2017 | 830 | 1.5 ± 0.01 | 0.38 | ||
| Selected | 2016 | 71 | 1.8 ± 0.04 | 0.36 | |
| 2017 | 71 | 1.8 ± 0.05 | 0.44 | ||
| Branching height | Total | 2016 | 846 | 1.5 ± 0.01 | 0.32 |
| 2017 | 829 | 1.4 ± 0.01 | 0.22 | ||
| Selected | 2016 | 71 | 1.8 ± 0.04 | 0.34 | |
| 2017 | 71 | 1.6 ± 0.02 | 0.20 | ||
| Stalk thickness | Total | 2016 | 846 | 1.5 ± 0.01 | 0.39 |
| 2017 | 829 | 1.5 ± 0.01 | 0.31 | ||
| Selected | 2016 | 71 | 1.9 ± 0.05 | 0.40 | |
| 2017 | 71 | 1.9 ± 0.03 | 0.29 |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAO FAOSTAT. Available online: http://www.fao.org/statistics/en/ (accessed on 10 February 2022).
- Kubota, S.; Konno, I.; Kanno, A. Molecular phylogeny of the genus Asparagus (Asparagaceae) explains interspecific crossability between the garden asparagus (A. officinalis) and other Asparagus species. Theor. Appl. Genet. 2012, 124, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A. Gathering food from the wild. In The Cultural History of Plants; Prance, S.G., Nesbitt, M., Eds.; Routledge: New York, NY, USA, 2005; pp. 29–44. ISBN 0203020901. [Google Scholar]
- Ellison, J.H. Asparagus breeding. In Breeding Vegetables Crops; Bassett, M., Ed.; AVI Publishing Company: Westport, CT, USA, 1986; pp. 521–569. [Google Scholar]
- Cumo, C. Encyclopedia of Cultivated Plants: From Acacia to Zinnia: From Acacia to Zinnia; Cumo, C., Ed.; ABC-CLIO: Santa Barbara, CA, USA, 2013; ISBN 1598847759. [Google Scholar]
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C. The bioactive compounds and biological functions of Asparagus officinalis L.—A review. J. Funct. Foods 2020, 65, 103727. [Google Scholar] [CrossRef]
- Iqbal, M.; Bibi, Y.; Iqbal Raja, N.; Ejaz, M.; Hussain, M.; Yasmeen, F.; Saira, H.; Imran, M. Review on therapeutic and pharmaceutically important medicinal plant Asparagus officinalis L. J. Plant Biochem. Physiol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Geoffriau, E.; Denoue, D.; Rameau, C. Assessment of genetic variation among asparagus (Asparagus officinalis L.) populations and cultivars: Agromorphological and isozymic data. Euphytica 1992, 61, 169–179. [Google Scholar] [CrossRef]
- Knaflewski, M. Genealogy of asparagus cultivars. Acta Hortic. 1996, 415, 87–91. [Google Scholar] [CrossRef]
- Brettin, T.S.; Sink, K.C. Allozyme variation and genetics in asparagus. J. Hered. 1992, 83, 383–386. [Google Scholar] [CrossRef]
- Lallemand, J.; Briand, F.; Breuils, F.; Denoue, D.; Rameau, C. Identification of asparagus varieties by isozyme patterns. Euphytica 1994, 79, 1–4. [Google Scholar] [CrossRef]
- Khandka, D.K.; Nejidat, A.; Golan-Goldhirsh, A. Polymorphism and DNA markers for asparagus cultivars identified by random amplified polymorphic DNA. Euphytica 1996, 87, 39–44. [Google Scholar] [CrossRef]
- Moreno, R.; Espejo, J.A.; Cabrera, A.; Millán, T.; Gil, J. Ploidic and molecular analysis of “Morado de Huétor” asparagus (Asparagus officinalis L.) population; A Spanish tetraploid landrace. Genet. Resour. Crop Evol. 2006, 53, 729–736. [Google Scholar] [CrossRef]
- Mercati, F.; Riccardi, P.; Harkess, A.; Sala, T.; Abenavoli, M.R.; Leebens-Mack, J.; Falavigna, A.; Sunseri, F. Single nucleotide polymorphism-based parentage analysis and population structure in garden asparagus, a worldwide genetic stock classification. Mol. Breed. 2015, 35, 59. [Google Scholar] [CrossRef]
- Nothnagel, T.; Budahn, H.; Krämer, I.; Schliephake, E.; Lantos, E.; Plath, S.; Krämer, R. Evaluation of genetic resources in the genus Asparagus for resistance to Asparagus virus 1 (AV-1). Genet. Resour. Crop Evol. 2017, 64, 1873–1887. [Google Scholar] [CrossRef]
- Kanno, A.; Yokoyama, J. Asparagus. In Wild Crop Relatives: Genomic and Breeding Resources: Vegetables; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–42. ISBN 978-3-642-20449-4. [Google Scholar]
- Chen, H.; Lu, Z.; Wang, J.; Chen, T.; Gao, J.; Zheng, J.; Zhang, S.; Xi, J.; Huang, X.; Guo, A.; et al. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci. Hortic. 2020, 264, 109168. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Rodriguez-Arcos, R.; Jiménez-Araujo, A.; López, S.; Gil, J.; Moreno, R.; Guillén-Bejarano, R. Saponin profile of wild Asparagus species. J. Food Sci. 2017, 82, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Regalado, J.J.; Moreno, R.; Castro, P.; Carmona-Martin, E.; Rodríguez, R.; Pedrol, J.; Larrañaga, N.; Guillén, R.; Gil, J.; Encina, C.L. Asparagus macrorrhizus Pedrol, Regalado et López-Encina, an endemic species from Spain in extreme extinction risk, is a valuable genetic resource for asparagus breeding. Genet. Resour. Crop Evol. 2017, 64, 1581–1594. [Google Scholar] [CrossRef]
- Almirall, A.; Bosch, L.; del Castillo, R.R.; Rivera, A.; Casañas, F. “Croscat” common bean (Phaseolus vulgaris L.), a prototypical cultivar within the “Tavella brisa” type. HortScience 2010, 45, 432–433. [Google Scholar] [CrossRef]
- Prohens, J.; Muñoz-Falcón, J.E.; Rodríguez-Burruezo, A.; Ribas, F.; Castro, Á.; Nuez, F. ‘H15′, an Almagro-type pickling eggplant with high yield and reduced prickliness. HortScience 2009, 44, 2017–2019. [Google Scholar] [CrossRef]
- Nogué, F.; Mara, K.; Collonnier, C.; Casacuberta, J.M. Genome engineering and plant breeding: Impact on trait discovery and development. Plant Cell Rep. 2016, 35, 1475–1486. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Moreno, R.; Castro, P.; Die, J.V.; Gil, J. Asparagus (Asparagus officinalis L.) Breeding. In Advances in Plant Breeding Strategies: Vegetable Crops; Springer: Berlin/Heidelberg, Germany, 2021; pp. 425–469. ISBN 978-3-030-66961-4. [Google Scholar]
- Riccardi, P.; Casali, P.E.; Mercati, F.; Falavigna, A.; Sunseri, F. Genetic characterization of asparagus doubled haploids collection and wild relatives. Sci. Hortic. 2011, 130, 691–700. [Google Scholar] [CrossRef]
- Castro, P.; Rubio, J.; Gil, J.; Moreno, R. Introgression of new germplasm in current diploid cultivars of garden asparagus from a tetraploid spanish landrace “Morado de Huétor”. Sci. Hortic. 2014, 168, 157–160. [Google Scholar] [CrossRef]
- Regalado, J.J.; Martín, E.C.; Madrid, E.; Moreno, R.; Gil, J.; Encina, C.L. Production of “super-males” of asparagus by anther culture and its detection with SSR-ESTs. Plant Cell. Tissue Organ Cult. 2016, 124, 119–135. [Google Scholar] [CrossRef]
- Plath, S.; Krämer, R.; Lantos, E.; Nothnagel, T. Breeding programs to transmit Asparagus virus 1 resistance. Acta Hortic. 2018, 1223, 17–24. [Google Scholar] [CrossRef]
- Garcia, V.; Castro, P.; Turbet-Delof, M.; Gil, J.; Moreno, R. Development and diversity analysis of an hexaploid pre-breeding asparagus population with introgressions from wild relative species. Sci. Hortic. 2021, 287, 110273. [Google Scholar] [CrossRef]
- Moreno, R.; Espejo, J.A.; Cabrera, A.; Gil, J. Origin of tetraploid cultivated asparagus landraces inferred from nuclear ribosomal DNA internal transcribed spacers’ polymorphisms. Ann. Appl. Biol. 2008, 153, 233–241. [Google Scholar] [CrossRef]
- Moreno, R.; Espejo, J.A.; Moreno, M.T.; Gil, J. Collection and conservation of “Morado de Huétor” Spanish tetraploid asparagus landrace. Genet. Resour. Crop Evol. 2008, 55, 773–777. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez, G.; Cermeño, P.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Identification of flavonoid diglycosides in several genotypes of asparagus from the Huétor-Tájar population variety. J. Agric. Food Chem. 2007, 55, 10028–10035. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jiménez-Araujo, A.; Rodriguez-Arcos, R.; Cermeño-Sacristan, P.; Espejo-Calvo, J.A.; Guillén-Bejarano, R. Optimization of a method for the profiling and quantification of saponins in different green asparagus genotypes. J. Agric. Food Chem. 2013, 61, 6250–6258. [Google Scholar] [CrossRef]
- Moreno, R.; Espejo, J.A.; Gil, J. Development of triploid hybrids in asparagus breeding employing a tetraploid landrace. Euphytica 2010, 173, 369–375. [Google Scholar] [CrossRef]
- Moreno, R.; Castro, P.; Rubio, J.; Rodríguez-Arcos, R.; Gil, J. Desarrollo de una nueva variedad de espárrago octoploide “HT-801”. Actas Hortic. 2012, 60, 105–108. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Pedregosa, F.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Identification of bioactive compounds of Asparagus officinalis L.: Permutation test allows differentiation among “triguero” and hybrid green varieties. Molecules 2021, 26, 1640. [Google Scholar] [CrossRef]
- Torres, A.M.; Weeden, N.F.; Martín, A. Linkage among isozyme, RFLP and RAPD markers in Vicia faba. Theor. Appl. Genet. 1993, 85, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Federici, C.T.; Roose, M.L. EST-SSR markers for asparagus genetic diversity evaluation and cultivar identification. Mol. Breed. 2008, 21, 195–204. [Google Scholar] [CrossRef]
- Moreno, R.; Carmona, E.; Lopez-Encina, C.; Rubio, J.; Gil, J. Aplicación de marcadores microsatélites en la mejora del espárrago. Actas Hortic. 2010, 55, 221–222. [Google Scholar]
- Amian, L.; Rubio, J.; Castro, P.; Gil, J.; Moreno, R. Introgression of wild relative Asparagus spp. germplasm into the Spanish landrace “Morado de Huétor”. Acta Hortic. 2018, 1223, 33–38. [Google Scholar] [CrossRef]
- Castro, P.; Gil, J.; Cabrera, A.; Moreno, R. Assessment of genetic diversity and phylogenetic relationships in Asparagus species related to Asparagus officinalis. Genet. Resour. Crop Evol. 2013, 60, 1275–1288. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Newton, A.C.; Akar, T.; Baresel, J.P.; Bebeli, P.J.; Bettencourt, E.; Bladenopoulos, K.V.; Czembor, J.H.; Fasoula, D.A.; Katsiotis, A.; Koutis, K.; et al. Cereal landraces for sustainable agriculture. Sustain. Agric. 2011, 2, 147–186. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Mousavizadeh, S.J.; Hassandokht, M.R.; Kashi, A.; Gil, J.; Cabrera, A.; Moreno, R. Physical mapping of 5S and 45S rDNA genes and ploidy levels of Iranian Asparagus species. Sci. Hortic. 2016, 211, 269–276. [Google Scholar] [CrossRef]
- Vázquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Cermeño-Sacristán, P.; Espejo-Calvo, J.A.; Guillén-Bejarano, R. Saponin profile of green asparagus genotypes. J. Agric. Food Chem. 2013, 61, 11098–11108. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Rubio, J.; Gil, J. Transferencia de genes entre la variedad local de espárrago tetraploide Morado de Huétor y las variedades comerciales diploides. Actas Hortic. 2010, 55, 219–220. [Google Scholar]
- Venezia, A.; Soressi, G.; Falavigna, A. Aspects related to utilization of wild Asparagus species in Italy. Agric Ric 1993, 141, 41–48. [Google Scholar]
- Nothnagel, T.; Budahn, H.; Krämer, I.; Schliephake, E.; Schreyer, L.; Krämer, R. Resistance to Asparagus virus 1 in the Wild Relative Asparagus amarus. J. Phytopathol. 2014, 162, 180–189. [Google Scholar] [CrossRef]
- Jaramillo, S.; Muriana, F.J.G.; Guillen, R.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Lopez, S. Saponins from edible spears of wild asparagus inhibit AKT, p70S6K, and ERK signalling, and induce apoptosis through G0/G1 cell cycle arrest in human colon cancer HCT-116 cells. J. Funct. Foods 2016, 26, 1–10. [Google Scholar] [CrossRef]
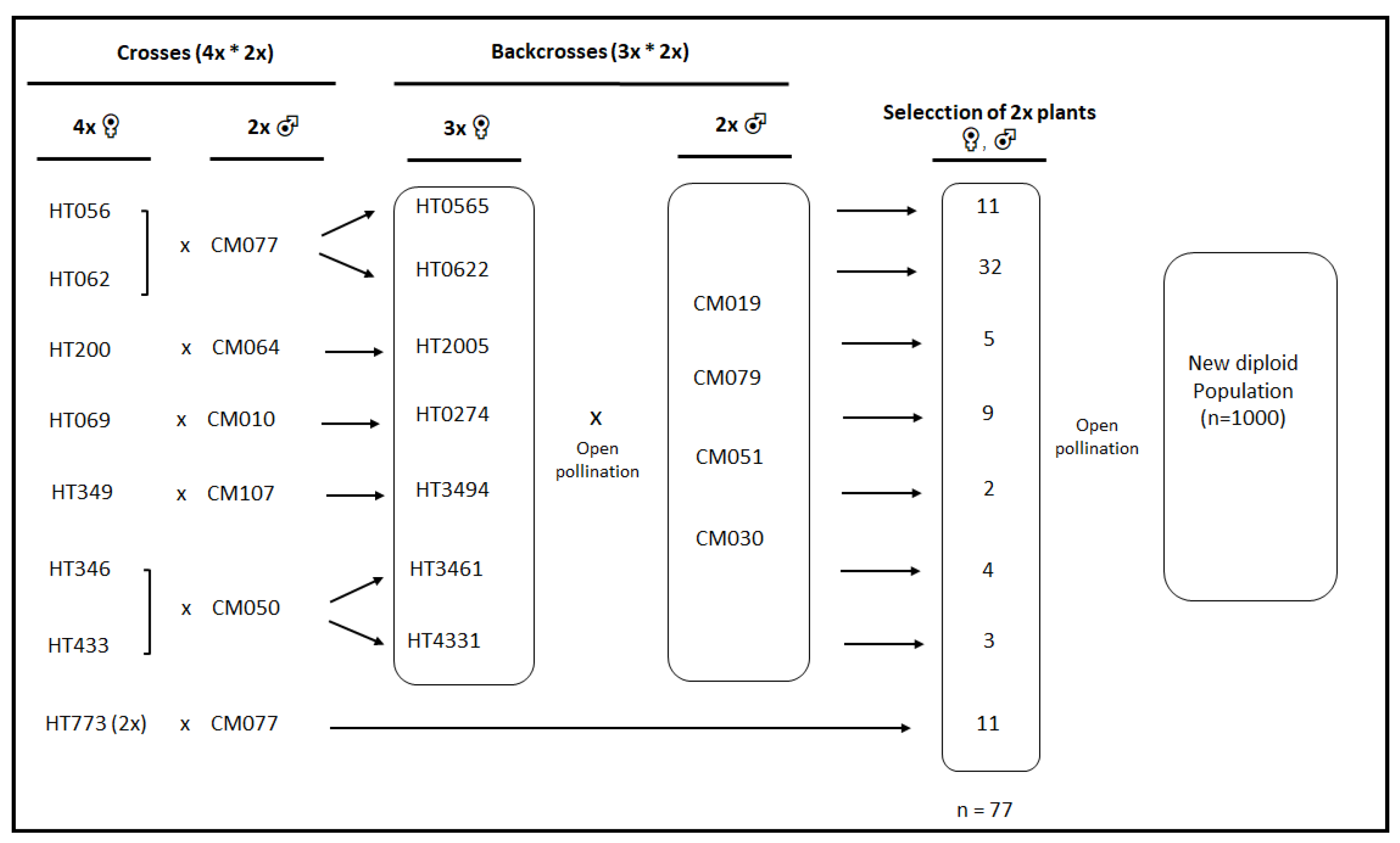
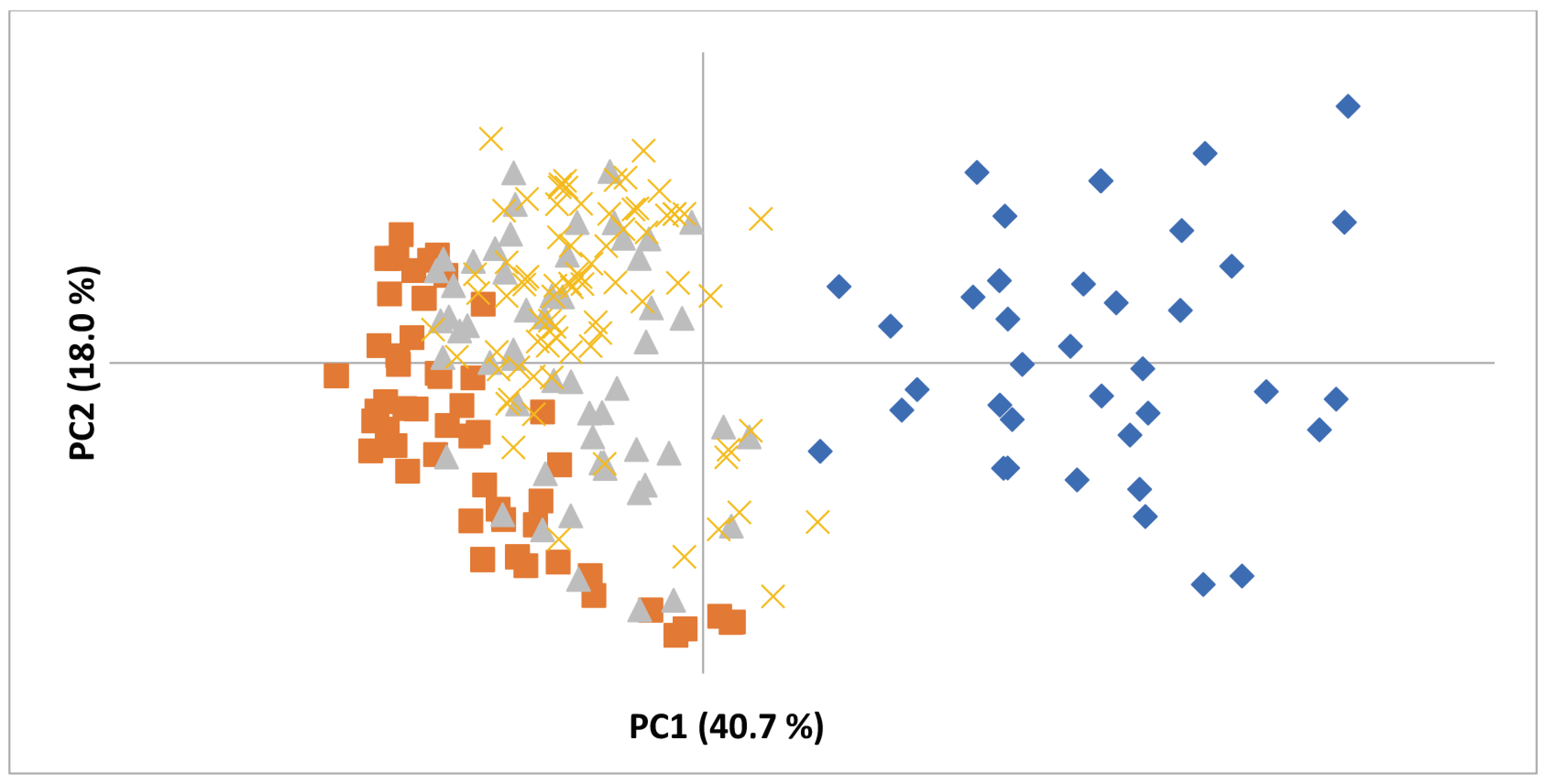
| EST-SSR Locus | ‘Morado de Huétor’ (n = 38) | Diploid Cultivars (n = 63) | Parental Popul. (n = 77) | New Diploid Popul. (n = 57) | ||||
|---|---|---|---|---|---|---|---|---|
| No. Alleles | PICm | No. Alleles | PICm | No. Alleles | PICm | No. Alleles | PICm | |
| AG7 | 8 | 0.77 | 3 | 0.52 | 4 | 0.64 | 4 | 0.61 |
| AG8 | 14 | 0.88 | 4 | 0.49 | 7 | 0.68 | 7 | 0.73 |
| TC1 | 14 | 0.87 | 3 | 0.61 | 6 | 0.66 | 6 | 0.73 |
| TC3 | 15 | 0.84 | 5 | 0.74 | 12 | 0.78 | 11 | 0.85 |
| TC7 | 8 | 0.81 | 6 | 0.72 | 7 | 0.71 | 7 | 0.75 |
| TC9 | 13 | 0.84 | 4 | 0.62 | 7 | 0.83 | 7 | 0.83 |
| Total | 72 | 25 | 43 | 42 | ||||
| Mean | 12.0 | 0.83 | 4.2 | 0.61 | 7.2 | 0.72 | 7.0 | 0.75 |
| Population | N (1) | Mean ± SE (2) | |
|---|---|---|---|
| New diploid | 57 | 12.38 ± 0.072 | |
| Parental diploid | 77 | 10.93 ± 0.057 | *** |
| Morado de Huétor | 38 | 18.78 ± 0.157 | *** |
| Diploid cultivars | 63 | 8.31 ± 0.070 | *** |
| Diploid selected plants | 67 | 12.12 ± 0.061 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, V.; Castro, P.; Millán, T.; Gil, J.; Moreno, R. Genetic Variability Assessment of a Diploid Pre-Breeding Asparagus Population Developed Using the Tetraploid Landrace ‘Morado de Huétor’. Horticulturae 2022, 8, 859. https://doi.org/10.3390/horticulturae8100859
García V, Castro P, Millán T, Gil J, Moreno R. Genetic Variability Assessment of a Diploid Pre-Breeding Asparagus Population Developed Using the Tetraploid Landrace ‘Morado de Huétor’. Horticulturae. 2022; 8(10):859. https://doi.org/10.3390/horticulturae8100859
Chicago/Turabian StyleGarcía, Verónica, Patricia Castro, Teresa Millán, Juan Gil, and Roberto Moreno. 2022. "Genetic Variability Assessment of a Diploid Pre-Breeding Asparagus Population Developed Using the Tetraploid Landrace ‘Morado de Huétor’" Horticulturae 8, no. 10: 859. https://doi.org/10.3390/horticulturae8100859
APA StyleGarcía, V., Castro, P., Millán, T., Gil, J., & Moreno, R. (2022). Genetic Variability Assessment of a Diploid Pre-Breeding Asparagus Population Developed Using the Tetraploid Landrace ‘Morado de Huétor’. Horticulturae, 8(10), 859. https://doi.org/10.3390/horticulturae8100859






