Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Experimental Design
2.3. Determinations
2.3.1. Plant Growth
2.3.2. Selenium Content
2.3.3. Mineral Content
2.3.4. Leaf Photosynthetic Pigments, Flavonoids, Total Phenols, and Total Antioxidant Capacity
2.4. Statistical Analysis Data
3. Results
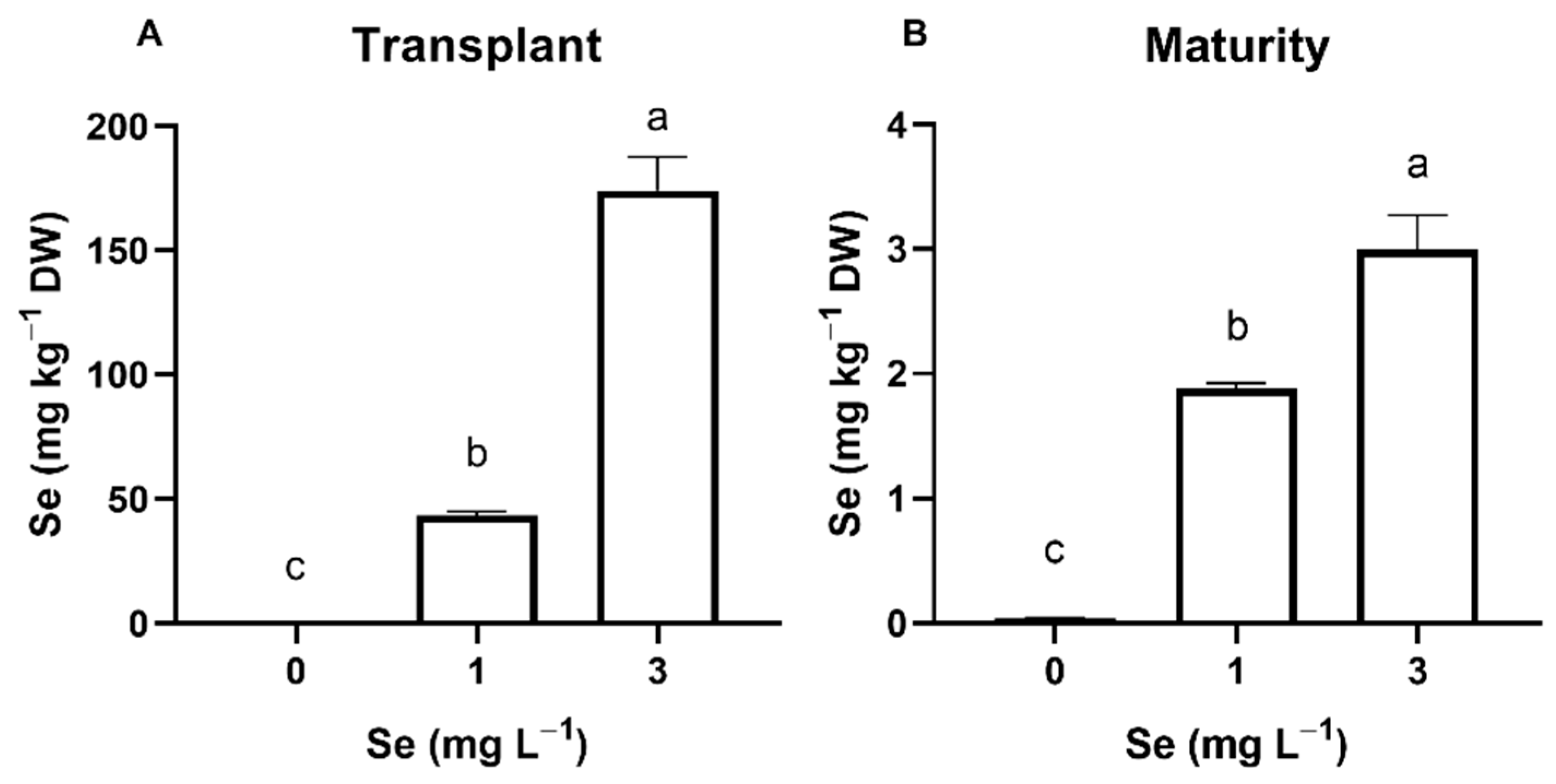
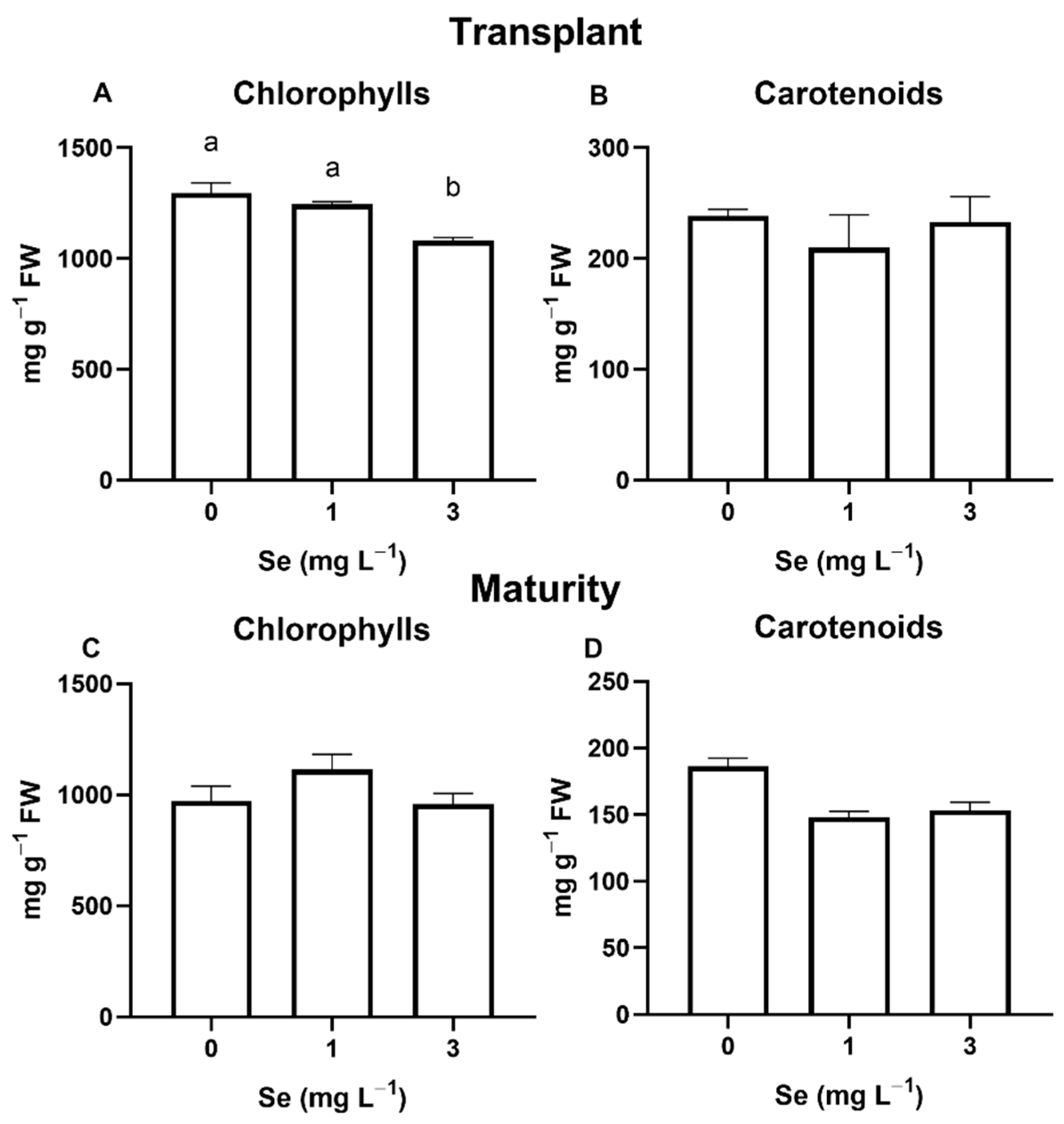
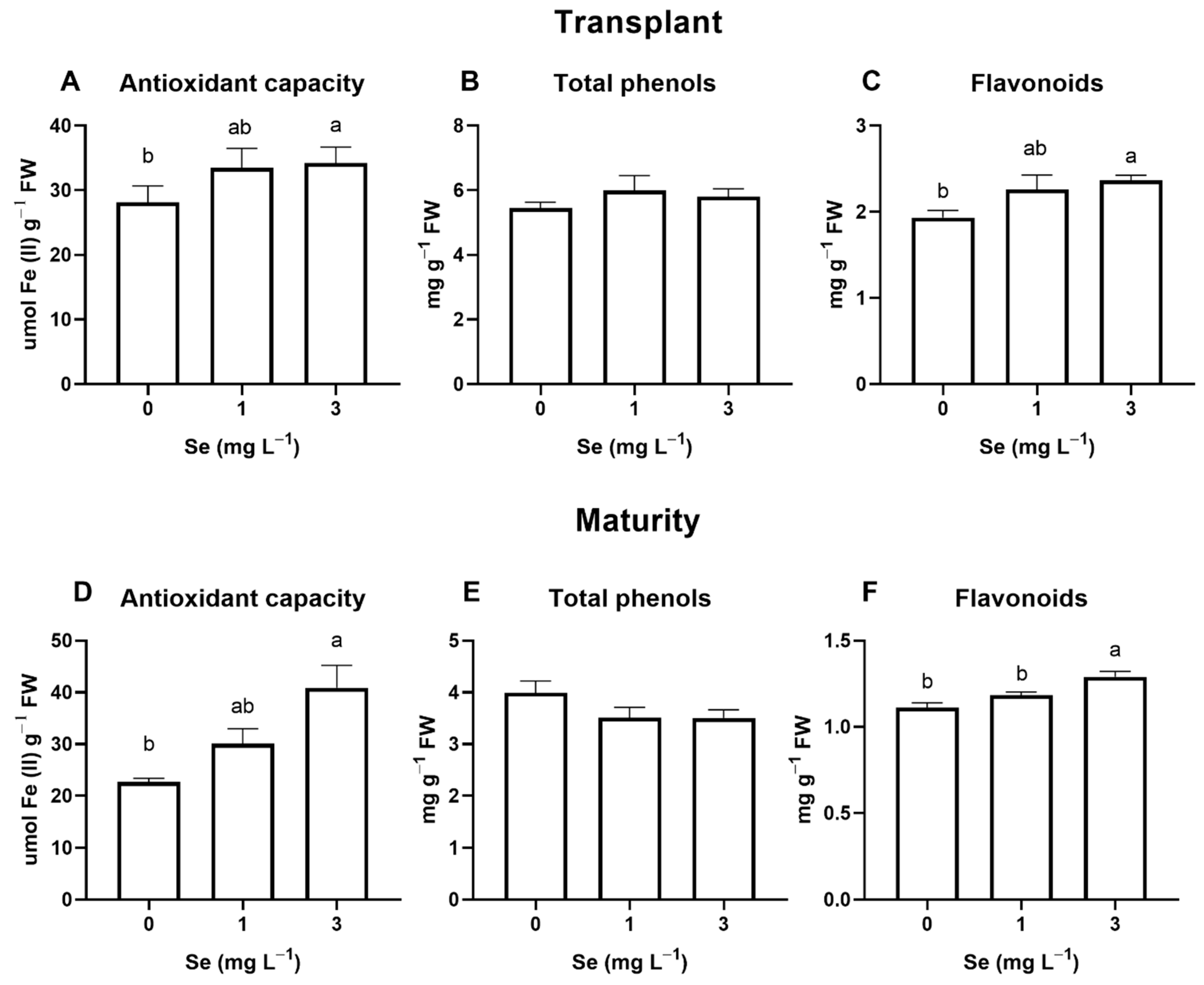
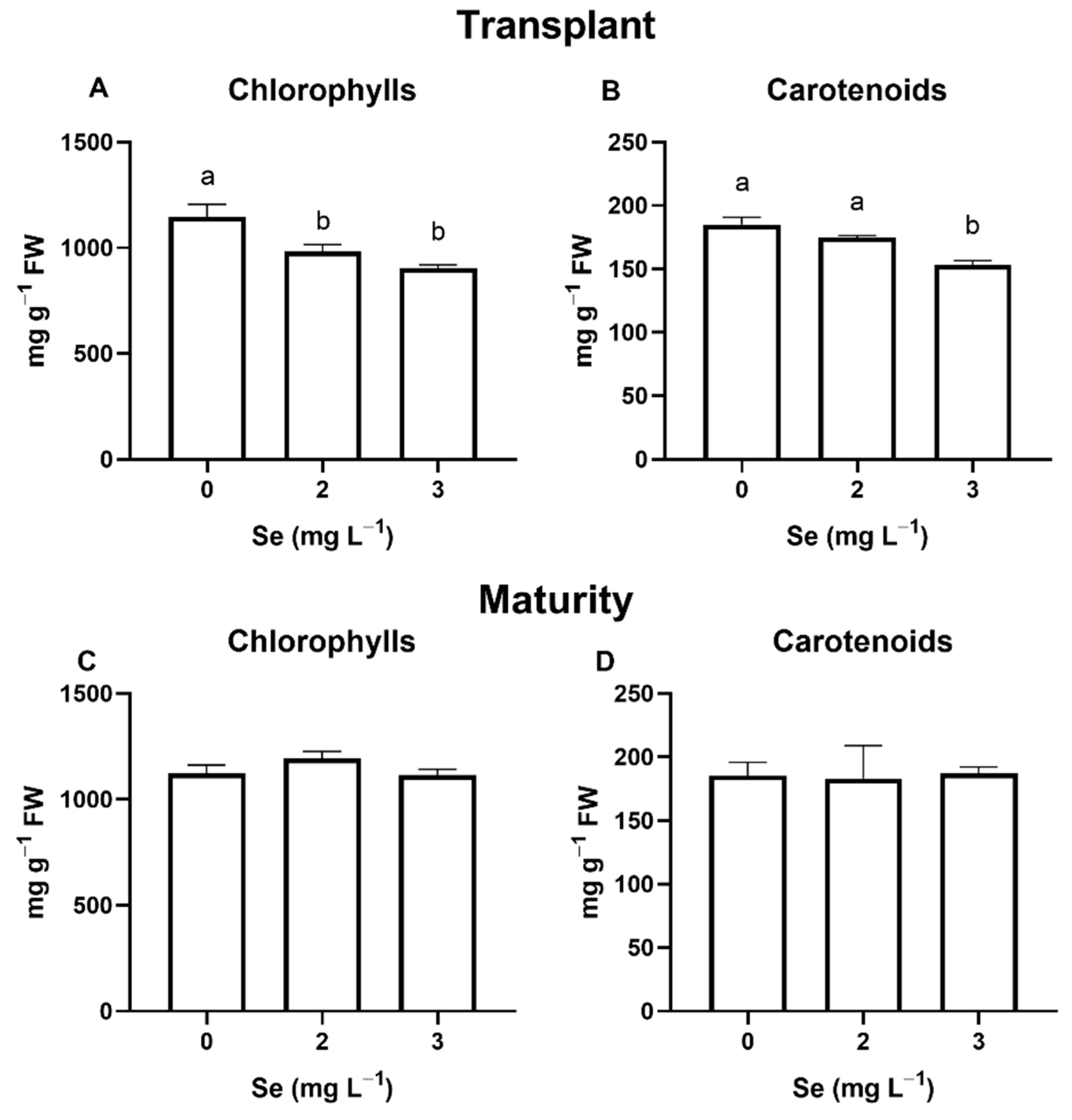
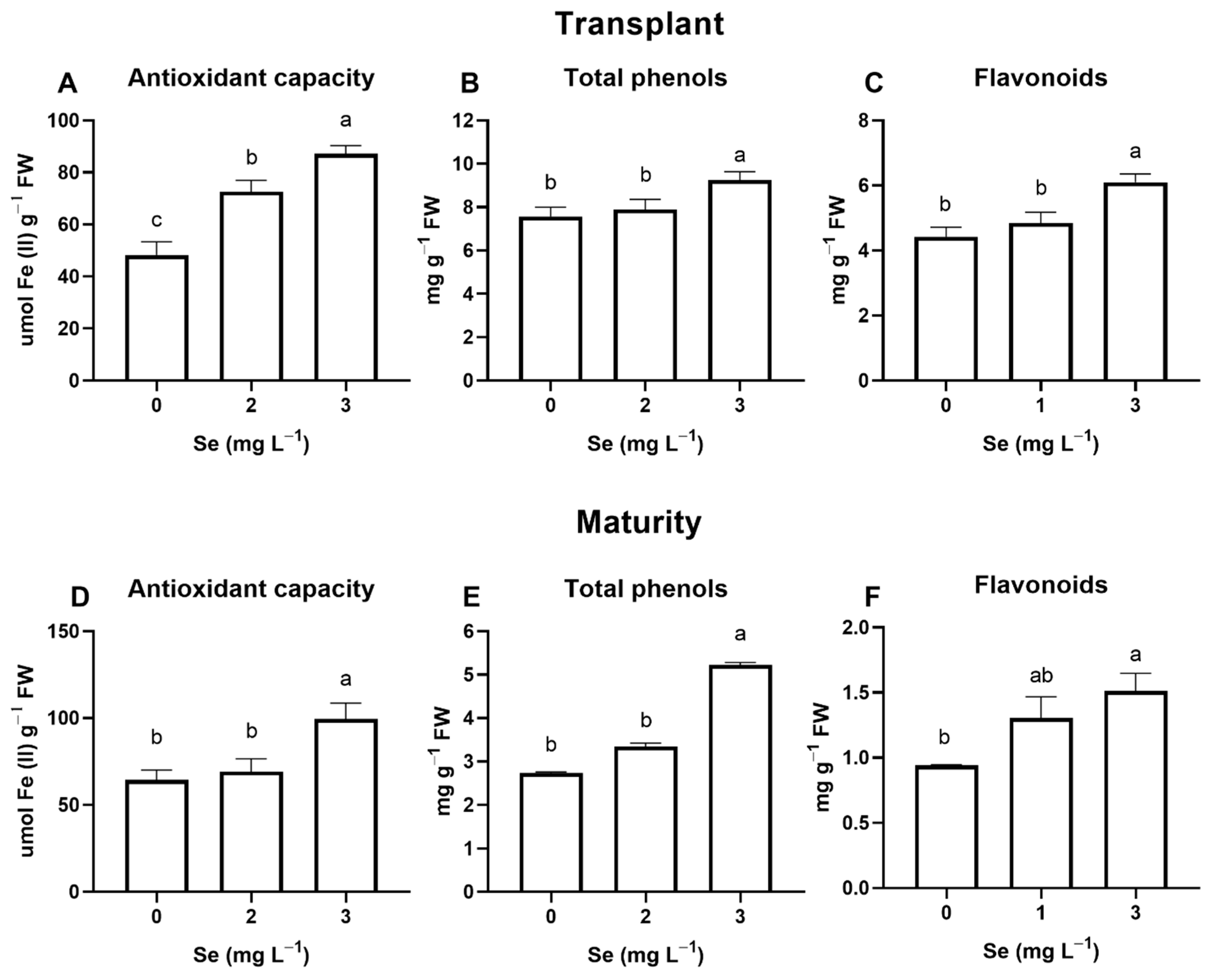
3.1. Lettuce Plants
3.1.1. Seedlings
3.1.2. Mature Plants
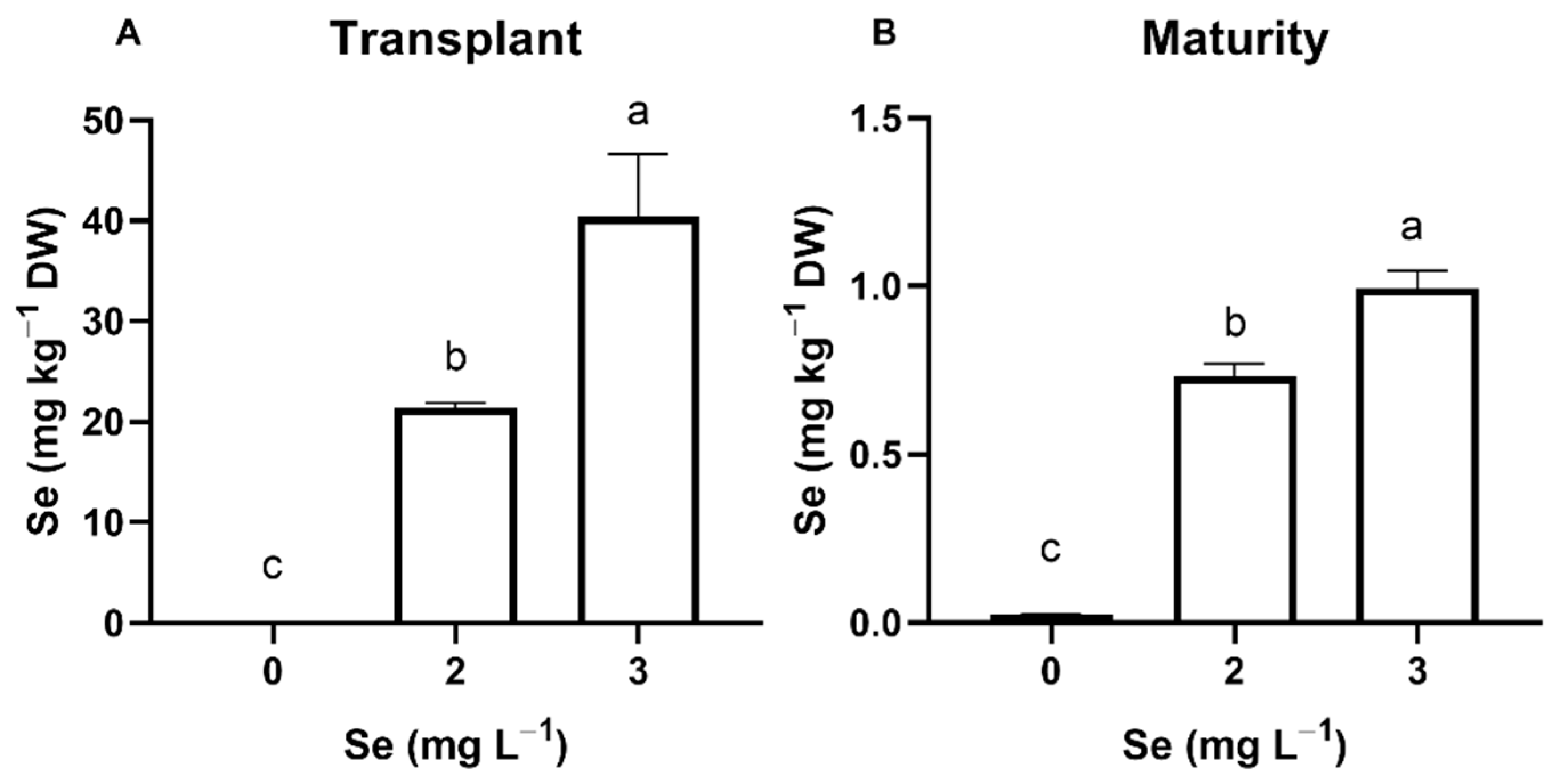
3.2. Basil Plants
3.2.1. Seedlings
3.2.2. Mature Plants
4. Discussion
4.1. Effect of Se Treatments on Plant Se Accumulation
4.2. Effect of Se Treatments on Plant Growth
4.3. Effect of Se Treatments on Leaf Mineral Content
4.4. Effect of Se Treatments on Leaf Quality Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bañuelos, G.S.; Lin, Z.Q. Use and Development of Biofortified Agricultural Products; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-6005-8. [Google Scholar]
- Birringer, M.; Pilawa, S.; Flohé, L. Trends in Selenium Biochemistry. Nat. Prod. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Selenium; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2014; Volume 12. [Google Scholar]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium Metabolism in Plants. Plant Cell Monogr. 2010, 17, 225–241. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding Selenium Metabolism in Plants and Its Role as a Beneficial Element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of Selenium and Its Impact on Food Chain Quality and Human Health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium Increases Seed Production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium—An Antioxidative Protectant in Soybean during Senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of Selenium Biofortification on the Bioactive Compounds and Antioxidant Activity of Wheat Microgreen Extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Ardebili, Z.O.; Ardebili, N.O.; Jalili, S.; Safiallah, S. The Modified Qualities of Basil Plants by Selenium and/or Ascorbic Acid. Turk. J. Bot. 2015, 39, 401–407. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum Basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium Uptake, Translocation and Speciation in Wheat Supplied with Selenate or Selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics. Plants 2019, 8, 68. [Google Scholar] [CrossRef]
- White, P.J. Selenium Metabolism in Plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Bian, Z.; Lei, B.; Cheng, R.; Wang, Y.; LI, T.; Yang, Q. Selenium Distribution and Nitrate Metabolism in Hydroponic Lettuce (Lactuca Sativa L.): Effects of Selenium Forms and Light Spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Incrocci, L.; Rosellini, I.; Pezzarossa, B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae 2021, 7, 590. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; de Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Duma, M.; Alsina, I.; Dubova, L.; Stroksa, L.; Smiltina, Z. The Effect of Sodium Selenite and Selenate on the Quality of Lettuce. In Proceedings of the 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production, FOODBALT-2011, Jelgava, Latvia, 5–6 May 2011; pp. 39–44. [Google Scholar]
- Puccinelli, M.; Malorgio, F.; Maggini, R.; Rosellini, I.; Pezzarossa, B. Biofortification of Ocimum Basilicum L. Plants with Selenium. Acta Hortic. 2019, 1242, 663–670. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Businelli, D.; D’Amato, R.; Onofri, A.; Tedeschini, E.; Tei, F. Se-Enrichment of Cucumber (Cucumis Sativus L.), Lettuce (Lactuca Sativa L.) and Tomato (Solanum Lycopersicum L. Karst) through Fortification in Pre-Transplanting. Sci. Hort. 2015, 197, 697–704. [Google Scholar] [CrossRef]
- Iwashita, Y.; Nishi, K. Cultivation of Selenium-Enriched Vegetables in Large Scale. Biomed. Res. Trace Elem. 2004, 15, 72–75. [Google Scholar] [CrossRef]
- Kowalska, I.; Smoleń, S.; Czernicka, M.; Halka, M.; Kęska, K.; Pitala, J. Effect of Selenium Form and Salicylic Acid on the Accumulation of Selenium Speciation Forms in Hydroponically Grown Lettuce. Agriculture 2020, 10, 584. [Google Scholar] [CrossRef]
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium Fortification Induces Growth, Antioxidant Activity, Yield and Nutritional Quality of Lettuce in Salt-Affected Soil Using Foliar and Soil Applications. Plant Soil 2017, 421, 245–258. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum Sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology Access Details: Access Details: [Subscription Number 772810551]. J. Herbs. Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- ASA-SSSA. Physical and Chemical Methods. In Methods of Soil Analysis; ASA-SSSA: Madison, WI, USA, 1996; ISBN 0471597627. [Google Scholar]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and Partitioning of Selenium in Basil (Ocimum Basilicum L.) Plants Grown in Hydroponics. Sci. Hort. 2017, 225, 271–276. [Google Scholar] [CrossRef]
- UNI EN 13657; Characterization of Waste—Digestion for Subsequent Determination of Aqua Regia Soluble Portion of Elements. Comite Europeen de Normalisation: Brussels, Belgium, 2004.
- UNI EN ISO 17294-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. Comite Europeen de Normalisation: Brussels, Belgium, 2016.
- Olsen, S.R.; Sommers, E.L. Phosphorus. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Adv. Photosynth. Res. 1984, 2, 9–12. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and Antiproliferative Activities in Different Maturation Stages of Broccoli (Brassica Oleracea Italica) Biofortified with Selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Funes-Collado, V.; Morell-Garcia, A.; Rubio, R.; López-Sánchez, J.F. Selenium Uptake by Edible Plants from Enriched Peat. Sci. Hort. 2013, 164, 428–433. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Lodine and Selenium Biofortification of Lettuce (Lactuca Sativa L.) by Soil Fertilization with Various Compounds of These Elements. Acta Sci. Pol. Hortorum 2016, 15, 69–91. [Google Scholar]
- Ramos, S.J.; Faquin, V.; Guilherme, L.R.G.; Castro, E.M.; Ávila, F.W.; Carvalho, G.S.; Bastos, C.E.A.; Oliveira, C. Selenium Biofortification and Antioxidant Activity in Lettuce Plants Fed with Selenate and Selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of Biofortification with Iodine and Selenium of Lettuce Cultivated in the NFT Hydroponic System. Sci. Hort. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Ferrarese, M.; Mahmoodi Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of Spinach Plants Applying Selenium in the Nutrient Solution of Floating System. Veg. Crop. Res. Bull. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; International Potash Institute: Zug, Switzerland, 1987; 687p. [Google Scholar]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Ríos, J.J.; Blasco, B.; Leyva, R.; Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M. Nutritional Balance Changes in Lettuce Plant Grown Under Different Doses and Forms of Selenium. J. Plant Nutr. 2013, 36, 1344–1354. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.Z. Selenium Assimilation and Nutrient Element Uptake in White Clover and Tall Fescue under the Influence of Sulphate Concentration and Selenium Tolerance of the Plants. J. Exp. Bot. 1992, 43, 549–555. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; Volume 8. [Google Scholar]
- Lee, S.-J.; Kang, H.-M.; Kim, I.-S. Effect of Sodium Selenate Supplied Condition by Fertigation on the Growth and Content of Minerals, Ascorbic Acid, Nitrate, and Selenium of Some Western Vegetables. J. Bio-Environ. Control 2008, 17, 43–50. [Google Scholar]
- Ríos, J.J.; Blasco, B.; Rosales, M.A.; Sanchez-Rodriguez, E.; Leyva, R.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Response of Nitrogen Metabolism in Lettuce Plants Subjected to Different Doses and Forms of Selenium. J. Sci. Food Agric. 2010, 90, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Comparative Effects of Selenite and Selenate on Growth and Selenium Accumulation in Lettuce Plants under Hydroponic Conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of Selenium Supplementation on Growth and Selenium Accumulation on Spinach (Spinacia Oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum Lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a New Definition of Quality for Fresh Fruits and Vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Enhanced Selenium Content in Sweet Basil (Ocimum Basilicum L.) by Foliar Fertilization. Veg. Crop. Res. Bull. 2008, 69, 63–72. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in Plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]




| Cultivation Event | Date | |
|---|---|---|
| Sowing date and selenate treatment | 21 May 2020 | |
| Transplanting/1st sampling | 13 June 2020 | |
| Maturity/2nd sampling | 3 July 2020 | |
| Climatic Parameters | Seedlings Cultivation | Open Field Cultivation |
| Mean air temperature (°C) | 27.0 | 21.6 |
| Mean air relative humidity (%) | 54.0% | 79.8% |
| Mean daily solar radiation (MJ m−2 day−1) | 15.9 | 23.0 |
| Cumulative solar radiation (MJ m−2) | 381.1 | 438.2 |
| Se (mg L−1) | P-PO43− (g kg−1 DW) | K (g kg−1 DW) | Na (g kg−1 DW) | Ca (g kg−1 DW) | Mg (g kg−1 DW) | |
|---|---|---|---|---|---|---|
| Transplant | 0 | 8.24 | 64.76 | 5.70 b | 8.15 | 3.75 b |
| 1 | 6.76 | 59.90 | 6.79 a | 8.19 | 3.74 b | |
| 3 | 6.96 | 62.60 | 7.22 a | 8.37 | 4.34 a | |
| ANOVA | ||||||
| Se | ns | ns | * | ns | * | |
| Maturity | 0 | 9.63 | 67.63 | 0.96 | 11.85 | 3.93 |
| 1 | 10.43 | 66.19 | 0.80 | 10.25 | 3.25 | |
| 3 | 10.09 | 60.51 | 0.73 | 10.06 | 3.25 | |
| ANOVA | ||||||
| Se | ns | ns | ns | ns | ns | |
| Se (mg L−1) | Cu (mg kg−1 DW) | Mn (mg kg−1 DW) | Fe (mg kg−1 DW) | Zn (mg kg−1 DW) | |
|---|---|---|---|---|---|
| Transplant | 0 | 1.00 b | 82.0 b | 294.0 b | 119.3 b |
| 1 | 1.33 ab | 88.0 ab | 248.0 b | 159.3 a | |
| 3 | 1.60 a | 91.3 a | 374.0 a | 161.3 a | |
| ANOVA | |||||
| Se | * | * | * | * | |
| Maturity | 0 | 24.7 b | 72.7 b | 865.3 b | 72.7 b |
| 1 | 30.7 a | 100.0 ab | 1147.3 a | 68.7 b | |
| 3 | 30.7 a | 127.3 a | 1162. a | 86.7 a | |
| ANOVA | |||||
| Se | ** | ** | * | * | |
| Se (mg L−1) | P-PO43− (g kg−1 DW) | K (g kg−1 DW) | Na (g kg−1 DW) | Ca (g kg−1 DW) | Mg (g kg−1 DW) | |
|---|---|---|---|---|---|---|
| Transplant | 0 | 6.20 a | 44.97 | 1.30 a | 16.19 | 2.84 |
| 1 | 5.84 a | 39.00 | 0.16 b | 13.91 | 2.54 | |
| 3 | 4.98 b | 40.43 | 0.23 b | 14.91 | 2.41 | |
| ANOVA | ||||||
| Se | * | ns | ** | ns | ns | |
| Maturity | 0 | 5.80 | 48.17 | 0.02 | 23.43 | 4.56 b |
| 1 | 3.96 | 41.50 | 0.01 | 24.41 | 4.87 ab | |
| 3 | 4.64 | 43.73 | 0.01 | 22.56 | 5.15 a | |
| ANOVA | ||||||
| Se | ns | ns | ns | ns | * | |
| Se (mg L−1) | Cu (mg kg−1 DW) | Mn (mg kg−1 DW) | Fe (mg kg−1 DW) | Zn (mg kg−1 DW) | |
|---|---|---|---|---|---|
| Transplant | 0 | 1.6 | 84.7 | 218.7 | 130.7 |
| 1 | 1.3 | 69.3 | 152.0 | 113.3 | |
| 3 | 2.0 | 84.7 | 179.3 | 133.3 | |
| ANOVA | |||||
| Se | ns | ns | ns | ns | |
| Maturity | 0 | 15.3 a | 120.7 a | 449.3 a | 94.0 a |
| 1 | 8.0 b | 78.7 b | 372.7 ab | 80.7 b | |
| 3 | 7.3 b | 82.7 b | 345.3 b | 82.7 b | |
| ANOVA | |||||
| Se | ** | * | * | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccinelli, M.; Malorgio, F.; Pintimalli, L.; Rosellini, I.; Pezzarossa, B. Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables. Horticulturae 2022, 8, 801. https://doi.org/10.3390/horticulturae8090801
Puccinelli M, Malorgio F, Pintimalli L, Rosellini I, Pezzarossa B. Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables. Horticulturae. 2022; 8(9):801. https://doi.org/10.3390/horticulturae8090801
Chicago/Turabian StylePuccinelli, Martina, Fernando Malorgio, Lucia Pintimalli, Irene Rosellini, and Beatrice Pezzarossa. 2022. "Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables" Horticulturae 8, no. 9: 801. https://doi.org/10.3390/horticulturae8090801
APA StylePuccinelli, M., Malorgio, F., Pintimalli, L., Rosellini, I., & Pezzarossa, B. (2022). Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables. Horticulturae, 8(9), 801. https://doi.org/10.3390/horticulturae8090801






