Abstract
The effects of 1.0 μL/L 1-methylcyclopropene (1-MCP) treatment on aroma quality and ester-biosynthesis-related gene expression of ‘Jinyan’ kiwifruit during room storage were examined, aiming to provide a theoretical basis and technical reference for the postharvest storage of kiwifruit. The results demonstrate that 1-MCP treatment conspicuously inhibited respiration rate, delayed a decrease in fruit firmness and increased soluble solid content (SSC) in ‘Jinyan’ kiwifruit. Compared to the control, the relative content of aroma components markedly changed in 1-MCP treatment kiwifruit during fruit ripening. The characteristic aroma of ‘Jinyan’ kiwifruit included ethyl butanoate, methyl butanoate, E-2-hexanal and hexenal, and 1-MCP treatment significantly reduced the ester content in kiwifruit. During the entire shelf life, the expression levels of AcLOX1, AcLOX5, AcLOX6, AcHPL and AcAAT were significantly inhibited in 1-MCP-treated fruit. However, the transcript level of AcADH was not suppressed by 1-MCP. The lower content of ester volatiles maybe ascribed to the suppression of AcLOXs, AcHPL and AcAAT.
1. Introduction
‘Jinyan’ kiwifruit (Actinidia chinensis Planch) is a yellow-fleshed, late-maturing cultivar bred through interspecific hybridization between Actinidia eriantha and Actinidia chinensis [1]. In recent years, the consumption of ‘Jinyan’ kiwifruit has increased mainly because of its strong aroma and special taste [2]. However, kiwifruit is a typical climacteric fruit and its ripening is accompanied by a respiration peak that shortens its shelf life [3,4]. Many studies have reported that 1-methylcyclopropene (1-MCP) could delay postharvest ripening and maintain the quality of kiwifruit. For example, 1-MCP significantly reduced the ethanol accumulation, affected the process of energy metabolism and prolonged the shelf life of ‘Bruno’ kiwifruit [5]. ‘Hardy’ kiwifruit had higher ascorbic acid and maintained higher hardness by 1-MCP treatment during cold storage [6].
Fruit quality is usually decided by external shape, color, internal soluble sugar, titratable acidity, aroma and so on. Aroma is one of the important flavor factors directly affecting sensory quality [7,8] that has received much attention. Studies have revealed that volatile compounds, which are commonly considered to be secondary metabolites, are biosynthesized by a series of enzymatic reactions during the growth, development and maturity processes of fruit [9,10]. At present, more than 300 volatile compounds have been identified in fruit [11,12]. Fruit aroma is determined by a complex mixture of many aromatic compounds, including esters, aldehydes, alcohols and terpenes. According to previous studies, fruit aroma quality depends on the types and concentrations of volatile components that affect organoleptic attributes [12]. Studies have also found that the synthesis of aroma significantly differs among varieties, postharvest handling and storage methods in fruit [2,13,14].
The lipoxygenase (LOX) pathway, which is derived from fatty acid (FA) metabolism, is one of the key pathways for volatiles synthesis in many fruits. As the main substrates, the unsaturated fatty acids, including linoleic acid and linolenic acid, are catabolized into hydroperoxides, C6 aldehyde, alcohol and ester via the LOX pathway [11,15,16]. C18 polyunsaturated fatty acids are converted to hydroperoxides by LOX and further form C6 aldehyde by hydroperoxide lyase (HPL). Then, C6 aldehydes are converted to C6 alcohols with the removal of hydrogen by alcohol dehydrogenase (ADH). Finally, alcohols and acyl-CoAs are combined to synthesize various esters by alcohol acyltransferase (AAT) [17,18]. LOX family genes have six members in kiwifruit, AcLOX1, AcLOX2, AcLOX3, AcLOX4, AcLOX5 and AcLOX6, which exhibit differential expression patterns during the ripening process [19]. AAT is a terminal gene of fatty acid metabolism for ester synthesis [17]. HPL and ADH are also involved in the production of volatiles [20,21].
As an effective ethylene receptor inhibitor, 1-MCP is widely applied in the postharvest storage and quality preservation of various climacteric fruits [22,23,24]. 1-MCP was also used in postharvest kiwifruit to maintain fruit quality, prolong shelf life and delay senescence [25,26]. However, aroma biosynthesis was markedly affected by 1-MCP treatment in postharvest apple [27], banana [28] and peach [29]. In ‘Yate’ kiwifruit, the total content of aroma compounds was reduced by 1-MCP treatment [28]. However, the effects of 1-MCP on the production of volatiles and relevant gene expression are not very clear in ‘Jinyan’ kiwifruit. In the present investigation, the effects of 1-MCP treatment on the production of volatiles and the gene expression patterns of AcLOXs, AcHPL, AcADH and AcAAT in ‘Jinyan’ kiwifruit during room storage were investigated. Based on these results, the possible regulatory mechanism of 1-MCP in volatile formation in postharvest ‘Jinyan’ kiwifruit was discussed, to provide a theoretical basis and technical reference for the postharvest storage and preservation of kiwifruit.
2. Materials and Methods
2.1. Plant Materials, Treatments and Sampling
‘Jinyan’ kiwifruit fruits were harvested at a physiological maturity stage (firmness: 1191.05 g ± 100.39 g, soluble solid content (SSC): 9.0 ± 0.4° Brix) from a commercial orchard with standard cultural practices in Fengxin County, Jiangxi Province, China. The fruits without visible signs of damage or infections on surface were selected and transported to postharvest laboratory in Jiangxi Agricultural University immediately. Then, careful selection was conducted again to discard fruits with physical injuries or disease symptoms. Finally, 600 fruits were randomly divided into two groups; one group of fruits was held in a gas-tight plastic container and treated with 1.0 μL/L 1-MCP (Sinopharm Chemical Reagent Beijing Co., Ltd, Beijing, China) for 12 h at room temperature (20 ± 1 °C) [30], and the other group of fruits was also held in a gas-tight plastic container for 12 h without any treatment at the same temperature as the control. Both the control and 1-MCP treatment fruits were packed with a plastic bag (0.06 mm), 100 fruits per bag and 3 bags for each treatment, then stored at room temperature (20 ± 1 °C) at 85–90% relative humidity. During storage, 15 fruits per treatment were sampled at 0, 2, 4, 6, 8, 10 and 12 days after storage. The same 15 fruits were used to determined respiration rate, fruit firmness and SSC. Firstly, 3 replicates were taken for respiration rate and 15 replicates for fruit firmness and SSC measurement, then the flesh was gathered and frozen in liquid nitrogen immediately and stored at −80 °C for further analysis.
2.2. Determination of Fruit Physiological Indexes
Kiwifruit respiration rate was measured using the respiration analyzer (GHX-3051H, Beijing, China) with 1040 μL/L CO2 as the standard for calibration. The carrier gas was the CO2-removed air, the gas flow rate was 0.5 L/min and the respiration rate was calculated as mg CO2·kg−1·h−1. Fruit firmness was monitored using a TAXL texture analyzer (Stable Micro Systems, Surrey, UK) with an 8.0 mm-diameter probe head which penetrated the fruit at a speed of 5 mm s−1 to 10 mm depth. After the removal of skin, 15 fruits were used to measure twice on the opposite sides at the equator of each fruit, and results are expressed in grams (g). For SSC determination, each fruit was sampled for 3–4 drops of juice, which were then mixed and measured by a digital refractometer (RA250-WE, Kyoto, Japan), and the data are expressed as ° Brix.
2.3. Fruit Volatile Analysis
Three grams of frozen flesh sample were ground in liquid nitrogen to a fine powder, then transferred to a 20 mL vial sealed with a PTFE (polytetrafluoroethylene) silicone pad and an aluminum cap. In order to minimize the loss of volatile components and avoid browning, 3 mL saturated sodium chloride (NaCl) solution was subsequently added into the vial. Then, the vial was vortexed with 30 s to homogenize the mixture and maximize volatile emissions. The volatile compounds of kiwifruit were analyzed using a gas chromatograph–mass spectrometer (GC–MS-TQ 8040, Shimadzu, Kyoto, Japan) fitted with headspace sampler (HSS 86.5, DANI, Cologno, Italy) and SH-Rtx-Wax column (30 m × 0.25 mm, 0.25 μm). The headspace sampler conditions were set as follows: the pipe, transmission and oven temperature were held at 90 °C, 85 °C and 80 °C, respectively. The other conditions were set as follows: the oven temperature was held at 40 °C for 2 min firstly, then increased gradually at a rate of 5 °C min−1 to 180° C and kept for 3 min, and after that increased again to 230 °C at 5 °C min−1. The split ratio of the GC–MS was 2:1. Helium was used as a carrier gas with a flow rate at 1.0 mL min−1. Electronic ionization was used at 70 eV. The mass spectrometer was operated from 30 to 400 amu in a full-scan mode. The temperatures of ion source and connecting parts were held at 230 °C and 250 °C, respectively. Since standards were not available, NIST (National Institute of Standards and Technology) 2008 libraries were used to identify volatile compounds of kiwifruit. Most compounds were identified and confirmed by contrast with their linear retention indices and EI mass spectra; the results are described as relative content according to peak area percentage.
2.4. RNA Extraction and cDNA Synthesis
The quick RNA isolation Kit (HuaYueYang Biological Technology Beijing Co., Ltd, Beijing, China) was used to extract total RNA from 1 g kiwifruit samples. Then, the RNA was used to synthesize first-strand cDNA by a PrimeScript RT reagent kit (Takara, Dalian, China) under the manufacturer’s instructions. The DNA erasure and reverse transcription were based on the following steps: 2 µL 5× gDNA Eraser Buffer, 7 μL DNA-free RNA and 1 µL gDNA Eraser were mixed and incubated at 42 °C for 2 min. Then, 4 μL RNase Free dH2O, 4 μL 5× PrimeScript Buffer II, 1 μL RT Primer mix and 1 μL PrimeScript RT Enzyme mix I were added to the above reaction solution, transiently centrifuged, and the reaction was run at 37 °C for 15 min, 85 °C for 5 s and 4 °C for 5 min. The cDNA was used as the template for real-time quantitative PCR (RT-qPCR) analysis.
2.5. RT-qPCR
The RT-qPCR mixture (10 μL total volume) contained 3.4 μL RNase-free water, 1 μL cDNA, 0.3 μL of each primer (10 mM) and 5 μL SYBR Premix Ex Taq (Takara, Dalian, China). The two-step RT-qPCR program was started with an initial step of 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s and 63 °C for 25 s. The melting point curve was created according to the fluorescence collected with 55 °C–95 °C. The standard curve was generated to minimize discrepancies in amplification efficiencies between the actin gene and target genes [31]. Control reactions for each primer pair were performed in each run. According to the reported sequences of related genes [19,32], RT-qPCR primers were designed using Primer 3 online (version 0.4.0, http://frodo.wi.mit.edu/primer3/input.htm, accessed on 20 March 2020). Relative expression levels of target genes were calculated by the 2−ΔΔCt method [33], and three independent biological replicates were run for each sample. The primers sequences used for RT-qPCR analysis are shown in Table S1. The amplification, melt curve and melt peak of RT-qPCR are shown in Figure S1.
2.6. Statistical Analysis
Data were calculated and analyzed using SPSS (Statistical Program for Social Sciences) version 20.0 (SPSS Inc., Chicago, IL, USA). Duncan’s new multiple range test was used to compare the significance of differences between treatments. The Pearson correlation coefficient was used to measure the strength of the linear correlation between total aroma content and expression level of biosynthesis-related genes without normalization before analysis. Figures were created with GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) and HemI (Heatmap Illustrator, version 1.0, The CUCKOO Workgroup, Wuhan, China).
3. Results
3.1. Effect of 1-MCP Treatment on Respiration Rate, Fruit Firmness and Soluble Solid Content
Kiwifruit is one of typical climacteric fruits and exhibits an obvious respiration peak during postharvest storage. The respiration rate showed the same change patterns both in control and 1-MCP-treated ‘Jinyan’ kiwifruit during the storage period, which increased gradually to peak at 84.84 mg CO2·kg−1·h−1 at 2 days in the control and 55.02 mg CO2·kg−1·h−1 in 1-MCP at 6 days first, followed by decreasing and subsequently slowly increasing, as shown in Figure 1A. Of note, the respiration rate of 1-MCP-treated fruit was significantly lower than the control fruit, suggesting that 1-MCP delayed the appearance of the respiration peak and inhibited the respiration rate.
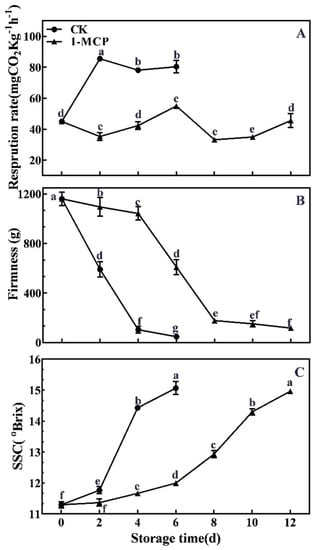
Figure 1.
Effects of 1-MCP treatment on respiratory rate (A), firmness (B) and soluble solid content (C) in ‘Jinyan’ kiwifruit during storage. Error bars indicate standard error (±SE). Different letters in the same figure represent a significant difference at p < 0.05 according to Duncan’s new multiple range test. d: days.
As shown in Figure 1B, the fruit firmness of the kiwifruit showed a gradual downward trend during postharvest storage. Fruit showed a dramatic softening before 4 days in control fruits (from 1161.15 g to 103.27 g), while 1-MCP-treated fruits had no obvious softening before 4 days but sharply decreased from 1042.48 g at 4 days to 164.487 g at 8 days, followed by slightly decreasing during the later storage period. Interestingly, the fruit firmness of 1-MCP-treated kiwifruit was significantly higher than the control fruit throughout the whole storage period, indicating that fruit softening was effectively inhibited by 1-MCP treatment.
Contrary to changes in fruit firmness, the SSC of kiwifruit gradually accumulated during the storage period, as shown in Figure 1C. The SSC of control fruit increased rapidly from 11.3° Brix at 2 days to 15.1° Brix at 6 days. The rate of increase in SSC was significantly inhibited by 1-MCP treatment, with fruit showing little change for the first 6 days, followed by a substantial increase to 15° Brix after 12 days. In general, 1-MCP-treated fruit maintained superior internal fruit quality.
3.2. Effect of 1-MCP Treatment on Volatile Production
Twenty-three volatile compounds were identified from ‘Jinyan’ kiwifruit during postharvest storage by GC–MS, including eleven esters, four alcohols, four aldehydes, one terpene, one ketone and two others, and ester and aldehyde were the most abundant volatiles (Table 1). Different aromatic profiles showed distinct changes in both control and 1-MCP-treated kiwifruits during storage. As shown in Figure 2, 1-MCP treatment inhibited ester production, delayed fruit aldehyde formation and significantly diminished the relative content of each of these compounds, while increasing alcohol relative content in kiwifruit. 1-MCP treatment also reduced aroma components, particularly four ester components, suggesting that 1-MCP treatment significantly influenced fruit volatile components and their relative content.

Table 1.
The aroma components in ‘Jinyan’ kiwifruit by gas chromatograph–mass spectrometer analysis.
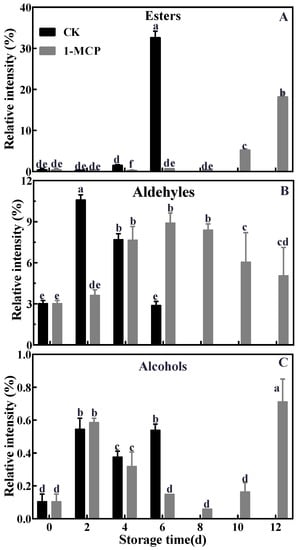
Figure 2.
Effects of 1-MCP on the relative contents of ester (A), aldehyde (B) and alcohol (C) in ‘Jinyan’ kiwifruit during storage. Error bars indicate standard error (±SE). Different letters in the same figure represent a significant difference at p < 0.05 according to Duncan’s new multiple range test. d: days.
As shown in Figure 2A, the relative content of esters was low in kiwifruit during the earlier stage of storage and then dramatically increased. The ester content increased significantly from 1.44% at 4 days to 32.60% at 6 days in control kiwifruit, while it only increased from 0.68% at 6 days to 18.18% at 12 days in 1-MCP-treated kiwifruit. This indicated that 1-MCP treatment significantly inhibited esters production during the ripening of postharvest kiwifruit. The relative content of fruit aldehydes increased first to a maximum of 10.61% at 2 days and 8.91% at 6 days in the control and 1-MCP treatment groups, respectively, and then declined (Figure 2B). During postharvest storage, the content of alcohols showed similar change patterns to aldehydes, which increased gradually at 2 d, then decreased and increased again at 6 days and 12 days for control and 1-MCP-treated fruits, respectively (Figure 2C). Furthermore, the most abundant aroma volatiles in ‘Jinyan’ kiwifruit were esters and aldehydes, including methyl butanoate, ethyl butanoate, E-2-hexenal and hexanal (Table 1). As shown in Figure 3, changes in methyl butanoate and ethyl butanoate showed a gradual upward trend in both control and 1-MCP-treated kiwifruit, and the peak appeared at 6 days and 12 days in the control and 1-MCP-treated fruits, respectively, but the peak content of ethyl butanoate and methyl butanoate was approximately 68.51% and 16.61% in 1-MCP-treated kiwifruits compared to controls. The two esters, as the characteristic aromatic components in kiwifruit, were highly consistent with total ester changes. Of note, 1-MCP treatment significantly inhibited and delayed the biosynthesis of ester volatiles in ‘Jinyan’ kiwifruit throughout the entire storage period. Hexanal and E-2-hexenal, the other characteristic aroma components of ‘Jinyan’ kiwifruit, were highly consistent with total aldehyde changes, and their relative content increased initially then decreased during postharvest storage. Hexanal concentration increased dramatically from 0.87% to 3.57% and E-2-hexenal increased from 1.89% to 6.96% in control fruit at 2 days after storage. However, the highest concentration of these two aldehydes in 1-MCP-treated fruit was just 2.97% and 6.04% at 6 days, respectively. These results indicate that aromatic components exhibited obvious differences from aldehydes during the initial period of storage compared to esters, including ethyl butyrate and methyl butyrate, with the ripening of ‘Jinyan’ kiwifruit.
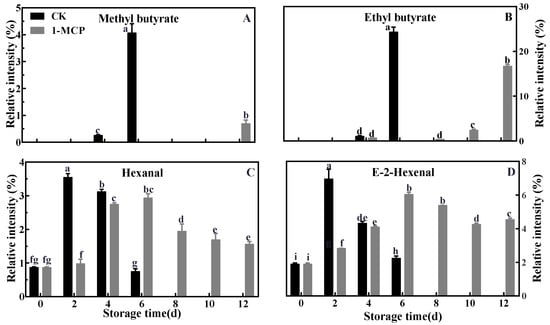
Figure 3.
Effects of 1-MCP treatment on the relative contents of ethyl butyrate (A), methyl butyrate (B), hexanal (C) and E-2-hexenal (D) in ‘Jinyan’ kiwifruit during storage. Error bars indicate standard error (±SE). Different letters in the same figure represent a significant difference at p < 0.05 according to Duncan’s new multiple range test. d: days.
3.3. Effect of 1-MCP Treatment on Transcription Levels of Volatile Biosynthesis-Related Genes
LOX is a key enzyme in the synthesis of flavor components in kiwifruit. There are six family members of LOX genes. Transcript levels of all LOX genes showed distinct patterns throughout the storage period (Figure 4). Transcript levels of AcLOX1, AcLOX5 and AcLOX6 were dramatically up-regulated at 6 days in control fruits, while their expression remained very low until 12 days in 1-MCP-treated kiwifruits during fruit ripening. Interestingly, the expression of these genes in ‘Jinyan’ kiwifruit was significantly inhibited in response to 1-MCP treatment. In contrast, expression levels of AcLOX2, AcLOX3 and AcLOX4 were down-regulated in control fruits, but 1-MCP treatment promoted their expression peak at 2 days of storage.
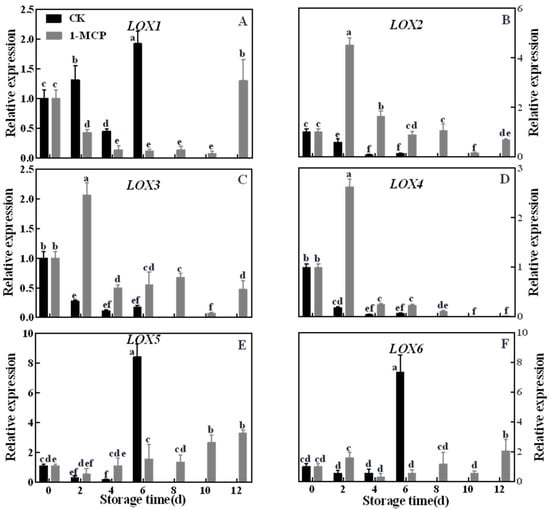
Figure 4.
Effects of 1-MCP treatment on the gene expression level of lipoxygenase (LOX)’s ((A) AcLOX1, (B) AcLOX2, (C) AcLOX3, (D) AcLOX4, (E) AcLOX5, (F) AcLOX6) in ‘Jinyan’ kiwifruit during storage. Error bars indicate standard error (±SE). Different letters in the same figure represent a significant difference at p < 0.05 according to Duncan’s new multiple range test. d: days.
The expression levels of AcHPL, AcADH and AcAAT in kiwifruit during storage are shown in Figure 5. The transcript level of AcHPL was dramatically up-regulated at 2 days in control fruits, then decreased, while its expression was obviously inhibited by 1-MCP, for which the highest transcript level was significantly lower than in control fruits (Figure 5A). The expression patterns of AcADH were similar in control and 1-MCP-treated kiwifruits, and 1-MCP had no significant effect on AcADH expression during storage of kiwifruits (Figure 5B). The expression level of AcAAT was sustained at a stable level during early storage, then up-regulated to 3.11 at 6 days in the control group and 1.50 at 12 days in 1-MCP-treated fruit (Figure 5C). It should be noted that 1-MCP treatment also inhibited the expression level of AcAAT in ‘Jinyan’ kiwifruit.
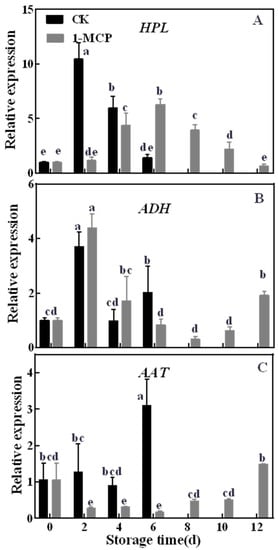
Figure 5.
Effects of 1-MCP treatment on the gene expression level of hydroperoxidelyase (HPL) (A), alcohol dehydrogenase (ADH) (B) and alcohol acyltransferase (AAT) (C) in ‘Jinyan’ kiwifruit during storage. Error bars indicate standard error (±SE). Different letters in the same figure represent a significant difference at p < 0.05 according to Duncan’s new multiple range test. d: days.
Finally, a correlation indicated that changes in the content of total aroma esters was significantly positively correlated with the expression of AcLOX1, AcLOX5, AcLOX6 and AcAAT, while significantly negatively correlated with AcLOX2, AcLOX3 and AcLOX4 (Figure 6). Meanwhile, the expression levels of AcLOX1, AcLOX5, AcLOX6 and AcAAT were also significantly positively correlated with each other, the same as AcLOX2, AcLOX3 and AcLOX4, which showed a co-expression pattern involved in aroma biosynthesis during kiwifruit storage.
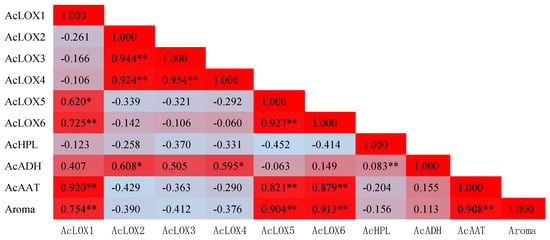
Figure 6.
Correlation coefficients between total aroma content and expression level of biosynthesis-related genes in ‘Jinyan’ kiwifruit (* correlation is significant at the 0.05 level; ** correlation is significant at the 0.01 level).
4. Discussion
In recent years, many studies have reported the application of 1-MCP on the storage and preservation of fruits and vegetables. Previous studies showed that 1-MCP treatment maintained the quality and extended the storage life of many climacteric fruits such as strawberry [34], pear [35] and kiwifruit [25,26]. In this study, 1-MCP treatment conspicuously inhibited respiration rate, delayed the decline in fruit firmness and the increase in SSC, delayed senescence and prolonged the shelf life of ‘Jinyan’ kiwifruit, all of which is in accordance with previous studies [26,36]. The function of ethylene on the ripening of postharvest fruits was significantly inhibited by 1-MCP treatment.
As a secondary metabolite, aroma is an important fruit quality which is being focused on by more and more consumers and researchers. There are more than 300 volatile compounds that have been identified in fruit. Fruit aroma quality depends on the types and concentrations of volatile components that affect organoleptic attributes. For example, the ‘fruit candy’ aroma is associated with ethyl hexanoate and ethyl butanoate [37]. Terpene is described as presenting tropical, mint-like, sweet attributes, and its generation increases during a fruit’s shelf life [38]. Previous reports indicated that kiwifruit possesses abundant volatile components, leading to unique aroma in ripen fruit. The characteristic aromas were identified in different varieties of kiwifruit [2,39]; ethyl butanoate and methyl butanoate were the key aroma volatile compounds in ‘Hongyang’ [40] and ‘Hort16A’ [37] kiwifruit. For ‘Jinyan’ kiwifruit, the most abundant aroma volatiles were esters and aldehydes, including ethyl butanoate, methyl butanoate, hexanal and E-2-hexenal. With the ripening of kiwifruit, the ester content increased continually while the aldehydes content decreased gradually, which indicated that the aldehydes were converted to esters during kiwifruit storage. Meanwhile, many studies also showed that postharvest treatment had significant effects on the aroma formation of kiwifruit. Propylene treatment induced the production of aroma volatile compounds in ‘Kosui’ kiwifruit [32]; the level of esters were also increased by ethylene treatment in ‘Hort16A’ kiwifruit during storage [37]. However, the volatile synthesis in apple [41,42] and banana [28] was markedly repressed due to 1-MCP treatment. In peach fruit, higher levels of C6 aldehydes and alcohols were found in 1-MCP fruits, while esters significantly decreased [29]. The results also show that 1-MCP treatment inhibited esters and aldehyde generation, then significantly reduced aroma components and relative content, and four kinds of esters disappeared in 1-MCP-treated kiwifruit. These findings indicate that 1-MCP treatment had obvious effects on the components and relative content of aroma in ‘Jinyan’ kiwifruit during storage. How to alleviate the negative role of 1-MCP on the aroma quality of postharvest fruit requires further study [21,43].
Numerous studies showed that aroma synthesis is closely related to the LOX pathway in many fruits during the ripening process, with key genes of LOX, HPL, ADH and AAT [44,45]. In this study, six AcLOX genes were divided into two groups according to their expression pattern during kiwifruit ripening. Expression levels of AcLOX1, AcLOX5 and AcLOX6 were up-regulated, which were consistent with the increase in ester components and severely suppressed by 1-MCP in ‘Jinyan’ kiwifruit during storage. On the contrary, expression levels of AcLOX2, AcLOX3 and AcLOX4 decreased with fruit ripening, while were increased rapidly after 1-MCP treatment, then down-regulated. A previous study suggested that the transcriptional abundances of AcLOX1 and AcLOX5 were up-regulated in response to kiwifruit ripening [19]. Previous studies showed that the production of aroma volatiles in kiwifruit was strongly dependent on ethylene [32]. Ethylene-mediated up-regulation of LOX genes has also been observed in melon for CmLOX3, CmLOX18 [46] and peach for PpaLOX3 [11] during fruit ripening and senescence. It is possible that AcLOX2, AcLOX3 and AcLOX4 were negatively regulated by ethylene.
The expression level of AcHPL, another gene involved in the LOX pathway, was also significantly inhibited by 1-MCP treatment, which was consistent with the change in aldehyde content both in the control and 1-MCP-treated kiwifruit during storage. In ‘Nanguo’ pears, the HPL expression was higher in CaCl2-treated group fruit, which was conducive to the synthesis of aldehydes [47]. These results suggest that HPL was a key gene in the synthesis of aldehydes, affecting the synthesis of esters. Meanwhile, 1-MCP treatment had no significant effect on the transcript level of AcADH, which was similar to the change in alcohol content. The increase in AcADH expression led to high alcohol content in 1-MCP-treated fruits at the end of storage. A previous study also suggested that the inhibition of alcohol production in the ‘Delbarde Estivale’ apple was consistent with a reduction in ADH activity and decrease in ADH gene expression [48]. Methyl jasmonate treatment increased the activities/expression of LOX, ADH, and AAT, then enhanced the content of volatile esters and unsaturated fatty acids in postharvest ’Nanguo’ pear [43].
Esters are biosynthesized through an esterification reaction between acyl-CoA and alcohol, catalyzed by AAT. Esters are the most abundant aromatic components in kiwifruit [2,39]. Our results show that the content of esters, including methyl butyrate and ethyl butyrate, increased dramatically at later storage periods in ‘Jinyan’ kiwifruit, which was consistent with the expression level of AcAAT gene during storage. The result was consistent with previous studies performed on peach [49,50]. Meanwhile, the transcript level of AcAAT was inhibited in 1-MCP-treated fruit. We speculate that AAT, as well as LOX and HPL, may play a key role in the ester synthesis of kiwifruit. The gene expression of AAT is controlled by ethylene in many climacteric fruits, such as pear [35] and apple [27], and the impact of ethylene on ester production in climacteric fruit is well accepted [45].
5. Conclusions
In summary, the work revealed that 1-MCP treatment was able to inhibit fruit softening and extend the storage life of ‘Jinyan’ kiwifruit during storage. Further investigation, based on GC–MS analysis, suggested the abundant aromas of ‘Jinyan’ kiwifruit were ethyl butanoate, methyl butanoate, E-2-hexanal and hexenal, and 1-MCP treatment significantly inhibited ester production during fruit ripening. During storage, the expression of AcLOX1, AcLOX5, AcLOX6, AcHPL and AcAAT increased and were also inhibited by 1-MCP treatment. The lower content of ester volatiles in 1-MCP-treated kiwifruit may be ascribed to the suppression of AcLOXs, AcHPL and AcAAT. Although this study will be useful for understanding how the mechanisms of 1-MCP affect the aroma quality of postharvest kiwifruit, the molecular mechanisms are still unclear. Thus, further studies should focus on transcript factors, gene functional verification and ethylene signal interactions on aroma formation during kiwifruit storage.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae7100381/s1, Figure S1: The amplification, melt curve and melt peak of RT-qPCR, Table S1: Sequences of primers used in qPCR.
Author Contributions
Conceptualization, M.C. and J.C.; methodology, X.A., Q.W. and Y.F.; formal analysis, X.A. and Q.W.; investigation, X.A., Q.W. and M.X.; data curation, X.C. and Z.L.; writing—original draft preparation, X.A. and Q.W.; writing—review and editing, M.C. and Q. W.; project administration, M.C.; funding acquisition, M.C. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangxi Province Superior Technology Innovation Team Construction Project (20171BCB24006) and the Natural Science Foundation of Jiangxi Province (20192BAB204018).
Data Availability Statement
The datasets supporting the conclusions of this article are included.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhong, C.H.; Wang, S.M.; Jiang, Z.W. ‘Jinyan’, an interspecific hybrid kiwifruit with brilliant yellow flesh and good storage quality. Hortscience 2012, 47, 1187–1190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.P.; Mo, X.Q.; Tang, D.M.; Ma, Y.H.; Xie, Y.X.; Yang, H.B.; Shi, M.Y.; Li, L.; Li, W.Y.; Yan, F.H.; et al. Comparative analysis of volatile and carotenoid metabolites and mineral elements in the flesh of 17 kiwifruit. J. Food Sci. 2021, 86, 3023–3032. [Google Scholar] [CrossRef]
- Asiche, W.O.; Mitalo, O.W.; Kasahara, Y.; Tosa, Y.; Mworia, E.G.; Owino, W.O. Comparative transcriptome analysis reveals distinct ethylene-independent regulation of ripening in response to low temperature in kiwifruit. BMC Plant Biol. 2018, 18, 47. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Q.Z.; Zhao, Q.F.; Zhang, X.P. Roles of antioxidant capacity and energy metabolism in the maturity-dependent chilling tolerance of postharvest kiwifruit. Postharvest Biol. Technol. 2020, 168, 111281. [Google Scholar] [CrossRef]
- Ali, M.; Raza, M.A.; Li, S.G.; Huan, C.; Zheng, X.L. 1-Methylcyclopropene treatment controls ethanol accumulation associated with regulation of mitochondrial energy metabolism in kiwifruit (Actinidia deliciosa) cv. ‘Bruno’during storage at room temperature. J. Food Biochem. 2020, 44, e13273. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Huang, M.; Crane, J.H.; Wang, Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.). J. Food Drug. Anal. 2018, 26, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.D.; Zhang, Y.Y.; Liu, X.C.; Xiao, Y.W.; Zhang, Z.Y.; Shi, Y.N.; Kong, W.B.; Yang, X.F.; Jiang, G.H.; Zhang, B.; et al. Cultivation conditions change aroma volatiles of strawberry fruit. Horticulturae 2021, 7, 81. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. The flavor of pomegranate fruit: A review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Cai, H.F.; An, X.J.; Han, S.; Jiang, L.; Yu, M.L.; Ma, R.J.; Yu, Z.F. Effect of 1-MCP on the production of volatiles and biosynthesis-related gene expression in peach fruit during cold storage. Postharvest Biol. Technol. 2018, 141, 50–57. [Google Scholar] [CrossRef]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Dou, T.X.; Shi, J.F.; Li, Y.; Bi, F.C.; Gao, H.J.; Hu, C.H.; Li, C.Y.; Yang, Q.S.; Deng, G.M.; Sheng, O.; et al. Influence of harvest season on volatile aroma constituents of two banana cultivars by electronic nose and HS-SPME coupled with GC-MS. Sci. Hortic. 2020, 265, 109214. [Google Scholar] [CrossRef]
- Lv, Y.H.; Chen, G.G.; Ouyang, H.; Sang, Y.Y.; Jiang, Y.; Cheng, S.B. Effects of 1-MCP treatment on volatile compounds and quality in Xiaobai apricot during storage at low temperature. J. Food Process. Preserv. 2021, 45, e15452. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, J.; Yan, X.X.; Liu, H.B.; Pan, S.Y. Comparative transcriptome analysis of genes involved in volatile compound synthesis in blueberries (Vaccinium virgatum) during postharvest storage. Postharvest Biol. Technol. 2020, 170, 111327. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Peng, B.; Yu, M.L.; Zhang, B.B.; Xu, J.L.; Ma, R.J. Differences in PpAAT1 activity in high- and low-aroma peach varieties affect γ-decalactone production. Plant Physiol. 2020, 182, 2065–2080. [Google Scholar] [CrossRef]
- Zlatić, E.; Zadnik, V.; Fellman, J.; Demšar, L.; Hribar, J.; Čejić, Ž.; Vidrih, R. Comparative analysis of aroma compounds in ‘Bartlett’ pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biol. Technol. 2016, 117, 71–80. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.R.; Li, X.; Yang, S.L.; Ferguson, I.B.; Chen, K.S. Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. J. Agric. Food Chem. 2009, 57, 2875–2881. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Shu, P.; Min, D.D.; Zhou, J.X.; Ai, W.; Li, J.Z.; Li, Z.L.; Zhang, X.H.; Shi, Z.D.; Sun, Y.J.; Li, F.J.; et al. The synergism of 1-methylcyclopropene and ethephon preserves quality of “Laiyang” pears with recovery of aroma formation after long-term cold storage. Front. Plant Sci. 2020, 11, 490. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Shi, T.; Sun, J.; Wu, X.X.; Weng, J.Y.; Wang, P.K.; Qie, H.L.; Huang, Y.H.; Wang, H.K.; Gao, Z.H. Transcriptome analysis of Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruit treated with heat and 1-MCP. Plant Physiol. Biochem. 2018, 133, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.J.; Wei, C.Q.; Zhao, Z.H.; Guan, J.F.; Wang, W.J. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Gorinstein, S. Shelf life extension and antioxidant activity of ‘Hayward’ kiwifruit as a result of prestorage conditioning and 1-methylcyclopropene treatment. J. Food Sci. Technol. 2015, 52, 2711–2720. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.X.; Wang, Y.T.; Liu, Y.F.; Yong, K.; Liu, Z.D. 1-MCP extends the shelf life of ready-to-eat ‘Hayward’ and ‘Qihong’ kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Thewes, F.R.; Anese, R.O.; Thewes, F.R.; Ludwig, V.; Klein, B.; Wagner, R.; Nora, F.R.; Rombaldi, C.V.; Brackmann, A. Dynamic controlled atmosphere (DCA) and 1-MCP: Impact on volatile esters synthesis and overall quality of ’galaxy’ apples. Food Packag. Shelf Life 2020, 26, 100563. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Song, Z.Y.; Li, Q.M.; Li, J.; Chen, W.X.; Li, X.P. Physiological and transcriptomic analysis reveals the roles of 1-MCP in the ripening and fruit aroma quality of banana fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef]
- Cai, H.F.; Han, S.; Jiang, L.; Yu, M.L.; Ma, R.J.; Yu, Z.F. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Huan, C.; Zhang, J.; Jia, Y.; Li, S.E.; Jiang, T.J.; Shen, S.L.; Zheng, X.L. Effect of 1-methylcyclopropene treatment on quality, volatile production and ethanol metabolism in kiwifruit during storage at room temperature. Sci Hortic. 2020, 265, 109266. [Google Scholar] [CrossRef]
- Pan, L.Y.; Zhao, X.Y.; Chen, M.; Fu, Y.Q.; Xiang, M.L.; Chen, J.Y. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A.T.; Nakano, R.; Ushijima, K.; et al. Low temperature storage stimulates fruit softening and sugar accumulation without ethylene and aroma volatile production in kiwifruit. Front. Plant Sci. 2019, 10, 888. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Q.; Yin, X.R.; Lin, Q.; Chen, J.Y.; Allan, A.C.; Xu, C.J.; Chen, K.S. Effect of hot air treatment on organic acid- and sugar- metabolism in ponkan (Citrus reticulata) fruit. Sci. Hortic. 2012, 147, 118–125. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, X.H.; Fu, M.R.; Chen, Q.M.; Muzammil, J.M. Chlorine dioxide fumigation generated by a solid releasing agent enhanced the efficiency of 1-MCP treatment on the storage quality of strawberry. J. Food Sci. Technol. 2018, 55, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Lia, M.; Zhi, H.H.; Dong, Y. The influence of pre- and postharvest 1-MCP application and oxygen regimes on textural properties, cell wall metabolism, and physiological disorders of late-harvest ‘Bartlett’ pears. Postharvest Biol. Technol. 2021, 173, 111429. [Google Scholar] [CrossRef]
- Ali, M.; Raza, M.A.; Li, S.E.; Zhou, L.C.; Huan, C.; Shen, S.L.; Zheng, X.L. 1-MCP regulates ethanol fermentation and GABA shunt pathway involved in kiwifruit quality during postharvest storage. Hortic. Plant J. 2021, 7, 23–30. [Google Scholar] [CrossRef]
- Günther, C.S.; Marsh, K.B.; Winz, R.A.; Harker, R.F.; Wohlers, M.W.; White, A.; Goddard, M.R. The impact of cold storage and ethylene on volatile ester production and aroma perception in ‘Hort16A’ kiwifruit. Food Chem. 2015, 169, 5–12. [Google Scholar] [CrossRef]
- Günther, C.S.; Matich, A.J.; Marsh, K.B.; Winz, R.A.; Harker, R.F.; Wohlers, M.W.; White, A.; Goddard, M.R. Development of a quantitative method for headspace analysis of methylsulfanyl-volatiles from kiwifruit tissue. Food Res. Int. 2011, 44, 1331–1338. [Google Scholar] [CrossRef]
- Lan, T.; Gao, C.X.; Yuan, Q.Y.; Wang, J.Q.; Zhang, H.X.; Sun, X.Y.; Lei, Y.S.; Ma, T.T. Analysis of the aroma chemical composition of commonly planted kiwifruit cultivars in China. Foods 2021, 10, 1645. [Google Scholar] [CrossRef]
- Du, D.D.; Xu, M.; Wang, J.; Gu, S.; Zhu, L.Y.; Hong, X.Z. Tracing internal quality and aroma of a red-fleshed kiwifruit during ripening by means of GC-MS and E-nose. RSC Advances 2019, 9, 21164–21174. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, V.; Shin, S.B.; Johnson, F.; Mattheis, J.P.; Zhu, Y.M. Differential suppression of ethylene biosynthesis and receptor genes in ‘Golden Delicious’ apple by preharvest and postharvest 1-MCP treatments. Plant Growth Reg. 2013, 32, 585–595. [Google Scholar] [CrossRef]
- Yang, X.T.; Song, J.; Du, L.N.; Forney, C.; Leslie, C.; Sherry, F.; Wismer, P.; Zhang, Z.Q. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Zhou, X.; Hao, Y.; Sun, H.J.; Zhou, Q.; Sun, Y.Y.; Ji, S.J. Methyl jasmonate pretreatment improves aroma quality of cold-stored ’Nanguo’ pears by promoting ester biosynthesis. Food Chem. 2020, 338, 127846. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Schwab, W.; Mayershofer, M.; Gonzalez-Aguero, M.; Defilippi, B.G. Volatile compound and gene expression analyses reveal temporal and spatial production of Lox-derived volatiles in pepino (Solanum muricatum Aiton) Fruit and LOX Specificity. J. Agric. Food Chem. 2017, 65, 6049–6057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.D.; Zhang, Q.Y.; Li, J.Z.; Gong, H.S.; Fan, X.G.; Yang, Y.Q.; Liu, X.F.; Yin, X.R. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2021, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.F.; Zhang, C.; Cao, S.X.; Wang, X.; Qi, H.Y. The effect of CmLOXs on the production of volatile organic compounds in four aroma types of melon (Cucumis melo). PLoS ONE 2015, 10, e0143567. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, J.W.; Chen, J.Y.; Song, T.; Jiang, Y.G.; Zhang, Y.F.; Wang, L.J.; Li, F.L. Preharvest spraying calcium ameliorated aroma weakening and kept higher aroma-related genes expression level in postharvest ‘Nanguo’ pears after long-term refrigerated storage. Sci. Hortic. 2019, 247, 287–295. [Google Scholar] [CrossRef]
- Harb, J.; Lara, I.; Saleh, O.; Streif, J. Khraiwesh, B. Treatments that suppress ethylene production or ethylene action modify ADH and AAT gene expression and aroma-related enzyme activities in ‘Delbarde Estivale’ apple: Consequences for the aroma profiles of fruit. J. Hortic. Sci. Biotech. 2015, 86, 182–188. [Google Scholar] [CrossRef]
- Xi, W.P.; Zhang, B.; Shen, J.Y.; Sun, C.D.; Xu, C.J.; Chen, K.S. Intermittent warming alleviated the loss of peach fruit aroma-related esters by regulation of AAT during cold storage. Postharvest Biol. Technol. 2012, 74, 42–48. [Google Scholar] [CrossRef]
- Peng, B.; Xu, J.L.; Cai, Z.X.; Zhang, B.B.; Yu, M.L.; Ma, R.J. Different roles of the five alcohol acyltransferases in peach fruit aroma development. J. Am. Soc. Hortic. Sci. 2020, 145, 374–381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).