Abstract
The present study describes the application of an acid tolerant and potentially probiotic L. paracasei SP3 strain, recently isolated from kefir grains, in the production of a novel functional beverage based on the fermentation of pomegranate juice. The fermentation ability of the novel strain was assessed during pomegranate juice fermentations at 30 °C for 24 h and storage at 4 °C for 4 weeks. Various parameters were assessed such as residual sugar, organic acid and alcohol levels, total phenolics content, antioxidant activity, astringency, cell viability, and consumer acceptance. Residual sugar was decreased by approximately 25%, while respectable amounts of lactic acid were determined (4.8 g/L) on the 28th day of storage, proving that the novel strain was effective at lactic acid fermentation. The concentration of ethanol was maintained at low levels (0.3–0.4 % v/v) and low levels of acetic acid were detected (0.6 g/L). The viability of L. paracasei SP3 cells retained high levels (>7 log cfu/mL), even by the 4th week. The total phenolic content (123.7–201.1 mg GAE/100 mL) and antioxidant activity (124.5–148.5 mgTE/100 mL) of fermented pomegranate juice were recorded at higher levels for all of the studied time periods compared to the non-fermented juice. The employment of the novel strain led to a significant reduction in the levels of hydrolysable tannins (42%) in the juice, reducing its astringency. The latter was further proven through sensorial tests, which reflected the amelioration of the sensorial features of the final product. It should be underlined that fruit juices as well as pomegranate juice comprised a very harsh food matrix for microorganisms to survive and ferment. Likewise, the L. paracasei SP3 strain showed a significant potential, because it was applied as a free culture, without the application of microencapsulation methods that are usually employed in these fermentations, leading to a product with possible functional properties and a high nutritive value.
1. Introduction
Improvements in human health through the attunement of diet have become a paramount strategy and target of the new Century. Awareness and employment of functional food as a key tool for the accomplishment of the above target has been well established, leading to the development of novel marketable food products [1,2,3]. Currently, the functional foods market is increasing worldwide as consumers seek not just “good” food, but request nutritive meals of exceptional quality for “farm to fork” [4,5]. Functional foods are foods that, besides the basic nutritional requirements, can provide enhanced health benefits to the consumer, leading to the promotion of their health state and a reduction of disease risk [6,7]. Requirements of this kind are satisfied by various nutrients, such as amino acids, major inorganic nutrients (calcium and iron), vitamins, and fatty acids, which are necessary for life, growth, and tissue repair [8]. On the other hand, functional foods are focused on beneficial improvements of one or more target functions in the body, beyond adequate nutritional effects, and on a reduction of chronic health disorder incidences [9]. Functional foods can be mainly classified as fortified/enhanced products (amino acid, vitamin, fatty acid, and mineral additions), natural products (fruits), and enriched products (probiotics and prebiotics) [10,11,12,13]. In this context, probiotic foods are included in the significant category of functional foods, as their incorporated probiotic strains interact harmoniously with the intestinal microbiota, providing prophylactic and therapeutic effects against various pathogens [7,14].
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Health benefits include anti-depression, anti-obesity, anti-diabetic, and anti-cholesterol activities, as well as immunostimulant action and the secretion of functional molecules [15,16,17]. After 2002, FAO/WHO published the “Guidelines for Evaluation of Probiotics in Food” and established the criteria for characterizing a strain as probiotic. These criteria deal with resistance to unfavorable conditions that the human body imposes, epithelium adhesion ability, antimicrobial activity, and safety assessment. The relevant clinical trials for probiotic characterization are known as placebo-controlled randomized clinical trials (RCTs) and are performed via oral, rectal, and vaginal routes [18,19]. As far as the technological properties of the products enriched with probiotic strains are concerned, the aroma and/or taste and/or acidity over the shelf life should not be adverse [20,21,22]. Additionally, the probiotic must survive food-processing stressors, such as fermentation, freeze-drying, variations in temperature, pH, and oxidative and osmotic stress during storage [23]. High viabilities of the probiotic species during food storage are critical and are required. Specifically, the minimum concentration of probiotic is approximately 106–107 cfu/mL at the time of food product consumption [14,20].
On the other hand, a significant matter rises as probiotic delivery has been mainly employed through dairy products, even though they exhibit some drawbacks, such as lactose intolerance, high fat content, and possible allergy effects [14]. These issues have forced the research community along with the food industry to seek other food substrates of probiotic delivery. In this vein, fruit juices seem to be a good alternative, while being a nutritious, health-promoting, and disease-preventive source of food [24]. Fruit juices display antioxidant, antimicrobial, and antimutagenic properties and provide consumers with beneficial macronutrients that seems to positively be involved in genome damage and repair [25,26]; hence, they can be considered appropriate substrates for the production of functional food. In addition, fruit juices are rich in bioactive compounds that can exert prebiotic properties, which enhance probiotic delivery and viability [14,27]. Apart from the application of living probiotic cells for the production of functional beverages, the incorporation of active biomolecules, antioxidants, and prebiotics can enhance food functionality [28]. Significantly, the combination of both living probiotic cells and fruit bioactive compounds can lead to the development of super foods.
In this vein, fermented fruit beverages meet functional production demands as they are minimally processed foods with an improved nutritional value, improved sensorial features, ameliorated shelf-life, and additional health-promoting derived components [29,30,31]. Specifically, pomegranate is a very good source of antioxidant phytochemicals as well as natural antimicrobial compounds [32]. Moreover, pomegranate juice has been applied in lactic acid fermentation, providing very promising results [31,33,34,35,36]. However, fruit juice is a very harsh matrix as various obstacles can prevent microorganism growth. For instance, low pH values and high viscosity are considered significant obstacles that a microorganism should overcome in order to accomplish a juice fermentation procedure [30]. As a result, the selection of the proper starter culture is of paramount importance and possible modifications/preparations of the fruit juice are considered critical [30,33]. Kefir grains are considered a very good source of probiotics, and scientific research has considering the isolation of novel LAB strains of multifunctional probiotic characteristics [37,38,39,40,41].
In this frame, acid tolerant L. paracasei SP3, recently isolated from kefir grains [42] will be applied for the first time in pomegranate juice fermentation, targeting the production of a functional beverage. The strategy adopted in the current study is the valorization of this novel L. paracasei SP3 in pomegranate juice fermentation in parallel with the quality of the produced beverage, according to consumers acceptability and nutritional value (antioxidant activity and total phenolic content). The level of astringency is also assessed though the determination of the hydrolysable tannin content.
2. Materials and Methods
2.1. Strain Selection and Growing Conditions
The bacterial strain Lactobacillus paracasei SP3, prior isolated from traditional kefir grains, as described before [42], was selected for the fermented pomegranate juice product according to its potential probiotic character and tolerance to acidic environments, as well as its potential postbiotic characteristics [43,44].
The bacterial strain was kept in the Laboratory of Food Processing of Democritus University of Thrace. It was stored at −80 °C in vials containing glycerol (50%) and distilled sterile water (50%), and was activated in de Man, Rogosa, and Sharpe broth (Merck, Darmstadt, Germany), and was placed overnight in a heat chamber (37 °C for 18 h). The growth of L. paracasei SP3 was performed under anaerobic conditions at 37 °C for 48 h in de Man, Rogosa, and Sharpe broth (Merck, Darmstadt, Germany). The biomass of the bacterial culture was harvested through centrifugation (5000 rpm, 10 min, 25 °C) under aseptic conditions (Sigma 3K12, Bioblock Scientific, Illkirch, France). All media were sterilized by autoclave for 15 min (120 °C, 1–1.5 atm) prior to use.
2.2. Production of Fermented Pomegranate Beverage
Pomegranate fruits were purchased from the local market of Nea Orestiada (Greece) and fresh juice was directly produced via mechanical crushing and pressing the seeds (after washing and peeling) for 10 min. Adjustment of the initial sugar concentration of the fresh juice was conducted to 80 g/L, through minimal (10%) addition of sterilized, deionized water (juice pH 3.2). Afterwards, 100 mL of the prepared pomegranate juice solution was transferred a previously sterilized conical flask and pasteurized at 80 °C for 5 min [45]. After cooling at room temperature,1 g of L. paracasei SP3 was added in 100 mL of a fermentation substrate in biological duplicates. For comparison reasons, a conical flask containing 100 mL of pomegranate juice with the same sugar adjustment and pH as the fermented sample was prepared as the control sample. The samples were maintained at 30 °C for 1 day and subsequently at 4 °C for 28 days.
2.3. Chemical Analysis of Produced Pomegranate Beverages
The ethanol concentration and residual sugar were quantified through high-performance liquid chromatography/HPLC (Shimadzu) using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan), as described previously [46]. The system consisted of a SCR-101N stainless steel column (7.9 m × 300 mm i.d., Sigma, St. Louis, MO, USA), a LC-9A pump, a CTO-10A oven set at 60 °C, and a RID-6A refractive index detector. Ultra-pure water obtained by a Milli-Q water (Millipore, Milan, Italy) purifying system (resistivity 18.2 MΩ cm−1) was used as the mobile phase with a flow rate of 0.8 mL/min, while 1-butanol (0.1% v/v) was used as the internal standard. Pomegranate beverage samples were double filtered through 0.2 μm microfilters and 20 μL was injected directly into the column.
Organic acids were quantified through ion-exchange liquid chromatography/HPLC (Shimadzu), as described before [47]. For the quantitative analysis, standard solutions of acetic and lactic acid (Merk, Taufkirchen, Germany) were prepared in pure water (Milli-Q, Merk, Taufkirchen, Germany). The determinations of the pomegranate samples were carried out by means of standard curves.
Folin−Ciocalteu reagent was applied for the evaluation of the total phenolic content (TPC) of pomegranate beverages and the content was determined based on colorimetric reduction, as described previously [45,48]. TPC was expressed as mg of gallic acid equivalents (GAE/100 mL juice). The ABTS radical cation decolorization assay was used in order to determine antioxidant activity (AA) [45,49]. AA was expressed as mg of Trolox equivalent (TE/100 mL juice).
All of the results are presented as the means of three repetitions plus standard deviations.
2.4. Hydrolysable Tannins Content
The content of hydrolysable tannins was determined by the method applied by Hagerman and Butier [50]. Briefly, 1 mL of pomegranate juice was mixed with 5 mL methanol. In 1 mL of the upper phase, 2 mL of an acetate buffer (pH 5.0) containing 0.1% w/v BSA and 0.99% w/v NaCl was added. Subsequently, centrifugation (Sigma 3K12, Bioblock Scientific, France) was applied at 3000 rpm for 15 min. A reagent was produced containing 1% w/v SDS and 5% w/v triethanolamine, and was applied (4 mL) in order to dissolve the tannins by precipitation for 1 h at 37 °C. Finally, 1 mL of reagent containing 0.27% FeCl36H2O and 0.1 NHCl was added to the solution and then the whole mixture was shaken for 30 min in a vortex (Scientific Industries, Genie-2, USA). The hydrolysable tannin content was determined with a spectrophotometer, (510 nm) and was expressed as catechin equivalents in mg/L by comparison with a standard curve [51]. All of the results are presented as the means of at least three repetitions plus standard deviations.
2.5. Microbiological Analysis
From each pomegranate beverage, a sample of 10 mL was collected aseptically at various time intervals (0/T0: the day of production, and days 1, 7, 14, 21, and 28) right after fermentation and during storage at 4 °C. In addition, a sample just before fermentation was collected in the same manner. Pomegranate beverage samples were serially diluted under aseptic conditions (open flame, sterilized prior in a Laminar cabinet) in 90 mL 1/4 strength Ringer’s solution and placed in sterile bag-mixers and homogenized in a stomacher (Bagmixer 400, Model VW, Interscience).
Viable cell counts of each species—yeasts and fungi, enterobacteria, coliforms, Staphylococci, and the selected L. paracasei strain—were enumerated by pouring plating (0.1 mL or 1 mL) of appropriate dilutions on the selective media under aseptic conditions, considering the instructions given by the manufacturer (Merck, Germany). In brief, the yeasts and fungi were enumerated on Sabouraud Chloramphenicol Agar (incubation at 30 °C for 72 h), enterobacteria were enumerated on Violet Red Bile Glucose Agar (VRBGA; incubation at 37 °C for 24 h), coliforms were enumerated on Violet Red Bile agar (VRBA; incubation at 30 °C for 24 h), and Staphylococci were enumerated on Baird Parker agar (BP; incubation at 37 °C for 48 h) [52].
Viable counts of the selected strain L. paracasei SP3 were enumerated on acidified MRS agar (Merck, Germany) at 37 °C for 72 h, anaerobically (Anaerobic jar, Anerocult C, Merck, Germany). All of the media were sterilized by autoclaving at 120 °C and at 1–1.5 atm for 15 min, and were then cooled before use.
In addition, the survival rate (SR%) of the selected starter culture (L. paracasei SP3) was assessed according to the following equation, aiming to evaluate the significance of viability rates:
The equation was based on cell counts (log CFU/g) of the selected Lactobacilli strain enumerated in each pomegranate beverage sample: No represents the viable cell counts (log CFU/g) from the day of production (T0) and N represents the viable cell counts (log CFU/g) of each day tested during cold storage [52]. The results of the survival rate were expressed as % of L. paracasei viability.
2.6. Assessors Acceptance Test: Preliminary Sensory Evaluation
The sensory attributes of the fermented pomegranate beverage were compared with the produced unfermented pomegranate juice after the end of the juice fermentation and at the final day of cold storage (4 °C) [38]. A panel of 25 (age average 22–58 years old) frequent consumers of fruit juices was conducted. The samples were placed in panel booths and served directly from refrigerated storage in glass containers usually applied for wine tasting (glasses of 155 mm height, total volume of 215 mL). Each glass was covered with a sterile Petri dish and filled with approximately ~25 mL of juice [53]. Each sample was coded with a different three-digit number and was served in a randomized order. Initially, the panel was asked to assess the intensity of the aroma and taste based on a 0–10 preference scale (0 represents an unacceptable product, while 10 represents a product of superior quality). Likewise, astringency was also evaluated. Finally, the panel was asked to comment on whether they would prefer one of the two beverages, and if they noticed any significant difference between the two.
2.7. Statistical Analysis
All experiments were repeated three times and the results are expressed as mean ± standard deviation. Microbial populations were expressed as Log10 CFU/mL. The data obtained from the ethanol, organic acids, and residual sugar concentrations; total phenolic content; antioxidant activity; and L. paracasei SP3 cell viability of the non-fermented and fermented pomegranate juice were analyzed for their mean differences using the analysis of variance (ANOVA) procedure, followed by Duncan’s post hoc multiple range test, in order to highlight the explicit differences between the various treatments. Analysis was performed by using IMB SPSS v20 (IBM Corp., Armonk, NY, USA) at an alpha level of 5%.
3. Results and Discussion
L. paracasei SP3 previously isolated from kefir grains with potential probiotic properties [42] was examined in pomegranate juice fermentation for an evaluation of its technological abilities with a satisfied outcome [54]. Fruit juices such as pomegranate juice have gained a lot of interest the last years as a substrate for probiotic delivery versus dairy products [12,55,56,57]. As the most important drawback of fruit juices is their low pH values, more and more potential probiotic or certain probiotic strains have been applied in these fermentations. The main reason for this is that these strains are considered pH tolerant, as one of the in vitro tests that should succeed is the examination of their resistance to low pH [37,55]. Therefore, L. paracasei SP3 can be considered as an acid tolerant strain because through in vitro tests, it exhibits satisfactory levels of viability (7.1~8.5 log cfu/mL) with pH values between 2.0 and 4.0 [43], and it could be appropriate for fruit juice fermentation, where the pH values are usually very low.
3.1. Ethanol, Organic Acids and Residual Sugar Concentrations
The levels of sugars, organic acids (lactic acid and acetic acid), and ethanol in pomegranate juice during fermentation (30 °C for 24 h) and storage (4 °C for 4 weeks) are presented in Table 1.

Table 1.
Analysis of sugars, organic acids, and ethanol in pomegranate juices fermented by L. paracasei SP3 after fermentation (24 h at 30 °C) and during 4 weeks of storage at 4 °C.
The concentration of lactic acid increased (statistically significantly) in all of the studied time periods, reaching a final value of 4.8 g/L, while acetic acid was only slightly produced (0.6 g/L). The total sugars were decreased significantly, reaching (25.4% reduction) their lowest value in the 4th week of storage (58.4 g/L), while alcohol was marginally detected (0.4 g/L) at the end of storage. All of the above results demonstrate that L. paracasei SP3 was effective in the lactic acid fermentation of pomegranate juice, even during cold storage for 4 weeks. Respectable amounts of lactic acid were produced, while the low alcohol content (<1% v/v) met the standards set for low or non-alcoholic beverages [58]. Acetic acid was produced in low concentrations, probable due to the enzymatic degradation of citric acid naturally present in pomegranate juice, as other researchers have already reported in the lactic acid fermentation of fruit juices [59]. The pH value of the fermented juices was monitored in all of the studied periods. No significant alteration was recorded. In particular, the initial pH value was 3.2 and varied between 3.2 ± 0.2 for all of the time periods, due to the high buffering capacity of the fermented juices, as other researchers have reported in similar experiments [60].
3.2. Microbial Stability and Starter Culture Survival Rate
Microbial stability is crucial in fruit juices, as spoilage microorganisms (especially yeast and fungi) may alter a product’s characteristics and subsequently alter the nutritional value of the juice [61].
In the present study, an initial thermal conditioning was employed to pomegranate juices aiming to minimize any effects of yeast or fungi, which are well known as natural fruit microflora [62]. Thus, an evaluation of the possible spoilage by yeasts, fungi, and coliforms was carried out in all pomegranate beverages (data not shown). The results showed that the initial thermal treatment was sufficient as no yeast or fungi were detected after pomegranate juice fermentation or during the four weeks of storage at 4 °C. Moreover, possible coliform, Staphylococci, or enterobacteria that could appear through processing (e.g., water treatments/human source) were not detected in any stages of production and storage [63].
The levels of viability of L. paracasei SP3 after fermentation and during the four weeks of cold storage (4 °C) are presented in Figure 1. Initially, the viability of the potentially probiotic L. paracasei SP3 was 9.2 log CFU/mL and was maintained at high levels throughout 4 weeks of storage (7.2 log CFU/mL at the 4th week of storage). These recorded viability values were above the limit of 6–7 log cfu/mL, which is required for probiotic products [14]. It is worth mentioning that no adjustment in the pH value of the juice was applied, while in the low pH juice environment, the survival rates of the starter culture remained high (Figure 2). This is a significant outcome, because it highlights that the novel strain can survive the harsh environment of pomegranate juice (low pH and antimicrobial compounds) and retain high viability rates for approximately 1 month of cold storage. In particular, the decline in the L. paracasei SP3 viability rate was determined to be approximately 20% during cold storage, which was a decisive issue of the industrialization potential, as minimal processing treatments are required for the production of pomegranate beverages [3].
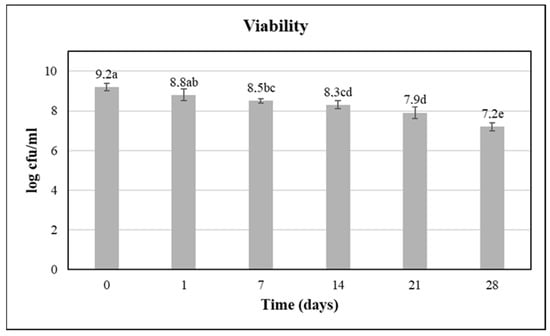
Figure 1.
Viability of L. paracasei SP3 (log CFU/mL) after production (D0) and during cold storage (4 °C), similar superscript letters in bars denote no significant differences at an alpha = 0.05 (ANOVA and Duncan Post Hoc Multiple Comparisons).
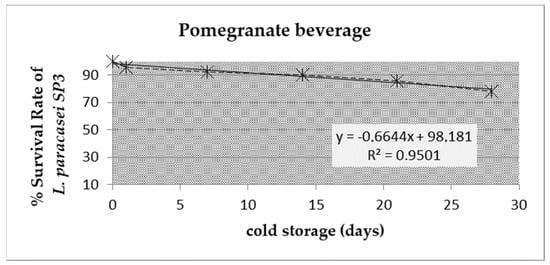
Figure 2.
Survival rate (SR %) of the starter culture (L. paracasei SP3) after manufacture and during cold storage (4 °C) for 28 days.
The preservation of the cell viability of potential probiotic strains has been reported by other researchers during the lactic acid fermentation of fruit juices, even in cold storage conditions. Specifically, other potentially probiotic L. paracasei strains applied for pomegranate juice and cornelian cherry juice fermentation have achieved high viability levels during cold storage [45,64]. This finding can be attributed to two main reasons, namely: (i) L. paracasei SP3 is an acid tolerant strain [42] and (ii) components of a plant origin may possess prebiotic properties and, consequently, ameliorate the growth of LAB [59,60]. For example, black rice is known to exhibit a prebiotic activity by promoting the growth of Bifidobacteria and Lactobacilli [65]. In addition, it has been reported that ellagitannins appear to be favorable for the viability of LAB [66]. Likewise, the composition of pomegranate juice with high levels of ellagitannins could enhance LAB growth and the survival rate.
3.3. Total Phenolic Content and Antioxidant Activity
The total phenolics content (TPC) and antioxidant activity (AA) of fermented (FJ) and unfermented (UFJ) juice was also monitored, and the results are presented in Table 2. The TPC value of freshly prepared pomegranate juice (fermentation time 0) was approximately 101 ± 10 mg GAE/100 mL. UFJ was also studied in the same period until the 4th week at 4 °C, in order to display the effect of juice fermentation by L. paracasei SP3.

Table 2.
Determination of the total phenolic content and antioxidant activity of unfermented (UFJ) and fermented pomegranate juice (FJ) with L. paracasei SP3 for the first 24 h at 30 °C and during storage at 4 °C for 4 weeks.
At the beginning of fermentation with the starter L. paracasei SP3, the values of TPC were boosted and were always statistically significant higher compared to the TPC of UFJ. Specifically, the TPC of FJ reached its highest value in the 3rd week of storage (201.1 mg GAE/100 mL), while the TPC of UFJ continuously decreased, reaching its lowest value in the 4th week of storage (58.4 mg GAE/100 mL). The same outcome was observed in the case of the antioxidant activity (AA) determination of FJ and UFJ. Specifically, the AA of the freshly prepared pomegranate beverage was about 107 ± 10 mg TE/100 mL (fermentation time 0). The values of AA of FJ were statistically significantly higher compared to the AA of UFJ in all of the studied periods. The highest value for AA of FJ was observed in the 1st week of storage (148.5 mg TE/100 mL), while the AA of UFJ decreased, reaching its lowest value in the 4th week of storage (53.4 mg TE/100 mL). Enhancements in the total phenolic content could be attributed in the enzymatic transformation of the initial complex phenolics by LAB, through decarboxylation and reduction reactions to other simpler compounds, which increased the quantitative amount of TPC [30,67,68,69]. Specifically, the metabolic activities of the L. paracasei strains seemed to effectively modify the levels and the composition of the bioactive phenolic compounds of pomegranate juice, due to certain enzymes that they contained (β-glucoside and β-galactoside), as others researchers have reported in similar studies [36,69,70].
3.4. Hydrolysable Tannins
Tannins affect sensorial properties of fruit juice, in both appearance and flavor, causing an astringent sensation in the juice [71]. Likewise, the reduction of astringency is a very important issue in many fruit juices, such as pomegranate. The main categories of tannins are condensed and hydrolysable tannins [72]. Ellagitannins are the major category of hydrolysable tannins in pomegranate juice. Their formation takes place, when ellagic acid binds with a carbohydrate, like punicalin and punicalagin [32,73]. During the industrial extraction process, they are introduced in high levels into the juice, due to their hydrophilic properties [74]. The application of lactic acid fermentation in fruit juices seems to overcome this problem to a satisfactory level [35]. This assumption was verified in the present study, according to the results presented in Figure 3.
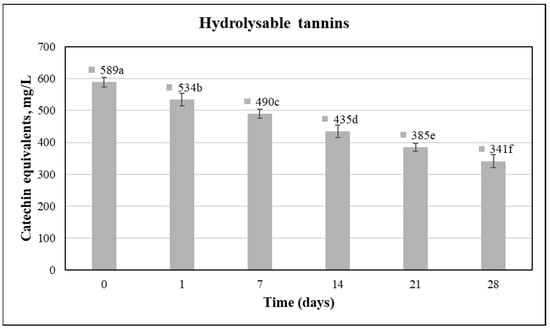
Figure 3.
Hydrolysable tannins of fermented pomegranate beverages; similar superscript letters in bars denote no significant differences at an alpha = 0.05 (ANOVA and Duncan Post Hoc Multiple Comparisons).
The levels of hydrolysable tannins were reduced statistically significantly (approximately 42%) during the whole period of fermentation and storage. Before fermentation, the concentration of the hydrolysable tannins level was 589 mg/L and after 28 days it was 341 mg/L. It should be also underlined that a statistically significant decrease was monitored in every time period examined, as well as during the fermentation of pomegranate juice and cold storage. In addition, no significant changes were recorded regarding the unfermented juices, where hydrolysable tannins remained almost constant during the time period examined. These results are in accordance with other researchers, who demonstrated that lactic acid fermentation is a significant tool for mitigating this drawback, even though the type of strain and the fruit juice applied are critical [35]. A main explanation for the decrease in the astringency of pomegranate is that transformed phenolic compounds (possible produced by the degradation of tannins) do not induce astringency. On the other hand, sensorial tests are considered crucial in order to further evaluate the level and the rate of astringency in fruit juices, including pomegranate, because others phenolic compounds besides tannins could provoke astringency [72,74].
3.5. Sensory Evaluation
The results regarding the preliminary sensory evaluation for the evaluation of the produced FJ and UFJ in terms of aroma, taste, and astringency are presented in Table 3.

Table 3.
Preliminary sensory evaluation of fermented (FJ) and non-fermented (UFJ) pomegranate juice after fermentation (24 h at 30 °C) and over 4 weeks of storage at 4 °C.
Statistically, significant differences were found for the 2nd, 3rd, and 4th week of cold storage between UFJ and FJ, where FJ was scored better by consumers. Regarding astringency, FJ was found to be more attractive by consumers and developed a statistically significant difference compared with UFJ from the 1st week of storage until the last one. This outcome agrees with the determined contents of the hydrolysable tannins (Figure 3). Likewise, L. paracasei SP3 seems to be a good alternative for the fermentation of pomegranate juice, leading to a final product with improved sensorial characteristics and a lower astringency. The latter is very important, as astringency is considered a drawback in the commercialization of some fruit juices [72,75,76,77]. These very hopeful results are in agreement with other researchers, who have reported that lactic acid fermentation enhances the aromatic profile and flavor of pomegranate juice [31]. However, this phenomenon depends on the type of strain used for fermentation and other parameters, such as fermentation temperature [78]. More research is needed in the future applying GC-MS analysis, in order to correlate this outcome with the certain volatile compounds that the fermented juice possess. Finally, the evaluators found that the fermented beverage would be preferable as it has attributes of good aroma and a smoother taste compared to the unfermented juice. At this stage, this outcome is quite encouraging and gives more value to the final product.
4. Conclusions
The outcome of the present study showed that the novel potentially probiotic L. paracasei SP3 was successfully applied in the fermentation of pomegranate juice (at 30 °C for 1 day and at 4 °C for 28 days). Specifically, fermented pomegranate juice (i) exhibited improved AA and TPC contents, (ii) high viability, (iii) high levels of organic acids, and (iv) acceptable sensorial features. In addition, the fermentation of pomegranate juice with L. paracasei SP3 significantly reduced the juice astringency, due to the lower levels of hydrolysable tannins. It should be underlined that fruit juices comprise a difficult substrate for fermentation, due mainly to their low pH values. In this context, the prospects of L. paracasei SP3 seem attractive, as in the frame of the present study, it was verified as an effective acid tolerant strain that retained its viability without the application of protective techniques, such as encapsulation.
Author Contributions
Conceptualization, S.P. and I.M.; methodology, I.M., A.T. and S.P.; software, A.B. and S.P.; validation, I.M., A.T. and A.B.; formal analysis, I.M. and A.T.; investigation, I.M.; resources, A.B. and S.P.; data curation, I.M. and A.T.; writing—original draft preparation, S.P.; writing—review and editing, S.P.; visualization, S.P.; supervision, S.P.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koirala, S.; Anal, A.K. Probiotics-Based Foods and Beverages as Future Foods and Their Overall Safety and Regulatory Claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Ali, A.; Rahut, D.B. Healthy Foods as Proxy for Functional Foods: Consumers’ Awareness, Perception, and Demand for Natural Functional Foods in Pakistan. Int. J. Food Sci. 2019, 2019, 6390650. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S. Minimally Processed Functional Foods: Technological and Operational Pathways. J. Food Sci. 2016, 81, R2309–R2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaño, D.; Gironés-Vilapana, A.; García-Viguera, C.; Moreno, D.A. Development of Functional Foods. In Innovation Strategies in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–207. [Google Scholar]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional Foods Modulating Inflammation and Metabolism in Chronic Diseases: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Reyes-Castillo, P.A.; González-Vázquez, R.; Torres-Maravilla, E.; Tello, M.; Bermúdez-Humarán, L.G.; Mayorga-Reyes, L. Probiotics against Viral Infections: Current Clinical Trials and Future Perspectives. Immuno 2021, 1, 468–498. [Google Scholar] [CrossRef]
- Mann, J.; Truswell, A.S. Essentials of Human Nutrition; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Birch, C.S.; Bonwick, G.A. Ensuring the Future of Functional Foods. Int. J. Food Sci. Technol. 2019, 54, 1467–1485. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Jibril, H.; Abubakar, S.A. Basis for Classification of Functional Foods: A Review. Bayero J. Pure Appl. Sci. 2021, 13, 138–144. [Google Scholar] [CrossRef]
- Horáčková, Š.; Rokytová, K.; Bialasová, K.; Klojdová, I.; Sluková, M. Fruit Juices with Probiotics—New Type of Functional Foods. Czech J. Food Sci. 2018, 36, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Jędrusek-Golińska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Micha\lowska, A.; Szymandera-Buszka, K. Recent Progress in the Use of Functional Foods for Older Adults: A Narrative Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, Formulations, Advanced Delivery and Health Benefits of Probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Korada, S.K.; Yarla, N.S.; Mishra, V.; Daim, M.A.; Sharma, B.; Gm, A.; Peluso, I.; Kamal, M.A. Single Probiotic versus Multiple Probiotics-a Debate on Current Scenario for Alleviating Health Benefits. Curr. Pharm. Des. 2018, 24, 4150–4153. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior, A.I.M.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C.; Ibarra, A. Effects of Probiotic-Containing Products on Stool Frequency and Intestinal Transit in Constipated Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Gastroenterol. 2017, 30, 629. [Google Scholar] [CrossRef]
- Kazakos, S.; Mantzourani, I.; Plessas, S. Assessment of Pomegranate Juice as an Alternative “Substrate” for Probiotic Delivery. Recent Advances and Prospects. Fermentation 2020, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Pogorzelski, E.; Wilkowska, A. Flavour Enhancement through the Enzymatic Hydrolysis of Glycosidic Aroma Precursors in Juices and Wine Beverages: A Review. Flavour Fragr. J. 2007, 22, 251–254. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Tassou, C.C. Effect of Lactobacillus Plantarum L125 Strain with Probiotic Potential on Physicochemical, Microbiological and Sensorial Characteristics of Dry-Fermented Sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A Review of the Microencapsulation Techniques for the Incorporation of Probiotic Bacteria in Functional Foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Chakkaravarthi, S.; Aravind, S.M. Chapter 12—Fruit Juice Added with Prebiotics and Probiotics. In Probiotics and Prebiotics in Foods; Gomes da Cruz, A., Ranadheera, C.S., Nazzaro, F., Mortazavian, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 219–232. ISBN 978-0-12-819662-5. [Google Scholar]
- Fenech, M.; Baghurst, P.; Luderer, W.; Turner, J.; Record, S.; Ceppi, M.; Bonassi, S. Low Intake of Calcium, Folate, Nicotinic Acid, Vitamin E, Retinol, β-Carotene and High Intake of Pantothenic Acid, Biotin and Riboflavin Are Significantly Associated with Increased Genome Instability—Results from a Dietary Intake and Micronucleus Index Survey in South Australia. Carcinogenesis 2005, 26, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Compounds as Beneficial Phytochemicals in Pomegranate (Punica Granatum L.) Peel: A Review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, T.V.; Rodrigues, S. Prebiotic in Fruit Juice: Processing Challenges, Advances, and Perspectives. Curr. Opin. Food Sci. 2018, 22, 55–61. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application—A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Gomes, I.A.; Venâncio, A.; Lima, J.P.; Freitas-Silva, O. Fruit-Based Non-Dairy Beverage: A New Approach for Probiotics. Adv. Biol. Chem. 2021, 11, 302–330. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) Plantarum Subsp. Plantarum Application. Fermentation 2022, 8, 6. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic Acid Fermentation Drives the Optimal Volatile Flavor-Aroma Profile of Pomegranate Juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Hegazi, N.M.; El-Shamy, S.; Fahmy, H.; Farag, M.A. Pomegranate Juice as a Super-Food: A Comprehensive Review of Its Extraction, Analysis, and Quality Assessment Approaches. J. Food Compos. Anal. 2021, 97, 103773. [Google Scholar] [CrossRef]
- Mustafa, S.M.; S Chua, L.; El-Enshasy, H.A.; Majid, F.A.A.; Malek, R.A. A Review on Fruit Juice Probiotication: Pomegranate. Curr. Nutr. Food Sci. 2016, 12, 4–11. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, R.K. Development of Probiotic Pomegranate Beverage and Its Physico-Chemical and Microbial Characterization. Int. J. Pure App. Biosci. 2017, 5, 35–41. [Google Scholar] [CrossRef]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation of Pomegranate Juice as a Tool to Improve Antioxidant Activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [Green Version]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the Probiotic Potential of Lactic Acid Bacteria Isolated from Kefir Grains: Evaluation of Adhesion and Antiproliferative Properties in in Vitro Experimental Systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Plessas, S.; Kiousi, D.E.; Rathosi, M.; Alexopoulos, A.; Kourkoutas, Y.; Mantzourani, I.; Galanis, A.; Bezirtzoglou, E. Isolation of a Lactobacillus Paracasei Strain with Probiotic Attributes from Kefir Grains. Biomedicines 2020, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk Kefir: Composition, Microbial Cultures, Biological Activities, and Related Products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [Green Version]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Chondrou, P.; Galanis, A.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Application of a Novel Potential Probiotic Lactobacillus Paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms 2018, 6, 121. [Google Scholar] [CrossRef] [Green Version]
- What Are Postbiotics? Types, Benefits, and Downsides. Available online: https://www.healthline.com/nutrition/postbiotics (accessed on 11 February 2022).
- Terpou, A.; Rai, A.K. 2 - Microbial Transformation for Improving Food Functionality. In Current Developments in Biotechnology and Bioengineering; Rai, A.K., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–45. ISBN 978-0-12-823506-5. [Google Scholar]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus Plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2019, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional Pomegranate Beverage Production by Fermentation with a Novel Synbiotic L. Paracasei Biocatalyst. Food Chem. 2020, 308, 125658. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Alexopoulos, A.; Bekatorou, A.; Bezirtzoglou, E. Kefir Immobilized on Corn Grains as Biocatalyst for Lactic Acid Fermentation and Sourdough Bread Making. J. Food Sci. 2012, 77, C1256–C1262. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Gentile, C.; Reig, C.; Corona, O.; Todaro, A.; Mazzaglia, A.; Perrone, A.; Gianguzzi, G.; Agusti, M.; Farina, V. Pomological Traits, Sensory Profile and Nutraceutical Properties of Nine Cultivars of Loquat (Eriobotrya Japonica Lindl.) Fruits Grown in Mediterranean Area. Plant Foods Hum. Nutr. 2016, 71, 330–338. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein Precipitation Method for the Quantitative Determination of Tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Electronic Tongue as an Objective Evaluation Method for Taste Profile of Pomegranate Juice in Comparison with Sensory Panel and Chemical Analysis. Food Anal. Methods 2016, 9, 1726–1735. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel Frozen Yogurt Production Fortified with Sea Buckthorn Berries and Probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Terpou, A.; Ganatsios, V.; Kanellaki, M.; Koutinas, A.A. Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality. Microorganisms 2020, 8, 764. [Google Scholar] [CrossRef]
- Plessas, S.; Ganatsios, V.; Mantzourani, I.; Bosnea, L. White Brined Cheese Production by Incorporation of a Traditional Milk-Cereal Prebiotic Matrix with a Candidate Probiotic Bacterial Strain. Appl. Sci. 2021, 11, 6182. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef] [Green Version]
- James, A.; Wang, Y. Characterization, Health Benefits and Applications of Fruits and Vegetable Probiotics. CyTA-J. Food 2019, 17, 770–780. [Google Scholar] [CrossRef]
- Tanganurat, P. Probiotics Encapsulated Fruit Juice Bubbles as Functional Food Product. GEOMATE Int. J. 2020, 19, 145–150. [Google Scholar] [CrossRef]
- Foster, R.K.; Marriott, H.E. Alcohol Consumption in the New Millennium–Weighing up the Risks and Benefits for Our Health. Nutr. Bull. 2006, 31, 286–331. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus Plantarum in Model Solutions and Fruit Juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Production of a Potentially Synbiotic Fermented Cornelian Cherry (Cornus Mas L.) Beverage Using Lactobacillus Paracasei K5 Immobilized on Wheat Bran. Biocatal. Agric. Biotechnol. 2019, 17, 347–351. [Google Scholar] [CrossRef]
- Salomão, B. de C.M. Chapter 16 - Pathogens and Spoilage Microorganisms in Fruit Juice: An Overview. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 291–308. ISBN 978-0-12-802230-6. [Google Scholar] [CrossRef]
- Vantarakis, A.; Affifi, M.; Kokkinos, P.; Tsibouxi, M.; Papapetropoulou, M. Occurrence of Microorganisms of Public Health and Spoilage Significance in Fruit Juices Sold in Retail Markets in Greece. Anaerobe 2011, 17, 288–291. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nouska, C.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Panayiotidis, M.I.; Galanis, A.; Plessas, S. Production of a Novel Functional Fruit Beverage Consisting of Cornelian Cherry Juice and Probiotic Bacteria. Antioxidants 2018, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and Prebiotics Activity of Anthocyanins from Black Rice (Oryza Sativa L.) in Vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [Green Version]
- Vivas, N.; Augustin, M.; Lonvaud-Funel, A. Influence of Oak Wood and Grape Tannins on the Lactic Acid Bacterium Oenococcus Oeni (Leuconostoc Oenos, 8413). J. Sci. Food Agric. 2000, 80, 1675–1678. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus Plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shah, N.P. Effect of Tea Extract on Lactic Acid Bacterial Growth, Their Cell Surface Characteristics and Isoflavone Bioconversion during Soymilk Fermentation. Food Res. Int. 2014, 62, 877–885. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 °C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Prommajak, T.; Leksawasdi, N.; Rattanapanone, N. Tannins in Fruit Juices and Their Removal. Chiang Mai Univ. J. Nat. Sci. 2020, 19, 76–90. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An Overview of the Perception and Mitigation of Astringency Associated with Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a Source of Bioactive Constituents: A Review on Their Characterization, Properties and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef]
- Bodbodak, S.; Farmani, B.; Nejaatian, M. Effect of Operating Parameters on Permeate Flux and Fouling Behavior during Clarification of Pomegranate Juice Using Ultrafiltration Polymeric Membrane. J. Food Res. 2021, 31, 185–197. [Google Scholar]
- Das, I.; Sasmal, S.; Arora, A. Effect of Thermal and Non-Thermal Processing on Astringency Reduction and Nutrient Retention in Cashew Apple Fruit and Its Juice. J. Food Sci. Technol. 2021, 58, 2337–2348. [Google Scholar] [CrossRef]
- Sedat Velioglu, Y.; Ekici, L.; Poyrazoglu, E.S. Phenolic Composition of European Cranberrybush (Viburnum Opulus L.) Berries and Astringency Removal of Its Commercial Juice. Int. J. Food Sci. Technol. 2006, 41, 1011–1015. [Google Scholar] [CrossRef]
- Peleg, H.; Noble, A.C. Effect of Viscosity, Temperature and PH on Astringency in Cranberry Juice. Food Qual. Prefer. 1999, 10, 343–347. [Google Scholar] [CrossRef]
- Allam, H. Impact of Processing on Flavor Volatiles and Physicochemical Properties of Pomegranate Juice. Suez Canal Univ. J. Food Sci. 2016, 3, 67–74. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).