Abstract
Flower thinning is often used during the planting of fruit trees to improve fruit quality and promote large fruit. Flower buds become an agricultural by-product of the planting process. Pitaya (Hylocereus undatus) is a popular fruit in many tropical regions, which is widely cultivated in Southeast Asian countries. Probiotics such as Lactobacillus plantarum have been shown to exhibit an anti-obesity effect by regulating gut microbiota. This study investigated the effect of polysaccharides from pitaya flower buds (PFW) extracted with water on the regulation of gut microbiota and body weight control in mice fed with a high-fat diet. The effects of PFW on the growth of L. plantarum were analyzed and the propagation of L. plantarum was promoted in an aqueous solution containing PFW. In an in vivo study, mice were fed with a high-fat diet supplemented with PFW for 12 weeks; PFW treatment effectively controlled body weight and reduced short bowel syndrome of mice induced by the high-fat diet. Gut microbiota sequencing revealed that Lachnospiraceae and Lactobacillaceae were the main bacteria targeted by PFW. Moreover, transcript analysis demonstrated that PFW alleviated obesity through amino acid metabolism, carbohydrate metabolism, and glycan metabolism. Overall, PFW is a valuable food supplement that can regulate gut microbiota and may have potential to ameliorate the physiological damage caused by a high-fat diet.
1. Introduction
Modern dietary patterns are gradually being westernized, and the problem of obesity is increasingly becoming serious worldwide. The harmful health effects of being obese at a young age have been discussed in copious studies. Brinkworth et al. (2009) [1] found that different diets (low-carbohydrate, high-carbohydrate, low-fat diet, high-fat diet, and high-fiber diet) affected the health and functional index of gut microbiota differently. They observed that a high-fat diet reduced feces weight and impacted the levels of short-chain fatty acids (SCFAs) and excretion rate of feces. Therefore, long-term intake of a high-fat diet increases the risk of gastrointestinal diseases. High-fat diets in mice cause intestinal metabolic disorders [2]. In Japan, functional constipation in 3–8-year-old children was reported to be related to a high-fat diet [3]; therefore, strategies for reducing the damage caused by a high-fat diet have attracted the interest of researchers.
The gastrointestinal tract naturally has a balanced gut microbiota; however, aging, stress, environment, and dietary habits change the gut microbiota composition and cause dominant flora to change. These changes in the intestinal ecological environment are closely related to the health of the host body and the potential disease risk factors. In addition, a low-calorie diet modulates the level of beneficial gut microbiota, including Lactobacillus bacteria [4], which also indicates that patients with diabetes and obesity have a high Firmicutes/Bacteroidetes ratio.
Although the importance of gut microbiota to health is well known, it is difficult to maintain the balance of gut microbiota over a long period of time due to the host’s living environment, dietary habits, and health status [5]. Supplementation of probiotics is sometimes a way to overcome this issue, however, probiotics viability through the gastrointestinal tract is not fully successful [6]. Prebiotics are a group of food components that are not absorbed in the gastrointestinal tract but degraded by gut microbiota, which selectively stimulate the growth and/or activity of the intestinal bacteria and improve the host’s health [6]. Prebiotics such as non-digestible polysaccharides are not easily digested by the human body but maintain a good gastrointestinal environment and may selectively stimulate beneficial gut microbiota. Therefore, supplementing prebiotics in a daily diet may promote gut microbiota and consequently human health [6].
Pitaya (Hylocereus undatus), also called dragon fruit, is a nutritious fruit adaptable to arid areas and is gradually becoming popular in the global market [7]. The exact native range of pitaya is uncertain but is considered to be in Central America. Since the late twentieth century, it has been widely planted as a fruit crop in many tropical regions, particularly in Southeast Asian countries. Several countries have invested in pitaya production, including Thailand, Indonesia, Taiwan, and Vietnam [7]. In Taiwan, the production of pitaya exceeds 66 million kilograms per year [7]. The pulp, peel, seeds, buds, and flowers of pitaya plants contain antioxidants, cellulose, vitamins, and minerals [8]. Active ingredients are extracted from fruit waste to enhance the value of by-products and reduce waste disposal problems. Pitaya farmers execute defloration during cultivation to concentrate nutrients on a few fruits to achieve better quality and yield. However, the removed flowers buds are discarded as agricultural wastes. Thus, centralized management and recycling mechanisms are essential to reduce environmental contamination [8]. The structural characteristics of a water-soluble polysaccharide derived from fruit pitaya has been discussed in a previous study, which had a molecular weight of 2.2 × 103 kDa; →4-β-D-GlcpA-1→, →6-β-D-Galp-1→, and →4-α-L-Rhap-1→ constituted the backbone and α-L-Araf-1→5-α-L-Araf-1→ formed the branch chain [9]. Previous studies showed that probiotics supplementation contributed to improve obesity. Lactobacillus plantarum has been shown to exhibit an anti-obesity effect in high-fat diet-induced obese mice by regulating gut microbiota and its metabolites [10,11], which can be considered a single probiotic agent for preventing or treating obesity. This study investigated whether the polysaccharides from pitaya flower buds extracted with water (PFW) promoted the growth of L. plantarum and modulated the gut microbiota of high-fat diet mice.
2. Materials and Methods
2.1. Material Preparation
Approximately 25 g of fresh flower buds of pitaya was mixed with 250 mL of deionized water and was extracted at 95 °C for 30 min. After extraction, the aqueous extract was filtered, and the filtrate was precipitated with 3 times the volume of alcohol (99%). The precipitated extracts were collected and decomposed the protein with protease and then freeze-dried to obtain the extracts sample PFW containing the polysaccharide sample. The yield of PFW from flower buds is 14%.
2.2. Cultivation of Lactobacillus plantarum
Ten percent of PFW (w/v) was dissolved in the dH2O. After sterilization by autoclave and cooling down, Lactobacillus plantarum (1%) was inoculated in MRS medium or 10% of PFW solution and cultured at 37 °C. The absorbance at 600 nm was measured at 0, 3, 6, 12, 24, 48, 60, and 72 h to confirm the number of bacteria. The growth curve was plotted to assess the growth of L. plantarum.
2.3. Animal Studies
C57BL/6 mice (8-week-old) were purchased from Lesco Biotechnology Co., Ltd. (Taipei, Taiwan). The breeding environment followed the guidelines for the management and use of laboratory animals, as stated by the Agricultural Committee of the Executive Yuan. The temperature was controlled at 20–26 °C, the relative humidity was 55 ± 10%, and the light cycle was 12:12 h (7:00–19:00 h). All mice were fed without restraint during the experiment. After a 1-week adaptation period, C57BL/6 mice were randomly divided into 3 groups (n = 5 each): group Std, the standard diet; group HF, mice fed with a high-fat diet; and group HF+PFW, mice fed with a high-fat diet supplemented with PFW (250 mg/kg body weight (bw)). The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Animal Care and Use Committee (IACUC) in National Chiayi University in Taiwan with IACUC approval No. 110026.
The control group Std was given LabDiet 5001, a standard commercial feed containing 10.7% total fat content. Groups HF and HF+PFW received high-fat feed that containing 30% ghee for 12 weeks. In group HF+PFW, 250 mg/kg bw of PFW was orally administered by oral gavage once a day for 4 weeks from week 9 to week 12. At the end of the study, the mice were weighed before sacrificing the mice with carbon dioxide. Cecum and colon tissues from mice were collected and the length of the colon measured with an iron ruler. Subsequently, colon tissue was cut with a sterile scalpel and the feces were collected from the colon of mice for further analysis.
2.4. Species Classification of Gut Microbiota by Next-Generation Sequencing
The 16S ribosomal DNA of samples was extracted for next-generation sequencing. Two grams of fecal specimens were promptly placed in the specimen preservation solution and stored at −80 °C. For DNA isolation, stool samples (180–220 mg) were placed into microbead tubes, and a Stool DNA kit (CatchGene, New Taipei City, Taiwan) was used for extraction and purification. The DNA samples were subjected to polymerase chain reaction (PCR) and the V3-V4 region was amplified by specific primer set (319F: 5′-CCTACGGGNGGCWGCAG-3′, 806R: 5′-GACTACHVGGGTATCTAATCC-3′) according to the 16S Metagenomic Sequencing Library Preparation procedure (Illumina). The PCR products were purified by electrophoresis, and 300 bp DNA fragments were recovered for sequencing. Sequencing was performed using the Illumina MiSeq PE300 platform [12]. After importing the sequencing raw data into QIIME2, the primer was removed to obtain input reads that can be used for the subsequent DADA2 denoising process. After the denoising operation is completed, Amplicon Sequence Variants (ASVs) are obtained, and the species classification is continued in the analysis [13]. Based on the 16S sequencing data, the GreenGenes database and the KEGG orthology copy the number relationship table, predicting the metabolic pathway functions of the KEGG database at 3 levels. The number is the copy of the number of reads in the sample that may be related to the function. The function of the gut microbiota affecting metabolism was predicted using KEGG level 2.
2.5. Statistical Analysis
Experimental results were performed in five repeats and expressed as the mean ± standard error of the mean (SEM). The results were examined using one-way analysis of variance (ANOVA) and Duncan’s multiple range tests. A p value ≤ 0.05 was considered significantly different. For β diversity of microbiota analysis, pairwise ANOSIM (analysis of similarities) with 999 permutations were conducted and evaluated using principal component analyses (PCA) based on different distance matrices, where p value was reported after the Benjamini–Hochberg multiple testing correction (q value).
3. Results and Discussion
3.1. Effect of the PFW on the Growth of L. plantarum
To evaluate whether PFW has the potential to modulate gut microbiota and obesity, whether PFW could promote the growth of probiotic L. plantarum was firstly tested in vitro. In the present study, the effects of PFW on the growth of L. plantarum were analyzed. The MRS medium, as a control group, was appropriate for the growth of L. plantarum. The results show that PFW treatment promotes the growth of L. plantarum, which provides nutrients for the reproduction of L. plantarum (Figure 1), indicating that PFW acts as a probiotic promoter and has potential for regulating gut microbiota. Proper supplementation of the nutrition of the gut microbiota requires enhancement of the probiotics to resist the growth of pathogens and maintain the intestinal tract in a good state. Probiotics are microorganisms that provide health benefits to the host. However, probiotics need to be supplemented with prebiotics, which are not easy to digest but can be utilized by the microorganisms to grow and colonize in the intestines [14]. Probiotic replenishing and prebiotic consumption are essential components of a healthy diet. Cummings et al. (2002) [15] proposed that fructose-based carbohydrates, inulin, and fructooligosaccharides selectively stimulate gut microbiota and significantly increase the content of bifidobacteria. Probiotic L. plantarum regulates gut microbiota partially through producing antimicrobial peptides (bacteriocins) that inhibit the growth of other microorganisms [16]. L. plantarum also promotes bile acid metabolism and exhibits better colonization and indestructibility [17]. The postbiotics produced by L. plantarum are cytotoxic to cancer cells without affecting normal cell survival [18].
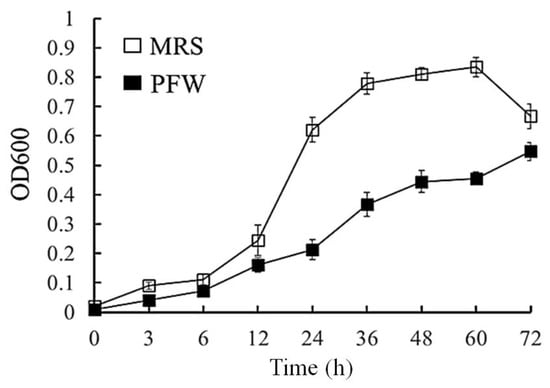
Figure 1.
Growth curves of Lactobacillus plantarum grown in MRS medium or PFW solution at 3, 6, 12, 24, 36, 48, 60, and 72 h of incubation. Data were expressed as mean ± standard error of the mean (SEM).
3.2. Effect of PFW on Body Weight and Colon Length of High-Fat Diet-Induced Mice
Long-term high fat intake can impair gastrointestinal signals such as cholecystokinin, Peptide YY, and glucagon-like peptide 1, leading to weight gain [19]. In this study, mice were fed a high-fat diet to explore the effect of PFW on the body weight gain of mice. After treating for 12 weeks, the weight change during the period showed that the mice in the high-fat diet group had significantly higher weight when compared with that of the standard diet mice. However, there was no significant difference between the weight of mice fed a high-fat diet supplemented with PFW and standard diet mice (Figure 2). After the mice were dissected, the total length of the cecum and large intestine was measured. The high-fat diet group was found to have significant intestinal atrophy, damage, and shortening; however, feeding of PFW ameliorated intestinal atrophy (Figure 3).
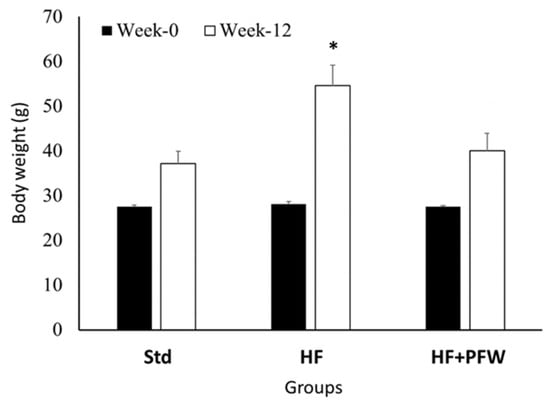
Figure 2.
Effect of PFW on body weight of high-fat diet-induced mice. Mice received standard diet or high-fat diet for 12 weeks. In total, 250 mg/kg bw of PFW was orally administered once a day for 4 weeks. Data were expressed as mean ± standard error of the mean (SEM). * p < 0.05 compared with the Std group. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
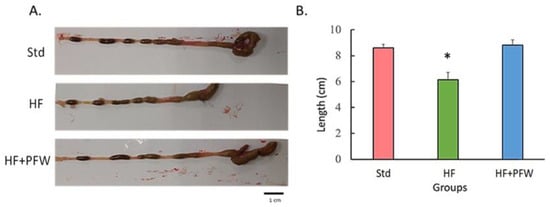
Figure 3.
Effect of the PFW on colon length of mice. After 12 weeks of high-fat diet induction, the colon tissue of mice was collected and the length calculated. (A) Representative images (scale bar, 1 cm). (B) Quantified the length of the colon. Data were expressed as mean ± SEM. * p < 0.05 compared with the Std group. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
Obesity is a growing global disease epidemic with multiple causes. Patients with obesity have lifelong weight-loss difficulties, suffer from metabolic disorders and poor social psychology. Moreover, owing to an altered food cycle, the activities of the biological clock are also affected [20]. The government has currently formulated many strategies to deal with one-third of the adult obesity issues by giving advice, guidance, and encouraging public health education and actively promoting the benefits of healthy eating [21,22]. Besides probiotics, prebiotics supplementation also has beneficial effects on host physiology [23]. In Canadian children aged between 7–12 years who were obese, supplementing with oligofructose-enriched inulin for 16 weeks after diagnosis changed the gut microbiota and reduced the frequency of obesity [24].
The intestinal length not only affects digestion and absorption, but also alters the living environment of the gut symbionts. C57BL/6 mice fed a high-fat diet for 3 weeks showed atrophy of the small intestine, colon, and lymphatic tissue, and a large amount of free fatty acids produced by a high-fat diet caused intestinal lipotoxicity. Although unsaturated fatty acids and mid-chain triglycerides are considered healthy free fatty acids that can prevent metabolic syndrome, they are toxic to the intestines [25]. These findings suggest that prebiotics such as some polysaccharides and oligosaccharides have potential for modulating gut microbiota composition. PWF treatment may combat obesity via regulating the intestinal environment and hence alleviate the colon shortening induced by a high-fat diet in mice.
3.3. Effect of the PFW on Gut Microbiota in High-Fat Diet-Induced Mice
The human genome contains 26,600 protein-encoding genes [26], while the human intestine is composed of more than 1000 different microbial species. Gut microbiota has a total of 4,000,000 protein-encoding genes, which indirectly affect the development of human diseases [27,28,29]. A high-fat diet alters the composition of gut microbiota and thereby accelerates obesity. Moreover, obesity leads to the dysregulation of the gut microbiota, intestinal permeability, villi and crypt length, intestinal cell tightness, and secretion of mucus [30].
To access whether PFW has the potential to regulate gut microbiota in vivo, fecal bacteria of mice were subjected to a 16S metagenomic analysis. The ASVs of fecal samples in the 3 groups were of good quality, and sufficient DNA sequence fragments were obtained for analysis. Principal components analysis (PCA) was used to analyze the difference in beta diversity of fecal bacteria, and it was found that the PFW group had the widest distribution of microbiota among the three groups. Weighted statistical analysis of the diversity of the gut microbiota revealed that PFW treatment effectively increased the diversity of gut microbiota (Figure 4). The HF+PFW group and Std showed a significant difference. The characteristics of fecal microorganisms revealed that the dysbiosis of the microbial phase induced by the high-fat diet mainly affects the growth of aerobic bacteria; subsequently, the HF+PFW group could restore the number of aerobic bacteria in the intestines (Figure 5). Thus, PFW resisted the decreased in the number and types of gut microbiota caused by the high-fat diet and resulted in more increased diversity of gut microbiota species than in the Std group.
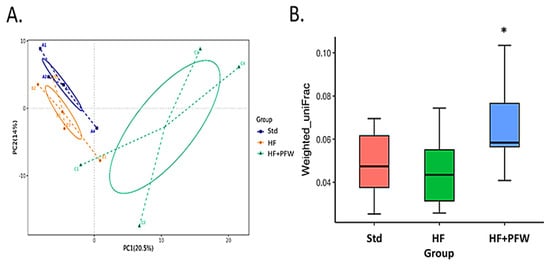
Figure 4.
The effect of PFW on gut microbiota distribution in mice fed a high-fat diet. (A) Principal components analysis (PCA) was used to determine the beta diversity of gut microbiota. Samples with more similar composition cluster closer together. (B) Beta diversity of the community was calculated by using the UniFrac distance matrix. * p < 0.05 compared with the Std group. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
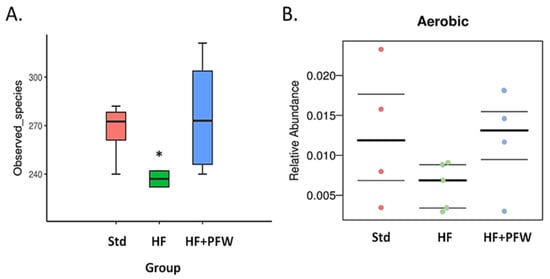
Figure 5.
The effect of PFW on gut microbiota composition in high-fat diet-induced mice. (A) Alpha diversity index of the gut microbiota. (B) Relative abundance of aerobic bacteria. * p < 0.05 compared with the Std group. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
Facultative anaerobic bacteria exist in the digestive tract, but the intestine is at the end of the digestive tract and is an anaerobic environment. Symbiotic microorganisms can be divided into Bacteroides, Firmicutes, Actinobacteria, and Proteobacteria and are mainly anaerobic bacteria. Many species have different enterotypes and cause various health effects [31].
Analysis of gut microbiota at the family level and investigation of feeding intensity revealed that PFW significantly reduced the ratio of Muribaculaceae and increased the ratio of Lachnospiraceae, Ruminococcaceae, and Lactobacillaceae (Figure 6). Lachnospiraceae and Ruminococcaceae can ferment carbohydrates from plant materials that cannot be converted to SCFAs by the host. SCFAs affect the absorption and metabolism of the host [32]. Since PFW treatment increased the ratio of Lachnospiraceae and Ruminococcaceae in the gut of mice, PFW may have potential for elevating the level of SCFAs via promoting the ratio of SCFAs producing gut bacteria. Lactobacillaceae has anti-obesity effects [33]. PFW is a good source of nutrition for Lactobacillaceae, which is consistent with the results of in vitro tests.
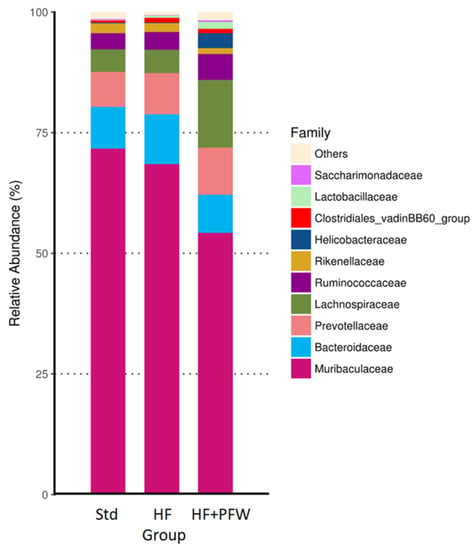
Figure 6.
Taxa analysis of gut microbiota composition in mice by relative abundance bar chart. Top 10 classifications for family level are shown. Taxa not in the top 10 are grouped together in the others category. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
From the perspective of the genus, the heat map analysis showed that high-fat diet induction reduced the ratio of Akkermansia, Mucispirillum, and Parasutterella and increased the number of Marvinbryantia, Bacteroides, and Ruminiclostridium-9. PFW treatment significantly increased the types and quantities of various gut microbiota, the most important of which are Lactobacillus, Lachnospiraceae-NK4A136-group, Ruminiclostridium-5, Ruminococcaceae-UCG-004, Lachnoclostridium, and Butyricicoccus (Figure 7). Clostridium has many different genera of microorganisms, including the ones that support human health (such as Clostridium botulinum and Clostridium acetobutylicum) and those that harm the human body (such as Clostridium difficile and Clostridium perfringens). Clostridiales-vadinBB60-group increased significantly due to the induction of the high-fat diet, but the PFW had no enhancement effect. However, the family level of Lachnospiraceae and Lactobacillaceae were significantly increased by the addition of PFW (Figure 8).
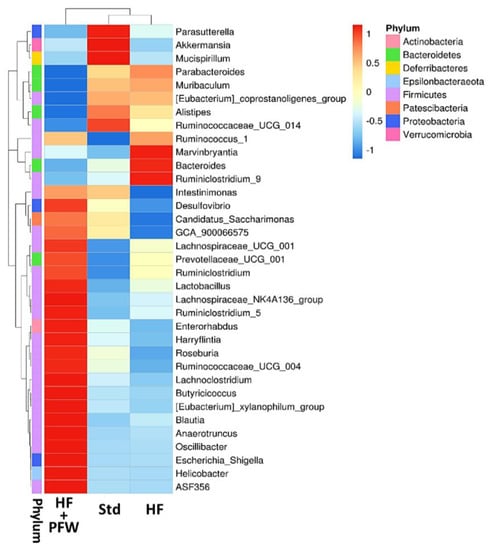
Figure 7.
Taxa analysis of gut microbiota composition in mice by heatmap. Heatmap represents relative abundance of taxa on a color gradient. The top 35 most abundant genus taxa are shown. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
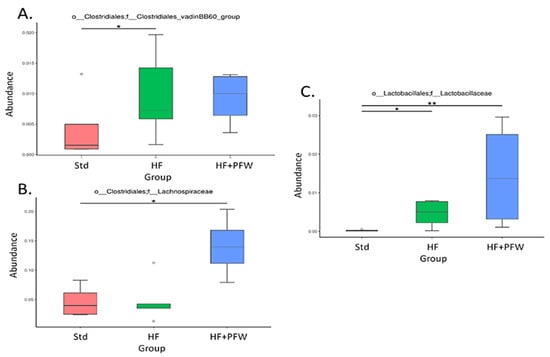
Figure 8.
Relative abundance of the gut microbiota in mice under PFW treatment. The abundance of Clostridiales (A), Lachnospiraceae (B), and Lactobacillaceae (C) changed significantly among the groups. * p < 0.05, ** p < 0.01 compared with the Std group. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
Bioactive components in natural products have been shown to be beneficial in preventing or improving the development of metabolic diseases, such as obesity, diabetes, and steatohepatitis. In addition, possible therapeutic manifestations and beneficial effects in the health of the host due to the regulation of the gut microbiota through prebiotics can come from nutrition such as food consumption. Natural bioactive compounds in pitaya may play roles in modulating metabolic disorders. For example, purified betacyanins isolated from the peel of pitaya have been demonstrated to have the ability to ameliorate obesity, insulin resistance, and hepatic steatosis in high-fat diet-induced obese mice [34]. In addition, the beneficial effect of betacyanins protects from diet-induced obesity and is associated with the adjustment of gut microbiota, especially its ability to elevate the relative abundance of Akkermansia [35].
3.4. The Effect of the PFW on the Metabolic Function of High-Fat Diet Mice
The miRNAs regulate the gut microbes and human transcriptome, and miRNAs in the gut mainly affect cell metabolism, not immune function [36]. Gut microbiota is highly correlated with host metabolism. The results show that high-fat diet induction reduced amino acid, carbohydrate, and glycan metabolism in mice causing obesity. However, the microorganisms regulated by PFW are involved in amino acid metabolism, carbohydrate metabolism, glycan metabolism, and energy metabolism in mice, thereby improving the symptoms of obesity in mice (Figure 9).
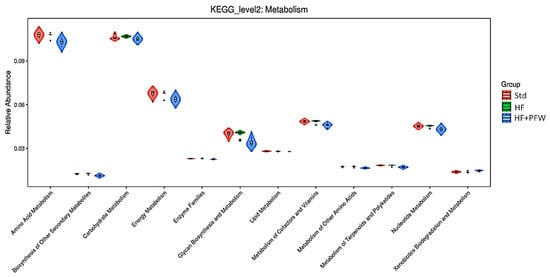
Figure 9.
Violin image of KEGG function prediction based on 16S rRNA gene. Comparison of the gut microbiota function prediction among different groups. The function of the gut microbiota affecting metabolism was predicted using KEGG level 2. Group Std, standard diet; group HF, high-fat diet; group HF+PFW, high-fat diet plus PFW treatment.
4. Conclusions
This study showed that supplementation with PFW restores the physiological changes caused by a high-fat diet. PFW is an effective material that selectively multiplies Lachnospiraceae and Lactobacillaceae. Moreover, it indirectly affects amino acid, carbohydrate, glycan metabolism, and energy consumption to ameliorate obesity. Simultaneously, it aided in controlling weight gain and preventing intestinal atrophy in vivo. Therefore, supplementation with PFW may reduce body weight gain due to a high-fat diet and improve the intestinal environment and gut microbiota composition. Moreover, enhancing the utilization of PFW to solve the problem of agricultural waste is worth developing to reduce waste disposal problems in the future.
Author Contributions
Conceptualization, B.-H.L. and W.-H.H.; methodology, Y.-Z.C.; writing—original draft preparation, K.-T.H.; writing—review and editing, Y.-L.T. and C.-Y.H.; study design, W.-H.H., B.-H.L. and Y.-C.L.; supervision, W.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research work and subsidiary spending were mainly supported by the Council of Agriculture, Executive Yuan (110AS-1.3.2-ST-aA) (110AS-10.1.7-AD-U1). This work is also supported by the Ministry of Science and Technology (MOST) in Taiwan under grant nos. MOST 109-2636-B-006-008 (Young Scholar Fellowship Program). This research was also supported in part by the Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Animal Care and Use Committee (IACUC) in National Chiayi University in Taiwan with IACUC approval No. 110026.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brinkworth, G.; Noakes, M.; Clifton, P.; Bird, A.R. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Wiggins, H.S.; Jenkins, D.J.; Houston, H.; Jivraj, T.; Drasar, B.S.; Hill, M.J. Influence of diets high and low in animal fat on bowel habit, gastrointestinal transit time, fecal microflora, bile acid, and fat excretion. J. Clin. Investig. 1978, 61, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujitani, A.; Sogo, T.; Inui, A.; Kawakubo, K. Prevalence of Functional Constipation and Relationship with Dietary Habits in 3- to 8-Year-Old Children in Japan. Gastroenterol. Res. Pract. 2018, 2018, 3108021. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; De Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.G.; Fitzgerald, G.F.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4586–4591. [Google Scholar] [CrossRef] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Overall Dragon Fruit Production and Global Marketing. Available online: https://ap.fftc.org.tw/article/1596 (accessed on 10 December 2021).
- Jalgaonkar, K.; Mahawar, M.K.; Bibwe, B.; Kannaujia, P. Postharvest Profile, Processing and Waste Utilization of Dragon Fruit (Hylocereus Spp.): A Review. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, L. Structure characteristics of a water-soluble polysaccharide purified from dragon fruit (Hylocereus undatus) pulp. Carbohydr. Polym. 2016, 146, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet–induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Park, E.-J.; Park, G.-S.; Ko, S.-H.; Park, J.; Lee, Y.-K.; Kim, J.-Y.; Lee, D.; Kang, J.; Lee, H.-J. Lactiplantibacillusplantarum ATG-K2 Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 12665. [Google Scholar] [CrossRef]
- Caporasoa, J.G.; Lauberb, C.L.; Waltersc, W.A.; Berg-Lyonsb, D.; Lozuponea, C.A.; Turnbaughd, P.J.; Fiererb, N.; Knighta, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J.; Michel, C. Microbiota of the Human Body, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 119–142. [Google Scholar]
- Cummings, J.H.; Macfarlane, G.T. Gastrointestinal effects of prebiotics. Br. J. Nutr. 2002, 87, S145–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potenti-alities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Alitheen, N.B.M.; Yeap, S.K.; Mutalib, N.E.A.; Rahim, R.A.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Duca, F.A.; Sakar, Y.; Covasa, M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem. 2013, 24, 1663–1677. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, J.; Bass, J. High-fat diet disrupts be-havioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.; Ytton, O.; White, M.; Monsivais, P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 2016, 13, e1001990. [Google Scholar]
- Biddle, A.; Stewart, L.; Blanchard, J.L.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intes-tinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S.; et al. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelial damage. Biochem. Biophys. Res. Commun. 2019, 522, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Nagpal, R.; Newman, T.M.; Wang, S.; Jain, S.; Lovato, J.F.; Yadav, H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J. Diabetes Res. 2018, 2018, 3462092. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2015, 64, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Carney, M.C.; Zhan, X.; Rangnekar, A.; Chroneos, M.Z.; Craig, S.J.; Makova, K.D.; Paul, I.M.; Hicks, S.D. Associations between stool micro-transcriptome, gut microbiota, and infant growth. J. Dev. Orig. Health Dis. 2021, 12, 876–882. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).