Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture
Abstract
:1. Introduction
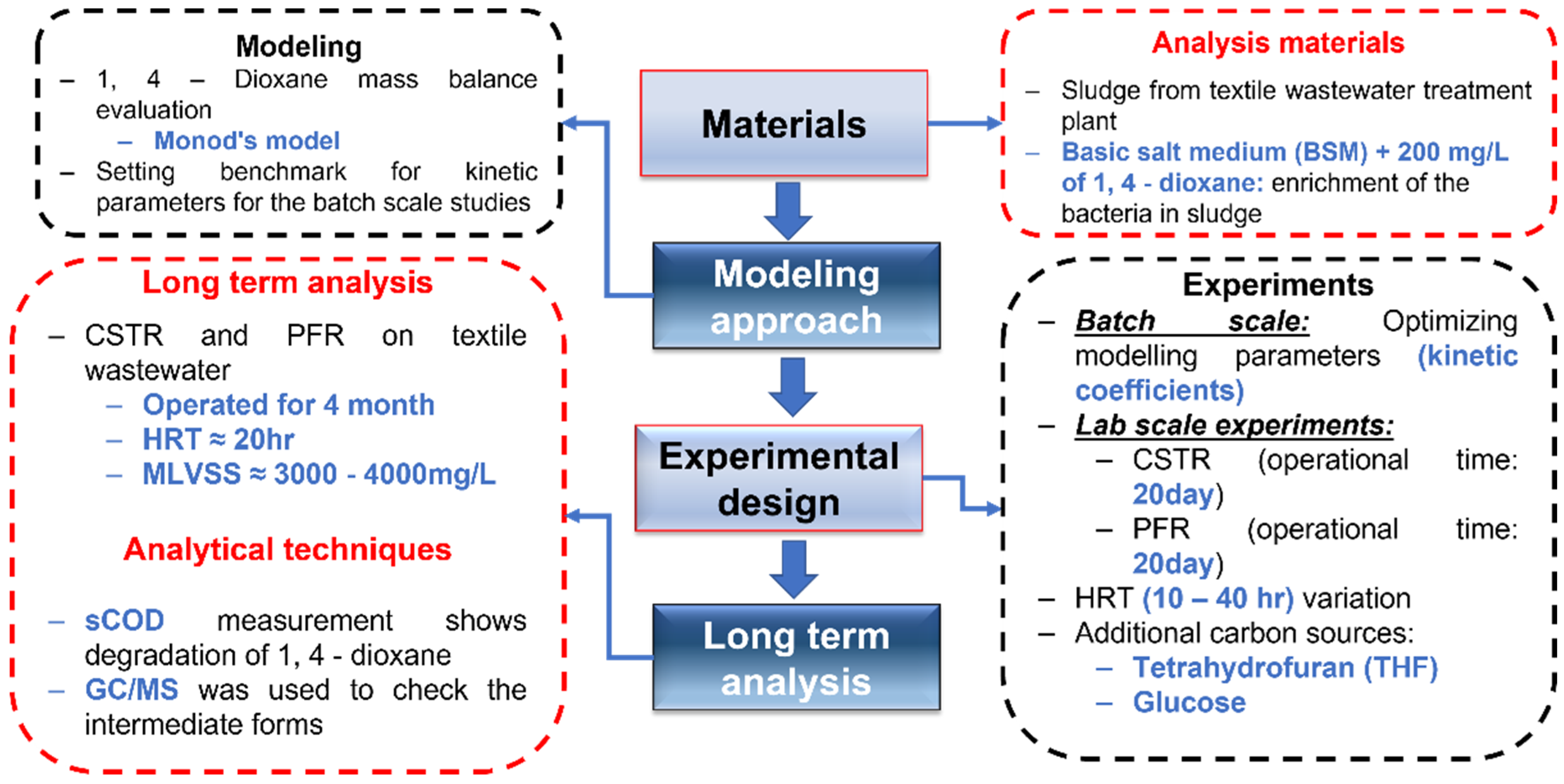
2. Materials and Methods
2.1. Materials
2.2. Modelling Approach
2.3. Experimental Design
2.3.1. Enrichment of Mixed Culture
2.3.2. Continuous Stirred Reactor (CSTR)
2.3.3. Plug Flow Reactor (PFR)
2.4. Analysis Procedure
3. Results and Discussion
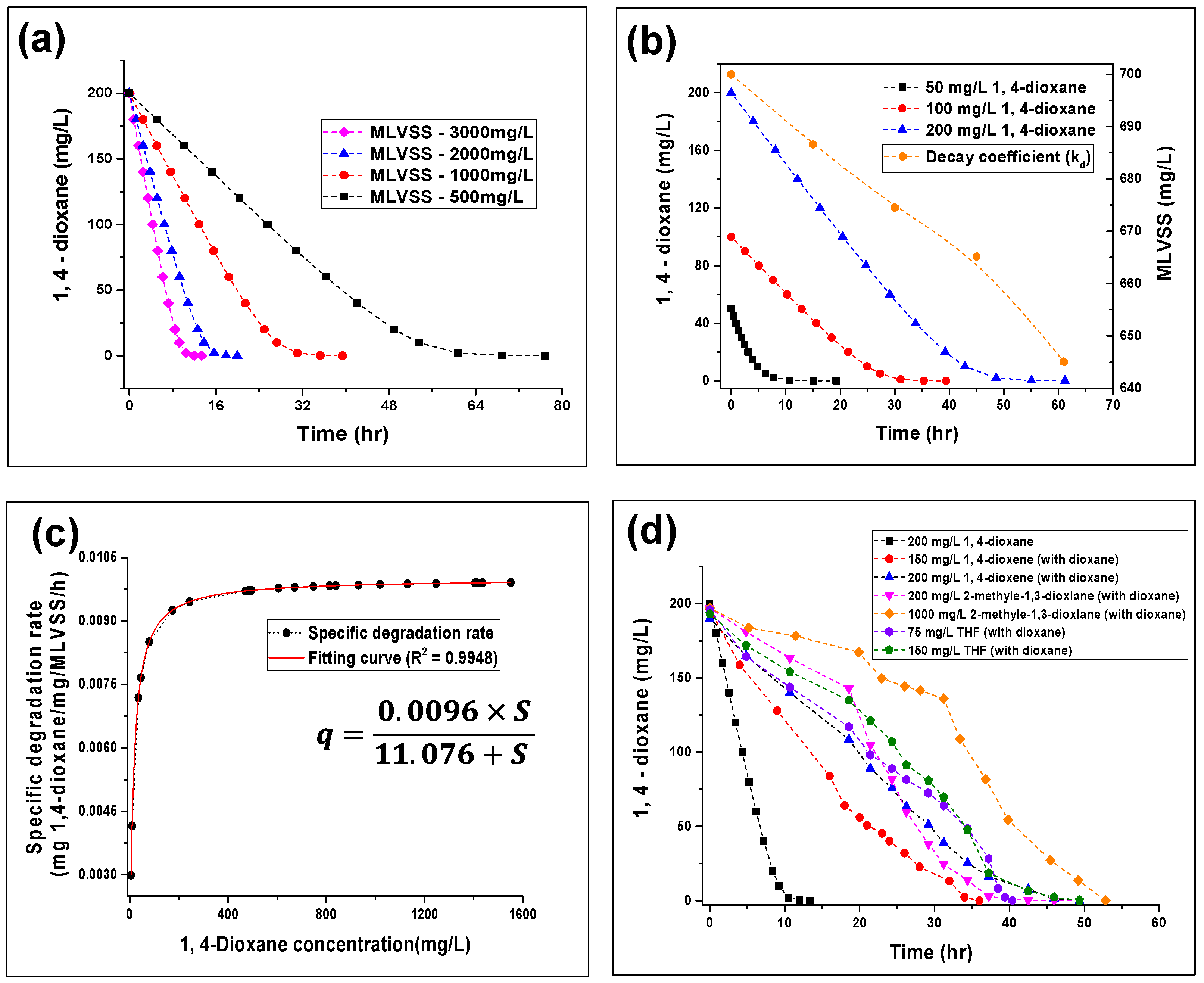
3.1. Batch Test Analysis for 1,4-Dioxane Biodegradation
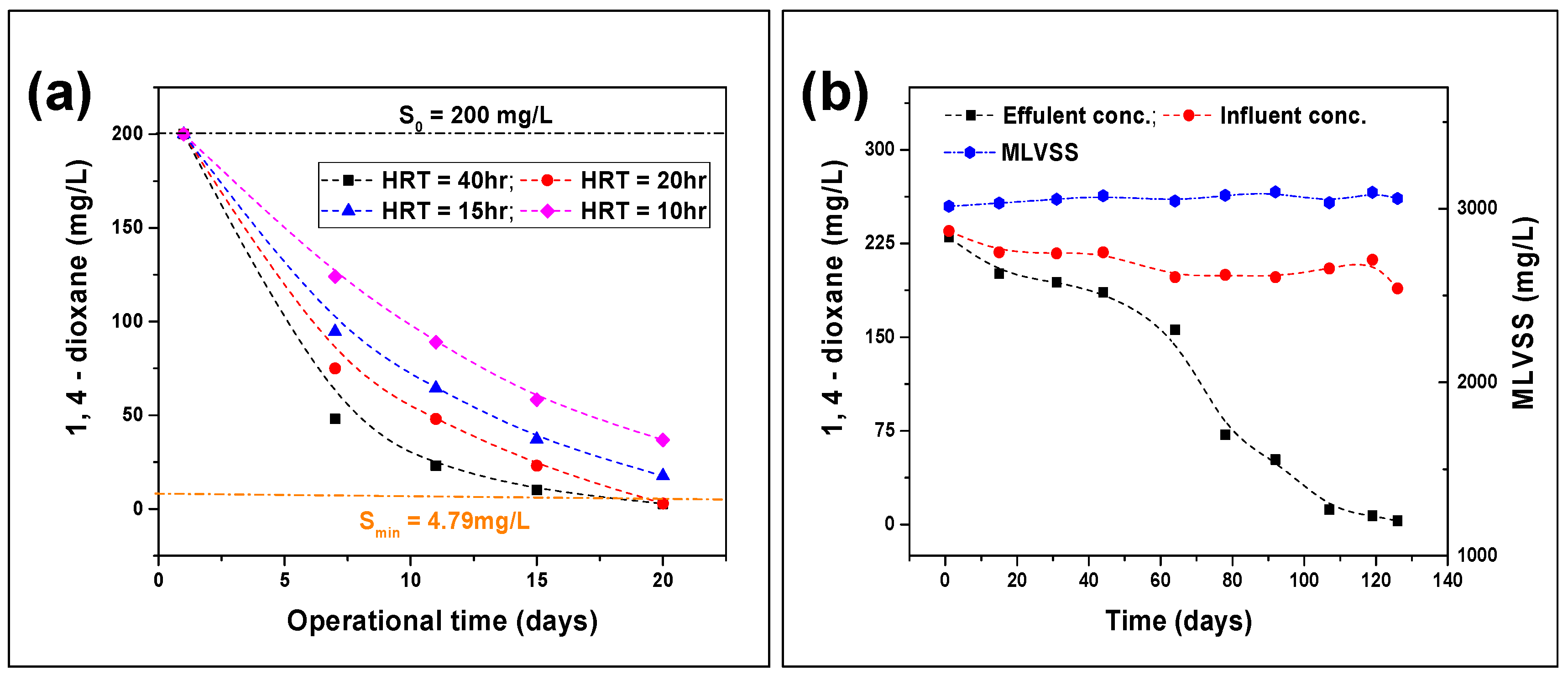
3.2. 1,4-Dioxane Biodegradation from Industrial Wastewater
3.2.1. Continuous Stirred Reactor (CSTR) Analysis
3.2.2. Plug Flow Reactor (PFR) Analysis
4. Conclusions
- In the batch-test analysis, a relatively low specific substrate utilization rate (qmax) of 0.0096 mg of 1,4-dioxane/mg MLVSS/h was observed, with a half-saturation coefficient (Ks) of 11.076 mg 1,4–dioxane/L. Moreover, an endogenous biomass decay rate (kd) of 0.03 day−1 and maximum specific microbial growth rate (μmax) of 0.099 day−1 were observed to optimize the degradation of 1,4–dioxane under a mixed culture condition, grown using 1,4–dioxane as the sole carbon source.
- GC/MS results showed that the presence of 2-methyl-1,3-dioxlane as a competitive inhibitor hindered the degradation of 1,4–dioxane. Moreover, the presence of structure analogs, such as THF and 1,4–dioxane increased the degradation time for 1,4–dioxane. However, no changes in the degradation of the inhibitor were observed, but increases in the degradation time were noted, which increased to 55 h from 13 h.
- In a long-term analysis involving CSTR and PFR tests, a HRT of 20 h is considered to as the optimal condition for the efficient degradation of 1,4–dioxane. Moreover, effluent concentrations of 3 mg/L and <1 mg/L of 1,4–dioxane were observed in the CSTR and PFR tests. The higher removal efficacy by the PFR was due to the production of a higher MLVSS level of 4000 mg/L, compared to 3000 mg/L in the CSTR in a competitive environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minh Tran, H.D.; Boivin, S.; Kodamatani, H.; Ikehata, K.; Fujioka, T. Potential of UV-B and UV-C irradiation in disinfecting microorganisms and removing N-nitrosodimethylamine and 1,4-dioxane for potable water reuse: A review. Chemosphere 2022, 286, 131682. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Smith, K.; Wang, Y.; Hu, H. Light-driven breakdown of 1,4-Dioxane for potable reuse: A review. Chem. Eng. J. 2019, 373, 508–518. [Google Scholar] [CrossRef]
- Sun, B.; Ko, K.; Ramsay, J.A. Biodegradation of 1,4-dioxane by a Flavobacterium. Biodegradation 2011, 22, 651–659. [Google Scholar] [CrossRef]
- Lee, K.H.; Wie, Y.M.; Lee, Y.-S. Characterization of 1,4-Dioxane Biodegradation by a Microbial Community. Water 2020, 12, 3372. [Google Scholar] [CrossRef]
- Lee, K.H.; Qasim, M.; Lee, K.G.; Inam, M.A.; Khan, I.A.; Khan, R.; Wie, Y.M. Use of ballasted flocculation (BF) sludge for the manufacturing of lightweight aggregates. J. Environ. Manag. 2022, 305, 114379. [Google Scholar] [CrossRef] [PubMed]
- Godri Pollitt, K.J.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C.; et al. 1,4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Barndõk, H.; Cortijo, L.; Hermosilla, D.; Negro, C.; Blanco, Á. Removal of 1,4-dioxane from industrial wastewaters: Routes of decomposition under different operational conditions to determine the ozone oxidation capacity. J. Hazard. Mater. 2014, 280, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Nannelli, A.; De Rubertis, A.; Longo, V.; Gervasi, P.G. Effects of dioxane on cytochrome P450 enzymes in liver, kidney, lung and nasal mucosa of rat. Arch. Toxicol. 2005, 79, 74–82. [Google Scholar] [CrossRef]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Stickney, J.A.; Sager, S.L.; Clarkson, J.R.; Smith, L.A.; Locey, B.J.; Bock, M.J.; Hartung, R.; Olp, S.F. An updated evaluation of the carcinogenic potential of 1,4-dioxane. Regul. Toxicol. Pharmacol. 2003, 38, 183–195. [Google Scholar] [CrossRef]
- Isaka, K.; Udagawa, M.; Sei, K.; Ike, M. Pilot test of biological removal of 1,4-dioxane from a chemical factory wastewater by gel carrier entrapping Afipia sp. strain D1. J. Hazard. Mater. 2016, 304, 251–258. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). Technical Fact Sheet for 1,4-Dioxane; EPA 505-F-17-011; Federal Facilities Restoration and Reuse Office: Washington, DC, USA, 2017.
- Hamilton, J.T. Pollution as news: Media and stock market reactions to the toxics release inventory data. J. Environ. Econ. Manag. 1995, 28, 98–113. [Google Scholar] [CrossRef]
- McElroy, A.C.; Hyman, M.R.; Knappe, D.R. 1,4-Dioxane in drinking water: Emerging for 40 years and still unregulated. Curr. Opin. Environ. Sci. Health 2019, 7, 117–125. [Google Scholar] [CrossRef]
- Patton, S.; Romano, M.; Naddeo, V.; Ishida, K.P.; Liu, H. Photolysis of mono-and dichloramines in UV/hydrogen peroxide: Effects on 1,4-dioxane removal and relevance in water reuse. Environ. Sci. Technol. 2018, 52, 11720–11727. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Gao, Y.; Hu, H.-Y.; Tang, F.; Yang, Z. Characterization and biotoxicity assessment of dissolved organic matter in RO concentrate from a municipal wastewater reclamation reverse osmosis system. Chemosphere 2014, 117, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Masaki, S.; Kodamatani, H.; Ikehata, K. Degradation of N-nitrosodimethylamine by UV-based advanced oxidation processes for potable reuse: A short review. Curr. Pollut. Rep. 2017, 3, 79–87. [Google Scholar] [CrossRef]
- Doederer, K.; Farré, M.J.; Pidou, M.; Weinberg, H.S.; Gernjak, W. Rejection of disinfection by-products by RO and NF membranes: Influence of solute properties and operational parameters. J. Membr. Sci. 2014, 467, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.A.; Lee, Y.-S.; Kim, J.-O. Optimization of preoxidation to reduce scaling during cleaning-in-place of membrane treatment. J. Hazard. Mater. 2020, 400, 123212. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, Y.-S.; Kim, J.-O. A comparison of variations in blocking mechanisms of membrane-fouling models for estimating flux during water treatment. Chemosphere 2020, 259, 127328. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, Y.-S.; Kim, J.-O. Identification of scaling during clean-in-place (CIP) in membrane water treatment process. Chemosphere 2019, 237, 124398. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Khan, I.A.; Lee, K.H.; Kim, J.-O. Sensitivity of physical membrane damage detection on low pressure membranes of commercialized specification. Desalination 2022, 527, 115568. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of Halide Impacts on the Efficiency of Contaminant Degradation by Sulfate and Hydroxyl Radical-Based Advanced Oxidation Processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Patton, S.; Li, W.; Couch, K.D.; Mezyk, S.P.; Ishida, K.P.; Liu, H. Impact of the Ultraviolet Photolysis of Monochloramine on 1,4-Dioxane Removal: New Insights into Potable Water Reuse. Environ. Sci. Technol. Lett. 2017, 4, 26–30. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Jin, X.-J.; Chen, J.; Ye, J.-X.; Jiang, N.-X.; Chen, J.-M. Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8. Int. Biodeterior. Biodegrad. 2016, 106, 133–140. [Google Scholar] [CrossRef]
- Xu, X.; Pliego, G.; Garcia-Costa, A.L.; Zazo, J.A.; Liu, S.; Casas, J.A.; Rodriguez, J.J. Cyclohexanoic acid breakdown by two-step persulfate and heterogeneous Fenton-like oxidation. Appl. Catal. B Environ. 2018, 232, 429–435. [Google Scholar] [CrossRef]
- Khan, S.J.; Walker, T.; Stanford, B.D.; Drewes, J.E. Advanced treatment for potable water reuse. In Advanced Oxidation Processes for Water Treatment; IWA Publishing: London, UK, 2018; pp. 581–606. [Google Scholar]
- Collins, J.; Bolton, J.R. Advanced Oxidation Handbook; American Water Works Association Denver: Denver, CO, USA, 2016. [Google Scholar]
- Mahendra, S.; Alvarez-Cohen, L. Kinetics of 1,4-Dioxane Biodegradation by Monooxygenase-Expressing Bacteria. Environ. Sci. Technol. 2006, 40, 5435–5442. [Google Scholar] [CrossRef]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Biodegradation of 1,4-Dioxane Using Trickling Filter. J. Environ. Eng. 2004, 130, 926–931. [Google Scholar] [CrossRef]
- Mohr, T.K.; DiGuiseppi, W.H.; Hatton, J.W.; Anderson, J.K. Environmental Investigation and Remediation: 1,4-Dioxane and Other Solvent Stabilizers; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Han, T.-H.; Han, J.-S.; So, M.-H.; Seo, J.-W.; Ahn, C.-M.; Min, D.H.; Yoo, Y.S.; Cha, D.K.; Kim, C.G. The removal of 1,4-dioxane from polyester manufacturing process wastewater using an up-flow Biological Aerated Filter (UBAF) packed with tire chips. J. Environ. Sci. Health Part A 2012, 47, 117–129. [Google Scholar] [CrossRef]
- Sei, K.; Kakinoki, T.; Inoue, D.; Soda, S.; Fujita, M.; Ike, M. Evaluation of the biodegradation potential of 1,4-dioxane in river, soil and activated sludge samples. Biodegradation 2010, 21, 585–591. [Google Scholar] [CrossRef]
- Shin, D.; Sung, D.Y.; Moon, H.S.; Nam, K. Microbial succession in response to 1,4-dioxane exposure in activated sludge reactors: Effect of inoculum source and extra carbon addition. J. Environ. Sci. Health Part A 2010, 45, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Parales, R.E.; Adamus, J.E.; White, N.; May, H.D. Degradation of 1,4-dioxane by an actinomycete in pure culture. Appl. Environ. Microbiol. 1994, 60, 4527–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-M.; Zhou, Y.-Y.; Chen, D.-Z.; Jin, X.-J. A newly isolated strain capable of effectively degrading tetrahydrofuran and its performance in a continuous flow system. Bioresour. Technol. 2010, 101, 6461–6467. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Hayashida, N.; Matsumura, R.; Omura, A.; Nakashimada, Y.; Kato, J. Isolation and characterization of tetrahydrofuran-degrading Rhodococcus aetherivorans strain M8. Process Biochem. 2012, 47, 1665–1669. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeon, J.R.; Murugesan, K.; Kim, E.J.; Chang, Y.S. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 2009, 20, 511–519. [Google Scholar] [CrossRef]
- Myers, M.A.; Johnson, N.W.; Marin, E.Z.; Pornwongthong, P.; Liu, Y.; Gedalanga, P.B.; Mahendra, S. Abiotic and bioaugmented granular activated carbon for the treatment of 1,4-dioxane-contaminated water. Environ. Pollut. 2018, 240, 916–924. [Google Scholar] [CrossRef]
- Shen, W.; Chen, H.; Pan, S. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms. Bioresour. Technol. 2008, 99, 2483–2487. [Google Scholar] [CrossRef]
- Zhang, S.; Gedalanga, P.B.; Mahendra, S. Advances in bioremediation of 1,4-dioxane-contaminated waters. J. Environ. Manag. 2017, 204, 765–774. [Google Scholar] [CrossRef]
- Lee, K.H.; Wie, Y.M.; Jahng, D.; Yeom, I.T. Effects of Additional Carbon Sources in the Biodegradation of 1,4-Dioxane by a Mixed Culture. Water 2020, 12, 1718. [Google Scholar] [CrossRef]
- Yan, J.-L.; Cui, Y.-W.; Huang, J.-L. Continuous flow reactors for cultivating aerobic granular sludge: Configuration innovation, principle and research prospect. J. Chem. Technol. Biotechnol. 2021, 96, 2721–2734. [Google Scholar] [CrossRef]
- Kent, T.R.; Bott, C.B.; Wang, Z.-W. State of the art of aerobic granulation in continuous flow bioreactors. Biotechnol. Adv. 2018, 36, 1139–1166. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-H.; Ventura, J.-R.S.; Yeom, I.T.; Lee, Y.; Jahng, D. Structural and kinetic characteristics of 1,4-dioxane-degrading bacterial consortia containing the phylum TM7. J. Microbiol. Biotechnol. 2016, 26, 1951–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tusher, T.R.; Shimizu, T.; Inoue, C.; Chien, M.-F. Enrichment and analysis of stable 1,4-dioxane-degrading microbial consortia consisting of novel dioxane-degraders. Microorganisms 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, D.; Diekmann, H. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl. Microbiol. Biotechnol. 1991, 36, 120–123. [Google Scholar] [CrossRef]
- Nakamiya, K.; Hashimoto, S.; Ito, H.; Edmonds, J.S.; Morita, M. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl. Environ. Microbiol. 2005, 71, 1254–1258. [Google Scholar] [CrossRef] [Green Version]
- Vainberg, S.; McClay, K.; Masuda, H.; Root, D.; Condee, C.; Zylstra, G.J.; Steffan, R.J. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol. 2006, 72, 5218–5224. [Google Scholar] [CrossRef] [Green Version]
- Skinner, K.; Cuiffetti, L.; Hyman, M. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 2009, 75, 5514–5522. [Google Scholar] [CrossRef] [Green Version]
- Sei, K.; Miyagaki, K.; Kakinoki, T.; Fukugasako, K.; Inoue, D.; Ike, M. Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 2013, 24, 665–674. [Google Scholar] [CrossRef]
- Tusher, T.R.; Shimizu, T.; Inoue, C.; Chien, M.-F. Isolation and characterization of novel bacteria capable of degrading 1,4-dioxane in the presence of diverse co-occurring compounds. Microorganisms 2021, 9, 887. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Yang, J.; Guo, H.; Su, D.; Yu, L. Degradation of 1,4-Dioxane by Xanthobacter sp. YN2. Curr. Microbiol. 2021, 78, 992–1005. [Google Scholar] [CrossRef]
- Hunt, M.; Rice, E. Microbiological examination. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Association, A.P.H. Microbiologic examination. Standard Methods for the Examination of Water and Wastewater, 20th ed.; United Book Press Inc.: Baltimore, MD, USA, 1998; pp. 1–140. [Google Scholar]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Mineralization of 1,4-dioxane in the presence of a structural analog. Biodegradation 2000, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Value |
|---|---|
| 1,4-dioxane (mg/L) | 185.5–225.5 |
| CODcr (mg O2/L) | 1092–1820 |
| BOD (mg O2/L) | 894–1617 |
| SS (mg/L) | 96–155 |
| pH | 8.9–9.4 |
| Temperature °C | 35.9–37.1 |
| Kinetic Parameters | Values |
|---|---|
| Ks (mg 1,4-Dioxane/L) | 11.076 |
| qmax (mg 1,4-dioxane/mg MLVSS/h) | 0.0096 |
| kd (mg MLVSS/mg MLVSS/day) | 0.03 |
| Yt (mg MLVSS/mg 1,4-dioxane) | 0.432 |
| μmax (day−1) | 0.099 |
| Influent Characteristics | Biomass Properties | Kinetic Parameters | Technology | Results | Explanation | Ref. | |
|---|---|---|---|---|---|---|---|
| Source | Conc. (mg/L) | Growth Features | Ks (mg/L), qmax (day−1), kd (day−1) | Conc. (mg/L) | |||
| Synthetic wastewater | 100 | X. Flavius DT8 (Activated WW sludge) | Ks = 17.5, qmax = 0.42 kd = 0.073 | Batch test | 1.83 |
| [26] |
| // | 50 | CB1190 | Ks = 160 kd = 1.1 | Batch test | - |
| [30] |
| 50 | B5 | Ks = 330 kd = 0.1 | Batch test | - | |||
| // | 100 | Cultured grown (Activated sludge) | - | Batch test | 0.8 |
| [34] |
| Industrial wastewater | 200–300 | Ozonation was used for the degradation process and optimized for better operation (pH optimization) | 5–65 |
| [7] | ||
| Synthetic wastewater | 1.09–1.25 | THF substrate (on aquifer) | Ks = 10.8 kd = 1.09 | Trickling filter | 0.043–0.078 |
| [31] |
| // | 6 mM | Actinomycete (pure culture) | - | Batch test | 0.55 μM |
| [36] |
| // | 900 | Bacterial strain PH-06 (river sediment) | - | Batch test | 100 |
| [39] |
| Industrial wastewater | ≈200 | Mixed culture (on WW sludge) | Ks = 11.07, qmax = 0.23 kd = 0.03 | CSTR | 3 |
| This study |
| PFR | 0.4 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Khan, I.A.; Inam, M.A.; Khan, R.; Wie, Y.M.; Yeom, I.T. Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture. Fermentation 2022, 8, 143. https://doi.org/10.3390/fermentation8040143
Lee KH, Khan IA, Inam MA, Khan R, Wie YM, Yeom IT. Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture. Fermentation. 2022; 8(4):143. https://doi.org/10.3390/fermentation8040143
Chicago/Turabian StyleLee, Kang Hoon, Imtiaz Afzal Khan, Muhammad Ali Inam, Rizwan Khan, Young Min Wie, and Ick Tae Yeom. 2022. "Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture" Fermentation 8, no. 4: 143. https://doi.org/10.3390/fermentation8040143
APA StyleLee, K. H., Khan, I. A., Inam, M. A., Khan, R., Wie, Y. M., & Yeom, I. T. (2022). Efficacy of Continuous Flow Reactors for Biological Treatment of 1,4-Dioxane Contaminated Textile Wastewater Using a Mixed Culture. Fermentation, 8(4), 143. https://doi.org/10.3390/fermentation8040143









