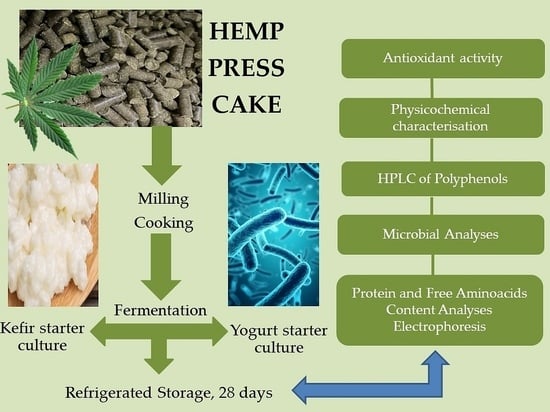
The Effect of Yogurt and Kefir Starter Cultures on Bioactivity of Fermented Industrial By-Product from Cannabis sativa Production—Hemp Press Cake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Preparation of Hemp-Based Yogurt-like and Kefir-like Beverages
2.3. Determination of Total Solid Content, Titratable Acidity, and pH
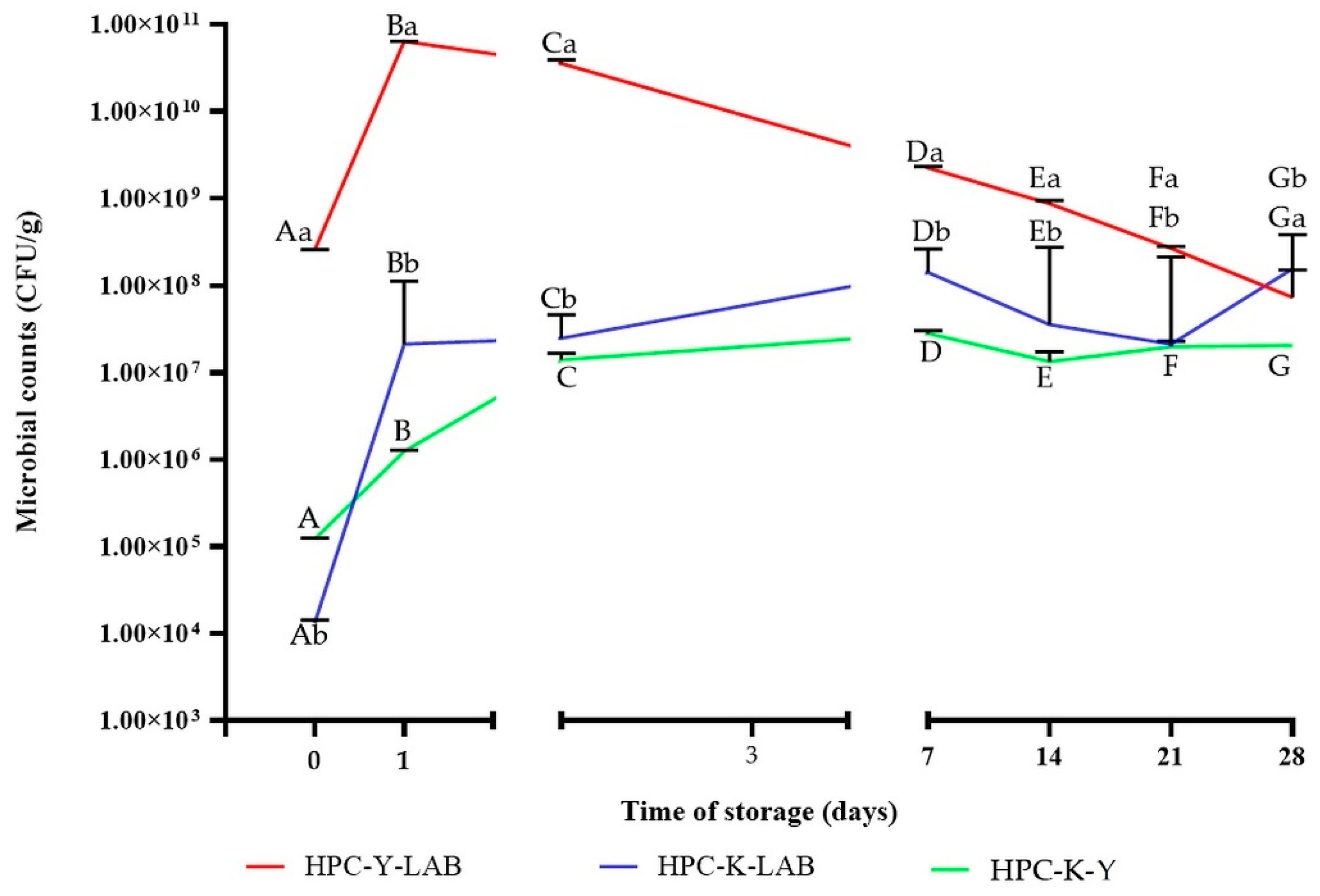
2.4. Microbiological Analyses during Storage Time
2.5. Preparation of Methanolic Extracts
2.6. Determination of the Reducing Sugar, Total Phenolic and Total Flavonoid Contents
2.7. HPLC Analyses
2.8. Determination of Antioxidant Potential
2.9. Determination of Protein Content, Free Amino Acid Content and Protein Profile by SDS Electrophoresis
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Changes in TSC, Protein Content, Acidity and Microbial Survivability
3.2. Changes in Reducing Sugar, Total Phenolic, Total Flavonoid and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPC | Hemp Press Cake |
| FAO | Food and Agriculture Organization |
| THC | Tetrahydrocannabinol |
| LAB | Lactic Acid Bacteria |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| TPTZ | Tripyridyl-s-triazine |
| HPLC | High-Performance Liquid Chromatography |
| SDS | Sodium Dodecyl Sulfate |
| TEMED | Tetramethylethylenediamine |
| DTT | Dithiothreitol |
| AOAC | Association of Official Agricultural Chemists |
| RSC | Reducing Sugars Content |
| DNS | 3,5-Dinitrosalicylic Acid |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| GAE | Gallic Acid Equivalents |
| QE | Quercetin Equivalents |
| FRAP | Ferric Reducing Antioxidant Power |
| AAE | Ascorbic Acid Equivalents |
| FAA | Free Amino Acids |
| SD | Standard Deviation |
| TSC | Total Solid Content |
| PC | Protein Content |
| TA | Titratable Acidity |
| HPC-Y | Hemp Press Cake-Yogurt |
| HPC-K | Hemp Press Cake-Kefir |
| HPC-Y-LAB | Hemp Press Cake-Yogurt-Lactic Acid Bacteria |
| HPC-K-LAB | Hemp Press Cake-Kefir-Lactic Acid Bacteria |
| HPC-K-Y | Hemp Press Cake-Kefir-Yeast |
| CFU | Colony Forming Unit |
| TFAA | Total Free Amino Acids |
| RP | Reducing Power |
| GABA | Gamma-Aminobutyric Acid |
| ADI | Acceptable daily intake |
| ASP | Asparagine |
| GLU | Glutamine |
| ALA | Alanine |
| HIS | Histidine |
References
- FAO. The State of Food and Agriculture, 2019: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; ISBN 9789251317891. [Google Scholar]
- Rajasekhar, K.; Fausto, S.; Byron, S.; Frank, C.; Chris, B.; Rodney, C.; Eric, W. Characterization of the nutritional and safety properties of hemp seed cake as animal feed ingredient. Int. J. Livest. Prod. 2021, 12, 53–63. [Google Scholar] [CrossRef]
- Vasantha Rupasinghe, H.P.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. Sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Kwiatkowski, P. Production and characterization of yogurt-like fermented beverage based on camelina (Camelina sativa L.) seed press cake. Appl. Sci. 2022, 12, 1085. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Śmietana, N.; Paradowska, D.; Drozłowska, E. Black cumin (Nigella sativa L.) seed press cake as a novel material for the development of new non-dairy beverage fermented with kefir grains. Microorganisms 2022, 10, 300. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Radočaj, O.; Dimić, E.; Tsao, R. Effects of hemp (Cannabis sativa L.) seed oil press-cake and decaffeinated green tea leaves (Camellia sinensis) on functional characteristics of gluten-free crackers. J. Food Sci. 2014, 79, C318–C325. [Google Scholar] [CrossRef]
- Kouakou, A.B.; Doué, G.G.; Mégnanou, R.-M.; Djoman, A.E.S. Shea shells and press-cake as new sources of bioactive phenolic compounds: GC MS profile and in Vitro antioxidant activity. EJBIO 2021, 2, 53–59. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis Sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Halle, I.; Schöne, F. Influence of rapeseed cake, linseed cake and hemp seed cake on laying performance of hens and fatty acid composition of egg yolk. J. Verbrauch. Lebensm. 2013, 8, 185–193. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Gunaratne, A.; Sui, Z.Q.; Corke, H. Effects of fermented edible seeds and their products on human health: Bioactive components and bioactivities. Compr. Rev. Food Sci. Food Saf. 2017, 16, 489–531. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The effect of fermentation with kefir grains on the physicochemical and antioxidant properties of beverages from blue lupin (Lupinus angustifolius L.) seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bernata, N.; Cháfera, M.; Chiralt, A.; González-Martínez, C. Probiotic fermented almond “milk” as an alternative to cow-milk yoghurt. Int. J. Food Stud. 2015, 4, 201–211. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.J.; Kang, J.; Zhang, C.M.; Kang, S.G. Antioxidant activity and main chemical components of a novel fermented tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Fritz, J.D.; Greaser, M.L. Changes in titin and nebulin in postmortem bovine muscle revealed by gel electrophoresis, western blotting and immunofluorescence microscopy. Food Sci. 1991, 56, 607–610. [Google Scholar] [CrossRef]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic potential of hemp blended drinks fermented byprobiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Tabor, S.; Kocira, S.; Czerwińska, E.; Kuboń, M.; Płóciennik, B.; Findura, P. Survivability of probiotic bacteria in model systems of non-fermented and fermented coconut and hemp milks. Sustainability 2019, 11, 6093. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Bendoraitiene, J.; Navikaite-Snipaitiene, V.; Ruzauskas, M. Technology and characterization of whole hemp seed beverages prepared from ultrasonicated and fermented whole seed paste. Int. J. Food Sc.i Technol. 2020, 55, 406–419. [Google Scholar] [CrossRef]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Yang, X.Q.; Gao, W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Docimo, T.; Caruso, I.; Ponzoni, E.; Mattana, M.; Galasso, I. Molecular Characterization of Edestin Gene Family in Cannabis sativa L. Plant. Physiol. Biochem. 2014, 84, 142–148. [Google Scholar] [CrossRef]
- Pihlanto, A.; Nurmi, M.; Pap, N.; Mäkinen, J.; Mäkinen, S. The effect of processing of hempseed on protein recovery and emulsification properties. Int. J. Food Sci. 2021, 2021, 8814724. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of GABA (γ-Aminobutyric Acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Lu, J. Metabolomic analysis reveals changes of bioactive compounds in mung beans (Vigna radiata) during γ-aminobutyric acid enrichment treatment. Foods 2022, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ai, Z.W.; Sun, X.M.; Liu, G.F.; Zhai, S.; Zhang, M.; Chen, H.; Feng, Z. Influence of arginine on the growth, arginine metabolism and amino acid consumption profiles of Streptococcus thermophilus T1C2 in controlled pH batch fermentations. J. Appl. Microbiol. 2016, 121, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Chiring Phukon, L.; Minhajul Abedin, M.; Sahoo, D.; Kumar Rai, A. Production and characterization of bioactive peptides in novel functional soybean chhurpi produced using Lactobacillus delbrueckii WS4. Food Chem. 2022, 387, 132899. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, M.; Shenana, M.; Abd-El-Aty, M.; Yousef, A.; Elkhtab, E. The production of novel functional yoghurt containing Angiotensin I-Converting enzyme (ACE)-Inhibitory activity. ZJAR 2016, 43, 1267–1278. [Google Scholar] [CrossRef]
- Undhad Trupti, J.; Das, S.; Solanki, D.; Kinariwala, D.; Hati, S. Bioactivities and ACE-Inhibitory peptides releasing potential of lactic acid bacteria in fermented soy milk. Food Prod. Processing Nutr. 2021, 3, 10. [Google Scholar] [CrossRef]
- Menzel, J.; Abraham, K.; Stangl, G.I.; Ueland, P.M.; Obeid, R.; Schulze, M.B.; Herter-Aeberli, I.; Schwerdtle, T.; Weikert, C. Vegan diet and bone health—results from the cross-sectional RBVD study. Nutrients 2021, 13, 685. [Google Scholar] [CrossRef]
- Letort, C.; Nardi, M.; Garault, P.; Monnet, V.; Juillard, V. Casein utilization by Streptococcus thermophilus results in a diauxic growth in milk. Appl. Environ. Microbiol. 2002, 68, 3162–3165. [Google Scholar] [CrossRef]
- Christensen, J.E.; Dudley, E.G.; Pederson, J.A.; Steele, J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1999, 76, 217–246. [Google Scholar] [CrossRef]
- Fernández, M.; Zúñiga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a functional beverage gaining momentum towards its heath promoting attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Babiker, E.E.; Uslu, N.; Ghafoor, K.; AL-Juhaimi, F.; Özcan, M.M.; Ahmed, I.A.M. Variations in bioactive properties, fatty acid compositions, and phenolic compounds of quinoa grain and oils roasted in a pan. J. Food Process. Preserv. 2022, 46, e16161. [Google Scholar] [CrossRef]
- López De Felipe, F.; Curiel, J.A.; Muñoz, R. Improvement of the fermentation performance of Lactobacillus plantarum by the flavanol catechin is uncoupled from its degradation. J. Appl. Microbiol. 2010, 109, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shah, N.P. Synergistic application of black tea extracts and lactic acid bacteria in protecting human colonocytes against oxidative damage. J. Agric. Food Chem. 2016, 64, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Medina-Meza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Lukšič, L.; Árvay, J.; Vollmannová, A.; Tóth, T.; Škrabanja, V.; Trček, J.; Germ, M.; Kreft, I. Hydrothermal treatment of tartary buckwheat grain hinders the transformation of rutin to quercetin. J. Cereal. Sci. 2016, 72, 131–134. [Google Scholar] [CrossRef]
- Baron, E. Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: An update on current evidence and cannabis science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical characterization of hempseed (seed of Cannabis sativa L.) oil from eight regions in China. J. Diet Suppl. 2010, 7, 117–129. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Pecorari, M.; Serafini, M.; Crozier, A. Bioavailability of C-linked dihydrochalcone and flavanone glucosides in humans following ingestion of unfermented and fermented rooibos teas. J. Agric. Food Chem. 2009, 57, 7104–7111. [Google Scholar] [CrossRef]
- Alves Magro, A.E.; de Castro, R.J.S. Effects of solid-state fermentation and extraction solvents on the antioxidant properties of lentils. Biocatal. Agric. Biotechnol. 2020, 28, 101753. [Google Scholar] [CrossRef]
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | 28 | |
| TSC (%) | |||||||
| HPC-K | 29.44 ± 0.04 Aa | 28.73 ± 0.51 Ba | 27.85 ± 0.13 Ca | 28.51 ± 0.18 Da | 28.45 ± 0.38 Ea | 26.83 ± 1.50 Fa | 28.79 ± 0.19 Ga |
| HPC-Y | 28.57 ± 0.36 Ab | 28.81 ± 0.05 Bb | 28.45 ± 0.52 Cb | 28.57 ± 0.52 Db | 28.81 ± 0.17 Eb | 28.99 ± 0.07 Fb | 29.22 ± 0.01 Gb |
| PC (%) | |||||||
| HPC-K | 9.34 ± 0.02 Aa | 9.33 ± 0.05 BCa | 9.23 ± 0.05 Da | 9.29 ± 0.08 ABa | 9.15 ± 0.05 Ea | 9.39 ± 0.04 Ca | 9.34 ± 0.05 Ea |
| HPC-Y | 9.52 ± 0.02 ABb | 9.38 ± 0.07 ABCb | 9.22 ± 0.04 Da | 9.45 ± 0.05 BCDb | 9.25 ± 0.06 Eb | 9.36 ± 0.01 Aa | 9.52 ± 0.07 Fb |
| pH (-) | |||||||
| HPC-K | 6.13 ± 0.01 Aa | 5.34 ± 0.05 Ba | 5.47 ± 0.01 Ca | 5.91 ± 0.00 Da | 5.38 ± 0.01 Ea | 5.72 ± 0.00 Fa | 6.49 ± 0.00 Ga |
| HPC-Y | 6.39 ± 0.00 Ab | 5.06 ± 0.01 Bb | 5.08 ± 0.00 Cb | 4.82 ± 0.05 Db | 4.94 ± 0.01 Eb | 4.98 ± 0.01 Fb | 4.82 ± 0.00 Gb |
| TA (mg/mL) | |||||||
| HPC-K | 0.64 ± 0.02 Aa | 1.05 ± 0.02 Ba | 1.07 ± 0.01 Ca | 0.75 ± 0.01 Da | 1.32 ± 0.05 Ea | 0.89 ± 0.02 Fa | 0.64 ± 0.05 Ga |
| HPC-Y | 0.70 ± 0.00 Ab | 1.23 ± 0.01 Bb | 1.14 ± 0.02 Cb | 1.04 ± 0.03 Db | 1.12 ± 0.02 Eb | 1.09 ± 0.00 Fb | 1.20 ± 0.06 Gb |
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | 28 | |
| TPC (mg GAE/g) | |||||||
| HPC-K | 53.36 ± 2.38 Aa | 36.08 ± 5.05 BCb | 30.66 ± 5.11 Da | 34.56 ± 2.86 BCa | 37.96 ± 3.26 BCa | 42.53 ± 6.10 ABCa | 57.76 ± 1.06 Aa |
| HPC-Y | 56.36 ± 2.11 Aa | 36.05 ± 2.77 Bb | 34.56 ± 4.68 Ba | 38.35 ± 1.78 Bb | 45.24 ± 2.82 Cb | 53.74 ± 1.69 Db | 53.89 ± 0.96 Db |
| TFC (mg QE/g) | |||||||
| HPC-K | 19.26 ± 2.99 ABa | 12.97 ± 7.66 Aa | 13.99 ± 5.11 ABa | 13.31 ± 2.70 ABa | 23.68 ± 3.40 BCa | 24.02 ± 7.85 Ca | 38.47 ± 6.50 Da |
| HPC-Y | 39.66 ± 1.56 Ab | 27.93 ± 8.17 Bb | 23.00 ± 1.53 Cb | 20.28 ± 0.59 Cb | 24.02 ± 0.75 Db | 29.29 ± 0.29 Eb | 20.79 ± 8.39 Fb |
| RSC (mg/g) | |||||||
| HPC-K | 677.11 ± 7.47 Aa | 233.32 ± 4.32 Ba | 236.91 ± 5.43 Ba | 236.91 ± 3.88 Ba | 258.88 ± 3.11 Ca | 240.50 ± 5.38 Ba | 236.91 ± 3.38 Ba |
| HPC-Y | 448.94 ± 8.07 Ab | 216.74 ± 2.05 Bb | 247.22 ± 4.58 Cb | 251.70 ± 6.77 Cb | 215.39 ± 1.55 Bb | 194.77 ± 2.69 Db | 188.50 ± 0.78 Db |
| TFAA (mg/g) | |||||||
| HPC-K | 3.57 ± 0.00 Aa | 2.44 ± 0.00 Ba | 2.71 ± 0.01 Ca | 2.22 ± 0.00 Da | 2.42 ± 0.00 Ea | 2.30 ± 0.01 Fa | 2.05 ± 0.01 Ga |
| HPC-Y | 3.46 ± 0.01 Ab | 3.02 ± 0.01 Bb | 3.20 ± 0.00 Cb | 3.29 ± 0.02 Db | 3.38 ± 0.01 Eb | 4.26 ± 0.01 Fb | 3.12 ± 0.01 Gb |
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 14 | 21 | 28 | |
| Gallic acid (µg/mL) | |||||||
| HPC-K | 108.00 ± 0.41 Aa | 110.83 ± 0.63 Ba | 112.91 ± 0.03 Ca | 107.58 ± 0.07 Da | 128.67 ± 0.23 Ea | 123.42 ± 0.08 Fa | 127.99 ± 0.23 Ga |
| HPC-Y | 106.82 ± 0.32 Ab | 106.41 ± 0.01 Bb | 97.86 ± 0.02 Cb | 84.49 ± 0.02 Db | 76.19 ± 0.24 Eb | 79.17 ± 0.01 Fb | 74.07 ± 0.06 Gb |
| Epicatechin (µg/mL) | |||||||
| HPC-K | 189.19 ± 0.84 Aa | 127.24 ± 0.83 Ba | 70.64 ± 0.00 Ca | - | - | - | - |
| HPC-Y | 180.82 ± 1.27 Ab | 158.84 ± 0.85 Bb | 128.16 ± 0.20 Cb | 116.00 ± 0.05 | 113.34 ± 0.51 | 162.52 ± 0.85 | 157.41 ± 1.04 |
| Coumaric acid (µg/mL) | |||||||
| HPC-K | 19.68 ± 0.02 Aa | 13.98 ± 0.03 Ba | 8.19 ± 0.00 Ca | - | - | - | - |
| HPC-Y | 21.14 ± 0.01 Ab | 15.30 ± 0.03 Bb | 13.27 ± 0.00 Cb | 8.57 ± 0.00 | - | - | - |
| Rutin (µg/mL) | |||||||
| HPC-K | 162.37 ± 0.58 Aa | 108.23 ± 0.00 Ba | - | - | - | - | - |
| HPC-Y | 175.51 ± 1.13 Ab | 106.91 ± 0.18 Bb | 101.44 ± 0.00 | - | - | - | - |
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | 28 | |
| DPPH (%) | |||||||
| HPC-K | 84.17 ± 0.79 Aa | 85.31 ± 1.13 Aa | 69.58 ± 0.79 Ca | 70.52 ± 1.26 Ca | 86.98 ± 0.18 ABa | 86.88 ± 0.54 ABa | 88.85 ± 0.72 Ba |
| HPC-Y | 83.13 ± 0.54 Aa | 88.13 ± 6.56 Ba | 80.10 ± 1.18 Cb | 80.10 ± 0.95 Cb | 82.40 ± 0.48 ACb | 88.13 ± 0.54 Ba | 87.19 ± 1.90 Ba |
| ABTS (%) | |||||||
| HPC-K | 42.10 ± 2.45 Aa | 40.95 ± 2.03 Aa | 37.62 ± 2.07 Ba | 34.95 ± 2.08 Ba | 45.19 ± 1.39 Ca | 48.67 ± 1.57 Da | 56.19 ± 1.35 Ea |
| HPC-Y | 47.67 ± 1.43 Aa | 45.24 ± 1.54 ABa | 43.95 ± 2.03 Bb | 43.81 ± 5.25 Bb | 45.48 ± 7.79 ABb | 52.95 ± 2.52 Ca | 51.00 ± 1.65 Ca |
| FRAP (mg AAE/g) | |||||||
| HPC-K | 2.21 ± 0.00 Aa | 2.02 ± 0.10 BCa | 2.05 ± 0.12 BDa | 1.86 ± 0.10 Da | 1.88 ± 0.09 Da | 2.27 ± 0.12 Ea | 2.16 ± 0.29 ABEa |
| HPC-Y | 1.83 ± 0.10 Aa | 1.79 ± 0.03 Aa | 1.58 ± 0.12 Bb | 1.54 ± 0.08 Ba | 2.05 ± 0.14 Ca | 2.05 ± 0.15 Ca | 2.26 ± 0.20 Db |
| RP (-) | |||||||
| HPC-K | 0.522 ± 0.001 Aa | 0.524 ± 0.001 Ba | 0.452 ± 0.000 Ca | 0.503 ± 0.001 Da | 0.589 ± 0.001 Ea | 0.626 ± 0.001 Fa | 0.592 ± 0.001 Ga |
| HPC-Y | 0.551 ± 0.001 Ab | 0.576 ± 0.001 Bb | 0.438 ± 0.001 Cb | 0.686 ± 0.001 Db | 0.540 ± 0.001 Eb | 0.658 ± 0.001 Fb | 0.617 ± 0.001 Gb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Waszkowiak, K.; Polanowska, K.; Mikołajczak, B.; Śmietana, N.; Hrebień-Filisińska, A.; Sadowska, J.; Mazurkiewicz-Zapałowicz, K.; Drozłowska, E. The Effect of Yogurt and Kefir Starter Cultures on Bioactivity of Fermented Industrial By-Product from Cannabis sativa Production—Hemp Press Cake. Fermentation 2022, 8, 490. https://doi.org/10.3390/fermentation8100490
Łopusiewicz Ł, Waszkowiak K, Polanowska K, Mikołajczak B, Śmietana N, Hrebień-Filisińska A, Sadowska J, Mazurkiewicz-Zapałowicz K, Drozłowska E. The Effect of Yogurt and Kefir Starter Cultures on Bioactivity of Fermented Industrial By-Product from Cannabis sativa Production—Hemp Press Cake. Fermentation. 2022; 8(10):490. https://doi.org/10.3390/fermentation8100490
Chicago/Turabian StyleŁopusiewicz, Łukasz, Katarzyna Waszkowiak, Katarzyna Polanowska, Beata Mikołajczak, Natalia Śmietana, Agnieszka Hrebień-Filisińska, Joanna Sadowska, Kinga Mazurkiewicz-Zapałowicz, and Emilia Drozłowska. 2022. "The Effect of Yogurt and Kefir Starter Cultures on Bioactivity of Fermented Industrial By-Product from Cannabis sativa Production—Hemp Press Cake" Fermentation 8, no. 10: 490. https://doi.org/10.3390/fermentation8100490
APA StyleŁopusiewicz, Ł., Waszkowiak, K., Polanowska, K., Mikołajczak, B., Śmietana, N., Hrebień-Filisińska, A., Sadowska, J., Mazurkiewicz-Zapałowicz, K., & Drozłowska, E. (2022). The Effect of Yogurt and Kefir Starter Cultures on Bioactivity of Fermented Industrial By-Product from Cannabis sativa Production—Hemp Press Cake. Fermentation, 8(10), 490. https://doi.org/10.3390/fermentation8100490