Abstract
Fermentation is one of the world’s oldest techniques for food preservation, nutrient enhancement, and alcohol manufacturing. During fermentation, carbohydrates such as glucose and starch are converted into other molecules, such as alcohol and acid, anaerobically through enzymatic action while generating energy for the microorganism or cells involved. Black tea is among the most popular fermented beverages; it is made from the dried tea leaves of the evergreen shrub plant known as Camellia sinensis. The adequate consumption of black tea is beneficial to health as it contains high levels of flavanols, also known as catechins, which act as effective antioxidants and are responsible for protecting the body against the development of illnesses, such as inflammation, diabetes, hypertension, cancer, and obesity. The prevalence of obesity is a severe public health concern associated with the incidence of various serious diseases and is now increasing, including in Malaysia. Advances in ‘omic’ research have allowed researchers to identify the pivotal role of the gut microbiota in the development of obesity. This review explores fermented black tea and its correlation with the regulation of the gut microbiota and obesity.
1. Introduction
The obesity epidemic has become a severe health problem in Malaysia and many other countries around the globe [1,2,3,4,5]. The prevalence of obesity is rising at an alarming rate worldwide, raising mortality and reducing quality of life [6,7]. Obesity is projected to affect around fifty percent of the world population by 2030, and Malaysia was reported to have the highest obese population (15%) among Asian countries in 2019 [8,9,10]. Research has focused on foods containing natural substances, as they cause fewer side effects; hence, they are increasingly utilized due to their health benefits [11]. Studies on fermented products have become the fastest-growing ventures, among other functional foods, due to increased consumer awareness of their multitude of beneficial effects on health [12,13,14]. The consumption of fermented-tea beverages is gaining popularity due to their probiotic nature and purported health benefits in many countries, including Malaysia [15,16,17,18,19]. Previous studies have reported several bioactivities of fermented black tea, including anti-oxidant, antimicrobial, anti-cancer, anti-diabetic, and anti-lipidemic properties [20,21,22,23,24]. The metabolites produced by microorganisms during fermentation are responsible for their sour taste and other bio-properties [25,26,27,28]. Recent progress in molecular biology, including the advent of platforms for next-generation sequencings, such as metagenomics or amplicon sequencing, has allowed the microbial consortium to be characterized, in turn allowing researchers to elucidate the connection between microbial population and obesity [29,30]. In his review, the relationship between fermented black tea and the regulation of the gut microbiota in obesity is discussed.
2. Fermentation
The fermentation process was found thousands of years ago and has been extensively practiced for its benefits in food preservation, nutrient enhancement, and alcohol manufacturing [31,32]. Traces of mixed fermentation in the form of an alcoholic beverages prepared from rice, fruits, and honey between 7000 and 6600 BC was found in pottery jars from the early Neolithic town of Jiahu, China, and was declared the earliest archaeological evidence of fermentation to have been discovered [33,34,35]. This makes fermented beverages, such as vinegar and wine, among the oldest fermented foods consumed by people [36,37]. Kimchi, for example, is a popular fermented food among Koreans and has become popular in other countries worldwide [38,39,40]. On the other hand, pickled cucumber is used not only when preparing burgers and sandwiches by Westerners, but also as a side dish in Asian countries [41,42]. Due to their distinct flavor and aroma, fermented foods and beverages become some of the first processed foods to be consumed by humans [31,43,44,45]. A brief timeline of fermentation’s history, from the earliest archaeological evidence of beverage fermentation until the introduction of pasteurization, is illustrated in Figure 1.
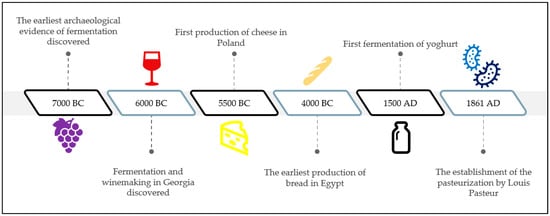
Figure 1.
A brief timeline of fermentation history.
During fermentation, carbohydrates, such as glucose and starch, are converted into other molecules, such as alcohol and acid, anaerobically through enzymatic action, while generating energy for the microorganism or cells involved [15,46]. Evidence was found in ancient organics from the pottery jars used for fermentation and winemaking in Georgia in 6000 BC [34,47]. Due to the colonization of the Mediterranean by the Romans, winemaking spread throughout other regions, such as Asia. In the late nineteenth century, Louis Pasteur, a French microbiologist, discovered that living microbes were responsible for souring alcohol during the fermentation process, leading to the establishment of the pasteurization technique, which involves the heating and cooling of liquids to kill microbes and prevent spoiling [48,49]. Pasteur was among the pioneering researchers in food preservation, who believed that the bacteria formed from microscopic inoculums were not generated spontaneously. His theory was later supported by Eduard Buchner, who discovered zymase, a mixture of enzymes produced by yeast during fermentation [49]. This discovery led Eduard Buchner to receive a Nobel Prize in chemistry in 1907 [50]. Table 1 summarizes the changes in the nutritional values of fermented products over the last ten years across the world.

Table 1.
Changes in nutritional values in fermented products.
3. Black Tea
Tea is an excellent alternative to energy drinks and coffee. Even though tea and coffee have multiple health benefits in common, such their caffeine and antioxidant contents [71,72], excessive coffee drinking (daily intake ≥400 mg for adults (4–5 cups of coffee) and ≥3 mg/kg for children [73]) may contribute to various adverse effects, such as headache [74], insomnia [75,76,77,78,79,80,81,82] and arrhythmia [75,82,83,84,85] due to caffeinism [76,82,86]. Compared to tea, coffee contains a higher concentration of caffeine, an energy-boosting psychostimulant. Studies showed that caffeine is widely used to promote alertness by increasing dopamine signaling in the brain [82], primarily via blocking adenosine [87,88,89,90], a known vasodilator and sleep-promoting receptor [91,92].
Tea is rich in natural bioactive compounds, such as flavonoids, methylxanthines, carbohydrates, and amino acids, which possess various health benefits [93,94,95]. Among these bioactive compounds, a high total content of flavonoids, a group of hydroxylated phenolic compounds found in different plants, including vegetables and fruits, was detected in tea [93]. In recent decades, flavonoids have become essential components in nutraceuticals [96,97,98,99]. They have been associated with human daily diet and health due to their therapeutic properties, such as antioxidants and anti-diabetic, anti-hypertensive, anti-cancer, and anti-inflammatory actions [72,73,79,80]. Flavonoids can be classified into subclasses based on their side-group position and substitutions, such as flavanols, flavonols, and flavanones [97,100,101,102,103]. Flavanols, also known as catechins, are the most abundant and vital constituents in black tea because the oxidation of catechins forms theaflavins and thearubigins, which are excellent antioxidative agents [103,104]. These antioxidants possess free-radical-scavenging properties; they can disrupt the oxidation reaction that causes oxidative stress in cells by donating an electron to the free radicals to form more stable phenoxyl radicals [72,73,86,87]. Oxidative stress is a hazardous process that occurs when free radicals are produced beyond the cell’s ability to eliminate them [105,106]. According to various studies, including clinical evidence, excessive oxidative stress can damage DNA, proteins, lipid, and membranes, leading to various disorders, such as diabetes, cardiovascular disease, and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s [105,106,107,108]. Antioxidants can scavenge and neutralize free radicals, thus reducing oxidative stress and assisting in the recovery process [89,90,92]. A previous study showed that theaflavins are the most effective antioxidants in black tea because they have a unique benzotropolone moiety that provides antioxidant protection to the favored oxidation site for electron donation [109,110,111,112,113], followed by catechins and thearubigins [114]. This finding was supported by He [115], who found that the free-radical scavenging activities in theaflavins are greater than in epigallocatechin gallate (EGCG), one of the most potent antiradical compounds found in foods. This circumstance is due to the presence of hydroxyl groups in theaflavins, which are essential for their radical-scavenging activities [116]. Theaflavins also showed anti-inflammatory properties by modulating the signal transducer and activating the NRF2 signaling pathway in vitro and in vivo, which is crucial for increasing the antioxidant defense [93,114,115,117,118,119,120,121]. Based on a systematic data-mining approach, Beresniak et al. [122] discovered that high black tea consumption was significantly associated with low diabetes prevalence; a single dose of black tea reduced peripheral vascular resistance, as well as the insulin response to the glucose load in both the upper and the lower extremities, in the 50 participating countries involved in the study. Hence, it can be concluded that flavonoids are the most vital compounds in black tea due to their crucial role in the bioactivities of black tea. Nevertheless, flavonoids’ biological activities might vary depending on their type, mode of action, and bioavailability [123].
Fermentation of Black Tea
Tea is made from the processed dried tea leaves of the evergreen shrub plant known as Camellia sinensis, a member of the Theaceae family, and is the world’s second most-consumed beverage after water [124,125,126,127]. C. sinensis is a native plant in Southeast Asia, specifically China, Myanmar, Laos, and Vietnam. According to Wang [128], tea was fortuitously discovered in 2737 BC by Shen Nung, an emperor of China, after he was poisoned. The efficacious use of tea to treat poisoning made tea a precious medicine during that era. However, the earliest physical evidence of tea consumption was found in tombs dating back to 207 BC [128,129]. Even with its extraordinary benefits, tea only gained in popularity and was recognized as a national beverage in China in 618 AD. Due to its benefits, tea spread and grew commercially worldwide [124,125,126,130]. Different types of tea are available commercially, such as black, green, and oolong tea. Although all kinds of tea are prepared from the same plant, different processing procedures and fermentation degrees produce various tea types [94,124]. Among these teas, only black tea is fully fermented and has the most significant production levels globally, accounting for 70% of total global tea production, followed by unfermented green tea, which accounts for 28%, and partially fermented oolong tea, which accounts for 2% [79,107,108,110].
The fermentation of sugared black tea by a tea fungus, a symbiotic relationship between Acetobacteria and osmophilic yeasts, produces a healthy beverage called kombucha, as illustrated in Figure 2 [131,132,133]. Kombucha originated in China and has been consumed since 220 BC. The name “Kombucha” is derived from a Korean physician named Kombu, who brought tea fungus to Japan in 414 AD to treat Emperor Inkyo, who was suffering from digestive problems [131,132,133,134]. Currently, kombucha is produced traditionally in many households worldwide, including Malaysia, and its consumption is widespread, principally in Korea, China, Europe, and the United States, because of its refreshing taste and beneficial effects on human health [131,133]. Tea and kombucha originated in China, as illustrated in Figure 3.

Figure 2.
Laboratory-fermented black tea (kombucha).
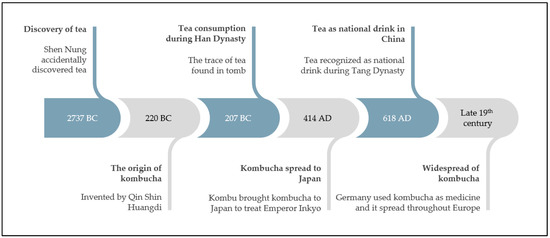
Figure 3.
A brief timeline of tea and kombucha history.
As the fermentation time increases, kombucha becomes mature, and the level of tartness of kombucha also changes throughout the process [32,135,136,137]. Kombucha’s flavor transforms from a refreshingly sour, mildly bubbling flavor to a mild vinegar-like taste throughout its fermentation due to the bacterial production of organic acid during alcohol conversion [26,138,139,140,141]. Even though kombucha is usually fermented for 7 to 21 days, it is recommended to limit its incubation time to 14 days as extended incubation times will increase the tartness and sourness of kombucha [142,143,144,145]. Kombucha contains a broad spectrum of microbial populations, including yeast (Zygosaccharomyces, Brettanomyces, Saccharomyces, and Pichia), acetic-acid bacteria (Komagataeibacter, Gluconobacter, and Acetobacter), and lactic-acid bacteria (Lactobacillus, Lactococcus, and Oenococcu). Table 2 summarizes the microbial population of kombucha throughout the world.

Table 2.
Microbial population in kombucha.
It has been proven that the consumption of black tea offers numerous benefits in terms of health; however, the excessive consumption of black tea may lead to indigestion due to the high concentration of tannins in black tea [151]. Tannins are excellent microbial inhibitors that could suppress lactic-acid bacteria and certain fungal activity; however, this effect may soon wear off with prolonged fermentation [152,153]. This issue, however, can be resolved by modifying its properties using the fermentation process, which can enhance its nutritional values at the same time [154]. A study by Chupeerach et al. [69] showed that fermentation considerably affects nutritional- and bioactive-component concentrations, which affects the properties of tea. On the other hand, Jolvis [94] showed that, during fermentation, tea leaves undergo an enzymatic oxidation process, in which the enzymes and chemical constituents of the leaves react with oxygen to form oxidized polyphenolic compounds. This process causes the total tannin content in the tea to gradually decrease with time during fermentation due to the action of the polyphenol oxidase enzyme, which oxidizes phenolics as they diffuse through cellular fluid [154,155].
In addition to tannin, black tea also contains abundant catechin, which acts as an effective antioxidant and is responsible for protecting the body against the development of illnesses [118]. Increases in these vitamins are favorable and beneficial for sustaining and maintaining good health [156,157,158,159,160]. In addition, fermentation is also shown to increase other nutrients, such as carbohydrates, fat, sodium, potassium, and minerals [69]. A similar finding was reported by Unban et al. [161], who showed increases in carbohydrate and fat contents in fermented tea compared to freshly made tea. According to Patel et al. [162], lipase activity by microorganisms breaks down lipid compounds, such as triglycerides, into fatty acids and glycerols through lipolysis, thus increasing the fat content in fermented tea. Due to high carbohydrate and fat contents, significantly higher energy was detected in fermented black tea than in fresh tea leaves [69].
On the other hand, the increase in sodium and potassium concentrations during fermentation may be related to the breakdown of covalent bonds in mineral–food-matrix complexes, which results in the improved bioavailability of the nutrients [69,163]. Furthermore, the fermentation of black tea was also shown to elevate mineral contents, such as iron and zinc, as a result of the metabolic activity of the microorganism [118,164]. However, their presence and nutritional value in different fermented black teas may vary, depending on the symbiotic culture employed, time and temperature of fermentation, sugar level, type of tea, and analysis methods used during the fermentation process [118].
4. Fermented Black Tea, Gut Microbiota, and Obesity
The prevalence of obesity, which is now an increasing trend, has become an epidemic globally, including in Malaysia [3,165,166,167,168]. Its association with the incidence of various serious diseases and health conditions, such as hypertension, heart disease, Type 2 diabetes, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis has been a significant issue for decades [20,169]. Obesity remains a serious public health concern that needs innovative nutritional and medicinal treatments, although various treatments for managing massive weight gain are currently practice. According to Ruiz Estrada et al. [167], obesity in Malaysia is rapidly increasing due to factors such as the high consumption of fast food and sugary soft drinks, long hours of sitting, poor national sports motivation, low water consumption, the high consumption of vitamins, and the dietary imbalance between the calories and carbohydrates consumed daily among Malaysians. Animal and human studies have shown compelling shreds of evidence on the significant role of the gut microbiota in the development of obesity [170]. This finding was supported by Aoun et al. [165] in a review involving animals and obese adult subjects, which found that a high-fat diet might trigger alterations in the gut microbiome’s structures and functions in the host gut. It is well known that in addition to being responsible for absorbing, storing, and digesting nutrients, the gut microbiota also helps maintain metabolic homeostasis, increasing the host’s immunity and gut barriers in humans [166,171]. Furthermore, John and Mullin [170] also suggested that preventing obesity and metabolic syndromes is possible with healthy gut-microbiota composition. Ironically, an unhealthy diet can result in gut dysbiosis, which could encourage the proliferation of the pathogenic microorganisms associated with chronic inflammation, contributing significantly to the pathogenesis of chronic metabolic and intestinal disorders, including obesity [165,172].
As one of the well-known products of fermented black tea, kombucha consumption has been proven to elevate the defense mechanism against pathogens. This is because, in addition to polyphenol compounds, which can be naturally found in plant products, the probiotics in kombucha produce a variety of organic acids, such as acetic acid and lactic acid, which possess antimicrobial and antioxidant properties [32,118,131,132,133,173,174]. This finding was supported by Jung et al. [20], according to whom a significant drop in Allobaculum and Turicibacter, two pathogens associated with non-alcoholic fatty liver disease (NAFLD), was observed in kombucha treatment. Furthermore, the Clostridium genus is associated with obesity, NAFLD, and non-alcoholic steatohepatitis (NASH) due to its ability to increase sugar and fat absorption; the Mucispirillum genus, which is a pro-inflammatory bacterium, was also revealed to decline after kombucha consumption [20,175]. By contrast, the kombucha-treatment group recorded a significant increase in beneficial probiotic bacteria, such as Lactobacillus, which possess anti-inflammatory properties [176,177].
The gut microbiota is a diverse community of microorganisms composed of various anaerobic bacteria, eukarya, and archaea, which inhabit the gastrointestinal tract through diet [178,179]. Over millennia, the gut microbiota and the host have co-evolved, resulting in a sophisticated and mutually beneficial interaction between them [20,180]. Previous studies revealed that the gut microbiota from Bacteroidetes, Firmicutes, and Actinobacteria phyla are crucial for sustaining immunological and metabolic homeostasis and defense pathogens [181,182]. These findings were supported by Baümler and Sperandio [183] and Gensollen et al. [184], who showed how the gut microbiota protects the gastrointestinal tract by providing resistance to pathogenic bacteria and fungi and regulating host immunity. Nevertheless, there is also a report on the pathogenesis of the gut microbiota. For example, a study showed that the occurrence of dysbiosis, in which the balance of the gut microbiota is disrupted and the number of pathobionts12 increases, resulting in infection and various inflammatory diseases, such as obesity, diabetes type 2, and fatty-liver disease [20,148,153]. A recent study by Costa et al. [185] postulated that gut dysbiosis could be treated or reduced by consuming fermented black tea. They also found that kombucha consumption aids in controlling and treating obesity and its associated complications and modulating the gut microbiota in vivo. The probiotic bacteria in kombucha, such as Lactobacillus and Bifidobacterium, help promote the proliferation of good microbes in the gastrointestinal tract to compete with the pathogenic microbes for nutrients and binding sites of the host cell [186]. Probiotic bacteria, which possess antimicrobial properties and contain high short-chain fatty acids (SCFAs) and other metabolites, strengthen the immune system and aid in balancing the human microbiota [186].
5. Conclusions
This mini review examined the benefits of adequate kombucha consumption in preventing and treating obesity. We highlighted the crucial role of the metabolites produced by microorganisms during the fermentation process in promoting beneficial microbes’ growth and inhibiting pathogenic-gut-microbes’ growth in the digestive system. Indeed, the bioactive compounds present in kombucha, such as catechins, can protect the body against various illnesses. Based on the evidence, it can be concluded that the consumption of kombucha can promote a healthy human gut due to its antimicrobial properties against enteric pathogens. However, pre-clinical and clinical research supporting fermented black tea’s effect on obesity and the gut is still lacking.
Author Contributions
Conceptualization: N.F.N. and N.E.M.; literature-data collection: N.F.N. and N.E.M.; writing—original draft: N.E.M. and N.B.A.; writing—review, and editing: N.E.M.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Universiti Malaysia Sabah (UMS) through Skim Pensyarah Lantikan Baru (SLB2116) grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cesare, M.D.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.L.; Pontiroli, A.E. Prevention of diabetes and cardiovascular disease in obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Sidik, S.; Lekhraj, R.; Foo, C.N. Prevalence, associated factors and psychological determinants of obesity among adults in Selangor, Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, W.; Xu, Y.; Wang, D.; Wang, Y.; Lv, W.; Xiong, M.; Yi, Y.; Wang, H.; Zhang, Q.; et al. Current landscape: The mechanism and therapeutic impact of obesity for breast cancer. Front. Oncol. 2021, 11, 704893. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Rubio-Almanza, M.; Cámara-Gómez, R.; Merino-Torres, J.F. Endocrinología, diabetes y nutrición obesity and type 2 diabetes: Also linked in therapeutic. Endocrinol. Diabetes Nutr. 2018, 66, 140–149. [Google Scholar] [CrossRef]
- Abdullah, Z.; Putri, K.Y.S.; Raza, S.H.; Istiyanto, S.B. Contrariwise obesity through organic food consumption in Malaysia: A signaling theory perspective. BMC Public Health 2022, 22, 99. [Google Scholar] [CrossRef]
- The Academy of Medical Sciences Addressing the Global Health Challenge of Obesity in Malaysia Workshop Report. 2017.
- Yale Global Online. Available online: https://archive-yaleglobal.yale.edu/content/world-population-2020-overview (accessed on 29 August 2022).
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Plasek, B.; Temesi, Á. The credibility of the effects of functional food products and consumers’ willingness to purchase/willingness to pay—Review. Appetite 2019, 143, 104398. [Google Scholar] [CrossRef]
- Sarkar, S. Potentiality of probiotic yoghurt as a functional food—A review. Nutr. Food Sci. 2019, 49, 182–202. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.Z. Beneficial effects of bifidobacterium Longum Subsp. Longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Kasron, N.; Manan, M.A.; Hafiz, M.N.; Azmin, M.; Saari, N.A.; Latip, M.A. Consumer acceptance of fermented drinks in Malaysia. Malaysian J. Soc. Sci. Humanit. 2021, 6, 306–314. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: A complementary and alternative medicine. Evid.-Based Complement. Altern. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in kombucha tea fermentation: A review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Vohra, B.M.; Fazry, S.; Sairi, F.; Babul-Airianah, O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of kombucha. Malaysian J. Fundam. Appl. Sci. Spec. Issue Int. Conf. Agric. 2018, 15, 298–302. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.S. Effect of kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Wang, S.; Sung, S.; Kim, N.; Lee, H.H.; Seo, Y.S.; Jung, Y. Hepatoprotective effect of kombucha tea in rodent model of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2019, 20, 2369. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit kombucha, black tea kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K. Kefir, kombucha, and sour beers. Probiotic Beverages 2021, 287–307. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a minimal microbial consortium to determine the origin of kombucha flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef]
- Abaci, N.; Senol Deniz, F.S.; Orhan, I.E. Kombucha—An ancient fermented beverage with desired bioactivities: A narrowed review. Food Chem. 2022, 14, 100302. [Google Scholar] [CrossRef]
- Osman, M.A.; Neoh, H.M.; Mutalib, N.S.A.; Chin, S.F.; Jamal, R. 16S RRNA gene sequencing for deciphering the colorectal cancer gut microbiome: Current protocols and workflows. Front. Microbiol. 2018, 9, 767. [Google Scholar] [CrossRef]
- Mataragas, M.; Alessandria, V.; Ferrocino, I.; Rantsiou, K.; Cocolin, L. A bioinformatics pipeline integrating predictive metagenomics profiling for the analysis of 16S RDNA/RRNA sequencing data originated from foods. Food Microbiol. 2018, 76, 279–286. [Google Scholar] [CrossRef]
- Anal, A. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; Sousa, P.H.M.D. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Tsaltas, D. Fermented foods and beverages. Innov. Tradit. Foods 2019, 257–291. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S.; Hoj, P.B. Grape and wine biotechnology: Challenges, opportunities and potential benefits. Aust. J. Grape Wine Res. 2005, 11, 83–108. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets west. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrition 2015, 7, 390–404. [Google Scholar] [CrossRef]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, H.-J. Consumption of kimchi, a salt fermented vegetable, is not associated with hypertension prevalence. J. Ethn. Foods 2014, 1, 8–12. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A holistic review on euro-asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Narzary, Y.; Brahma, J.; Brahma, C.; Das, S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. J. Ethn. Foods 2016, 3, 284–291. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Copetti, M.V. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early neolithic wine of georgia in the south caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics history. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Mani, A. Food preservation by fermentation and fermented food products. Int. J. Acad. Res. Dev. 2018, 1, 51–57. [Google Scholar]
- Jaenicke, L. Centenary of the award of a nobel prize to eduard buchner, the father of biochemistry in a test tube and thus of experimental molecular bioscience. Angew. Chemie Int. Ed. 2007, 46, 6776–6782. [Google Scholar] [CrossRef]
- Santosh, O.; Kaur Bajwa, H.; Singh Bisht, M.; Nirmala, C. Quality evaluation of biscuits fortified with bamboo shoot for their sensory properties. J. Pharmacogn. Phytochem. 2021, 10, 330–337. [Google Scholar]
- Badwaik, L.S.; Borah, P.K.; Borah, K.; Das, A.J.; Deka, S.C.; Sharma, H.K. Influence of fermentation on nutritional compositions, antioxidant activity, total phenolic and microbial load of bamboo shoot. Food Sci. Technol. Res. 2014, 20, 255–262. [Google Scholar] [CrossRef]
- Karademir, E.; Yalçin, E. Effect of fermentation on some quality properties of cornelian cherry tarhana produced from different cereal/pseudocereal flours. Qual. Assur. Saf. Crop. Foods 2019, 11, 127–135. [Google Scholar] [CrossRef]
- Vieira, C.P.; Álvares, T.S.; Gomes, L.S.; Torres, A.G.; Paschoalin, V.M.F.; Conte, C.A. Kefir grains change fatty acid profile of milk during fermentation and storage. PLoS ONE 2015, 10, e0139910. [Google Scholar] [CrossRef] [PubMed]
- Nwokoro, O.; Chukwu, B.C. Studies on akamu, a traditional fermented maize food. Rev. Chil. Nutr. 2012, 39, 180–184. [Google Scholar]
- Assohoun, M.C.N.; Djeni, T.N.; Koussémon-Camara, M.; Brou, K. Effect of fermentation process on nutritional composition and aflatoxins concentration of doklu, a fermented maize based food. Food Nutr. Sci. 2013, 4, 1120–1127. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Zhang, J.; Mi, Z.; Gesudu, Q.; Sun, T. Dynamic evaluation of the nutritional composition of homemade koumiss from inner mongolia during the fermentation process. J. Food Process. Preserv. 2019, 43, e14022. [Google Scholar] [CrossRef]
- Rohimah, A.; Setiawan, B.; Roosita, K.; Palupi, E. The effects of soaking treatments and fermentation process on nutritional and aflatoxin contents of fermented peanut cake (black oncom). Pol. J. Nat. Sci. 2021, 36, 59–78. [Google Scholar]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Ji, Y.; Lin, J.; Chen, X.; Qi, B. Improvement of nutritional value, bioactivity and volatile constituents of quinoa seeds by fermentation with lactobacillus casei. J. Cereal Sci. 2018, 84, 83–89. [Google Scholar] [CrossRef]
- Ryu, J.A.; Kim, E.; Kim, M.J.; Lee, S.; Yoon, S.R.; Ryu, J.G.; Kim, H.Y. Physicochemical characteristics and microbial communities in gochujang, a traditional Korean fermented hot pepper paste. Front. Microbiol. 2021, 11, 3543. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a potential probiotic, lactobacillus plantarum l7, for the preparation of a rice-based fermented beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Devi, P.B.; Rajendran, S. Impact of starter culture on nutraceutical and functional properties of underutilized millet-legume co-fermented Indian traditional product. LWT 2021, 149, 111818. [Google Scholar] [CrossRef]
- Rahmawati, I.S.; Suntornsuk, W. Effects of fermentation and storage on bioactive activities in milks and yoghurts. Procedia Chem. 2016, 18, 53–62. [Google Scholar] [CrossRef]
- Júnior, S.S.; Tavano, O.; Demonte, A.; Rossi, E.; Pinto, R. Nutritional evaluation of soy yoghurt in comparison to soymilk and commercial milk yoghurt. Effect of fermentation on soy protein. Acta Aliment. 2012, 41, 443–450. [Google Scholar] [CrossRef]
- Teng, D.; Gao, M.; Yang, Y.; Liu, B.; Tian, Z.; Wang, J. Bio-modification of soybean meal with bacillus subtilis or aspergillus oryzae. Biocatal. Agric. Biotechnol. 2012, 1, 32–38. [Google Scholar] [CrossRef]
- Mo, H.; Kariluoto, S.; Piironen, V.; Zhu, Y.; Sanders, M.G.; Vincken, J.-P.; Wolkers-Rooijackers, J.; Nout, M.J.R. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem. 2013, 141, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Franeck, A.; Wünsch, R.; Dwiputri, M.C.; Feroniasanti, Y.L. Effect of fermentation to total titrable acids, flavonoid and antioxidant activity of butterfly pea kombucha. J. Phys. Conf. Ser. 2019, 1241, 012014. [Google Scholar] [CrossRef]
- Castillo, M.D.d.; Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Martín-Cabrejas, M.A.; Farah, A. Coffee Antioxidants in Chronic Diseases. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 20–56. ISBN 978-1-78801-497-7. [Google Scholar]
- Gan, R.Y.; Zhang, D.; Wang, M.; Corke, H. Health benefits of bioactive compounds from the genus ilex, a source of traditional caffeinated beverages. Nutrients 2018, 10, 1682. [Google Scholar] [CrossRef]
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; Vieille, S.L.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The ambiguous role of caffeine in migraine headache: From trigger to treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Kharaba, Z.; Sammani, N.; Ashour, S.; Ghemrawi, R.; Al Meslamani, A.Z.; Al-Azayzih, A.; Buabeid, M.A.; Alfoteih, Y. Caffeine consumption among various university students in the UAE, exploring the frequencies, different sources and reporting adverse effects and withdrawal symptoms. J. Nutr. Metab. 2022, 2022, 5762299. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Smith, A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Choi, J. Motivations influencing caffeine consumption behaviors among college students in Korea: Associations with sleep quality. Nutrients 2020, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Samaha, A.; Al Tassi, A.; Yahfoufi, N.; Gebbawi, M.; Rached, M.; Fawaz, M.A. Data on the relationship between caffeine addiction and stress among lebanese medical students in lebanon. Data Br. 2020, 28, 104845. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.J.; Coates, A.M.; Kohler, M.; Banks, S. Caffeine consumption and sleep quality in Australian adults. Nutrients 2016, 8, 479. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Lee, J.H. Association between energy drink intake, sleep, stress, and suicidality in Korean adolescents: Energy drink use in isolation or in combination with junk food consumption. Nutr. J. 2016, 15, 87. [Google Scholar] [CrossRef]
- Lieberman, H.R. Why are certain caffeine-containing products associated with serious adverse effects? Mayo Clin. Proc. 2020, 95, 1562–1564. [Google Scholar] [CrossRef]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.S. Effect of caffeine consumption on the risk for neurological and psychiatric disorders: Sex differences in human. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef]
- Ellermann, C.; Hakenes, T.; Wolfes, J.; Wegner, F.K.; Willy, K.; Leitz, P.; Rath, B.; Eckardt, L.; Frommeyer, G. Cardiovascular risk of energy drinks: Caffeine and taurine facilitate ventricular arrhythmias in a sensitive whole-heart model. J. Cardiovasc. Electrophysiol. 2022, 33, 1290–1297. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Depaula, J.; Farah, A. Caffeine consumption through coffee: Content in the beverage, metabolism, health benefits and risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Svenningsson, P. Adenosine—Dopamine interactions. Neurology 2003, 61, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Manalo, R.V.M.; Medina, P.M.B. Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic caenorhabditis elegans. Front. Neurosci. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Logan, J.; Alexoff, D.; Fowler, J.S.; Thanos, P.K.; Wong, C.; Casado, V.; Ferre, S.; Tomasi, D. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl. Psychiatry 2015, 5, e549. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B.; Andreou, A.P. Caffeine and primary (migraine) headaches—Friend or foe? Front. Neurol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Fried, N.T.; Elliott, M.B.; Oshinsky, M.L. The role of adenosine signaling in headache: A review. Brain Sci. 2017, 7, 30. [Google Scholar] [CrossRef]
- Lazarus, M.; Oishi, Y.; Bjorness, T.E.; Greene, R.W. Gating and the need for sleep: Dissociable effects of adenosine A1 and A2A receptors. Front. Neurosci. 2019, 13, 740. [Google Scholar] [CrossRef]
- Gargi, S.; Nilanjan, S.; Moutusi, N.; Subhasis, M. Bioactive components of tea. Arch. Food Nutr. Sci. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Pou, K.R.J. Fermentation: The key step in the processing of black tea. J. Biosyst. Eng. 2016, 41, 85–92. [Google Scholar] [CrossRef]
- Rasheed, Z. Molecular evidences of health benefits of drinking black tea. Int. J. Health Sci. 2019, 13, 1. [Google Scholar]
- Kaleem, M.; Ahmad, A. Flavonoids as Nutraceuticals. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 137–155. ISBN 978-0-12-814625-5. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Polyphenols and Flavonoids. In Modern Nutrition in Health and Disease: Eleventh Edition; Ross, A.C., Caballero, B.H., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Wolters Kluwer Health Adis (ESP), 2012; pp. 494–505 ISBN 9781605474618.Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Koech, K.R.; Wachira, F.N.; Ngure, R.M.; Wanyoko, J.K.; Bii, C.C.; Karori, S.M.; Kerio, L.C. Antimicrobial, Synergistic and Antioxidant Activities of Tea Polyphenols. 2013. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochem.-Source Antioxid. Role Dis. Prev. 2018, 7, 49–74. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in parkinson’s disease (review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.W.; Lo, C.Y.; Li, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; Ho, C.T. Stability of black tea polyphenol, theaflavin, and identification of theanaphthoquinone as its major radical reaction product. J. Agric. Food Chem. 2005, 53, 6146–6150. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wan, X. Introductory of Basic Chemistry and Health Effects of Tea. In Tea as a Food Ingredient; Yin, J., Fu, Z., Xu, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–14. ISBN 9781003152828. [Google Scholar]
- Sharma, N.; Phan, H.T.; Chikae, M.; Takamura, Y.; Azo-Oussou, A.F.; Vestergaard, M.C. Black tea polyphenol theaflavin as promising antioxidant and potential copper chelator. J. Sci. Food Agric. 2020, 100, 3126–3135. [Google Scholar] [CrossRef]
- Koch, W. Theaflavins, Thearubigins, and Theasinensins. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 975–1003. ISBN 978-981-15-4147-6. [Google Scholar]
- He, H.F. Research progress on theaflavins: Efficacy, formation, and preparation. SNF Swedish Nutr. Found. 2017, 61, 1344521. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cattani, C.; Rao, R.V.; Wang, S.; Phillips, P. Tea category identification using a novel fractional fourier entropy and jaya algorithm. Entropy 2016, 18, 77. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wan, Z.; Li, G.; Chen, L.; Guo, Y. Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signalling pathway in vivo and in vitro. Biomed. Pharmacother. 2021, 134, 111097. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Nisar, M.F.; Li, M.; Zhang, C.; Wan, C. Theaflavin chemistry and its health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 6256618. [Google Scholar] [CrossRef] [PubMed]
- Beresniak, A.; Duru, G.; Berger, G.; Bremond-Gignac, D. Relationships between black tea consumption and key health indicators in the world: An ecological study. BMJ Open 2012, 2, e000648. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Sharma, V.; Jagan, L.; Rao, M.; Jagan, L. A thought on the biological activities of black tea. Crit. Rev. Food Sci. Nutr. 2009, 49, 379–404. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Kumar, A.; Ravi, R.; Simon, P.; Rai, P.; Francis, A.; Baliga, M.S. Hepatoprotective effects of green tea and its polyphenols: Preclinical observations. Polyphenols Hum. Health Dis. 2014, 1, 715–721. [Google Scholar] [CrossRef]
- Shivashankara, A.R.; Rao, S.; George, T.; Abraham, S.; Colin, M.D.; Palatty, P.L.; Baliga, M.S. Tea (Camellia sinensis L. Kuntze) as hepatoprotective agent: A revisit. Diet. Interv. Liver Dis. Foods, Nutr. Diet. Suppl. 2019, 183–192. [Google Scholar] [CrossRef]
- Stodt, U.W.; Blauth, N.; Niemann, S.; Stark, J.; Pawar, V.; Jayaraman, S.; Koek, J.; Engelhardt, U.H. Investigation of processes in black tea manufacture through model fermentation (oxidation) experiments. J. Agric. Food Chem. 2014, 62, 7854–7861. [Google Scholar] [CrossRef]
- Wang, N. A comparison of Chinese and British tea culture. Asian Cult. Hist. 2011, 3, 13. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Zhang, J.; Yang, Y.; Yang, X.; Xu, B.; Yang, W.; Tong, T.; Jin, S.; Shen, C.; Rao, H.; et al. Earliest tea as evidence for one branch of the silk road across the tibetan plateau. Sci. Rep. 2016, 6, 18955. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef]
- Bhat, R. Fermentation of black tea broth (kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Dufresne, C.; Farnworth, E. Tea, kombucha, and health: A review. Food Res. Int. 2000, 33, 409–491. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- de Noronha, M.C.; Cardoso, R.R.; dos Santos D’Almeida, C.T.; Vieira do Carmo, M.A.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black tea kombucha: Physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef]
- Kumar, V.; Joshi, V.K. Kombucha: Technology, microbiology, production, composition and therapeutic value. Int. J. Food Ferment. Technol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha tea: Metabolites. In Fungal Metabolites; Springer: Cham, Switzerland, 2017; pp. 965–978. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial dynamics between yeasts and acetic acid bacteria in kombucha: Impacts on the chemical composition of the beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, Chemical and antioxidant activities of different types kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Phetxumphou, K.; Vick, R.; Blanc, L.; Lahne, J. Processing condition effects on sensory profiles of kombucha through sensory descriptive analysis. J. Am. Soc. Brew. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S. Kombucha tea: A promising fermented functional beverage. Fermented Beverages Sci. Beverages 2019, 5, 401–432. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Lee, K.R.; Jo, K.; Ra, K.S.; Suh, H.J.; Hong, K.-B. Kombucha fermentation using commercial kombucha pellicle and culture broth as starter. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial composition of SCOBY starter cultures used by commercial kombucha brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and chemical profiles of commercial kombucha products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef] [PubMed]
- Besky, S. Empire and indigestion: Materializing tannins in the indian tea industry. Soc. Stud. Sci. 2020, 50, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.; Khan, F.; Nisar, M.F.; Niaz, K. Tannins (Hydrolysable tannins, condensed tannins, phlorotannins, flavono-ellagitannins). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 132–146. [Google Scholar]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Pasha, C.; Reddy, G. Nutritional and medicinal improvement of black tea by yeast fermentation. Food Chem. 2005, 89, 449–453. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.L.; et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef]
- McNulty, H.; Ward, M.; Hoey, L.; Hughes, C.F.; Pentieva, K. Addressing optimal folate and related B-vitamin status through the lifecycle: Health impacts and challenges. Proc. Nutr. Soc. 2019, 78, 449–462. [Google Scholar] [CrossRef]
- Park, J.; Hosomi, K.; Kawashima, H.; Chen, Y.-A.; Mohsen, A.; Ohno, H.; Konishi, K.; Tanisawa, K.; Kifushi, M.; Kogawa, M.; et al. Dietary vitamin B1 intake influences gut microbial community and the consequent production of short-chain fatty acids. Nutrients 2022, 14, 2078. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Shetty, K.; Khanongnuch, C. Nutritional biotransformation in traditional fermented tea (miang) from North Thailand and its impact on antioxidant and antimicrobial activities. J. Food Sci. Technol. 2019, 56, 2687. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Rai, D.; Shivam; Shahane, S.; Mishra, U. Lipases: Sources, production, purification, and applications. Recent Pat. Biotechnol. 2018, 13, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Anggrahini, S.; Efendi, Z. Effect of natural and lactobacillus plantarum fermentation on in-vitro protein and starch digestibilities of sorghum flour. Food Biosci. 2013, 2, 46–52. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and water soluble vitamin content in the kombucha drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotifcs prebiotics and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Estrada, M.A.R.; Kheng, K.S.; Ating, R. The evaluation of obesity in Malaysia. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- John, G.K.; Mullin, G.E. The gut microbiome and obesity. Curr. Oncol. Reports 2016, 18, 45. [Google Scholar] [CrossRef]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut microbiota as a therapeutic target for metabolic disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.C.; Van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A lactobacillus and bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammadi, A.R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Zeid, A.A.; Enan, G. Chemical constitution and antimicrobial activity of kombucha fermented beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Pfeiffer, N.; Loh, G.; Klaus, S.; Blaut, M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. MBio 2014, 5, e01530-14. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Kurrey, N.K.; Halami, P.M. In vitro anti-inflammatory activity among probiotic lactobacillus species isolated from fermented foods. J. Funct. Foods 2018, 47, 19–27. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.P.; Zhu, K.X.; Ding, Q.; Li, C.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—Mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Baümler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.d.C.; Vilela, D.L.d.S.; Fraiz, G.M.; Lopes, I.L.; Coelho, A.I.M.; Castro, L.C.V.; Martin, J.G.P. Effect of kombucha intake on the gut microbiota and obesity-related comorbidities: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).