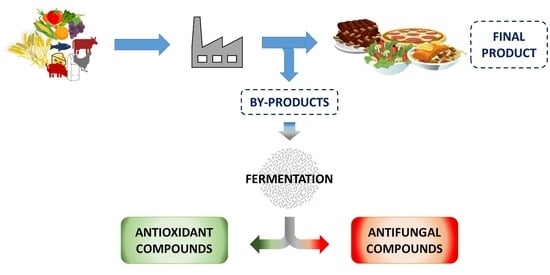
Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review
Abstract
:1. Introduction
2. Fermentation Processes
3. Preservative Compounds Obtained from Food By-Products Fermentation
3.1. Antioxidants
3.2. Antifungals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.T.; Wei, Y.H.; Chi-Wei Lan, J. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 12288. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doriya, K.; Jose, N.; Gowda, M.; Kumar, D.S. Solid-state fermentation vs submerged fermentation for the production of L-Asparaginase. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2016; Volume 78, pp. 115–135. [Google Scholar]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef]
- Klempová, T.; Slaný, O.; Michal, Š.; Marcin, S.; Milan, Č. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid-state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2019, 8, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Zhang, W.; Dong, Q.; Tang, C.; Cao, F.; Su, E. Submerged fermentation of Ginkgo biloba seed powder using Eurotium cristatum for the development of ginkgo seeds fermented products. J. Sci. Food Agric. 2021, 101, 1782–1791. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Valorization of egg shell biowaste and brewery wastewater for the enhanced production of fumaric acid. Waste Biomass Valorization 2015, 6, 535–546. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Feng, X.; Khaskheli, A.A.; Xiang, Y.; Huang, W. The effects of alcohol fermentation on the extraction of antioxidant compounds and flavonoids of pomelo peel. LWT Food Sci. Technol. 2017, 89, 763–769. [Google Scholar] [CrossRef]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of grapefruit by-products as solid support for solid-state fermentation to produce antioxidant bioactive extracts. Waste Biomass Valorization 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-state fermentation—Assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Diaconeasa, Z.; Socaciu, C. Liberation and recovery of phenolic antioxidants and lipids in chokeberry (Aronia melanocarpa) pomace by solid-state bioprocessing using Aspergillus niger and Rhizopus oligosporus strains. LWT 2018, 87, 241–249. [Google Scholar] [CrossRef]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of phenolic antioxidants from grape, apple and pitahaya residues via solid state fungal fermentation and carbohydrase treatment. LWT Food Sci. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Isolation and identification of an antioxidant peptide prepared from fermented peanut meal using Bacillus subtilis fermentation. Int. J. Food Prop. 2014, 17, 1237–1253. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Duhan, J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Da Costa Maia, I.; Thomaz dos Santos D’Almeida, C.; Guimarães Freire, D.M.; D’Avila Costa Cavalcanti, E.; Cameron, L.C.; Furtado Dias, J.; Simões Larraz Ferreira, M. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. LWT 2020, 133, 110136. [Google Scholar] [CrossRef]
- Bangar, S.P.; Sandhu, K.S.; Purewal, S.S.; Kaur, M.; Kaur, P.; Siroha, A.K.; Kumari, K.; Singh, M.; Kumar, M. Fermented barley bran: An improvement in phenolic compounds and antioxidant properties. J. Food Process. Preserv. 2021, e15543. [Google Scholar] [CrossRef]
- Mao, M.; Wang, P.; Shi, K.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lv, F. Effect of solid state fermentation by Enterococcus faecalis M2 on antioxidant and nutritional properties of wheat bran. J. Cereal Sci. 2020, 94, 102997. [Google Scholar] [CrossRef]
- Dei Piu’, L.; Tassoni, A.; Serrazanetti, D.I.; Ferri, M.; Babini, E.; Tagliazucchi, D.; Gianotti, A. Exploitation of starch industry liquid by-product to produce bioactive peptides from rice hydrolyzed proteins. Food Chem. 2014, 155, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Alvarado Pérez, Y.; Muro Urista, C.; Maciel Cerda, A.; Álvarez Sánchez, J.; Riera Rodríguez, F. Antihypertensive and antioxidant properties from whey protein hydrolysates produced by encapsulated Bacillus subtilis cells. Int. J. Pept. Res. Ther. 2019, 25, 681–689. [Google Scholar] [CrossRef]
- Rochín-Medina, J.J.; Ramírez-Medina, H.K.; Rangel-Peraza, J.G.; Pineda-Hidalgo, K.V.; Iribe-Arellano, P. Use of whey as a culture medium for Bacillus clausii for the production of protein hydrolysates with antimicrobial and antioxidant activity. Food Sci. Technol. Int. 2018, 24, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammoh, S.; Alu’datt, M.H.; Tranchant, C.C.; Al-U’datt, D.G.; Alhamad, M.N.; Rababah, T.; Kubow, S.; Haddadin, M.S.Y.; Ammari, Z.; Maghaydah, S.; et al. Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus delbrueckii subsp. lactis. LWT 2020, 129, 109501. [Google Scholar] [CrossRef]
- Yu, H.C.; Hsu, J.L.; Chang, C.I.; Tan, F.J. Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci. Biotechnol. 2017, 26, 1217–1225. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Martí-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.-J.; Barba, F.J. Impact of fermentation on the recovery of antioxidant bioactive compounds from sea bass byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Sun, J.; Dong, P.; Xue, C.; Mao, X. Conversion of turbot skin wastes into valuable functional substances with an eco-friendly fermentation technology. J. Clean. Prod. 2017, 156, 367–377. [Google Scholar] [CrossRef]
- Ruthu., N.; Murthy, P.S.; Rai, A.K.; Bhaskar, N. Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J. Food Sci. Technol. 2014, 51, 1884–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choksawangkarn, W.; Phiphattananukoon, S.; Jaresitthikunchai, J.; Roytrakul, S. Antioxidative peptides from fish sauce by-product: Isolation and characterization. Agric. Nat. Resour. 2018, 52, 460–466. [Google Scholar] [CrossRef]
- Ismail, S.A. Microbial valorization of shrimp byproducts via the production of thermostable chitosanase and antioxidant chitooligosaccharides. Biocatal. Agric. Biotechnol. 2019, 20, 101269. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. Technol. 2008, 99, 9013–9016. [Google Scholar] [CrossRef] [PubMed]
- Christ-Ribeiro, A.; Graça., C.S.; Kupski, L.; Badiale-Furlong, E.; De Souza-Soares, L.A. Cytotoxicity, antifungal and anti mycotoxins effects of phenolic compounds from fermented rice bran and Spirulina sp. Process Biochem. 2019, 80, 190–196. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic acid fermentation to re-cycle apple by-products for wheat bread fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzo, L.; Luz, C.; Ritieni, A.; Mañes, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Rodriguez, L.; Romano, R.; Mañes, J.; Meca, G. A natural strategy to improve the shelf life of the loaf bread against toxigenic fungi: The employment of fermented whey powder. Int. J. Dairy Technol. 2020, 73, 88–97. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Príncep, A.; Tornos, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.-J.; Barba, F.J.; Mañes, J. Isolation, Identification and investigation of fermentative bacteria from sea bass (Dicentrarchus labrax): Evaluation of antifungal activity of fermented fish meat and by-products broths. Foods 2020, 9, 576. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, Y.C.; Jao, C.L. Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour. Technol. 2007, 98, 1224–1230. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative production of bioproducts from organic waste through solid-state fermentation. Front. Sustain. Food Syst. 2019, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Arte, E.; Rizzello, C.G.; Verni, M.; Nordlund, E.; Katina, K.; Coda, R. Impact of enzymatic and microbial bioprocessing on protein modification and nutritional properties of wheat bran. J. Agric. Food Chem. 2015, 63, 8685–8693. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Y.; Huan, C.C.; Xu, L.; Ji, G.; Yan, Z. Comparison of poly-γ-glutamic acid production between sterilized and non-sterilized solid-state fermentation using agricultural waste as substrates. J. Clean. Prod. 2020, 255, 120248. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Oliveira, R.A.; Bon, E.P.S.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.F.; Tonon, R.V. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö.E.; Guðjónsdóttir, M.; Barba, F.J.; Marti-Quijal, F.J.; et al. Use of spectroscopic techniques to monitor changes in food quality during application of natural preservatives: A review. Antioxidants 2020, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Garcia, C.; Fessard, A.; Barba, F.; Munekata, P.; Lorenzo, J.; Remize, F.; Roselló-Soto, E.; Garcia, C.; Fessard, A.; et al. Nutritional and microbiological quality of tiger nut tubers (Cyperus esculentus), derived plant-based and lactic fermented beverages. Fermentation 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Embiriekah, S.; Bulatović, M.; Borić, M.; Zarić, D.; Arsić, S.; Rakin, M. Selection of Lactobacillus strains for improvement of antioxidant activity of different soy, whey and milk protein substrates. J. Hyg. Eng. Des. 2016, 16, 64–69. [Google Scholar]
- Skrzypczak, K.; Gustaw, W.; Kononiuk, A.; Sołowiej, B.; Waśko, A. Estimation of the antioxidant properties of milk protein preparations hydrolyzed by Lactobacillus helveticus T80, T105 and B734. Czech J. Food Sci. 2019, 37, 260–267. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Rendueles, M.; Díaz, M. Synbiotic fermentation for the co-production of lactic and lactobionic acids from residual dairy whey. Biotechnol. Prog. 2017, 33, 1250–1256. [Google Scholar] [CrossRef]
- Pescuma, M.; De Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Luo, J.; Huang, W.; Guo, W.; Ge, R.; Zhang, Q.; Fang, F.; Feng, Q.; Cao, J.; Wu, Y. Novel strategy to stimulate the food wastes anaerobic fermentation performance by eggshell wastes conditioning and the underlying mechanisms. Chem. Eng. J. 2020, 398, 125560. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.-J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [Green Version]
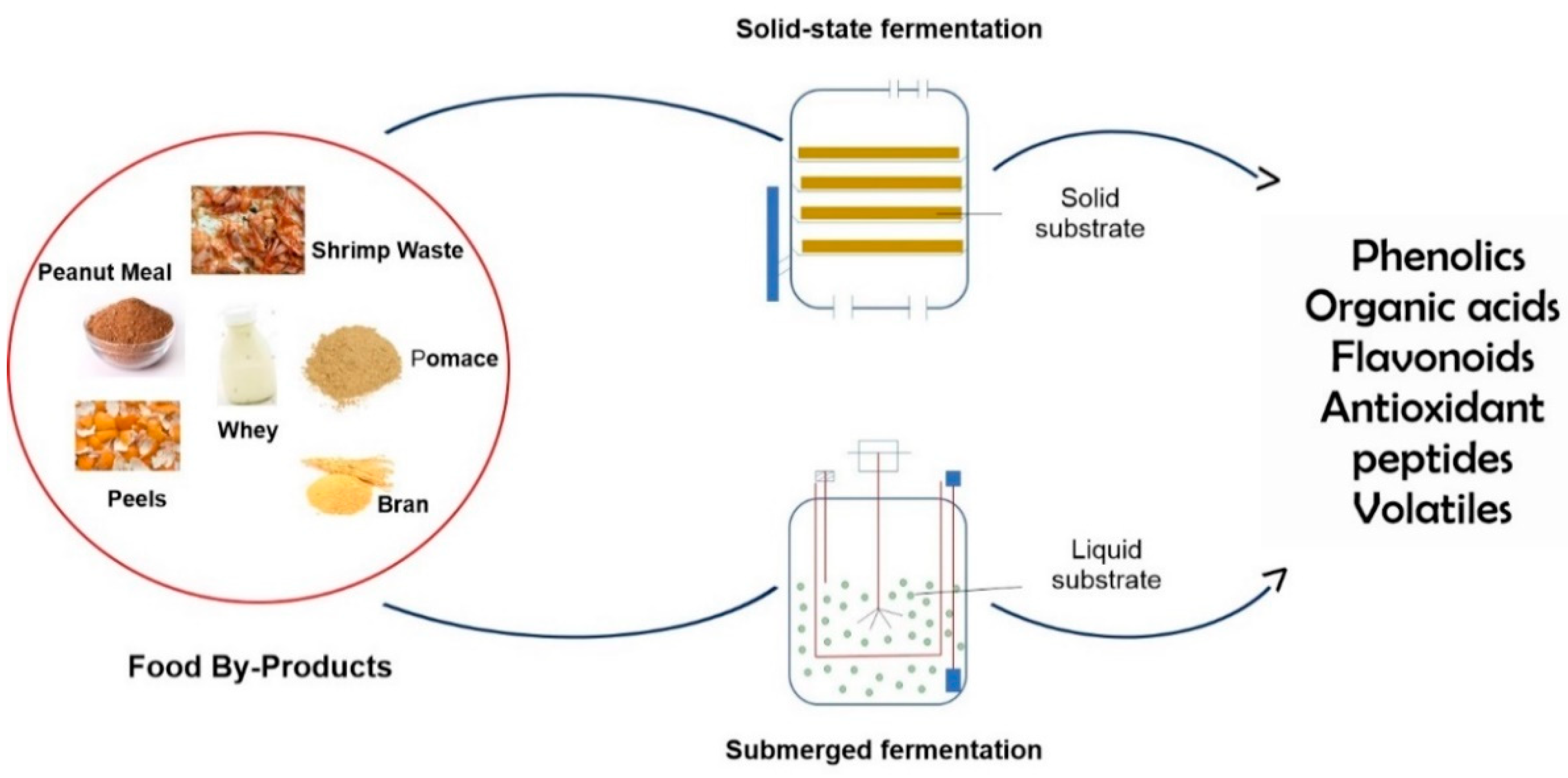
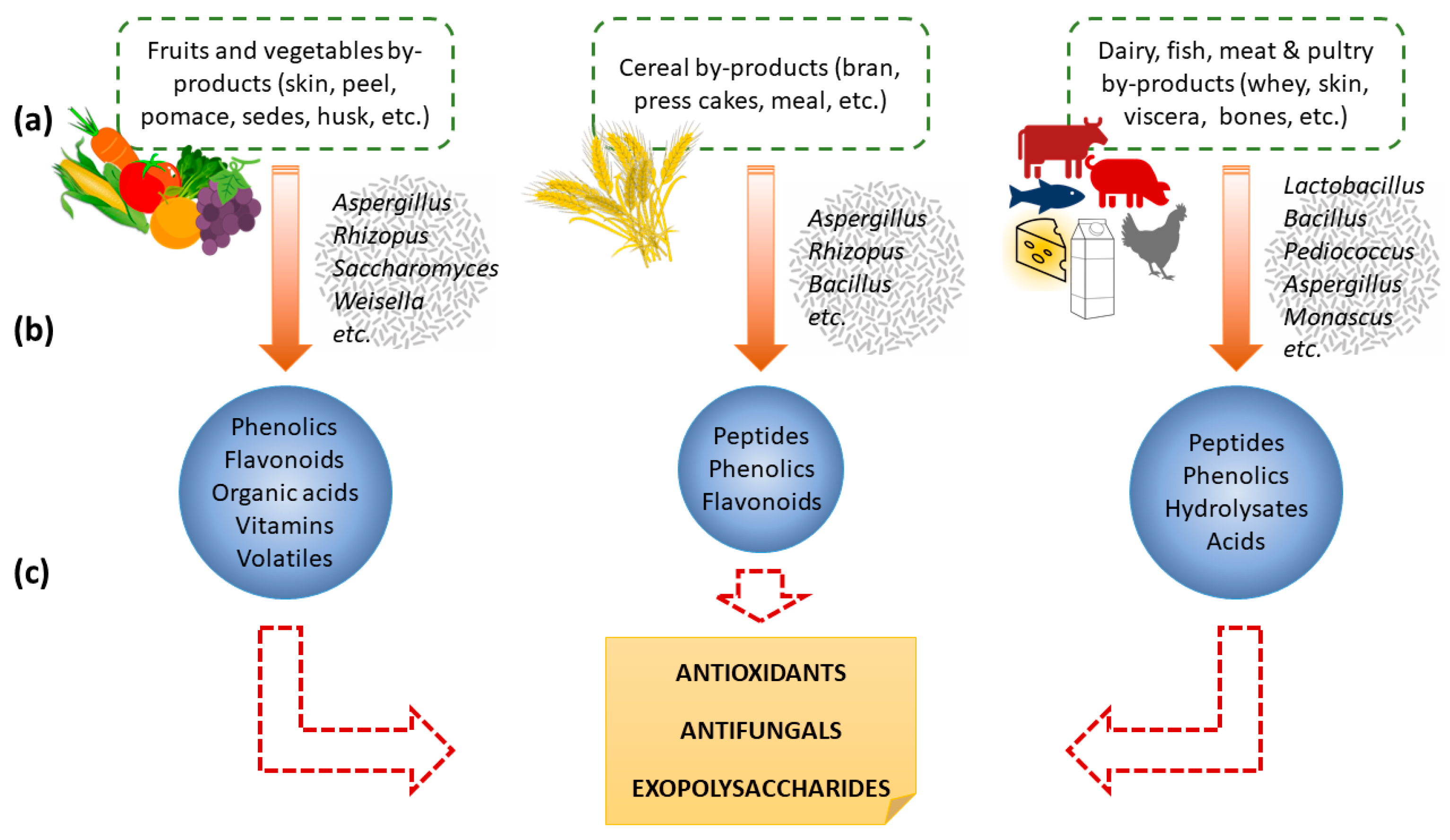
| Food By-Product (Substrate) | Microorganism | Fermentation | Compounds Obtained | Reference |
|---|---|---|---|---|
| Pomelo peels | F33 activated yeast | SmF | Flavonoids | [12] |
| Grapefruit by-products | Aspergillus niger | SSF | Antioxidant compounds | [13] |
| Hass avocado seeds | A. niger GH1 | SSF | Phenolic compounds | [14] |
| Plum by-products (pomaces, spent fruit pulp and peels) | A. niger and Rhizopus oligosporus | SSF | Phenolic compounds (mainly isoquercitrin, cinnamic acids and rutin) and flavonoids | [15] |
| Chokeberry pomace | A. niger and R. oligosporus | SSF | Polyphenols and flavonoids | [16] |
| Grape, apple and pitahaya by-products | Rhizomucor miehei NRRL5282 | SSF | Phenolic compounds | [17] |
| Grape pomace | Actinomucor elegans and Umbelopsis isabellina | SSF | γ-linolenic acid, carotenoids, phenolic compounds and flavonoids | [18] |
| Wheat and oat bran | Saccharomyces cerevisiae | SSF | Polyphenols | [19] |
| Peanut meal | Bacillus subtilis | SmF | Antioxidant peptides | [20] |
| Peanut press cake | Aspergillus awamori | SSF | Phenols and flavonoids | [21] |
| Brewer’s spent grain | A. awamori, Aspergillus oryzae, Aspergillus terreus, A. niger and R. oryzae | SSF | Phenolic compounds (mainly ferulic, p-coumaric and caffeic acids) | [22] |
| Barley bran | A. oryzae | SSF | Phenolic compounds | [23] |
| Wheat bran | Enterococcus faecalis M2 | SSF | Phenols, flavonoids and alkylresorcinols | [24] |
| Rice starch extraction by-product | Bacillus spp. | SmF | Antioxidant peptides | [25] |
| Whey proteins | Bacillus subtilis | SmF | Antioxidant peptides | [26] |
| Whey (cheese manufacturing by-product) | Bacillus clausii | SmF | Antioxidant peptides | [27] |
| Camel milk whey | Lactobacillus delbrueckii subsp. lactis | SmF | Antioxidant peptides | [28] |
| Porcine liver proteins | Monascus purpureus | SmF | Antioxidant peptides | [29] |
| Chicken eggshell membrane | Lactiplantibacillus plantarum | SmF | Antioxidant peptides | [30] |
| Sea bass by-products | L. plantarum | SmF | Antioxidant compounds | [31] |
| Turbot skin | A. oryzae | SmF | Antioxidant peptides | [32] |
| Carp heads | Pediococcus acidilactici and Enterococcus faecium | SmF | Antioxidant and antifungal peptides | [33] |
| Fermented fish sauce by-product | - | SmF | Antioxidant peptides | [34] |
| Shrimp waste (cephalothoraxes and carapaces) | Bacillus cereus | SSF | Antioxidant chitooligosaccharides | [35] |
| Shrimp waste | P. acidolactici | SmF | Antioxidant compounds | [36] |
| Rice bran | R. oryzae | SmF | Phenolic compounds | [37] |
| Rice bran | R. oryzae | SmF | Phenolic compounds | [38] |
| Apple by-products | Weissella cibaria and S. cerevisiae | SmF | Dietary fibers and volatile compounds | [39] |
| Whey (cheese manufacturing by-product) | L. plantarum | SmF | Antifungal compounds | [40] |
| Whey (cheese manufacturing by-product) | L. plantarum | SmF | Antifungal compounds | [41] |
| Sea bass by-products | L. plantarum | SmF | Antifungal compounds | [42] |
| Shrimp and crab shell | B. cereus | SmF | Antifungal compounds | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. https://doi.org/10.3390/fermentation7030106
Martí-Quijal FJ, Khubber S, Remize F, Tomasevic I, Roselló-Soto E, Barba FJ. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation. 2021; 7(3):106. https://doi.org/10.3390/fermentation7030106
Chicago/Turabian StyleMartí-Quijal, Francisco J., Sucheta Khubber, Fabienne Remize, Igor Tomasevic, Elena Roselló-Soto, and Francisco J. Barba. 2021. "Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review" Fermentation 7, no. 3: 106. https://doi.org/10.3390/fermentation7030106
APA StyleMartí-Quijal, F. J., Khubber, S., Remize, F., Tomasevic, I., Roselló-Soto, E., & Barba, F. J. (2021). Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation, 7(3), 106. https://doi.org/10.3390/fermentation7030106