Abstract
To lower the risk of obesity, diabetes, and other related diseases, the WHO recommends that consumers reduce their consumption of sugars. Here, we propose a microbiological method to reduce the sugar content in red beet juice, while incurring only slight losses in the betalain content and maintaining the correct proportion of the other beet juice components. Several yeast strains with different metabolic activities were investigated for their ability to reduce the sugar content in red beet juice, which resulted in a decrease in the extract level corresponding to sugar content from 49.7% to 58.2%. This strategy was found to have the additional advantage of increasing the chemical and microbial stability of the red beet juice. Only slight losses of betalain pigments were noted, to final concentrations of 5.11% w/v and 2.56% w/v for the red and yellow fractions, respectively.
1. Introduction
In the last two decades, there has been increasing consumer interest in food safety and nutrition. This has been reflected in a growing body of research examining the relation between diet and health [1]. As a result, there is currently a renewed emphasis on the importance of fruits and vegetables, as “functional foods” with phytochemical nutrients capable of preventing or postponing the onset of chronic diseases [2]. Red beets (Beta vulgaris L.) are a rich source of sugars and bioactive compounds, including phenolics, cyclic amines, and various minerals. Red beets usually contain about 9.6 g/100 g carbohydrates. Their caloric value is 42 kcal per 100 g. In contrast to many popular fruits, the main sugar in red beet is sucrose [3,4]. Red beets can be eaten raw, baked, boiled, pickled, or used to make juice and soup, which is popular in many Eastern and Central European countries. The bioactive substances in red beet exhibit numerous beneficial properties, such as anti-inflammatory, antimicrobial, anticancer, and antiviral effects [5].
Betalains are nitrogenous pigments which are characteristic of plants belonging to the order Caryophyllales. B. vulgaris roots are the best known edible source of betalains among the plants in the Caryophyllales order. There are two types of betalains: yellow (betaxanthins) and red-violet (betacyanins). The pure yellow and red-violet colors combine in nature to make orange and red shades. Betalains show good bioactive potential. These compounds exhibit strong antioxidant properties, inhibiting lipid peroxidation and protecting red blood cells against oxidative hemolysis [6]. Betalain extracts are listed as additive 73.40 in the 21 CFR section of the Food and Drug Administration (FDA) in the USA and under the E-162 code in the European Union [7]. The data show that a betacyanins/betaxanthins proportion of 2.08 is associated with good inner color and other sensory values [8]. However, treatment processes have been found to reduce the bioactivity value of red beet. Both betacyanins and betaxanthins are prone to degradation in the presence of oxygen, light, and elevated temperature, which act in a synergistic way [9]. Water activity and pH level also influence the stability of betalains, leading to the loss of pigment [8].
Red beet juice is a popular product of beets. To produce red beet juice, ripe beets are crushed and pressed. The juices are sometimes mixed, enabling the production of tailor-made blends. Red beet juice offers huge growth potential for the food industry, which could be explored through the development of new ingredients, processes, and products. Juiced red beets are ten times more nutrient-rich than raw red beet, because the sugars and other nutritive compounds are highly concentrated [10]. However, the high sugar content may be a disadvantage, in terms of ensuring a healthy balanced diet. Overconsumption of sugar is a major contributor to obesity, diabetes, and tooth decay. World Health Organization (WHO) guidelines advise adults and children should reduce their consumption of sugars to less than 10% of their daily energy intake and suggest a further reduction to below 5% of daily energy intake [11,12,13].
Various fermentation processes can be used to reduce the sugar content of red beet juice, as well as improve its nutritional value and stability. Numerous studies have investigated the extraction of red beet juice, with optional boiling, spontaneous fermentation or fermentation using bacterial starters with probiotic properties [14,15,16,17]. Both red beets and lactic fermented products offer nutritional benefits. However, according to Sawicki and Wiczkowski [15], heat-treatment and lactic fermentation may reduce the content of betalains by up to 88%. Moreover, fermented red beet juices are not always palatable to consumers. These facts have given impetus to the recent expansion of non-dairy lactic fermented juices on the market.
There is a need for safe, biological methods to reduce the sugar content in red beet juice, while stabilizing the natural betalain ratio and improving the sensory and nutritional quality of the juice. An interesting possibility is red beet juice fermentation using yeast strains. The activities of different yeast species and strains have an impact on the sensory profiles of juices. Classical Saccharomyces cerevisiae strains can metabolize sugar in two ways: aerobically or anaerobically. When yeast metabolizes a sugar under anaerobic conditions, ethanol and carbon dioxide are the main fermentation products. Under both aerobic and anaerobic conditions, the preliminary final product of sugar utilization is pyruvate. Under anaerobic conditions, pyruvate is reduced to ethanol. In turn, in the presence of oxygen, pyruvate is converted to acetyl-coenzyme A and oxidized to carbon dioxide in the tricarboxylic acid cycle [18]. The Crabtree effect plays important role in yeast metabolism. This mechanism occurs in S. cerevisiae (Crabtree-positive) yeasts when oxygen concentrations exceed a certain limit. The yeast utilizes glycolysis as the terminal electron acceptor instead of oxygen, despite the presence of sufficient dissolved oxygen [19]. In non-Saccharomyces strains, this effect does not function. As a result, non-conventional (Crabtree-negative) yeasts show rather weak fermentation activity, but they can create more flavor precursors. In general, each of the non-Saccharomyces yeasts shows unique fermentation characteristics [20]. Consequently, yeasts have the potential to improve the organoleptic values of red beet juice.
The purpose of this study was to evaluate the potential of conventional and non-conventional yeast strains with different sugar and enzymatic profiles to reduce the sugar content while maintaining the compactness and the proportion of betalain fractions in red beet juice. There has been little research concerning the reduction of sugar content by yeast cultures while stabilizing the natural betalain ratio. The following parameters were controlled: sugar content; extract content during fermentation; ethanol formation; changes in the concentration/proportion of betalain dyes.
2. Materials and Methods
2.1. Red Beet Juice
Red beet juice was obtained from Vin-Kon S.A in Konin (Poland). Red beet roots (Beta vulgaris L., cv. Detroit dark red) were used to make the juice. The red beets were grown locally (Poland, 52°13′24.17″ N, 18°15′4.36″ E) in the season of 2018. The roots of the red beet were washed and crushed using type-J63 hammer grinders (ZPOW, Jaslo, Poland) and type-C5 mills (Bucher, Zürich, Switzerland). The pulp was transferred to Bucher type HP5000 basket presses and pressed at 150 bar. The extracted juice was stabilized using citric acid (E330). The extract content of the juice was measured as 15 degrees Bx (symbol °Bx), and the pH level was 4.5. One degree Brix is 1 g of saccharose in 100 g of solution. The juice was kept in a refrigerator (4 °C) for a maximum of 5 days before use.
To investigate the betalain content after heat treatment, the red beet juice was treated at various temperatures, from 40 °C to 121 °C, for 60 min. This constant period was chosen to investigate the effect of temperature on the betalain content over a comparable time. To determine the effect of each of the tested yeasts (conventional and non-conventional) on extract reduction and betalain content, the red beet juice before fermentation was sterilized at 121 °C for 20 min. In the case of the strong fermentative yeasts, raw beet juice was used without any heat treatment.
2.2. Sugar Content
The saccharide profiles of the red beet juice were analyzed using Megazyme kits (Sucrose Fructose/D-Glucose Assay Kit, Raffinose/D-Galactose Assay Kit, L-Rhamnose Assay Kit, D-Xylose Assay Kit) (Megazyme Inc., County Wicklow, Ireland) and using a Multiskan GO UV spectrophotometer (Thermo Fisher Scientific, Munich, Germany), according to the manufacturer’s instructions, as previously described by Modelska et al. [21]. Total sugar content was determined using the Luff-Schoorl method, in accordance with the Polish Standard PN-90/A-75101/07 and the Grain and Feed Trade Association (GAFTA) Method 10.1. [22,23]. This method is based on hot reduction of an alkaline copper salt solution by direct titration, using a reducing sugar solution in the presence of methylene blue as an indicator. The reduction of the Cu(II) ions present in the Luff solution by the saccharides in the analysed sample was initiated at the boiling point. The volume of sodium thiosulphate (VI) corresponding to the amount of copper (II) reduced by saccharides was calculated as the difference between the volumes obtained from two (blank and specific) titrations. Based on these results, the content of reducing saccharides was determined in each sample [24].
2.3. Yeast Strains
Eight strains of yeasts representing various genera and species were used in the study. Four strains of the genus Saccharomyces represented commercial fermentative strains commonly used in breweries, distilleries, and wineries. The other four strains were non-Saccharomyces yeasts of weak fermentative nature, belonging to the genera Kluyveromyces, Scheffersomyces and Metschnikowia (Table 1). These yeasts are capable of pentose fermentation (Scheffersomyces sp.) or various biocontrol mechanisms (Kluyveromyces sp., Metschnikowia sp.) [25,26,27], which may improve sugar reduction and contribute to microbial stabilization.

Table 1.
Yeast strains used in the study.
The yeast inoculums were prepared in YPD [2% w/v peptone, 2% w/v glucose, 1% w/v yeast extract] broth (Merck, Darmstadt, Germany) after incubation at 30 °C for 24 h on a rotary shaker at 150 rpm.
2.4. Assimilation Profiles
The assimilation profiles of each of the tested yeast strains were determined using the API 20 C AUX identification system (bioMérieux, Lyon, France), according to the manufacturer’s instructions, as previously described by Pawlikowska et al. [27]. The ability of the yeasts to assimilate fructose and rhamnose (not present in API set) was evaluated using the conventional method for yeast identification [28].
2.5. Enzymatic Profiles
The enzymatic profiles of the tested yeast strains were estimated using API ZYM tests (bioMerieux). Inoculation and evaluation were carried out based on the manufacturer’s instructions and recommendations. Only the suspensions that showed visible changes in the color of the medium were considered to demonstrate enzymatic activity. Enzyme activity was graded from 0 to 5 by comparing the developed color to the API-ZYM color reaction chart, where ‘0’ indicates a negative reaction and ‘5’ indicates a high positive reaction [27].
2.6. Red Beet Juice Fermentation
Sterile glass bottles (volume 500 mL) were filled with 300 mL of the red beet juice. All samples were inoculated with 15 mL of yeast inoculum (5% v/v). The glass bottles were closed with fermentation airlocks and silicone stoppers, then incubated without agitation at 30 °C. The end of fermentation was estimated after stabilization of the extract content. The control sample was the sterilized or raw red beet juice without yeast inoculation. Inoculation was carried out using suspensions of the tested yeasts prepared to equal the 1.0 McFarland standard (1 °McF). Yeast cell density was measured using a DEN-1 densitometer (Merck). Yeast cells before and after fermentation were observed microscopically using a BX41 light microscope (Olympus, Tokyo, Japan) equipped with a digital camera.
2.7. Fermentation Efficiency
Standard analytical measurements were performed over the course of fermentation. The fermentation process was periodically monitored by measuring the apparent extract on a Rudolf Research J157 automatic refractometer (Rudolf Research Analytical, Hackettstown, NJ, USA) [29]. The ethanol content was determined using the classical distillation method, with a Rudolf Research DDM 2910 oscillatory densimeter (Rudolf Research Analytical) A digital oscillatory densimeter technique is suitable for measuring the density of ethanol solutions at different concentration [30].
2.8. Betalain Content
Stability tests for betalain dyes were performed across a wide range of temperatures (40–121 °C). Betalain pigments were assayed by differential spectrophotometry following the Nilsson method [31]. The samples were diluted with a phosphate buffer (pH 6.5), and the contents of betacyanins (red pigments) and betaxanthins (yellowish pigments) were determined at 476, 538, and 600 nm using a Spectroquant® Prove 300 spectrophotometer (Merck, Darmstadt, Germany) [32].
2.9. Sugar Index
We used the sugar index as a simple parameter to determine the stability of the red beet pigments relative to the reduction of sugar content in the tested red beet juices. The Sugar Index was calculated according to the formula I = (S/P), where I is the Sugar Index, S is the sugar content [g/100 g], and P is the betalain content [g/100 g].
2.10. Statistics
All experiments were performed in triplicate and the data shown are representative. In the statistical analysis, the mean and the standard deviation of five technical measurements were calculated. The mean values of the betalain content were compared using one-way repeated measures analysis of variance (ANOVA; OriginPro 8.1, OriginLab Corp., Northampton, MA, USA). The results were compared to those for the control samples. Values with different letters presented in the figures show statistically significant differences: a, p ≥ 0.05; b, 0.005 < p < 0.05; c, p < 0.005.
3. Results and Discussion
3.1. Sugar Content in Red Beet Juice
Table 2 presents the carbohydrate content in the raw red beet juice before fermentation.

Table 2.
Carbohydrate content in the raw red beet juice.
The total concentration of carbohydrates was 153 g/L. This value corresponded to the extract content of 15 °Bx. Therefore, the extract measurement was used as an indicator of the loss of sugars in the fermentation trials conducted by yeasts.
3.2. Assimilation Profiles
Red beet is known to contain various carbohydrates, such as sucrose, glucose, fructose, starch, and dietary fiber [33,34]. It has been confirmed in our study. This stimulated us to research the assimilation profiles of the yeast strains used in this study. The tested yeasts represent different genera, with various metabolic activities and potential applications. Saccharomyces cerevisiae is a model Crabtree-positive yeast widely used in biological processes and is responsible for alcohol production. Its metabolism has evolved to enable oxidative fermentation, meaning that it conducts fermentative metabolism even in the presence of oxygen and excess glucose. This feature provides its ecological niche, which is the ability to rapidly consume glucose and produce ethanol with antiseptic properties [35]. Other yeasts, so-called non-Saccharomyces (such as Kluyveromyces, Pichia, Scheffersomyces, and Metschnikowia) have weak fermentative activity, producing lower levels of ethanol. However, they have the ability to form various by-products, such as acetic acid, higher alcohols, esters, and acetoin [36]. These weak-fermentative but aromatic yeasts represent multi-enzyme pathways for the synthesis of fine chemicals and small molecular weight compounds of medicinal and nutritional importance [37].
Of the sugars used to characterize the tested yeasts, three were utilized by all the strains: glucose, fructose and saccharose. Few of the strains assimilated xylose and raffinose. Other sugars, such as arabinose, rhamnose, raffinose and xylose, were also assimilated, but there was variation between species. The narrowest assimilation profile was observed for the strain M. pulcherrima (VIII) (Table 3).

Table 3.
Assimilation profiles of the tested yeast strains.
3.3. Enzymatic Fingerprinting
Enzymatic fingerprinting was used to assess the ability of the yeasts to both assimilate various carbon compounds and create new sensory features in the fermented red beet juice. Red beet juice may contain unpleasant flavors, e.g., geosmin [38]. Therefore, the use of appropriate yeast strains can significantly improve its sensory qualities. Enzyme systems of microbial strains are usually mixtures of several enzymes. Some, including glycosidases, may act synergistically. The activity of one group of enzymes can influence another. For example, the activities of α- and β-glucosidase stimulate the action of α- and β-glucanases [25]. Hydrolysis of glycosyl-glucosides by yeast glucosidases enhances the content of aroma profiles in different plant materials [39,40,41]. Arylamidases (proteases) contribute to release amino acids as precursors of aromatic compounds. These enzymes catalyze the hydrolysis of the N-terminal amino acids from peptides or amides. Therefore, cystine arylamidase, leucine arylamidase, valine arylamidase, and acid phosphatase or naphthol-AS-BI-phosphohydrolase each have a significant role in enhancing aroma profiles during fermentation [42]. In the present study, we investigated the enzymatic profiles of all the tested strains, with special attention to the activities of proteases, esterases, phosphatases, and glycosidases (Table 4). According to the enzymatic profiles obtained from the API ZYM system, all the tested strains showed leucine arylamidase activity (score 5). S. cerevisiae were characterized by high acid phosphatase (score 5), naphtol-AS-BI-phosphohydrolase (score 3–5), and valine arylamidase (score 3–4). Other enzymatic activities were weaker and more variable. The enzymatic activities of the non-Saccharomyces yeasts varied. K. marxianus (V) showed the widest enzymatic spectrum. Both K. lactis (VI) and M. pulcherrima (VIII) showed high α-glucosidase activities (score 5). Similar results have been reported in other studies on the enzymatic profiles of S. cerevisiae and non-conventional yeasts. In research conducted by [43], S. cerevisiae isolates displayed alkaline phosphatase and acid phosphatase activities, and also exhibited two esterases. High leucine arylamidase activity was detected in all the tested strains. In other studies of non-conventional yeasts, strains from the genera Pichia and Metschnikowia, showed high α- and β-glucosidase activities. Esterase activity, which is involved in the formation of volatile aromatic ester compounds, was detected in non-Saccharomyces strains [44,45]. Kluyveromyces sp. and Metschnikowia sp. are able to produce various aroma compounds, such as fruit esters, carboxylic acids, ketones, furans, and alcohols [46,47,48]. Of these compounds, 2-phenyl ethanol with a characteristic rose aroma is the most important in commercial non-food applications [49]. The formation of a wide range of aroma profiles with decreasing carbohydrate concentration could become an important strategy for the development of new functional drinks with low-sugar content [50]. One of the easiest ways to reduce sugar is to replace it with sweeteners. However, research conducted in the Netherlands by the NIZO group suggests that rich aroma profiles can be leveraged as an alternative strategy, as a sweet aroma can enhance the perceived sweet taste of various types of food [51,52]. Another interesting result is the high activity of M. pulcherrima strain (VIII) for leucine arylamidase. Most of the pulcherriminic acid synthesized by M. pulcherrima clade is derived from the leucine present in the environment. The presence of leucine as a pulcherriminic acid precursor may have an additional ecological role in biocontrol mechanisms (pulcherrimin formation) [27,53]. Therefore, fermentation with M. pulcherrima yeast could provide the additional benefit of increasing the microbiological stability of red beet juice, especially with regard to fungal contamination [54,55].

Table 4.
Enzymatic fingerprinting of the tested yeast strains.
3.4. Red Beet Juice Fermentation
The high sugar content in the red beet juice accounts for its high caloric content. We therefore decided to research the possibility of reducing the content of saccharides while maintaining the content of betalains—health-promoting phytochemicals—in the juice.
We investigated fermentation with various yeast strains. Figure 1 presents the ethanol concentrations obtained on the third day of fermentation and at the end of the fermentation process. The tested strains showed various fermentation activities. Much better dynamics of ethanol production were noted for the Crabtree-positive S. cerevisiae strains (I, II, III). The Crabtree-negative, non-Saccharomyces yeasts (V, VI, VII, VIII) fermented much more slowly. The amount of ethanol produced ranged from 4.52% v/v for K. marxianus (V) to 6.08% v/v for Saccharomyces cerevisiae Lalvin (IV). The most active strains (I, II, III) finished fermentation after 3–5 days, and the weak fermentative strains finished fermentation after 2–8 weeks (Table 5).
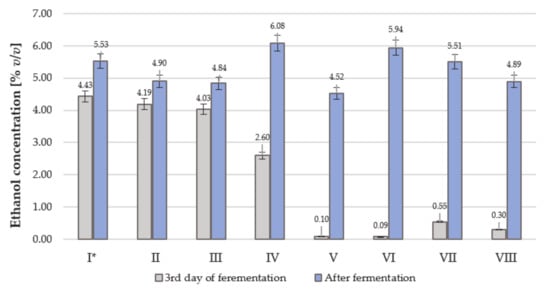
Figure 1.
Ethanol content in the sterilized red beet juice on the 3rd day and at the end of the fermentation process. The end of fermentation was estimated after stabilization of the extract content. At the start of fermentation the ethanol content was 0% v/v. * Symbols of yeast strains: I, II, III, IV, V, VI, VII, VIII—as in Table 1.

Table 5.
Extract reduction after fermentation trials.
The metabolic pathways of the central carbon metabolism are the same in different yeast species. However, several differences have been noted between Crabtree-positive and Crabtree-negative yeast strains, including different kinetics of sugar uptake and rates of glycolysis [56,57,58].
The extract content halved in the juice sample fermented with S. cerevisiae (II) compared to the control, from 15 °Bx in the control (unfermented) sample to 6.18 °Bx. It should be emphasized that the red beet juice had not been additionally supplemented. Red beet juice is known to contain not only nutrients (carbohydrates, proteins, etc.) but also natural stabilizers (saponins, polyphenols, flavonoids, etc.) with a wide range of antimicrobial effects [34,59,60]. However, the fermentation process with the tested yeasts took place even in the presence of these phytochemicals.
3.5. Control of Betalains and Sugar Index
Beetroot is one of the richest sources of betanin pigment, which is what gives its red or yellow color. The redness of beetroot varieties depend on the ratio of betacyanin (red) and betaxanthins (yellow) [34,61]. Betalains are generally used as color additives in food, due to their non-precarious, non-toxic, non-carcinogenic and non-poisonous nature. All betalain fractions have numerous nutritional and health benefits [61,62]. However, temperature, oxygen, and light are known to exhibit detrimental effects on betalain integrity [63]. Betalains are unstable under oxygen atmosphere, and antioxidants such as ascorbic acid offer only slight protection against oxidation in colour formulations. Betalain content has likewise been reported to be inversely related to light intensity. Under anaerobic conditions, the effect of light was found to be negligible [7]. To evaluate the influence of yeast fermentation processes on pigment stability in the raw red beet juice, we first assessed the effect of heat treatment. Various temperatures, from mild (40 °C) to very high (121 °C), were used to evaluate the effect of heating on the betalain content and the proportions of the individual fractions. The constant time 60 min was chosen to investigate the effect of temperature over comparable time. The results are presented in Figure 2. As expected, the betalains were found to be very sensitive to thermal treatment at the highest temperature. In particular, the instability of the red-violet fraction of betalains was inversely related to the temperature values. After incubation at 40 °C, the total betalain (betacyanins and betaxanthins) concentration was almost 10%, but at the highest temperature 121 °C this value decreased to 3.3% (loss 77%). We also observed that the desirable proportion of betacyanins to betaxanthins (about 2) was not maintained after the sterilization process. These results are similar to the literature data [7].
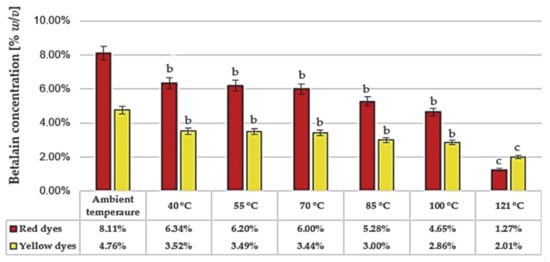
Figure 2.
Betalain content (red and yellow fractions) in raw red beet juice after 60 min of incubation at various temperatures. The results were compared to the control sample—the raw red beet juice. Values with different letters presented in the figures show statistically significant differences: a, p ≥ 0.05; b, 0.005 < p < 0.05; c, p < 0.005.
The betalain contents were also investigated after sterilization and controlled fermentation trials, with well-defined yeast strains representing various fermentation activities. Before inoculation with yeast, the fermentation medium was sterilized (121 °C, 15 min) to avoid uncontrolled microbial processes. The control sample was the raw red beet juice before sterilization and fermentation (Figure 3).
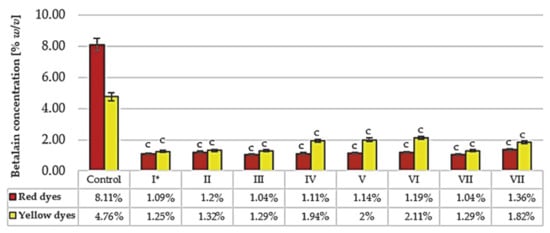
Figure 3.
Betalain content after sterilization (121 °C) and fermentation with selected yeast strains. * Symbols: I, II, III, IV, V, VI, VII, VIII—as in Table 1. The control sample was the raw red beet juice before sterilization and fermentation. The results were compared to the control sample. Values with different letters presented in the figures show statistically significant differences: a, p ≥ 0.05; b, 0.005 < p < 0.05; c, p < 0.005.
The total betalain contents after the fermentation trials varied from 2.34% to 3.29%, and decreased significantly in comparison to the control sample. The loss of the betalain fractions ranged from 75 to 82%. The desired proportion of betacyanins and betaxanthins (about 2) was not maintained [8]. We suppose that the main reason for this change was the sterilization process. The effects of incubation at various temperatures were shown in Figure 2. Yeast fermentation did not improve this negative effect. Similar results have been obtained in other studies after boiling and spontaneous juice fermentation, which resulted in high levels of betalain degradation (61–88%) [15]. The loss of betalains in the fermentation samples also may be due in part to the adsorption of dyes on the yeast cell wall. It is well known that yeast cells have the ability to adsorb various phytochemicals [64,65]. The absorption of the red pigment on the yeast cell wall was also confirmed microscopically, with the cells after fermentation having a distinct violet-red outline (Figure 4A,B).
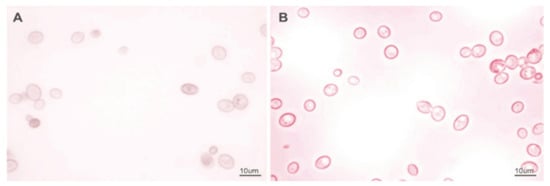
Figure 4.
S. cerevisiae cells under a light microscope. (A) cells suspended in isotonic saline; (B) cells after fermentation in sterilized red beet juice (scaling bar: 10 µm).
Table 6 presents Sugar Index values, representing the effect of yeast metabolic activity on the levels of sugars and betalains in sterilized red beet juice after fermentation. The Sugar Index was calculated as the ratio of the sugar content (which ranged after fermentation from 1.16 to 2.84 g/L) to the betalain concentration. In the case of the post-fermentation media, the results were 9–20-times lower in comparison to the raw red beet juice.

Table 6.
Sugar index values after fermentation of sterilized red beet juice.
The betalain content was also evaluated following fermentation of the raw red beet juice by the yeast strains with the best fermentation activity (I, II, III) (Figure 5).
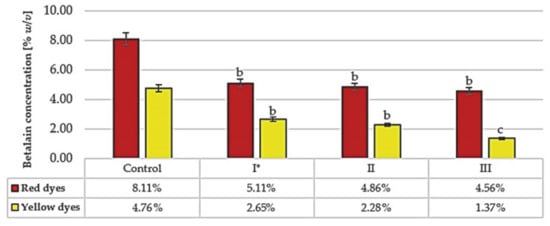
Figure 5.
Betalain content in raw beet juice after fermentation with selected yeast strains (I, II, III). * Symbols of yeast strains: I, II, III—as in Table 1. The control sample was the raw red beet juice before fermentation. The results were compared to the control sample. Values with different letters presented in the figures show statistically significant differences: a, p ≥ 0.05; b, 0.005 < p < 0.05; c, p < 0.005.
After 3–5 days of fermentation trials, the contents of both betalain fractions were strain-dependent and ranged from 5.93% to 7.78%. Therefore, the loss of betalain ranged from 22 to 41%. These values represent much smaller decreases compared to the losses resulting after 60 min of thermal treatment (Figure 2) or after the fermentation of sterilized red beet juice (Figure 3). It is worth noting that a favorable ratio of betacyanins and betaxanthins was maintained after fermentation of the raw red beet juice.
Table 7 presents sugar index values, representing the effect of yeast metabolic activity on the levels of sugars and betalains in the fermented raw red beet juice.

Table 7.
Sugar index values after fermentation of raw red beet juice.
Controlled fermentation with Crabtree-positive yeasts led to a significant improvement in the sugar index values, calculated as the ratio of the sugar content to the betalain concentration. The sugar indexes were reduced significantly and were 25–43-fold lower in comparison to the raw red beet juice. The sugar index values were more favorable than those obtained for sterilized beet juice, in which the sugar indexes reduced only 9–20-times.
4. Conclusions
The consumption of red beetroot products, including juice, is high in nutritional values. However, it is ten times more nutrient-rich than the root, which can be disadvantage due to the high sugar content and high caloric value. High sugar consumption has been shown to contribute to obesity and related lifestyle diseases. One common way to reduce sugar is to replace it with artificial sweeteners. Non-nutritive sweeteners often impart additional taste qualities considered as off-flavors. Instead, we propose a healthy alternative: the biological reduction of sugar content by yeast strains with different metabolic pathways and fermentation activities. Each of the tested species offered different advantages, from fast fermentation (Saccharomyces sp.) to slower ethanol formation (non-Saccharomyces yeasts). The activities of the different yeast species and strains may have a positive impact on the sensory profiles of juice, increasing its complexity and organoleptic richness. Fast fermenting yeasts belonging to S. cerevisiae reduced sugar content while maintaining the amounts of betalain fractions in the raw red beet juice. This strategy, based on the use of Crabtree-positive yeasts in a controlled fermentation process, could contribute to increase the microbiological and chemical stability of raw red beet juice. However, more research is needed on to select a proper strain or consortium of yeast strains for use in the production of low-sugar red beet juice with the desired sensory features.
Author Contributions
Conceptualization, J.B. and D.K.; methodology, P.D.; investigation, D.D., S.N., M.S., data curation, S.N., M.M.-F. and J.O.; writing—original draft preparation, D.D.; writing—review and editing, J.B. and D.K..; visualization, J.B. and M.M.-F.; supervision, D.K.; project administration, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Industrial Doctorate” project implemented at the Faculty of Biotechnology and Food Sciences of Lodz University of Technology, contract No. 40/DW/2017/01/1, financed in the years 2017–2021 by the Ministry of Science and Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The Authors gratefully thank (i) all the partners of this research for contributing to develop the themes in this manuscript and (ii) the Faculty of Biotechnology and Food Sciences of Lodz University of Technology, which contributed to promote this subject in the framework of research activities funded through the Ministry of Science and Education. We would like to thank Vin-Kon S.A., Konin, Poland for its skilled technical support during the realization of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Toydemir, G.; Nur Memis, K.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Rodier, F.; Willenbrink, J. Accumulation of sucrose in vacuoles isolated from red beet tissue. Planta 1979, 144, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Babarykin, D.; Smirnova, G.; Pundinsh, I.; Vasiljeva, S.; Krumina, G.; Agejchenko, V. Red Beet (Beta vulgaris) Impact on Human Health. J. Biosci. Med. 2019, 07, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Singh, A.; Masih, D. Red beetroot: A source of natural colourant and antioxidants: A review. J. Pharmacogn. Phytochem. 2019, 8, 162–166. [Google Scholar]
- Kayın, N.; Atalay, D.; Türken Akçay, T.; Erge, H.S. Color stability and change in bioactive compounds of red beet juice concentrate stored at different temperatures. J. Food Sci. Technol. 2019, 56, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Carle, R.; Schweiggert, R.M. Handbook on Natural Pigments in Food and Beverages, 1st ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Gasztonyi, M.N.; Daood, H.; Hájos, M.T.; Biacs, P. Comparison of red beet (Beta vulgaris var. conditiva) varieties on the basis of their pigment components. Proc. J. Sci. Food Agric. 2001, 81, 932–933. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Vasconcellos, J.; Conte-Junior, C.; Silva, D.; Pierucci, A.P.; Paschoalin, V.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. 2016, 25, 79–84. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Non-Communicable Diseases; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Taxes on Sugary Drinks: Why Do it? WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kyung, Y.Y.; Woodams, E.E.; Hang, Y.D. Fermentation of beet juice by beneficial lactic acid bacteria. LWT Food Sci. Technol. 2005, 38, 73–75. [Google Scholar] [CrossRef]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Jafar, N.B.; Ghaleb, Z.T.; Fadhil, Z.H. Production of fermented red beet juice using probiotic lactobacilli bacteria. Ann. Trop. Med. Public Health 2019, 22, 73–75. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Kregiel, D. Succinate Dehydrogenase of Saccharomyces cerevisiae—The Unique Enzyme of TCA Cycle—Current Knowledge and New Perspectives. In Dehydrogenases, 1st ed.; Canuto, R.A., Ed.; INTECH: Rijeka, Croatia, 2012; pp. 211–234. [Google Scholar] [CrossRef] [Green Version]
- Imura, M.; Nitta, K.; Iwakiri, R.; Matsuda, F.; Shimizu, H.; Fukusaki, E. Comparison of metabolic profiles of yeasts based on the difference of the Crabtree positive and negative. J. Biosci. Bioeng. 2020, 129, 52–58. [Google Scholar] [CrossRef]
- Joshi, V.K.; Sharma, S.; Devi, M.P. Influence of different yeast strains on fermentation behaviour, physico-chemical and sensory qualities of plum wine. Indian J. Nat. Prod. Resour. 2009, 8, 445–451. [Google Scholar]
- Modelska, M.; Berlowska, J.; Kregiel, D.; Cieciura, W.; Antolak, H.; Tomaszewska, J.; Binczarski, M.; Szubiakiewicz, E.; Witonska, I.A. Concept for recycling waste biomass from the sugar industry for chemical and biotechnological purposes. Molecules 2017, 22, 1544. [Google Scholar] [CrossRef] [Green Version]
- Polish Standard PN-90/A-75101/07. Przetwory Owocowe i Warzywne -- Przygotowanie Próbek i Metody Badań Fizykochemicznych. Oznaczanie Zawartości Cukrów i Ekstraktu Bezcukrowego. Available online: https://www.pkn.pl/en/polish-standard (accessed on 9 May 2021). (In Polish).
- The Grain and Feed Trade Association (GAFTA) Method 10.1. Available online: https://www.gafta.com/write/MediaUploads/Contracts/2018/METHOD_10.1_SUGAR_-_LUFF_SCHOORL_METHOD.pdf (accessed on 9 May 2021).
- Chwil, M.; Kostryco, M. Bioactive compounds and antioxidant activity of Rubus idaeus L. leaves. Acta Sci. Pol. Hortorum Cultus 2018, 17, 135–147. [Google Scholar] [CrossRef]
- Ghanbari, R.; Rezaie, S.; Noorbakhsh, F.; Khaniki, G.J.; Soleimani, M.; Aghaee, E.M. Biocontrol effect of Kluyveromyces lactis on aflatoxin expression and production in Aspergillus parasiticus. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Papini, M.; Nookaew, I.; Uhlén, M.; Nielsen, J. Scheffersomyces stipitis: A comparative systems biology study with the Crabtree positive yeast Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2019, 112, 1425–1445. [Google Scholar] [CrossRef] [Green Version]
- Mickelsen, P.A.; McCarthy, L.R.; Propst, M.A. Further modifications of the auxanographic method for identification of yeasts. J. Clin. Microbiol. 1977, 5, 5. [Google Scholar] [CrossRef]
- Šavel, J.; Kosin, P.; Broz, A.; Sigler, K. Convenient Monitoring of Brewery Fermentation Course by Refractometry. Kvas. Prum. 2009, 55, 94–99. [Google Scholar] [CrossRef]
- OIV-MA-BS-06. Density of Alcohols and Alcohlic Beverages Method for Determining Electronic Densimetry (Principle Based on Measuring the Period of Oscillation). Available online: https://www.oiv.int/public/medias/2667/oiv-ma-bs-06.pdf (accessed on 13 June 2021).
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp. vulgaris var. rubra L.). Lantbr. Ann. 1970, 36, 179–219. [Google Scholar]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech. J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Richardson, K. Preliminary evaluation of the leaf and root nutrient composition of a fresh market beetroot variety. J. Plant. Nutr. 2014, 20, 408–420. [Google Scholar]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Dai, Z.; Huang, M.; Chen, Y.; Siewers, V.; Nielsen, J. Global rewiring of cellular metabolism renders Saccharomyces cerevisiae Crabtree negative. Nat. Commun. 2018, 9, 3059. [Google Scholar] [CrossRef] [Green Version]
- Ji Ho, C.; Hwan Yeo, S.; Park, J.-H.; Choi, H.S.; Gang, J.-E.; In Kim, S.; Tae Jeong, S.; Ra Kim, S. Isolation of aromatic yeasts (non-Saccharomyces cerevisiae) from Korean traditional nuruks; and identification of fermentation characteristics. Agric. Sci. 2013, 4, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.A. Biotechnology of non-Saccharomyces yeasts-the basidiomycetes. Appl. Microbiol. Biotechnol. 2013, 97, 7563–7577. [Google Scholar] [CrossRef]
- Lu, G.; Fellman, J.K.; Edwards, C.G.; Mattinson, D.S.; Navazio, J. Quantitative determination of geosmin in red beets (Beta vulgaris L.) using headspace solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1021–1025. [Google Scholar] [CrossRef]
- Su, E.; Xia, T.; Gao, L.; Dai, Q.; Zhang, Z. Immobilization of β-glucosidase and its aroma-increasing effect on tea beverage. Food Bioprod. Process. 2010, 88, 83–89. [Google Scholar] [CrossRef]
- Zhang, X.B.; Du, X.F. Effects of exogenous enzymatic treatment during processing on the sensory quality of summer tieguanyin oolong tea from the Chinese Anxi county. Food Technol. Biotechnol. 2015, 53, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fang, K.; Ni, H.; Li, T.; Li, L.J.; Li, Q.B.; Chen, F. Aroma enhancement of instant green tea infusion using β-glucosidase and β-xylosidase. Food Chem. 2020, 315, 126287. [Google Scholar] [CrossRef] [PubMed]
- Dodor, D.E.; Tabatabai, M.A. Arylamidase activity as an index of nitrogen mineralization in soils. Commun. Soil Sci. Plant. Anal. 2007, 38, 2197–2207. [Google Scholar] [CrossRef]
- Pennacchia, C.; Blaiotta, G.; Pepe, O.; Villani, F. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J. Appl. Microbiol. 2008, 105, 1919–1928. [Google Scholar] [CrossRef]
- Mujdeci, G.N.; Ozbas, Z.Y. Technological and enzymatic characterization of the yeasts isolated from natural fermentation media of Gemlik olives. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, H.D. Isolation and investigation of potential non-Saccharomyces yeasts to improve the volatile terpene compounds in Korean muscat bailey a wine. Microorganisms 2020, 8, 1552. [Google Scholar] [CrossRef]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef]
- Rajkumar, A.S.; Morrissey, J.P. Rational engineering of Kluyveromyces marxianus to create a chassis for the production of aromatic products. Microb. Cell Fact. 2020, 19, 2–7. [Google Scholar] [CrossRef]
- Kregiel, D. Health safety of soft drinks: Contents, containers, and microorganisms. Biomed Res. Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Brattinga, C.; de Kok, P.M.T.; Bult, J.H.F. Sugar reduction in flavoured beverages: The robustness of aroma-induced sweetness enhancement. Proc. Flavour Sci. 2018, 199–206. [Google Scholar] [CrossRef]
- Askew, K. Sugar Reduction through Smell: ‘Aromas Can Be Used to Produce Long-Lasting Sweetness-Enhancing Effects’. Food Navigator. 2020. Available online: https://www.foodnavigator.com/Article/2020/01/21/Sugar-reduction-through-smell-Aromas-can-be-used-to-produce-long-lasting-sweetness-enhancing-effects (accessed on 5 May 2021).
- Pawlikowska, E.; Kolesińska, B.; Nowacka, M.; Kregiel, D. A New Approach to Producing High Yields of Pulcherrimin from Metschnikowia Yeasts. Fermentation 2020, 6, 114. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef] [Green Version]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Kregiel, D. Physiology and metabolism of Crabtree-negative yeast Debaryomyces occidentalis. Food Chem. Biotechnol. 2008, 72, 35–44. [Google Scholar]
- Kregiel, D.; Berlowska, J.; Ambroziak, W. Growth and metabolic activity of conventional and non-conventional yeasts immobilized in foamed alginate. Enzym. Microb. Technol. 2013, 53, 229–234. [Google Scholar] [CrossRef]
- Berłowska, J.; Binczarski, M.; Dziugan, P.; Wilkowska, A.; Kręgiel, D.; Witońska, I. Sugar Beet Pulp as a Source of Valuable Biotechnological Products. Adv. Biotechnol. Food Ind. 2018, 14, 359–392. [Google Scholar] [CrossRef]
- Mroczek, A.; Kapusta, I.; Janda, B.; Janiszowska, W. Triterpene saponin content in the roots of red beet (Beta vulgaris L.) cultivars. J. Agric. Food Chem. 2012, 60, 12387–12402. [Google Scholar] [CrossRef]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Panghal, A.; Virkar, K.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Development of probiotic beetroot drink. Curr. Res. Nutr. Food Sci. 2017, 5. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food. Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Vernhet, A.; Sieczkowski, N.; Brillouet, J.M. Interactions of Condensed Tannins with Saccharomyces cerevisiae Yeast Cells and Cell Walls: Tannin Location by Microscopy. J. Agric. Food Chem. 2015, 63, 7539–7545. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).