Abstract
Preparation of soymilk-based product with probiotics is reasonably a novel approach in the field of fermented functional foods. The aim of this study was to develop riboflavin enriched fermented soy curds with either or combination of the two riboflavin producing probiotic strains of Lactobacillus plantarum i.e., MTCC 25432 (BBC32B) and MTCC 25433 (BBC33), and to compare the technological and functional properties of its developed products. Acidification rate and lactic acid production were enhanced with L. plantarum and its combination in a shorter time to reach pH 4.7. Hardness and cohesiveness were significantly (p < 0.05) higher for fermented soymilk by co-culture of L. plantarum followed by individual strains. Similarly, higher G′ (6.25 × 102 Pa), G” (2.30 × 103 Pa) and G* (8.00 × 102 Pa) values observed for the combination of both L. plantarum strains showed that the gel formed was firmer and had solid character. The riboflavin content of product developed with a combination of test cultures was significantly higher (342.11 µg/L) than individual cultures and control. The final product had a higher probiotic count (more than 9 log cfu/mL), which is also required for functional food containing probiotics.
1. Introduction
The consumption of probiotic-containing beverages and fermented foods deliver health benefits to host, and has now become a growing trend worldwide [1]. Probiotics have established their efficacy in providing beneficial effects in different health-related conditions [2]. Several dairy-based fermented food products have been known to deliver probiotics because of their direct association with lactic fermentation [3]. But, these foods are having the disadvantage of being not suitable for lactose intolerant persons [4]. Hence, there is a need for evaluating the non-dairy food matrices for the delivery of probiotics. Soymilk or soy aqueous extract has been considered as a suitable medium for the development of probiotic-containing fermented functional foods [5,6]. Besides acceptable evaluation as a possible probiotic vehicle, soy-based products have more consideration because of their health-improving functional properties such as hypolipidemic, anti-atherogenic, antioxidant, and anti-allergenic [7,8,9].
Acidification of the foods due to fermentation is an important attribute as it inhibits the growth of some non-essential microorganisms. It is also essential to get good sensory properties such as aroma, flavor, and texture of the final product [10]. Because of this attribute, soymilk has been accepted as a suitable medium for probiotic growth, acidification, and their survival [11]. The protein contents of soy-product enhanced the growth of several probiotic strains such as Lactobacillus acidophilus, Streptococcus thermophilus, and Lactobacillus casei [12]. In 2003, Shimakama [13] first reported the fermented soy-beverages containing probiotic with a good sensory acceptance and health benefit potential. Gel formation of soymilk proteins is considered an important process step for developing non-dairy fermented product. However, very few studies have reported the rheological properties and textural profile of fermented soy products [14,15,16].
For any probiotic organism, the ability of producing the bio-effector molecule such as vitamins is an added advantage. The product developed with such organism will not only help in improving gut health but may also be a nutritional source for mitigation of related deficiencies. Riboflavin (vitamin B2) is an important vitamin which is essential for metabolic functions such as cellular growth, energy production, and redox potential [5]. Adeficiency of riboflavin can cause health issues such as development of ulcers and cataracts, cheilosis, anaemia, hyperhomocysteinemia, and dementia [17]. In a country like India where diets are largely plant based, the deficiency of riboflavin is prevalent in both children and adults [18].
We have previously studied the riboflavin producing potential of a few L. plantarum strains in broth as well as food matrix [19]. The in vitro probiotic potential and safety profile of these strains have also been studied comprehensively [20]. Current study has been undertaken to develop the fermented soycurd using our previously studied probiotic strains; Lactobacillus plantarum MTCC 25433 (BBC33), L. plantarum MTCC 25432 (BBC32B) individually or in combination of both. Rheological characteristics and texture properties of the fermented soyproduct were estimated in correlation with the previous reports. Impact of soymilk medium on the growth of probiotic strains and simultaneously, pH and titrable acidity, were also measured during fermentation. Moreover, the functional attributes (antioxidant, antimicrobial, and riboflavin production) were also estimated for the developed fermented soycurds.
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
The strains used in this study, L. rhamnosus GG, L. plantarumMTCC 25433 (BBC33) and L. plantarumMTCC 25432 (BBC32B) were obtained from the microbiology laboratory, National Institute of Food Technology Entrepreneurship and Management (NIFTEM), Kundli, India. All the strains were subcultured thrice in De Man, Rogosa, and Sharpe (MRS) broth (HiMedia laboratories, Mumbai, India) for biological activation followed by incubation at 37 °C for 24 h and then activated strains were used to ferment the soymilk. Preliminary experiments were conducted to decide the concentration of inoculums. Simultaneously, stocks of the cultures were prepared using sterile glycerol (60%) with final 1:1 ratio and stored at −80 °C. Nutrient broth (NB) and nutrient agar (NA) (HiMedia laboratories, Mumbai, India) were used for the cultivation of pathogen indicators (Staphylococcus aureus ATCC 6538, Enterococcus faecalis ATCC 14506, Escherichia coli ATCC 11775 obtained from American Type Culture Collection (ATCC), Manassas, VA, USA and Pseudomonas aeruginosaATCC 27853, Salmonella entericaATCC 13076 and Klebsiella pneumoniae ATCC 13883 obtained from Microbiology lab, NIFTEM, Kundli, India).
2.2. Preparation and Fermentation of Soymilk
Preparation of soymilk was done as per the standard method reported by Nelson [21] with some modifications. Briefly, soybeans (250 g) were soaked in distilled water and kept at room temperature for 12 h. Then, water was drained off from the soybeans and hydrated beans were peeled off manually to remove their testa. Beans were peeled and then placed in a mixer grinder (Bajaj Electronics, New Delhi, India) and grinded for 5 min with 500 mL of distilled water. Further, slurry was filtered using two layers of muslin cloth and final volume was set at 1000 mL with distilled water. Obtained soymilk was sterilized by autoclaving for 15 min and fermentation was done separately using 1% overnight activated inoculum of the strains GG (control), MTCC 25432, MTCC 25433, and a combination of MTCC 25432 and MTCC 25433 at 37 °C for 12 h. The soy curd developed after fermentation was transferred to refrigerator (4 °C) for further analysis.
2.3. Growth Rate Evaluation of Strains in Soymilk
Growth rate study of the probiotic strains used for fermentation was done to investigate their growth pattern in soymilk. In brief, 5 mL of soymilk was inoculated (at 1%) with tested strain and 500 µL of sample was collected at different time points up to 24 h for plating on MRS agar. 10th fold dilution (0.5 mL sample and 4.5 mL autoclaved phosphate buffer saline (PBS)) followed by serial dilution of the sample was performed to get the countable colonies on plate and then spread plating was done using 100 µL of diluted sample [22]. A threshold growth difference (Δlogcfu≥ 1) after 6 h was considered as the successful growing capability of the strains in soymilk. Growth rate (Δlogcfu) in soymilk was calculated by the following formula;
where T1 was growth at particular hour and T0 was growth at 0 h.
Δlogcfu = log cfu T1− log cfuT0
2.4. pH and Titratable Acidity Estimation
The pH and titratable acidity of the soycurd fermented with different probiotic strains was done to the exact pH and lactic acid (LA) content of the samples. The pH of soycurd was measured using pH meter (EUTECH Instruments, Mumbai, India) from 0 to 24 h and at 7, 14, and 28th day of storage. Similarly, acidity of the samples was determined using standardized method of Gatade [23] at same time points and days of storage. Briefly, the coagulum was broken by stirring and 2 mL sample of the fermented soycurd was taken in a 50 mL beaker and equal volume of distilled water was added. A few drops of phenolphthalein indicator were then added to the samples and titration was performed against 0.1N NaOH until light pink color appeared. The volume of NaOH consumed to change the color of soymilk was noted and % lactic acid (LA) was calculated using the formula; %LA = 90 × N × titer value × 100/sample volume × 1000.
2.5. Rheological Measurements
Rheological measurements of the fermented soycurdwere investigated to monitor the viscoelastic and gelation properties using a standard method of Ferragut [24] with some modifications. Briefly, the rheological properties of samples were monitored with Anton PaarRheometer (Model: MCR 302, Enzesfeld-Lindabrunn, Austria) using a parallel plate arrangement with 2.5-mm gap at 20 °C temperature. After moderate stirring, a small sample of fermented soycurd was deposited on the middle of inset plate. First, amplitude sweep test was performed using a frequency of 1 Hz at ascertain viscoelastic range (0.01 to 100 Pa) and then frequency sweep test (0.1 to 10 Hz at maximum strain of 0.06) was performed. The data obtained for rheological measurements were analyzed using instrument software. The dynamic moduli (G′, G″ and G*) and eta (η) values were also calculated from frequency sweep test.
2.6. Texture Profile Analysis (TPA)
TPA of the fermented soycurd was measured using texture analyzer (TA.XT2i, Stable Microsystems, Godalming, UK) equipped with 25-kg load cell at 20 °C. To calculate the most texture profiles after analysis, profile curves were used to calculate hardness, gumminess, adhesiveness, cohesiveness, and springiness of the samples [25].
2.7. Riboflavin Extraction from Fermented Soycurd
Riboflavin extraction from fermented soycurd was done using the method reported by Del-Valle [26] with slight modification. Briefly, 5 mL of fermented soymilk was mixed with equal volume of acetic acid (1%), autoclaved at 121 °C for 30 min, and centrifuged at 10,000× g for 15 min. Supernatant was collected, filtered using 0.22 µm filters (Merck, Germany), and kept at −20 °C until riboflavin was estimated using high-performance liquid chromatography (HPLC). Complete extraction procedure was performed in dark to avoid the light exposure of riboflavin present in the sample.
2.8. Riboflavin Quantification In Fermented Soycurd
Chromatographic analysis of the supernatant obtained from fermented soycurd was performed by Waters HPLC (Model 2707, Waters India Pvt. Ltd., Kolkata, India) having reverse-phase C18 column (Kromasil, SIGMA, St. Louis, MO, USA, 5μ 100A, 250 × 4.6 mm), an in-line degasser, auto-sampler, binary 515 pump control (module II) and fluorescence detector with excitation wavelength 440 nm and emission wavelength 520 nm) to estimate riboflavin. Analysis of the sample was done by isocratic elution with flow rate of 1 mL/min using a freshly prepared mobile phase of methanol/water (35:65 v/v) mixture [27]. Pure riboflavin (Sigma, USA) was used as control.
2.9. DPPH Scavenging Activity
The antioxidant activity of the fermented soycurd was analyzed by the method reported by Bhushan [20] with slight modifications. Briefly, 250 μL supernatant obtained from fermented soycurd was mixed to equal amount of DPPH (0.1 mM) working solution in an amber color Eppendorf tube and incubated at 37 °C for 30 min. After incubation, the absorbance of the solution was measured at 515 nm using SICAN 2301 Double Beam Spectrophotometer (Inkarp Instruments Pvt. Ltd., Hyderabad, India) against supernatant obtained from unfermented soycurd as blank. The following formula was used to calculate the antioxidative activity of the samples:
DPPH scavenging (%) = (A515 nm blank − A515 nm sample/A515 nm blank) × 100.
2.10. Antibacterial Activity
Antibacterial activity of the fermented soycurd was measured using agar well diffusion assay reported by Schillinger [28]. In brief, hard Nutrient Agar (1.5%) plates were prepared and allowed to solidify at room temperature. 100 μL aliquot of the overnight activated test pathogen was mixed in soft Nutrient Agar (0.7% agar) and poured on pre-solidified hard Nutrient Agar. Further, inoculated plates were allowed to dry at room temperature and equidistant wells (5 mm) were punched using sterile glass borer. Wells were filled with 100 μL of supernatant obtained from fermented soycurd and unfermented soycurd as control. The plates were first incubated at 4 °C for 1 h to get diffusion of the sample, followed by overnight incubation at 37 °C. The antibacterial activity was recorded by measuring the zone of inhibition (in mm).
2.11. Sensory Evaluation of Fermented Soycurd
Sensory evaluation of the soycurd fermented with different probiotic strain (s) was done using fuzzy logics with 15 numbers of judges of faculty and students aged between 23 to 50 years of NIFTEM, Kundli, India. members were instructed to use fuzzy logic scale (1 = not satisfactory, 2 = fair, 3 = good, 4 = very good, and 5 = excellent)to evaluate the acceptability of sensory attributes such as color, aroma, taste, and mouth feel and they were asked to give their response after tasting fermented soycurd and give score to each sample as per their feel. All judges cleaned their oral cavity with mineral water before each serving and were fastened for at least 3 h before evaluation. The key steps of this evaluation study were; (i) testing of all the samples in triplicate; (ii) sum of the sensory scores of every sample; (iii) computation of overall function on the standard fuzzy scale and (iv) estimation of the same values and scoring of the samples. The data analysis was done in MATLAB by the standard method reported by Chowdhury and Das [29].
2.12. Statistical Analysis
All the values were expressed as means ± SD and statistical analyses were analyzed by GraphPad Prism (version 5.01) (La Jolla, CA, USA). Differences between the means were tested for statistical significance using analysis of variance (ANOVA) followed by Tukey’s post-hoc test. All of the discussed experiments were conducted in triplicate and differences were considered statistically significant at p < 0.05 to p < 0.0001.
3. Results and Discussion
3.1. Probiotic Growth Pattern after Soymilk Fermentation
During growth evaluation, aseptic conditions were maintained during incubation and no unwanted growth was observed in soy samples plated on NA plate. Probiotic strains L. plantarum MTCC 25432 (BBC32B), L. plantarum MTCC 25433 (BBC33) and combination of MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) showed significantly higher (p < 0.0001) log CFU/mL in comparison to control L. rhamnosus GG. The outcomes of Del-Valle [5] and Bhushan [26] were also on the same line, where lactobacilli exhibited different growth patterns during soymilk fermentation. A constant increase in the mean log CFU values was noticed till 12th h and then no such growth was recorded (Figure 1). Similar data were reported by Del-Valle [26] when soymilk was fermented with lactobacilli for vitamin enrichment.
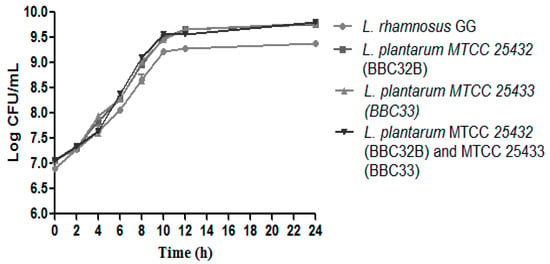
Figure 1.
Growth pattern evaluation: A higher growth pattern of the probiotic strains L. plantarum MTCC 25432 (BBC32B), L. plantarum MTCC 25433 (BBC33) and combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) in comparison to control L. rhamnosus GG in term of log CFU/mL. The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.0001).
3.2. pH and Titratable Acidity Estimation
Acidification of the food material due to low pH and high LA production upon fermentation is a common but important process [30]. In support of this statement, probiotic strains were found to be grown well in soymilk and marked acidification seen after 4 h of fermentation. At the 12th hour of fermentation, a significant decrease in initial pH (6.6) of soymilk was measured with fermentation of L. plantarum MTCC 25433 (BBC33) (6.0), L. plantarum MTCC 25432 (BBC32B) (5.9), and a combination of L. plantarum MTCC 25432 (BBC32B) and L. plantarum MTCC 25433 (BBC33) (6.1). Less reduction in pH was noticed for the soymilk fermented with L. rhamnosus GG (6.3) strain in comparison to other strains (Figure 2A). The stability in pH was found after 24 h of fermentation (Figure 2B). As anticipated, the continual increase in % LA production was recorded upto 12 h (Figure 2C), which was later on found stable upto 7th, 14th, and 28thday of storage (Figure 2D), and depicted the long-time shelf life of product.
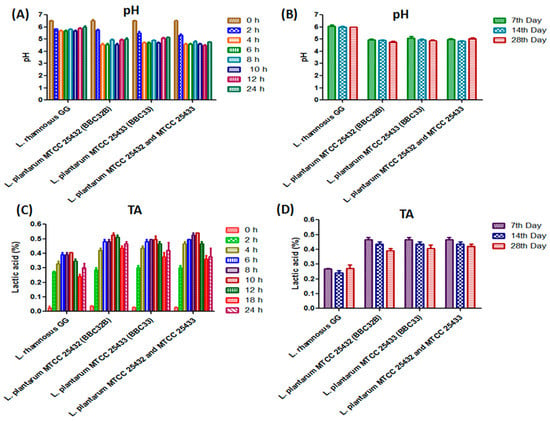
Figure 2.
Lactic acid production and pH stability; A sudden fall in pH of the soymilk fermented with L. plantarum MTCC 25433 (BBC33), L. plantarum MTCC 25432 (BBC32B) and combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) as compared to L. rhamnosus GG after 4th h (A). Almost equal pH stability up to 28 days after fermentation (B). A continues increase in lactic acid production in the soymilk fermented with L. plantarumMTCC 25433 (BBC33), L. plantarumMTCC 25432 (BBC32B) and combination of L. plantarumMTCC 25432 (BBC32B) and MTCC 25433 (BBC33) as compared to L. rhamnosus GG after 2nd h to 10th h of fermentation (C) and then a stable lactic acid production upto 28 days (D). The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.0001).
3.3. Rheological Properties
The rheological properties of the fermented soycurd samples were investigated using oscillatory testing in correlation with unfermented soycurd as control as shown in Table 1 and Table 2. Frequency sweep tests indicated the viscoelastic characteristics of fermented soycurd. Where storage modulus (G′) and loss modulus (G″) characterize the degree of solid/elasticity and liquid/viscous nature of the sample respectively. Starter cultures to ferment soymilk exhibited a fine gel-like behavior, therefore G′ found higher than G″ which was directly supported by the finding of Donkor [15]. High G′ value and low angular frequency values were noticed indicating the semi-solid character of every sample. The Bifidobacterium and Lactobacillus are best known to alter the rheological measurements of milk after fermentation [31]. A significant correlation between low-temperature storage and these microorganisms were found in the study of Kristo and Tamime [32,33] which showed a significant (p < 0.05) effect on the rheological parameters of supplemented soy yoghurt. Eta (η) values among all the pure culture were found higher for L. rhamnosus GG (23902 Pa) followed by L. plantarum MTCC 25432 (BBC32B) (2710.5), L. plantarum MTCC 25433 (BBC33) (1963.8 Pa) and a combination of both L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) (967.34 Pa). G′ and G″ values of the soycurd fermented by L. plantarum MTCC 25432 (BBC32B) were found to be higher as compared to the soycurd fermented with other cultures as shown in Table 1 and the exact same angular frequency was noticed for all samples. Similar to the frequency sweep test, stress sweep test also exhibited the variations in the parameters of the samples as shown in Table 2. Probiotic strain L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) in combination showed higher shear rate (2.56), shear stress (10), viscosity (3.91), speed (2.45), and torque (245) in comparison to other probiotic strains (L. rhamnosus GG, L. plantarum MTCC 25432 (BBC32B) and L. plantarum MTCC 25433 (BBC33)) which showed nearly equal stress sweep parameters. Some variations in the rheological measurements attributed the different magnitude of force applied in the mechanical evaluation. Therefore, G* obtained for all samples exhibited structure network which was not broken (Figure 3A–D). Similar outcomes were observed when higher interactions of the strains upon fermentation of samples in comparison to control [24]. Increased viscosity due to combination of inoculum strains has also been attributed to significant rheological properties of the samples [34,35] which also favor our finding.

Table 1.
Rheological properties; Frequency sweep test indicated the eta (η) values among the pure cultures of L. rhamnosus GG (23902 Pa) followed by L. plantarum MTCC 25432 (BBC32B) (2710.5), L. plantarum MTCC 25433 (BBC33) (1963.8 Pa) and combination of both L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) (967.34 Pa) which indicated the probiotic acceptability of the strains based on the rheology. The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.0001).

Table 2.
Rheological properties; Stress sweep test indicated the torque values among the pure cultures of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) (245) combination followed by L. rhamnosus GG (236), L. plantarum MTCC 25433 (BBC33) (214) and L. plantarumMTCC 25432 (BBC32B) (213) which indicated the probiotic acceptability of the strains based on the rheology. The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.0001).
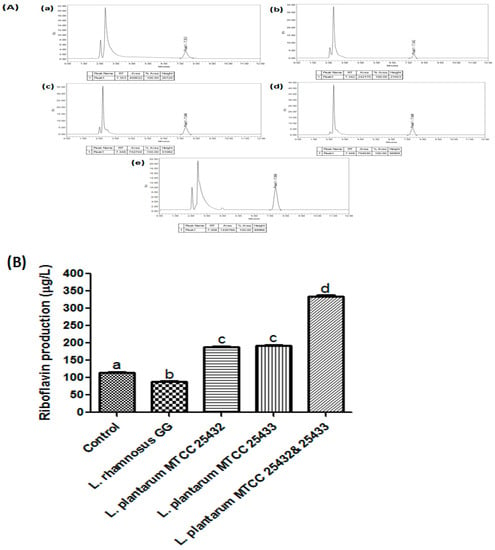
Figure 3.
Riboflavin estimation; (A), Chromatograms obtained after HPLC analysis of the unfermented soymilk (a) and the soymilk fermented with L. rhamnosus GG (b), L. plantarum MTCC 25432 (BBC32B) (c), L. plantarumMTCC 25433 (BBC33) (d), and a combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) (e). (B), Significantly highest riboflavin production in the soymilk fermented with a combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) strains in comparison to other strain of L. plantarum MTCC 25432 (BBC32B) and L. plantarum MTCC 25433 (BBC33) in relation of riboflavin level in the soycurd fermented with L. rhamnosus GG and unfermented soymilk. The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.0001) among all the pure cultures.
3.4. Textural Characteristics
The results of texture profile analysis exhibited that starter cultures of probiotic strains and their combination marked some crucial effects on the textural characteristics of fermented soycurd in comparison to unfermented soymilk as control as shown in Table 3. A special parameter for the assessment of textural measurement of soymilk “hardness” is essential to determine the force compression [36]. The hardness (in grams) of the soycurd ranged from 99.975 to 109.559. It may depend on the varying a single culture as well as combinations of the cultures which mimic the action of jaw compression in comparison to control soymilk (85.25). It has been reported previously that exopolysaccharide (EPS) produced by probiotic strain bacteria increased water retention, viscosity, and interaction of other components of soymilk which resulted in enhanced hardness of protein matrix in the final product [37,38]. Likely, a combination of probiotic strains L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) in this study showed significantly higher (109.559 g) hardness in comparison to individual probiotic strains. The hardness (99.995 g) of the sample fermented by L. plantarum MTCC25432 (BBC32B) was comparable to control unfermented soymilk. The increased hardness of two strains in combination may be attributed to molecular crosstalk between these that resulted into production of some hardening bioactive components, and resultantly improved the texture of the final product. Noticeably, the strain MTCC 25433 (BBC33) was earlier reported for exopolysaccharide production [20]. Other mechanisms responsible for gumminess in fermented soy-product reported as isoelectric point of soy protein similar to casein protein in milk [39]. Glycinin protein of soymilk has been accepted as a hexameric protein consisting five different subunits and each subunit containing acidic and basic chains linked with disulfide bond [40] which resulted in a tight and rigid structure during fermentation [39]. Therefore, higher gumminess was found in the soycurd fermented with probiotic strains in comparison to control (unfermented soymilk) (Table 3). The increased viscosity was found to be related to the improvement of texture which makes soyyoghurt susceptible to rearrangements its network, shrinkage, and whey expulsion [41,42]. Texture measurements including adhesiveness, cohesiveness, and springiness are also important for yoghurt structure [43]. Therefore, all above mentioned parameters were measured for the soycurd samples fermented with different probiotic strains and combination, which showed a significant correlation in the outcomes. Average cohesiveness values (0.03 to 0.09) were recorded after fermentation of soymilk to control unfermented soymilk (0.023). Adhesiveness is defined as the negative force required to remove the material adhered to the mouth during the first bite and represented the work (gram second) necessary to pull the plunger away from the sample. The adhesiveness of all the samples ranged from −4 to −51 gs indicated the significant adhesive value of the fermented soymilk in comparison to control unfermented soymilk (−63.52). These lower values are similar and well acceptable among the consumers as the values reported by [44]. Similarly, springiness was observed in the range from 0.04 to 0.5 for different samples also determined the significantly and accepted product as structure reforming product in comparison to control unfermented soymilk (0.035).

Table 3.
Textural analysis characteristics; Texture analysis parameters (Hardness, cohesiveness, adhesiveness and gumminess) of the soycurd samples fermented with different probiotic strains and combination, showed a significant correlation of the pure cultures of L. rhamnosus GG, L. plantarum MTCC 25432 (BBC32B), L. plantarum MTCC 25433 (BBC33) and combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 which indicated the probiotic acceptability of these strains. The values are expressed as mean ± SEM of three independent experiments with SD and significant difference (p < 0.0001).
3.5. Riboflavin Estimation in Fermented Soycurd
Riboflavin estimation after bio-fortification by a different probiotic strain of the soycurd was done by HPLC and a significant difference (p < 0.0001) was noticed in riboflavin production. The total riboflavin present in the samples was estimated using the peak values obtained from the chromatogram obtained after HPLC analysis (Figure 3A). We recorded the highest riboflavin production (342.11 µg/L) in the soymilk fermented with a combination of L. plantarumMTCC 25432 (BBC32B) and MTCC 25433 (BBC33) strains, in comparison to either of the strain, L. plantarumMTCC 25432 (BBC32B) (190.34 µg/L) and L. plantarum MTCC 25433 (BBC33) (L195.25 µg/L). Interestingly, riboflavin level in the soycurd fermented with L. rhamnosus GG was reduced from 119.64 µg/L (unfermented control) to 87.89 µg/L (post-fermentation) (Figure 3B). Similar observations have earlier been reported by Capozzi [45]. Previously, some studies have reported the fortification of soymilk, but with low riboflavin levels [27]. Recently, Yepez [46] reported that fermentation with L. plantrumCL725 doubled the riboflavin concentration of soymilk after 12 h incubation at 37 °C. Another in situ fortification approach, using probiotic fermentation of whole oat grains was reported using four probiotic strains of L. plantarum that improved the nutritional values of fermented product. Depending on previously published and current work, we claim that Lactobacillus strains can efficiently be used for the riboflavin bio-enrichment of soymilk.
3.6. Antioxidant Property
Oxidative free radicals are the major cause of oxidative stress and mainly generated by environmental factors and internal metabolism. These free radicals may lead to several life-threatening chronic diseases, carcinogenesis, and cellular (DNA) damage [47,48]. Probiotics strains were reported to produce a potential and emerging supply of antioxidants which has been evaluated by testing using DPPH [20,49]. With this support, antioxidative potential of the soycurd fermented either with the strain L. rhamnosus GG, L. plantarumMTCC 25432 (BBC32B), L. plantarumMTCC 25433 (BBC33) and L. plantarumMTCC 25432 (BBC32B), and MTCC 25433 (BBC33) in combination was estimated with respect to ascorbic acid as a positive control. The significantly higher (p < 0.05) scavenging activity was recorded for fermented soycurd in comparison to control unfermented soymilk (Figure 4) with all the tested strains. However, the samples (supernatant of fermented soymilk) showed the highest antioxidant potential. As supported with the previous report on a folate-producing Lactobacillus strain, cell-free supernatant of probiotic strains exhibited significantly (p < 0.0001) higher scavenging activity [50]. Recently, Levit and his team [51] reported L. plantarum strain as higher riboflavin producing strains and its derivative form (FAD) showed oxidation-quenching properties [52] which could be considered as a probiotic strain for future in vivo trials on riboflavin deficiency.
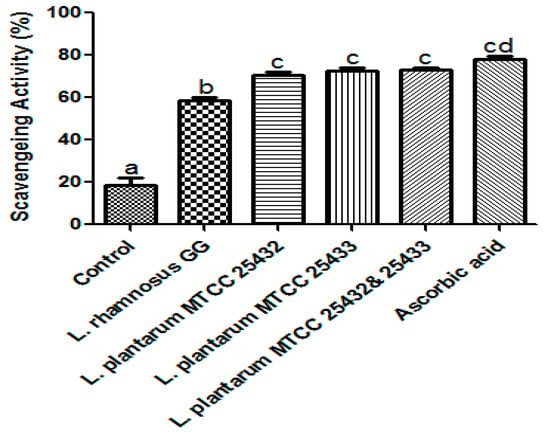
Figure 4.
Antioxidant attribute; Significantly higher antioxidative potential of the soycurd fermented with the strain L. rhamnosus GG, L. plantarum MTCC 25432 (BBC32B), L. plantarum MTCC 25433 (BBC33) and L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) combination in relation to ascorbic acid as a positive control and unfermented soymilk as negative control. The values are expressed as mean ± SEM of three independent experiments with significant difference (p < 0.05).
3.7. Antibacterial Activities
Inhibition of the growth of pathogenic strains may occur due to the direct involvement of antimicrobial agents and/or organic acids produced during fermentation [53]. Therefore, antimicrobial bioactive peptides were identified after LC-MS/MS analysis of the soymilk fermented with Lactobacillus plantarum C2 which indicated the production of antimicrobial peptides produced from soymilk protein after fermentation [54]. On the same line, antibacterial activity of the fermented soycurd exhibited the growth inhibition of tested pathogenic indicators in correlation with unfermented soymilk as control (Table 4). The highest antibacterial activity was noticed with the soymilk fermented by L. plantarum MTCC 25432 (BBC32B) followed by L. plantarum BBCC and combination of L. plantarum MTCC 25432 (BBC32B) and MTCC 25433 (BBC33) against Staphylococcus aureus ATCC 6538, Enterococcus faecalis ATCC 14506, Escherichia coli ATCC 11775, Pseudomonas aeruginosa ATCC 27853, Salmonella enterica ATCC 13076 and Klebsiella pneumoniae ATCC 13883 in comparison to control (unfermented soymilk). While, strain L. rhamnosus GG did not show any antimicrobial effect against any pathogen, owing to its low potential of soymilk fermentation reported earlier in growth experiments (Table 4). Similarly, some previous studies support our outcomes where L. plantarum strains were able to produce some antimicrobial compounds [55]. Mishra and coworkers [56] reported the antibacterial activity of flavored fermented soymilk against pathogenic strains E. coli, B. subtilis, L. monocytogenes, S. typhi, and S. aureus, while unfermented soymilk control did not show any inhibition. Antimicrobial activities of the cell-free supernatants of fermented soymilks have also been reported by Hati [57] against B. subtilis and E. coli strains upto three days of storage.

Table 4.
Size of zone of inhibition (mm)
3.8. Sensory Evaluation
The response sheets from fifteen panelists summed up to get the outcomes of sensory scores for quality attributes of the soycurd fermented with different probiotic strains. The sensory quality attributes of fermented soycurd were ranked as 1, 2, 3, 4 and 5 for “not satisfactory,” “fair,” “good,” “very good,” and “excellent” respectively. As per the sensory scores calculated by fuzzy logic, the highest values were recorded under the “good” and “very good” category as shown in Table 5. This implied the overall quality of the fermented soymilk samples. The order of the samples was sequenced as followed; soymilk fermented with L. plantarum MTCC 25432 (BBC32B) (excellent) <L. plantarum MTCC 25433 (BBC33) (very good) <L. plantarum MTCC 25432 (BBC32B) and L. plantarum MTCC 25433 (BBC33) (very good) <L. rhamnosus GG (satisfactory). A previous report on litchi juice quality attributes support our outcomes where quality of litchi juice was selected and calculated based on the fuzzy logic and overall scores for quality attributes of litchi juice were noticed as followed; taste > color > aroma >mouthfeel [58]. Similarly, in 2017, Vranac [59] reported the sensory evaluation score of apple juice.

Table 5.
Sensory scores of the soycurd produced by different probiotics.
4. Conclusions
It is important that some non-dairy mediums, which support the growth and survival of cultures, are explored for the delivery of probiotic organisms. Based on the outcomes from the current study, we infer that selected strains of L. plantarum were found to be suitable for the fermentation of soymilk and to produce some essential biofunctional component such as riboflavin during fermentation. The functional activities like antibacterial and antioxidative activities of fermented soycurd may add value to product and these could be due to production and cumulative effect of specific bioactive components in comparison to unfermented soymilk. Therefore, these selected strains of L. plantarum may be used for the development of health beneficial fermented soymilk product. The best possible conditions for development of functional soy-product were researched that maybe followed for future development of such a product on a pilot scale. The survivability of the probiotic lactobacilli during fermentation and acceptable textural and rheological properties showed that soymilk, which otherwise be considered an unusual medium for lactobacilli growth, may potentially be used to develop a fermented functional soy product.
Author Contributions
Data curation, K.S.N., S.G., V.S. and A.K.; Funding acquisition, V.M.; Methodology, K.S.N. and A.K.; Project administration, B.B.; Supervision, V.M.; Writing—original draft, K.S.N. and V.S.; Writing—review & editing, B.B. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Science and Technology, India [grant # DST/INT/South Africa/P-15/2016] under Indo-South African collaboration.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Support of Rishi Bhatia in Microbiology lab and Naveen Kumar in Food Engineering lab is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verbeke, W. Consumer acceptance of functional foods: Sociodemographic cognitive and attitudinal determinants. Food Qual. Prefer. 2005, 16, 45–57. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3636. [Google Scholar] [CrossRef]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy v/s non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Bhushan, B.; Tomar, S.K.; Chauhan, A. Techno-functional differentiation of two vitamin B12 producing Lactobacillus plantarum strains: An elucidation for diverse future use. Appl. Microbiol. Biotechnol. 2017, 101, 697–709. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M.; Akiyama, M. Flavonoids as anticancer agents: Structure-activity relationship study. Current Medicinal Chemistry. Anti-Cancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Gasmalla, M.A.A.; Tessema, H.A.; Salaheldin, A.; Alahmad, K.; Hassanin, H.A.M.; Aboshora, W. Health benefits of milk and functional dairy products. MOJ Food Process. Technol. 2017, 4, 108–111. [Google Scholar]
- Singh, B.P.; Bhushan, B.; Vij, S. Antioxidative, ACE inhibitory and antibacterial activities of soymilk fermented by indigenous strains of lactobacilli. Legume Sci. 2020. [Google Scholar] [CrossRef]
- De-Vuyst, L. Technology aspects related to the application of functional starter cultures, application of functional starter cultures. Food Technol. Biotechnol. 2000, 38, 105–112. [Google Scholar]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Probiotic strains as starter cultures improve angiotensin converting enzyme inhibitory activity in soy yoghurt. J. Food Sci. 2005, 70, 375–381. [Google Scholar] [CrossRef]
- Champagne, C.P.; Green-Johson, J.; Raymond, Y.; Barrete, J.; Buckley, N. Selection of probiotic bacteria for the fermentation of a soy beverage in combination with Streptococcus thermophilus. Food Res. Int. 2009, 42, 612–621. [Google Scholar] [CrossRef]
- Shimakama, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacteriumbreves strain Yakult fermented soymilk as a probiotic food. Int. J. Food Microbiol. 2003, 81, 131–136. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Impact of gelation conditions and structural breakdown on the physical and sensory properties of stirred yogurts. J. Dairy Sci. 2006, 89, 2374–2385. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Rheological properties and sensory characteristics of set-type soy yogurt. J. Agric. Food Chem. 2007, 55, 9868–9876. [Google Scholar] [CrossRef]
- Cayot, P.; Schenker, F.; Houze, G.; Sulmont-Rosse, C.; Colas, B. Creaminess in relation to consistency and particle size in stirred fat-free yogurt. Int. Dairy J. 2008, 18, 303–311. [Google Scholar] [CrossRef]
- Pinto, J.T.; Rivlin, R.S. Riboflvin (Vitamin B2). Handbook of Vitamins, 5th ed.; Cancer Metabolism, Glutamine Usage, Ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Singla, R.; Garg, A.; Surana, V.; Aggarwal, S.; Gupta, G.; Singla, S. Vitamin B12 deficiency is endemic in Indian population: A perspective from North India. Indian J. Endocrinol. Metab. 2019, 23, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Kumkum, C.R.; Kumari, M.; Ahire, J.J.; Dicks, L.M.T.; Mishra, V. Soymilk bio-enrichment by indigenously isolated riboflavin-producing strains of Lactobacillus plantarum. LWTFood Sci. Technol. 2020, 119, 108871. [Google Scholar] [CrossRef]
- Bhushan, B.; Sakhare, S.M.; Narayan, K.S.; Kumari, M.; Mishra, V.; Dicks, L.M.T. Characterization of riboflavin-producing strains of Lactobacillus plantarum as potential probiotic candidate through in vitro assessment and Principal Component Analysis. Probiotics Antimicrob. Proteins 2020. [Google Scholar] [CrossRef]
- Nelson, A.I.; Steinberg, M.P.; Wei, L.S. Illinois process for preparation of soymilk. J. Food Sci. 1976, 41, 57–61. [Google Scholar] [CrossRef]
- Dave, R.I.; Shah, N.P. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J. Dairy Sci. 1996, 79, 1529–1536. [Google Scholar] [CrossRef]
- Gatade, A.A.; Ranveer, R.C.; Sahoo, A.K. Effect of treatments, cmc and storage conditions on sensorial quality of mango flavored soymilk. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 6–9. [Google Scholar]
- Ferragut, V.; Criz, N.S.; Trujillo, A.; Guamis, B.; Capellas, M. Physical characteristics during storage of soy yogurt made from ultra high-pressure homogenized soy milk. J. Food Eng. 2009, 92, 63–69. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, H.N. Mango soy fortified set yoghurt: Effect of stabilizer addition on physicochemical, sensory and textural properties. Food Chem. 2004, 87, 501–507. [Google Scholar] [CrossRef]
- Del-Valle, M.J.; Laino, J.E.; de-Giori, G.S.; LeBlanc, J.G. Factors stimulating riboflavin produced by Lactobacillus plantarum CRL 725 grown in a semi-defined medium. J. Basic Microbiol. 2016. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Duenas, M.T.; Lopez, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U. Antimicrobial activity of L. sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef]
- Chowdhury, T.; Das, M. Sensory evaluation of aromatic foods packed in developed starch based films using fuzzy logic. Int. J. Food Stud. 2015, 4, 29–48. [Google Scholar] [CrossRef]
- Rhee, S.K.; Pack, M.Y. Effect of environmental pH on fermentation balance of Lactobacillus bulgaricus. J. Bacteriol. 1980, 144, 217–221. [Google Scholar] [CrossRef]
- Ozturkoglu-Budak, S.; Akal, H.C.; Buran, I.; Yetişemiyen, A. Effect of inulin polymerization degree on various properties of synbiotic fermented milk including Lactobacillus acidophilus La-5 and Bifidobacteriumanimalis Bb-12. J. Dairy Sci. 2019, 102, 6901–6913. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G.; Tzanetakis, N. Modelling of rheological, microbiological and acidification properties of a fermented milk product containing a probiotic strain of Lactobacillus paracasei. Int. Dairy J. 2013, 13, 517–528. [Google Scholar] [CrossRef]
- Tamime, Y. Probiotic Dairy Products; Blackwell: Oxford, UK, 2005; Volume 1. [Google Scholar]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996; pp. 295–349. [Google Scholar]
- Sodini, I.; Lucas, A.; Oliveira, N.M.; Remeuf, F.; Codrrieu, G. Effect of milk base and starter culture on acidification, texture, and probiotic cell counts in fermented milk processing. J. Dairy Sci. 2002, 85, 2479–2488. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesnaik, A.S. The texturometer-a new instrument for objective texture measurement. J. Food Sci. 1963, 28, 390. [Google Scholar] [CrossRef]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Welman, D.A.; Maddox, S.I. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Yang, M.; Li, L. Physicochemical, textural and sensory characteristics of probiotic soy yogurt prepared from germinated soybean characteristics of probiotic soy yogurt. Food Technol. Biotechnol. 2010, 48, 490–496. [Google Scholar]
- Adachi, M.; Kanamori, J.; Masuda, T.; Yagasaki, K.; Kitamura, K.; Mikami, B.; Utsumi, S. Crystal structure of soybean 11S globulin: Glycinin A3B4 homohexamer. Proc. Natl. Acad. Sci. USA 2003, 100, 7395–7400. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: Comparative study of the utilization of barley beta-glucan, guar gum and inulin. Int. J. Food Sci. Technol. 2008, 43, 824–833. [Google Scholar] [CrossRef]
- Oliveira, S.P.R.; Florence, R.C.A.; Perego, P.; Oliveira, N.M.; Converti, A. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. Int. J. Food Microbiol. 2011, 145, 22–27. [Google Scholar] [CrossRef]
- Domagala, J.; Sady, M.; Grega, T.; Bonczar, G. Rheological properties and texture of yogurts when oat-maltodextrin is used as a fat substitute. Int. J. Food Proper. 2006, 9, 1–11. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt Science and Technology; CRC Press: New York, NY, USA, 1999; Volume 2. [Google Scholar]
- Capozzi, V.; Russo, P.; Duenas, M.T.; Lopez, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Yepez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligne, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: Invitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Shah, C.; Mokashe, N.; Mishra, V. Preparation, characterization and in vitro antioxidative potential of synbiotic fermented dairy products. J. Food Sci. Technol. 2016, 53, 1984–1992. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; de-Giori, G.S.; de-LeBlanc, A.D.M.; LeBlanc, J.G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucosit is in mice. Nutrition 2018, 54, 165–172. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. BBAGen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S.; Singh, D.; Kumari, P.; Minj, J. Antimicrobial activity of bioactive peptides derived from fermentation of soy milk by Lactobacillus plantarum C2 against common food borne pathogens. Int. J. Ferment. Foods. 2015, 4, 91–99. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. Growth and bioactive peptide s production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT Food Sci. Technol. 2017, 86, 293–301. [Google Scholar] [CrossRef]
- Lin, T.H.; Pan, T.M. Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J. Microbiol. Immunol. Infect. 2017, 52, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.K.; Hati, S.; Das, S.; Prajapati, J.B. Biofunctional Attributes and Storage Study of Soy Milk Fermented by Lactobacillus rhamnosus and Lactobacillus helveticus. Food Technol. Biotechnol. 2019, 57, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, N.; Mandal, S. Comparative growth behavior and biofunctionality of lactic acid bacteria during fermentation of soy milk and bovine milk. Probiotics Antimicrobi. Proteins 2018, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.S.; Bashir, K.; Mishra, V. Development of Synbiotic Litchi Juice Drink and its Physiochemical, Viability and Sensory Analysis. J. Food Process. Technol. 2017, 8, 12. [Google Scholar]
- Vranac, A.; Akagic, A.; Gasi, F.; Spaho, N.; Kurtovic, M.; Meland, M. Sensory evaluation of blended cloudy apple juices. In Proceedings of the 28th International Scientific-Expert Conference of Agriculture and Food Industry, Sarajevo, Bosnia and Herzegovina, 27–29 September 2017; Volume 67, pp. 493–504. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).