Determination of Foam Stability in Lager Beers Using Digital Image Analysis of Images Obtained Using RGB and 3D Cameras
Abstract
1. Introduction
2. Materials and Methods
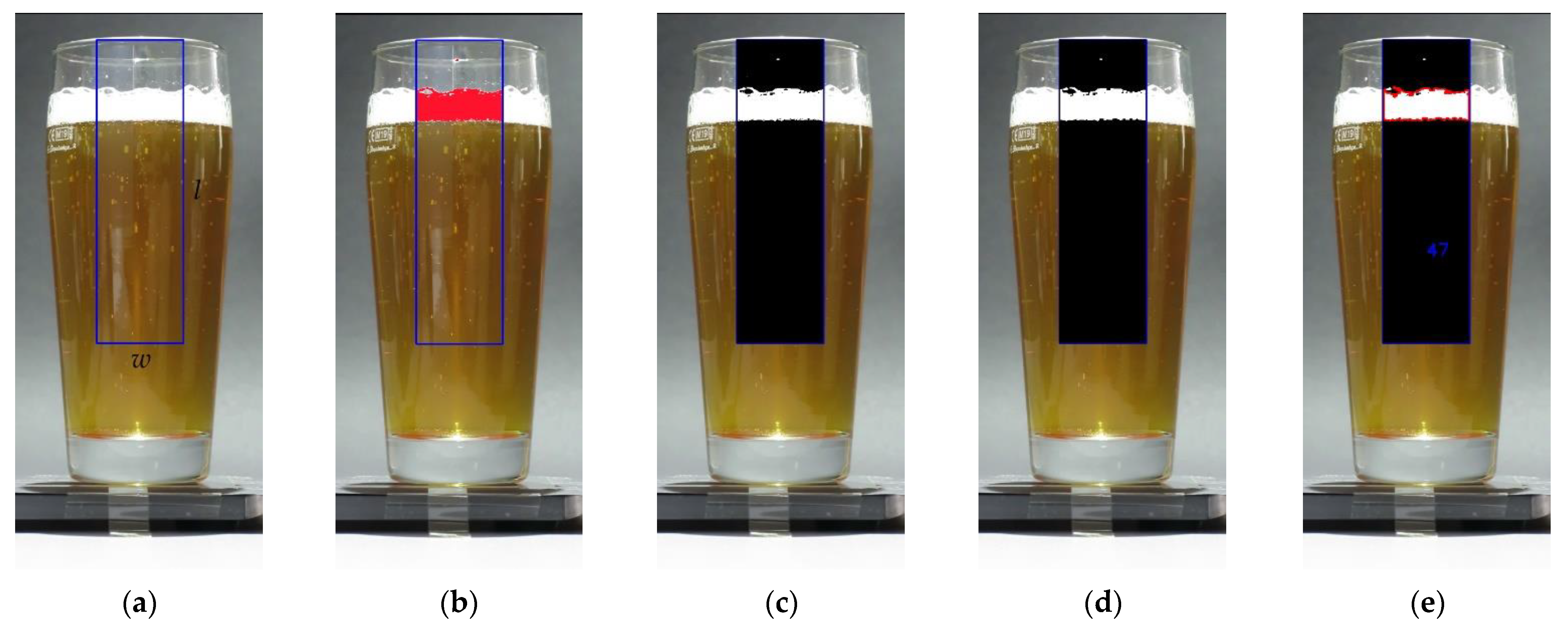
2.1. Image Analysis of Video
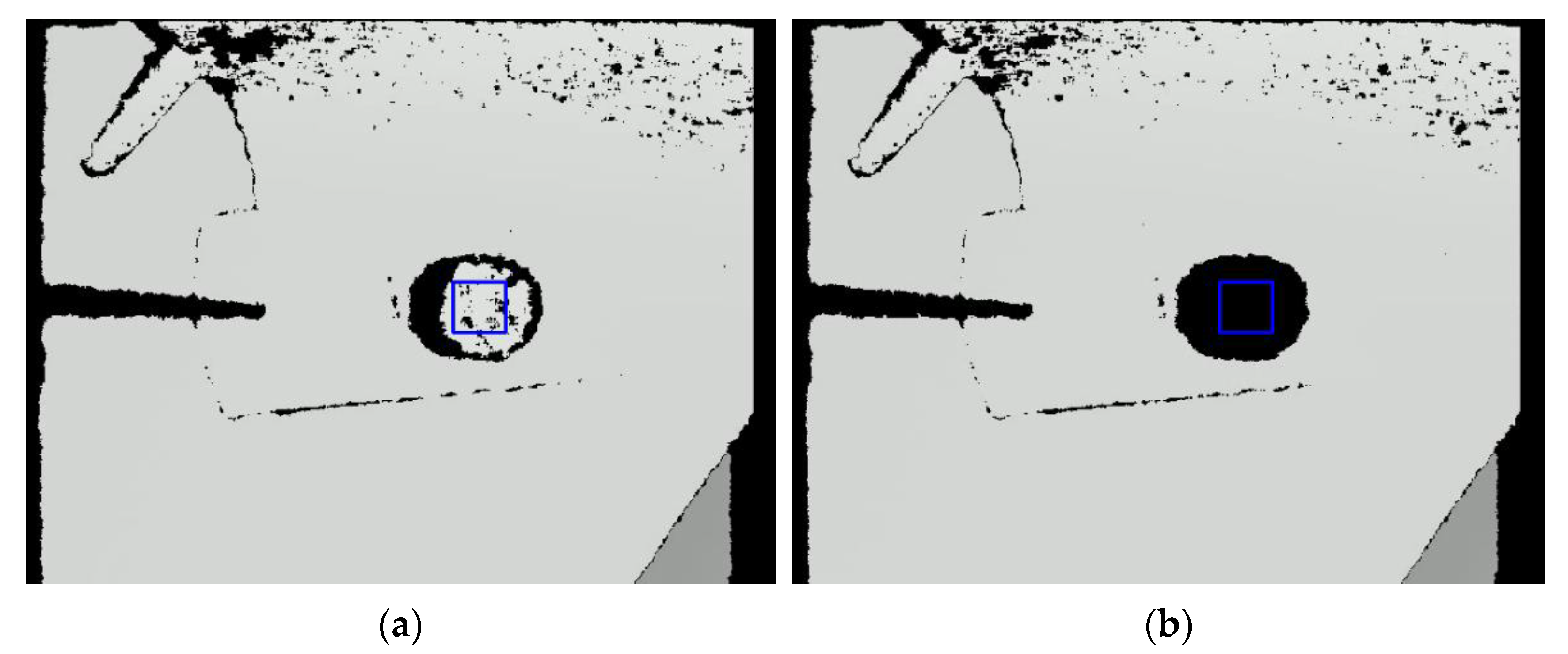
2.2. Depth Measurement Using a 3D Camera
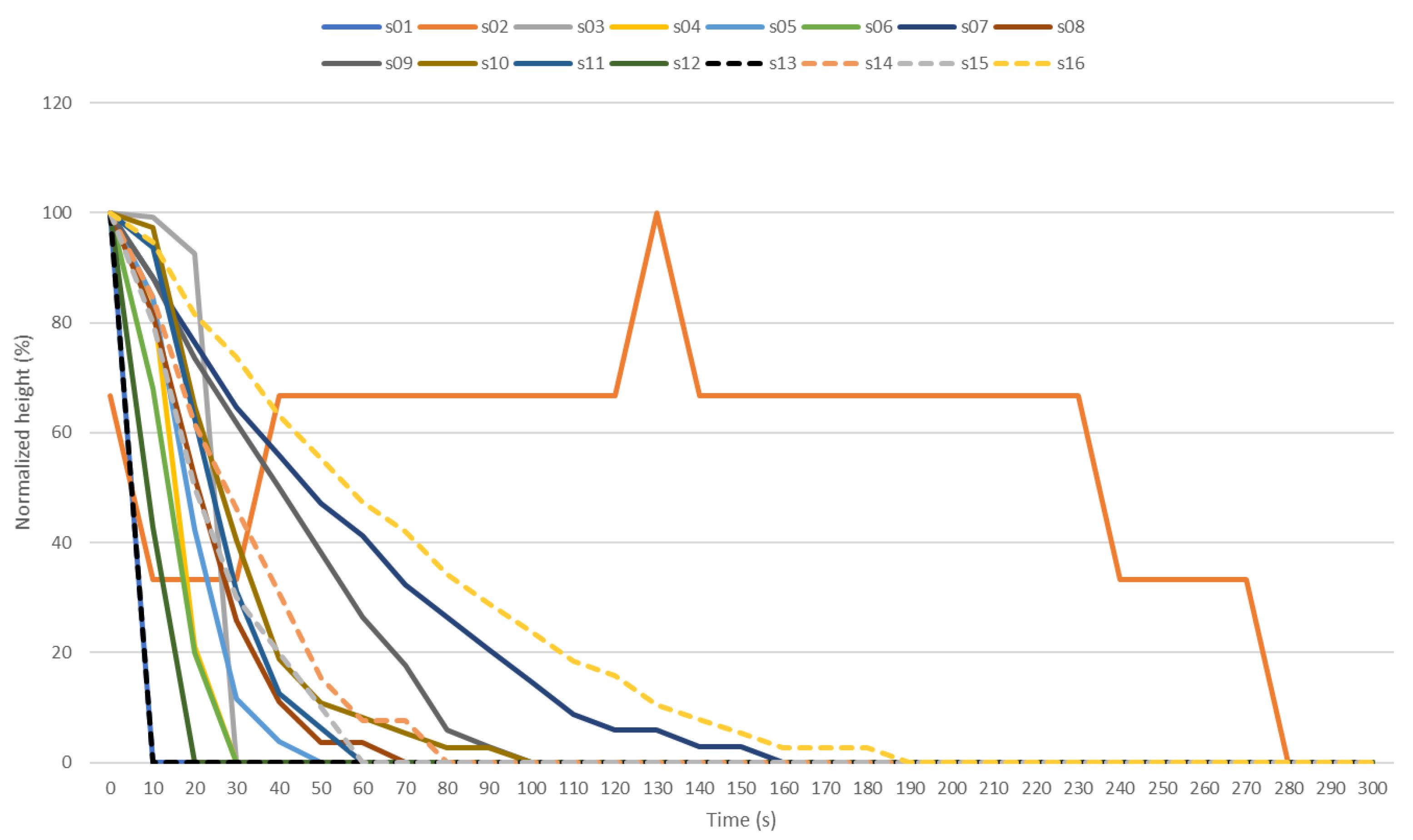
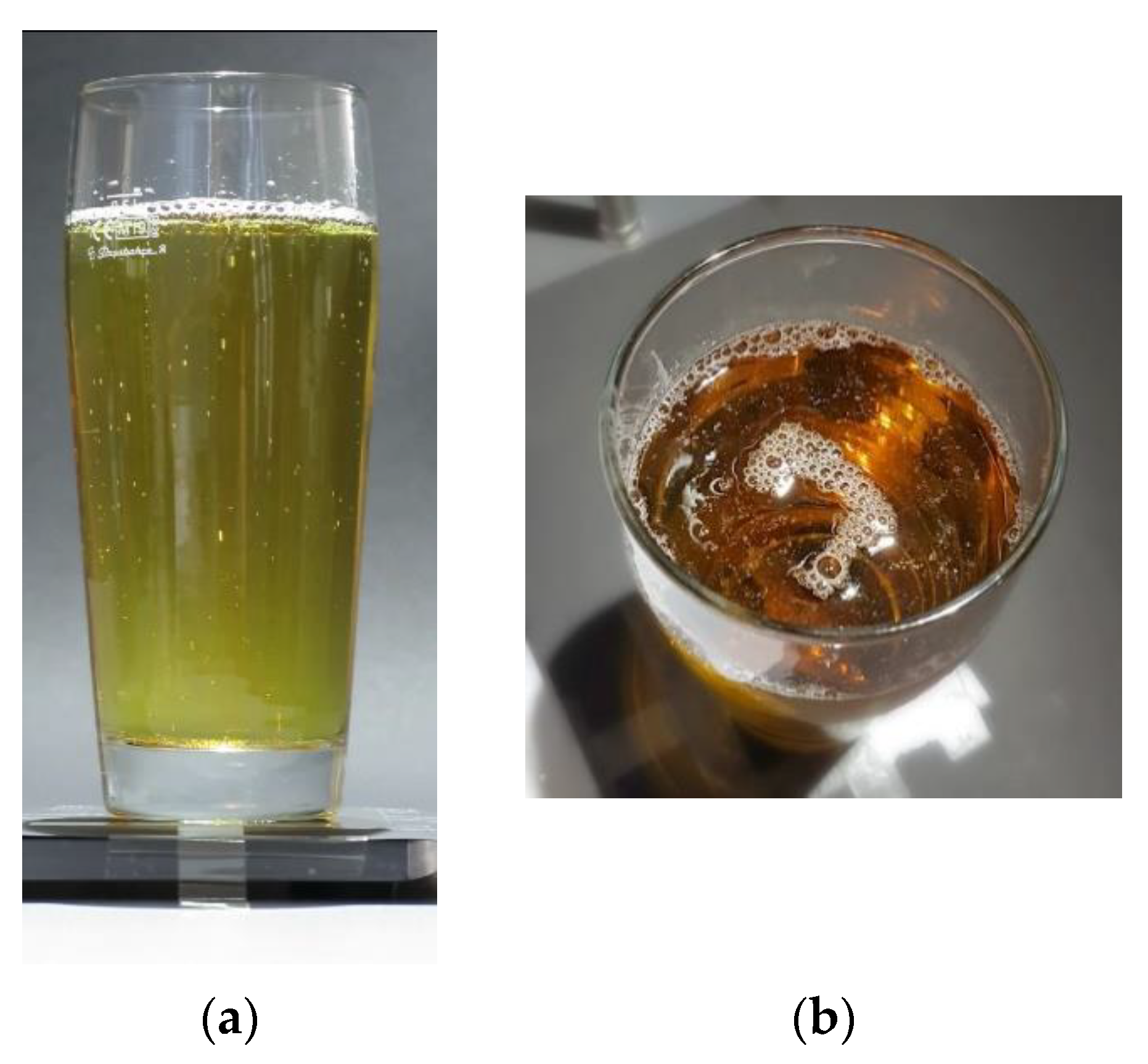
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Time (s) | s01 | s02 | s03 | s04 | s05 | s06 | s07 | s08 | s09 | s10 | s11 | s12 | s13 | s14 | s15 | s16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 43 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 10 | - | 21 | 33 | 34 | 52 | 43 | 68 | 56 | 82 | 74 | 61 | 22 | 0 | 77 | 84 | 88 |
| 20 | - | 11 | 10 | 11 | 22 | 11 | 52 | 30 | 61 | 45 | 26 | 0 | - | 56 | 61 | 86 |
| 30 | - | 32 | 0 | 0 | 6 | 0 | 40 | 10 | 50 | 29 | 12 | - | - | 42 | 55 | 73 |
| 40 | - | 36 | - | - | 0 | 1 | 33 | 0 | 39 | 15 | 1 | - | - | 34 | 29 | 63 |
| 50 | - | 46 | - | - | - | 0 | 28 | 1 | 30 | 12 | 0 | - | - | 28 | 18 | 55 |
| 60 | - | 61 | - | - | - | - | 23 | 0 | 38 | 9 | - | - | - | 22 | 11 | 48 |
| 70 | - | 50 | - | - | - | - | 18 | - | 29 | 6 | - | - | - | 18 | 0 | 40 |
| 80 | - | 54 | - | - | - | - | 14 | - | 9 | 4 | - | - | - | 16 | - | 34 |
| 90 | - | 82 | - | - | - | - | 10 | - | 3 | 2 | - | - | - | 12 | - | 29 |
| 100 | - | 79 | - | - | - | - | 8 | - | 0 | 0 | - | - | - | 9 | - | 23 |
| 110 | - | 79 | - | - | - | - | 7 | - | 0 | - | - | - | - | 7 | - | 19 |
| 120 | - | 89 | - | - | - | - | 6 | - | 3 | - | - | - | - | 0 | - | 14 |
| 130 | - | 89 | - | - | - | - | 5 | - | 3 | - | - | - | - | - | - | 11 |
| 140 | - | 75 | - | - | - | - | 4 | - | 0 | - | - | - | - | - | - | 11 |
| 150 | - | 100 | - | - | - | - | 4 | - | - | - | - | - | - | - | - | 8 |
| 160 | - | 86 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | 6 |
| 170 | - | 79 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | 4 |
| 180 | - | 68 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 8 |
| 190 | - | 61 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 6 |
| 200 | - | 57 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 5 |
| 210 | - | 61 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 4 |
| 220 | - | 57 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 4 |
| 230 | - | 50 | - | - | - | - | 1 | - | - | - | - | - | - | - | - | 3 |
| 240 | - | 32 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | 3 |
| 250 | - | 11 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 2 |
| 260 | - | 25 | - | - | - | - | 1 | - | - | - | - | - | - | - | - | 2 |
| 270 | - | 11 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 1 |
| 280 | - | 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| 290 | - | 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| 300 | - | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| Time (s) | s01 | s02 | s03 | s04 | s05 | s06 | s07 | s08 | s09 | s10 | s11 | s12 | s13 | s14 | s15 | s16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 67 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 10 | 0 | 33 | 89 | 84 | 85 | 68 | 88 | 81 | 88 | 97 | 94 | 43 | 0 | 85 | 80 | 95 |
| 20 | - | 33 | 0 | 21 | 42 | 20 | 76 | 52 | 74 | 65 | 63 | 0 | - | 62 | 50 | 82 |
| 30 | - | 33 | - | 0 | 12 | 0 | 65 | 26 | 62 | 41 | 31 | - | - | 46 | 30 | 74 |
| 40 | - | 67 | - | - | 4 | - | 56 | 11 | 50 | 19 | 13 | - | - | 31 | 20 | 63 |
| 50 | - | 67 | - | - | 0 | - | 47 | 4 | 38 | 11 | 6 | - | - | 15 | 10 | 55 |
| 60 | - | 67 | - | - | - | - | 41 | 4 | 26 | 8 | 0 | - | - | 8 | 0 | 47 |
| 70 | - | 67 | - | - | - | - | 32 | 0 | 18 | 5 | - | - | - | 8 | 0 | 42 |
| 80 | - | 67 | - | - | - | - | 26 | - | 6 | 3 | - | - | - | 0 | - | 34 |
| 90 | - | 67 | - | - | - | - | 21 | - | 3 | 0 | - | - | - | 0 | - | 29 |
| 100 | - | 67 | - | - | - | - | 15 | - | 0 | 0 | - | - | - | 0 | - | 24 |
| 110 | - | 67 | - | - | - | - | 9 | - | 0 | - | - | - | - | 0 | - | 18 |
| 120 | - | 67 | - | - | - | - | 6 | - | 0 | - | - | - | - | 0 | - | 16 |
| 130 | - | 100 | - | - | - | - | 6 | - | - | - | - | - | - | - | - | 11 |
| 140 | - | 67 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | 8 |
| 150 | - | 67 | - | - | - | - | 3 | - | - | - | - | - | - | - | - | 5 |
| 160 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 3 |
| 170 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 3 |
| 180 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 3 |
| 190 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 200 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 210 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 220 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 230 | - | 67 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 240 | - | 33 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 250 | - | 33 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 260 | - | 33 | - | - | - | - | 0 | - | - | - | - | - | - | - | - | 0 |
| 270 | - | 33 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| 280 | - | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| 290 | - | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
| 300 | - | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 |
References
- Ekberg, J.; Gibson, B.; Joensuu, J.J.; Krogerus, K.; Magalhães, F.; Mikkelson, A.; Seppänen-Laakso, T.; Wilpola, A. Physicochemical characterization of sahti, an‘ancient’ beer style indigenous to Finland. J. Inst. Brew. 2015, 121, 464–473. [Google Scholar] [CrossRef]
- Bamforth, C.W. The foaming properties of beer. J. Inst. Brew. 1985, 91, 370–383. [Google Scholar] [CrossRef]
- BJCP. Beer Style Guidelines, Edited by Gordon Strong, Kristen England. Available online: www.bjcp.org (accessed on 6 March 2021).
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Bubbles, Foam Formation, Stability and Consumer Perception of Carbonated Drinks: A Review of Current, New and Emerging Technologies for Rapid Assessment and Control. Foods 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, J.J. Multivariate Analyses of Beer Foam Stand. J. Inst. Brew. 2006, 112, 17–24. [Google Scholar] [CrossRef]
- Prins, A.; van Marle, J.T. Foam formation in beer: Some physics behind it. Monogr. Eur. Brew. Conv. 1999, 27, 26–36. [Google Scholar]
- Ronteltap, A.; Hollemans, M.; Bisperink, C.G.J.; Prims, A.R. Beer foam physics. Tech. Q. Master Brew. Assoc. Am. 1991, 28, 25–32. [Google Scholar]
- Fisher, S.; Hauser, G.; Sommer, K. Influence of dissolved gases on foam. Monogr. Eur. Brew. Conv. 1999, 27, 37–46. [Google Scholar]
- Evans, D.E.; Sheehan, M.C. Don’t Be Fobbed Off: The Substance of Beer Foam—A Review. J. Am. Soc. Brew. Chem. 2002, 60, 47–57. [Google Scholar] [CrossRef]
- ASBC. Methods of Analysis. Method Beer-22. Foam Collapse Rate. Approved 1962, Rev. 1975; American Society of Brewing Chemists: St. Paul, MN, USA, 2018. [Google Scholar] [CrossRef]
- Klopper, W.J. Foam stability and foam cling. In Proceedings of the Eur. Brew. Conv. Congr., Salzburg, Austria; Elsevier Scientific: Amsterdam, The Netherlands, 1973; pp. 363–371. Available online: https://www.kruss-scientific.com/en/explore/research-and-development/research-of-foam (accessed on 21 January 2021).
- Rudin, A. Measurement of the foam stability of beers. J. Inst. Brew. 1957, 63, 506–509. [Google Scholar] [CrossRef]
- Rasmussen, J.N. Automated analysis of foam stability. Carlsberg Res. Commun. 1981, 46, 25–36. [Google Scholar] [CrossRef]
- Jackson, G.; Bamforth, C. The measurement of foam-lacing. J. Inst. Brew. 1982, 88, 378–381. [Google Scholar] [CrossRef]
- Constant, M. A practical method for characterizing poured beer foam quality. J. Am. Soc. Brew. Chem. 1992, 0, 37–47. [Google Scholar] [CrossRef]
- Vundla, W.; Torline, P. Steps toward the formulation of a model foam standard. J. Am. Soc. Brew. Chem. 2007, 65, 21–25. [Google Scholar] [CrossRef]
- Amerine, M.A.; Martini, L.; Mattei, W.D. Foaming Properties of Wine. Ind. Eng. Chem. 1942, 34, 152–157. [Google Scholar] [CrossRef]
- Wilson, P.; Mundy, A. An improved method for measuring beer foam collapse. J. Inst. Brew. 1984, 90, 385–388. [Google Scholar] [CrossRef]
- Evans, D.E.; Surrel, A.; Sheehy, M.; Stewart, D.C.; Robinson, L.H. Comparison of foam quality and the influence of hop α-acids and proteins using five foam analysis methods. J. Am. Soc. Brew. Chem. 2008, 66, 1–10. [Google Scholar] [CrossRef]
- Evans, D.E.; Oberdieck, M.; Red, K.S.; Newman, R. Comparison of the Rudin and NIBEM Methods for Measuring Foam Stability with a Manual Pour Method to Identify Beer Characteristics That Deliver Consumers Stable Beer Foam. J. Am. Soc. Brew. Chem. 2012, 70, 70–78. [Google Scholar] [CrossRef]
- Smith, R.J.; Davidson, D.; Wilson, R.J. Natural foam stabilizing and bittering compounds derived from hops. J. Am. Soc. Brew. Chem. 1998, 56, 52–57. [Google Scholar] [CrossRef]
- Cimini, A.; Pallottino, F.; Menesatti, P.; Moresi, M. A low-cost image analysis system to upgrade the rudin beer foam head retention meter. Food Bioprocess Technol. 2016, 9, 1587–1597. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Li, G.; Collmann, R.; Condé, B.; Torrico, D. Development of a robotic pourer constructed with ubiquitous materials, open hardware and sensors to assess beer foam quality using computer vision and pattern recognition algorithms: RoboBEER. Food Res. Int. 2016, 89, 504–513. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.D.; Howell, K.; Dunshea, F.R. Assessment of Beer Quality Based on a Robotic Pourer, Computer Vision, and Machine Learning Algorithms Using Commercial Beers. J. Food Sci. 2018, 83, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Fuentes, S.; Howell, K.; Torrico, D.D.; Dunshea, F.R. Integration of non-invasive biometrics with sensory analysis techniques to assess acceptability of beer by consumers. Physiol. Behav. 2019, 200, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lukinac, J.; Mastanjević, K.; Mastanjević, K.; Nakov, G.; Jukić, M. Computer Vision Method in Beer Quality Evaluation—A Review. Beverages 2019, 5, 38. [Google Scholar] [CrossRef]
- Middle European Brewing Analysis Commission (MEBAK); Band II.n Brautechnische Middle European Brewing Analysis Commission (MEBAK). Band II.n Brautechnische Analysenmethoden, 3th ed.; Selbstverlag der MEBAK: Freising-Weihenstephan, Germany, 1997. [Google Scholar]
- Python Programming Language. Available online: https://www.python.org/ (accessed on 21 January 2021).
- OpenCV. Available online: https://opencv.org/ (accessed on 21 January 2021).




| Time (s) | s01 | s02 | s03 | s04 | s05 | s06 | s07 | s08 | s09 | s10 | s11 | s12 | s13 | s14 | s15 | s16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | - | 46 | 91 | 176 | 229 | 207 | 308 | 264 | 320 | 308 | 280 | 187 | 32 | 169 | 124 | 318 |
| 10 | - | 40 | 32 | 67 | 137 | 103 | 231 | 172 | 269 | 236 | 183 | 60 | 8 | 138 | 108 | 285 |
| 20 | - | 37 | 12 | 28 | 80 | 45 | 193 | 120 | 212 | 155 | 96 | 24 | - | 110 | 85 | 280 |
| 30 | - | 43 | 3 | 10 | 49 | 25 | 163 | 77 | 181 | 111 | 62 | - | - | 91 | 79 | 246 |
| 40 | - | 44 | - | - | 37 | 26 | 147 | 58 | 152 | 72 | 35 | - | - | 80 | 52 | 219 |
| 50 | - | 47 | - | - | - | - | 135 | 59 | 126 | 63 | 33 | - | - | 72 | 41 | 198 |
| 60 | - | 51 | - | - | - | - | 123 | 58 | 148 | 55 | - | - | - | 64 | 34 | 179 |
| 70 | - | 48 | - | - | - | - | 111 | 57 | 124 | 46 | - | - | - | 58 | 23 | 158 |
| 80 | - | 49 | - | - | - | - | 101 | - | 69 | 41 | - | - | - | 56 | - | 143 |
| 90 | - | 57 | - | - | - | - | 93 | - | 51 | 35 | - | - | - | 50 | - | 129 |
| 100 | - | 56 | - | - | - | - | 86 | - | 44 | 29 | - | - | - | 46 | - | 115 |
| 110 | - | 56 | - | - | - | - | 84 | - | 45 | - | - | - | - | 43 | - | 103 |
| 120 | - | 59 | - | - | - | - | 82 | - | 53 | - | - | - | - | 34 | - | 91 |
| 130 | - | 59 | - | - | - | - | 80 | - | 52 | - | - | - | - | - | - | 82 |
| 140 | - | 55 | - | - | - | - | 78 | - | - | - | - | - | - | - | - | 81 |
| 150 | - | 62 | - | - | - | - | 77 | - | - | - | - | - | - | - | - | 73 |
| 160 | - | 58 | - | - | - | - | 75 | - | - | - | - | - | - | - | - | 69 |
| 170 | - | 56 | - | - | - | - | 74 | - | - | - | - | - | - | - | - | 64 |
| 180 | - | 53 | - | - | - | - | 72 | - | - | - | - | - | - | - | - | 73 |
| 190 | - | 51 | - | - | - | - | 72 | - | - | - | - | - | - | - | - | 68 |
| 200 | - | 50 | - | - | - | - | 73 | - | - | - | - | - | - | - | - | 66 |
| 210 | - | 51 | - | - | - | - | 73 | - | - | - | - | - | - | - | - | 64 |
| 220 | - | 50 | - | - | - | - | 72 | - | - | - | - | - | - | - | - | 63 |
| 230 | - | 48 | - | - | - | - | 71 | - | - | - | - | - | - | - | - | 62 |
| 240 | - | 43 | - | - | - | - | 72 | - | - | - | - | - | - | - | - | 60 |
| 250 | - | 37 | - | - | - | - | 69 | - | - | - | - | - | - | - | - | 58 |
| 260 | - | 41 | - | - | - | - | 70 | - | - | - | - | - | - | - | - | 58 |
| 270 | - | 37 | - | - | - | - | 68 | - | - | - | - | - | - | - | - | 56 |
| 280 | - | 35 | - | - | - | - | - | - | - | - | - | - | - | - | - | 54 |
| 290 | - | 35 | - | - | - | - | - | - | - | - | - | - | - | - | - | 54 |
| 300 | - | 34 | - | - | - | - | - | - | - | - | - | - | - | - | - | 53 |
| Time (s) | s01 | s02 | s03 | s04 | s05 | s06 | s07 | s08 | s09 | s10 | s11 | s12 | s13 | s14 | s15 | s16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 158 | 159 | 161 | 172 | 171 | 177 | 181 | 176 | 178 | 167 | 177 | 170 | 134 | 168 | 165 | 191 |
| 10 | 41 | 158 | 160 | 169 | 167 | 169 | 177 | 171 | 174 | 166 | 175 | 162 | 36 | 166 | 163 | 189 |
| 20 | - | 158 | 152 | 157 | 156 | 157 | 173 | 163 | 169 | 154 | 165 | 156 | - | 163 | 160 | 184 |
| 30 | - | 158 | - | 153 | 148 | 152 | 169 | 156 | 165 | 145 | 155 | - | - | 161 | 158 | 181 |
| 40 | - | 159 | - | - | 146 | - | 166 | 152 | 161 | 137 | 149 | - | - | 159 | 157 | 177 |
| 50 | - | 159 | - | - | 145 | - | 163 | 150 | 157 | 134 | 147 | - | - | 157 | 156 | 174 |
| 60 | - | 159 | - | - | - | - | 161 | 150 | 153 | 133 | 145 | - | - | 156 | 155 | 171 |
| 70 | - | 159 | - | - | - | - | 158 | 149 | 150 | 132 | - | - | - | 156 | 155 | 169 |
| 80 | - | 159 | - | - | - | - | 156 | - | 146 | 131 | - | - | - | 155 | - | 166 |
| 90 | - | 159 | - | - | - | - | 154 | - | 145 | 131 | - | - | - | 155 | - | 164 |
| 100 | - | 159 | - | - | - | - | 152 | - | 144 | 130 | - | - | - | 155 | - | 162 |
| 110 | - | 159 | - | - | - | - | 150 | - | 144 | - | - | - | - | 155 | - | 160 |
| 120 | - | 159 | - | - | - | - | 149 | - | 144 | - | - | - | - | 155 | - | 159 |
| 130 | - | 160 | - | - | - | - | 149 | - | - | - | - | - | - | - | - | 157 |
| 140 | - | 159 | - | - | - | - | 148 | - | - | - | - | - | - | - | - | 156 |
| 150 | - | 159 | - | - | - | - | 148 | - | - | - | - | - | - | - | - | 155 |
| 160 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 154 |
| 170 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 154 |
| 180 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 154 |
| 190 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 200 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 210 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 220 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 230 | - | 159 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 240 | - | 158 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 250 | - | 158 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 260 | - | 158 | - | - | - | - | 147 | - | - | - | - | - | - | - | - | 153 |
| 270 | - | 158 | - | - | - | - | - | - | - | - | - | - | - | - | - | 153 |
| 280 | - | 157 | - | - | - | - | - | - | - | - | - | - | - | - | - | 153 |
| 290 | - | 157 | - | - | - | - | - | - | - | - | - | - | - | - | - | 153 |
| 300 | - | 157 | - | - | - | - | - | - | - | - | - | - | - | - | - | 153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyarko, E.K.; Glavaš, H.; Habschied, K.; Mastanjević, K. Determination of Foam Stability in Lager Beers Using Digital Image Analysis of Images Obtained Using RGB and 3D Cameras. Fermentation 2021, 7, 46. https://doi.org/10.3390/fermentation7020046
Nyarko EK, Glavaš H, Habschied K, Mastanjević K. Determination of Foam Stability in Lager Beers Using Digital Image Analysis of Images Obtained Using RGB and 3D Cameras. Fermentation. 2021; 7(2):46. https://doi.org/10.3390/fermentation7020046
Chicago/Turabian StyleNyarko, Emmanuel Karlo, Hrvoje Glavaš, Kristina Habschied, and Krešimir Mastanjević. 2021. "Determination of Foam Stability in Lager Beers Using Digital Image Analysis of Images Obtained Using RGB and 3D Cameras" Fermentation 7, no. 2: 46. https://doi.org/10.3390/fermentation7020046
APA StyleNyarko, E. K., Glavaš, H., Habschied, K., & Mastanjević, K. (2021). Determination of Foam Stability in Lager Beers Using Digital Image Analysis of Images Obtained Using RGB and 3D Cameras. Fermentation, 7(2), 46. https://doi.org/10.3390/fermentation7020046









