Abstract
Most reported members of Microascaceae that have been reported originate from the terrestrial environment, where they act as saprobes or plant pathogens. However, our understanding of their species diversity and distribution in the marine environment remains vastly limited, with only 22 species in nine genera having been reported so far. A survey of the fungal diversity in intertidal areas of China’s mainland has revealed the discovery of several Microascaceae strains from 14 marine algae and 15 sediment samples. Based on morphological characteristics and LSU-ITS-tef1-tub2 multilocus phylogeny using Bayesian inference and maximum likelihood methods, 48 strains were identified as 18 species belonging to six genera. Among these, six new species were discovered: Gamsia sedimenticola, Microascus algicola, M. gennadii, Scedosporium ellipsosporium, S. shenzhenensis, and S. sphaerospermum. Additionally, the worldwide distribution of the species within this family across various marine habitats was briefly reviewed and discussed. Our study expands the knowledge of species diversity and distribution of Microascaceae in the marine environment.
1. Introduction
The family Microascaceae (Microascales, Sordariomycetes, and Ascomycota) was originally erected in 1951 by Luttrell to accommodate the genus Microascus, characterised as having beaked ascocarps with evanescent asci disposed irregularly throughout the centrum [1]. Later in 1970, Malloch reviewed known species, introduced two new species, and then illustrated the new concepts of this family, including ascocarps, ascospores, and complex hyaline to brightly coloured, conidiophores that are phialide bearing [2]. Over the past several decades, advances in morphology and multilocus phylogenetic analyses have led to a better understanding of the taxonomy and species diversity of this family. Species in this family are mainly characterised by their annellidic asexual morphs with dry aseptate conidia, as well as their sexual morphs that form cleistothecial or perithecial, carbonaceous ascomata producing reniform, lunate, or triangular ascospores with or without germ pores [3]. Currently, about 290 species in 23 genera are accepted in Microascaceae [4]. The majority of these species are found in terrestrial environments and act as saprobes or pathogens of plants, and also as opportunistic pathogens of humans with some exhibiting intrinsic resistance to antifungal agents [5].
However, there is limited information available on the species diversity and distribution of Microascaceae in the marine environment. In 1973, a new species, Scopulariopsis halophilica Tubaki, was introduced and described from the macroalgae Undaria pinnatifida collected from Japan [6]. This is, to our knowledge, the first microascaceous fungus to be isolated from the marine environment. Since then, several studies using culture-dependent approaches have documented the wide distribution of microascaceous fungi from intertidal zones of European and tropical regions, and marginal seas of China, with a total of 22 species reported in nine genera (Acaulium, Cephalotrichum, Microascus, Petriella, Pseudallescheria, Pseudoscopulariopsis, Scedosporium, Scopulariopsis, and Wardomyces) [7,8,9,10]. Several culture-independent approaches using ITS1 or ITS2 rDNA metabarcoding have uncovered substantial occurrences of this family in the seas surrounding China [11,12,13]. This is fortified by the identification of 109 microascaceous OTUs (operational taxonomic units), which were annotated into 13 genera, and extracted from intertidal sediments from China’s mainland [14]. Five microascaceous OTUs were also detected from the seawater samples collected from the Western Pacific Ocean [15]. These findings indicate the potential global distribution of microascaceous fungi within the ocean.
Marine fungi are capable of producing a variety of secondary metabolites such as those with antimicrobial properties [16]. The Microascaceae fungi recovered from the marine environment have demonstrated great potential in industrial and agricultural applications. A new monoterpenoid compound, Scopuquinolone B, was first isolated from a coral-derived Scopulariospis sp. isolate LF580 [17]. Proteomic analysis of Sc. brevicaulis from marine sponges identified thousands of proteins and diverse biosynthetic enzyme complexes [18]. Several species, e.g., Micoascus trigonosporus, displayed a highly lethal effect on agricultural pests and a significant inhibitory effect on pathogens of plant diseases [19]. It is necessary to obtain more Microascaceae fungi from the marine environment and to promote more research about the physiology, biochemistry, metabolites, and ecological functions of this group of fungi.
Most of the known culturable fungal species have been found on various substrates collected from the intertidal region, such as seagrasses, seaweeds, mangrove plants, sediments, and driftwood [7,8,9,10], indicating that the intertidal region provides an ideal shelter to host numerous and diverse fungi. In a survey of fungal diversity in the intertidal areas of China’s mainland, 48 microascaceous strains were isolated from 14 marine algae and 15 sediment samples collected from 22 locations. Our aims for this study are (1) to identify species and clarify the phylogenetic relationships of these fungi, (2) to identify and describe new species of Microascaceae, and (3) to review species diversity, host/habitat, and geographic distribution of Microascaceae in the marine environment.
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
Algae and sediment samples were collected from intertidal zones of Fujian, Guangdong, Jiangsu, Liaoning, Shandong, and Zhejiang provinces, and Tianjin city (Figure 1). For algae, six asymptomatic thalli of each algae species from each site were collected and put into sterilised plastic bottles. Species identification of algae was carried out following morphological descriptions by Zeng [20]. For sediment samples, a 200 g sediment sample was collected from each site and placed into a sterilised sampling bag. The samples were placed into a low-temperature storage box with gel packs and brought back to the laboratory as soon as possible.
Fungi were isolated from algae using the dilution-plate method [21] and tissue-isolation method, with some modifications. Each algae sample was washed with sterilised seawater, cut into segments of about 0.5 × (0.2–0.5) cm, and surface sterilised by dipping in 70% ethanol for 5 s followed by immersion in 4% NaOCl for 60 s and washed with sterile distilled water for 10 s [22]. Using the dilution-plate method, approximately 1 g of the segments were placed in a sterilised mortar and ground to tissue homogenate with 10 mL of sterilised seawater. The suspension was moved into a sterilised tube, diluted to a series of concentrations (10−1, 10−2, 10−3, and 10−4), and, finally, spread onto isolation media with three replicates. Using the tissue-isolation method, after cutting into segments and surface sterilization, 6–10 algal segments were spread on the isolation media with three replicates. Five types of isolation media, namely Martin medium (MM; each 1 L medium containing peptone 5.0 g, dextrose 10.0 g, monopotassium phosphate 1.0 g, magnesium sulfate 0.5 g, chloramphenicol 0.1 g, rose Bengal 0.033 g, agar 20.0 g, and seawater 1 L), 1/10 potato dextrose agar (1/10 PDA; each 1 L medium containing potato 20.0 g, dextrose 2.0 g, agar 20.0 g, and seawater 1 L), 1/5 malt extract agar (1/5 MEA; each 1 L medium containing malt extract 4.0 g, peptone 0.2 g, dextrose 4.0 g, agar 20.0 g, and seawater 1 L), corn meal agar (CMA; each 1 L medium containing corn meal 30.0 g, agar 20.0 g, and seawater 1 L), and yeast extract peptone glucose agar (YPG; each 1 L medium containing yeast extract 1.25 g, peptone 1.25 g, dextrose 4.0 g, agar 20.0 g, and seawater 1 L) containing ampicillin (500 mg/L) and streptomycin (500 mg/L), were selected and used for fungal isolation in this study.

Figure 1.
Locations of the 14 marine algae (green dot) and 15 sediments (black dot) samples from the intertidal areas of mainland China (a), the thallus of algal hosts of the microascaceous fungi (b), and the typical algal habitats and the typical mudflat, sand beach, and gravel beach in the intertidal zones (c).
Fungi were isolated from sediments using the dilution-plate method [21] and the direct-isolation method, with some modifications. In the dilution-plate method, 10 g of sediment from each sample were suspended in 90 mL of sterile seawater in a 100 mL triangular glass flask and cultivated by shaking at 150 rpm/min at 25 °C for 20 min. The suspension was diluted to a series of concentrations (10−1, 10−2, 10−3, and 10−4) and plated on five types of isolation media with three replicates. Using the direct-isolation method, approximately 1 g of each sediment sample was spread directly on five types of isolation media with three replicates.
All plates were incubated at room temperature and examined every 2 days using an Olympus SZX7 stereomicroscope for fungal hyphae. Individual colonies were picked up with a sterilised needle and transferred onto fresh PDA plates on the benchtop. All the cultures were incubated at room temperature after 7 days and then were purified using an optimised protocol for single-spore isolation [23]. All isolates examined in this study were deposited in Wei Li’s personal culture collection (WL). Type specimens of new species were deposited in the Fungarium of the Institute of Microbiology (HMAS), with the ex-type living cultures in the China General Microbiological Culture Collection Center (CGMCC).
2.2. Morphological Observation
The isolates studied were incubated on synthetic nutrient-poor agar plates (SNA) [24], PDA, and oatmeal agar (OA). After 7 days of incubation in the dark, culture characteristics, including colony morphology, pigmentation, and odour were observed. Colours were assessed according to the colour charts of Kornerup and Wanscher (1978) [25]. Micromorphological characteristics were examined and photo-documented using water as a mounting medium under an Olympus BX53 microscope with differential interference contrast (DIC) optics. For each species, respectively, 30 conidiophores, 30 conidiogenous cells, 30 chlamydospores, and 50 microconidia were mounted and measured randomly.
2.3. DNA Extraction and Amplification
Genomic DNA was extracted from fungal mycelia grown on PDA, using a modified CTAB protocol as described in Guo et al. (2000) [26]. Four loci, including partial large-subunit ribosomal RNA (LSU), 5.8S nuclear ribosomal RNA gene with the two flanking internal transcribed spacer (ITS) regions, partial translation elongation factor (tef1), and partial β-tubulin (tub2), were amplified using primer pairs LR0R/LR5 [27], ITS5/ITS4 [28], EF1-983F/EF1-2218R [29], and Bt2a/Bt2b [30], respectively. Amplification reactions were performed in a reaction volume containing 12.5 μL of 2 × Taq PCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China), 1 μL each of 10 μM primers, and 1 μL of the undiluted genomic DNA, adjusted to a final volume of 25 μL with distilled deionised water (Dongsheng, EDC810, China). PCR parameters were as follows: 94 °C for 10 min, followed by 35 cycles of 94 °C for 30 s, 50 (for LSU)/54 (for ITS)/57 (for tef1 and tub2) °C for 30 s, 72 °C for 30 s, and a final elongation step at 72 °C for 10 min. The PCR products were visualised on 1% agarose electrophoresis gel. Sequencing was performed bidirectionally and conducted by the BGI Write Company (Beijing, China). Consensus sequences were obtained using SeqMan of the Lasergene software package v. 14.1 (DNAstar, Madison, WI, USA).
2.4. Phylogenetic Analyses
The sequences of the Microascaceae strains examined in this study and the reference strains are listed in Table 1. For each locus, sequences were aligned using MAFFT v. 7 [31], and the alignments were manually adjusted where necessary. The best-fitting nucleotide-substitution models according to the Akaike Information Criterion (AIC) were selected using jModelTest v. 2.1.7 [32,33]. Alignments derived from this study were deposited in TreeBASE (submission ID 30581), and taxonomic novelties were deposited in FungalNames.

Table 1.
Strains examined in this study, with information on the source, origin and GenBank accessions of the sequences.
Phylogenetic analyses of the combined dataset were performed using Bayesian inference (BI) and maximum-likelihood (ML) methods. The BI analyses were conducted using MrBayes v. 3.2.1 [34] following the protocol of Wang et al. (2019) [35], with optimisation of each locus treated as a partition in combined analyses, based on the Markov Chain Monte Carlo (MCMC) approach [36]. All characters were equally weighted, and gaps were treated as missing data. The stationarity of the analyses was determined by examining the standard deviation of split frequencies (<0.01) and –ln likelihood plots in AWTY [37]. The ML analyses were conducted using PhyML v. 3.0 [38], with 1000 bootstrap replicates. The general time reversible model was applied with an invariable gamma-distributed rate variation (GTR+I+G).
3. Results
3.1. Phylogenetic Analyses
Analyses of the Microascaceae phylogeny were conducted by using a combined LSU (897 bp), ITS (665 bp), tef1 (924 bp) and tub2 (539 bp) dataset. For the BI analysis, the GTR+I+G model was selected for the LSU, ITS, tef1 and tub2 loci. The phylogeny showed that our isolates were clustered into 18 species in six genera of Microascaceae, namely Cephalotrichum (3 species), Gamsia (1), Microascus (7), Scedosporium (5), Scopulariopsis (1), and Wardomyces (1), including six new species (Figure 2).
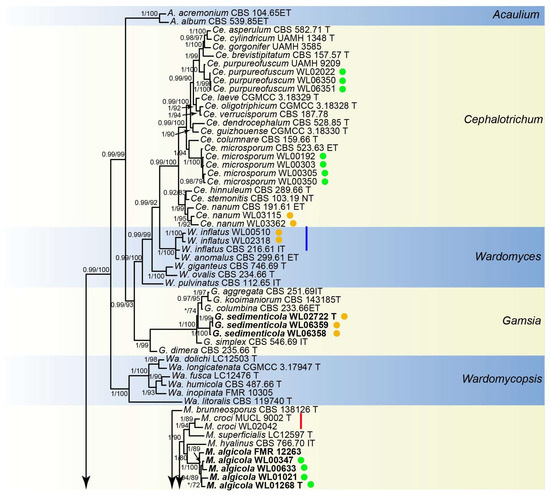
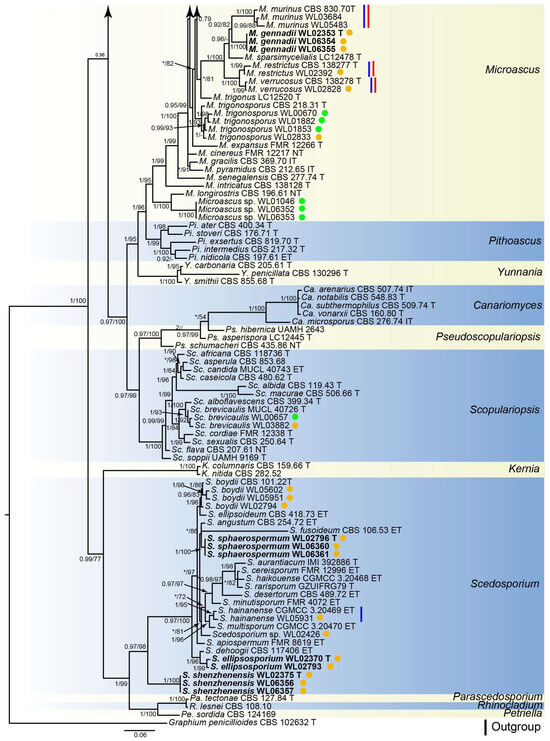
Figure 2.
Fifty percent majority rule consensus tree from a Bayesian analysis based on a four-locus combined dataset (LSU-ITS-tef1-tub2) showing the phylogenetic relationships of genera within the family Microascaceae. The Bayesian posterior probabilities (PP > 0.9) and PhyML bootstrap support values (BS > 50%) are displayed at the nodes (PP/BS). The tree was rooted to Graphium penicillioides (CBS 102632 T). Ex-type cultures are indicated with “T”, epi-type with “ET”, iso-type with “IT”, and neo-type with “NT”. New species introduced in this paper are marked in bold. New records in the marine environment are marked in the blue line and new records in China in the red line. Strains newly isolated in this study are marked in green dots (isolates from algae) and brown dots (isolates from sediment).
3.2. Species List and Taxonomy
Cephalotrichum Link, Mag. Gesell. naturf. Freunde, Berlin 3(1-2): 20 (1809).
Cephalotrichum microsporum (Sacc.) P.M. Kirk, in Kirk and Spooner, Kew Bull. 38(4): 578 (1984).
Examined isolates: CHINA, Shandong Province, Qingdao city, from an unidentified red alga collected from a sand beach, April 2010, Y.T. Peng and K.M. Sun (WL00192); Weihai city, from unidentified green algae, June 2010, K.M. Sun and W. Li (WL00303, WL00305); Weihai city, from an unidentified brown alga, June 2010, K.M. Sun and W. Li (WL00350).
Cephalotrichum nanum (Ehrenb.) S. Hughes, Can. J. Bot. 36: 744 (1958).
Examined isolates: CHINA, Zhejiang Province, Ningbo city (121°46′36.72″ E, 29°58′15.42″ N), from intertidal sediment of a mudflat, July 2014, X.M. Bian and W. Li (WL03115, WL03362).
Cephalotrichum purpureofuscum (S. Hughes) S. Hughes, Can. J. Bot. 36: 744 (1958).
Examined isolates: CHINA, Guangdong Province, Shenzhen city, from Sargassum canfusum collected from a gravels beach, May 2014, M.M. Wang and W. Li (WL02022, WL06350, WL06351).
Gamsia M. Morelet, Ann. Soc. Sci. Nat. Arch. Toulon et du Var 21: 105 (1969).
Gamsia sedimenticolaM.M. Wang, W. Li and L. Cai sp. nov., Figure 3.
FungalNames: FN 571622.
Etymology: named after the habitat of the type specimen, sediment.
Typus: CHINA, Shandong Province, Qingdao city (120°19′09.12″ E, 36°03′38.64″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (HMAS352501, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25342 = WL02722).
Sexual morph not observed. Asexual morph on PDA and OA conidiophores reduced to conidiogenous cells, polyblastic, subcylindrical to cylindrical with a swollen apical part, 2–2.5 × 2–3.5 μm, and hyaline to somewhat darkening at the apex, smooth- and thin-walled, with 1–3 apical conidiogenous loci. Conidia 0–1-septate, ovoid to broadly ellipsoidal, with a rounded-to-pointed apex, flat at the base, pale to dark brown, 7–9 × 5–6.5 μm, smooth and thick walled, and often with a conspicuous longitudinal germ slit.
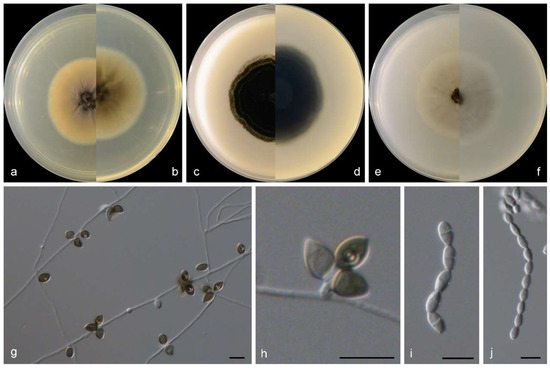
Figure 3.
Morphological characters of Gamsia sedimenticola (from ex-type WL02722). (a–f): colonies on PDA, OA and SNA after 7d; (g,h): Conidiogenous cells; (i,j): Conidia in chains. Bars: (g–j) = 10 μm.
Culture characteristics—colonies on PDA are 30–40 mm diam in 7 d at 25 °C, flat, felty to floccose, and olive green near the centre, with a white regular margin; reverse pale olive green near the centre and white near the margin. Colonies on OA are 30–40 mm diam in 7 d at 25 °C, margin regular, flat, and dark olive green; the reverse is dark olive green. Colonies on SNA are 30–35 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia, and pale olive green near the centre, with a white regular margin; reverse pale olive green near the centre, white near the margin.
Other examined isolates: CHINA, Shandong Province, Qingdao city (120°19′09.12″ E, 36°03′38.64″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (WL06358); ibid. (WL06359).
Notes: The genus Gamsia was erected to accommodate the Wardomyces species that form 1-septate annelloconidia [39]. Sandoval-Denis et al. demonstrated that the lack of well-differentiated conidiophores and the conidial arrangement with large apical clusters justifies the separation of Gamsia from Wardomyces [40]. Five species are currently accepted in Gamsia [39,40,41,42,43], and one new species, G. sedimenticola, was added in this study. Phylogenetically, G. sedimenticola is closely related to G. aggregate (Malloch) Kiffer and M. Morelet, G. columbina (Demelius) Sand.-Den., Guarro and Genéand G. kooimaniorum Sand.-Den. (Figure 2), but differs from G. aggregate and G. kooimaniorum by 29 and 24 bp in the LSU-ITS sequences, and from G. columbina by 34 bp in the LSU-ITS-tef1-tub2 dataset, respectively. Morphologically, the four species exhibit marked differences in characters of aerial conidiophores (reduced to conidiogenous cells, polyblastic, subcylindrical to cylindrical with a swollen apical part, 2–2.5 × 2–3.5 μm in G. sedimenticola vs. borne singly or in small clusters on short branches, flask-shaped, 10–35 × 3–4 μm in G. aggregate, usually unbranched, 0−1-septate, 3–15 × 1.5–3 μm in G. columbina and unbranched or rarely laterally branched once, produced abundantly borne laterally and singly on the aerial hyphae, 0−1(−2)-septate, 12–28 × 1.5–7 μm in G. kooimaniorum), and the shape, size, and colour of conidia (7–9 × 5–6.5 μm, ovoid to broadly ellipsoidal, pale to dark brown in G. sedimenticola vs. 8–10.5 × 3.5–5 μm, ellipsoidal, rounded or apiculate/hyaline in G. aggregate, 5–10.5 × 2.5–5.5 μm, oval, smooth/hyaline in G. columbina and 5.5–10.5 × 4–7 μm, ovoid to broadly ellipsoidal, and pale to dark brown in G. kooimaniorum) [40,41,42,43]. In addition, G. sedimenticola was isolated from intertidal sediment in China, differing from G. aggregate from the dung of a carnivore in the USA, G. columbina from air, soil, decaying wood, and milled rice in Austria, Germany, Japan, and the Netherlands, and G. kooimaniorum from the soil in Netherlands [40,41,42,43].
MicroascusZukal, Verh. Kaiserl.-Königl. zool.-bot. Ges. Wien 35: 342 (1886).
Microascus algicolaM.M. Wang, W. Li and L. Cai sp. nov., Figure 4.
FungalNames: FN 571623.
Etymology: named after the host of type specimen, algae.
Typus: CHINA, Shandong Province, Qingdao city, from Grateloupia filicina collected from a gravels beach, unknown date, M.M. Wang and W. Li (HMAS352502, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25343 = WL01268).
Sexual morph not observed. Asexual morphs on PDA, OA, and SNA conidiophores are simple, straight, septate, branched or rarely unbranched, and hyaline. Conidiogenous cells in whorls of 2–3 on the apex of conidiophores, or rarely solitary on aerial hyphae, lageniform to ampulliform, straight or slightly curved, pale brown, 5–11 × 2.5–4 µm. Conidia in long chains, ellipsoidal, smooth to slightly rough, thick walled, hyaline to pale brown, and 3.5–6 × 3–5 µm.
Culture characteristics—colonies on PDA are 35–45 mm diam in 7 d at 25 °C, flat, felty to floccose, and greyish brown near the centre, with a pale-brown regular margin; the reverse is greyish brown near the centre, white near the margin. Colonies on OA are 35–45 mm diam in 7 d at 25 °C, margin regular, flat, and dark olive green in the centre, with a white regular margin; the reverse is dark olive green near the centre and white near the margin. Colonies on SNA are 30–35 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia, and pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre and with a white regular margin.
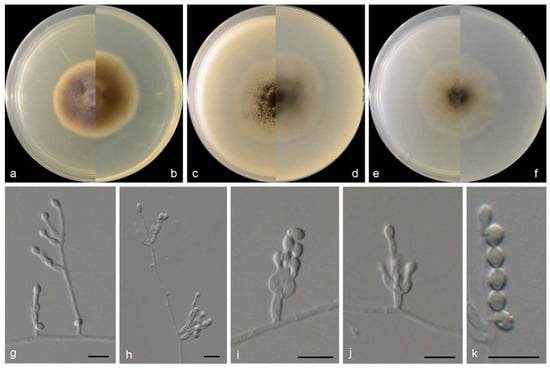
Figure 4.
Morphological characters of Microascus algicola (from ex-type WL01268). (a–f): colonies on PDA, OA and SNA after 7 d; (g–j): Conidiophores and conidiogenous cells; (k): Conidia in chains. Bars: (g–k) = 10 μm. Bars: (g–k) = 10 μm.
Other examined isolates: CHINA, Shandong Province, Weihai city, from an unidentified brown alga collected from a sand beach, June 2010, K.M. Sun and W. Li (WL00347); Qingdao city, from Colpomenia sinuosa from a sand beach, October 2011, Y.T. Peng, C.H. Feng, and C.L. Li (WL00633), from Ahnfeltiopsis flabelliformis from a sand beach, December 2014, M.M. Wang (WL01021).
Notes: phylogenetically, Microascus algicola is most closely related to M. hyalinus (Malloch and Cain) Sand.-Den., Gené and Guarro (Figure 2), but differs by 233 bp in the four loci dataset. Morphologically, M. algicola could be distinguished in the shape and size of conidia (ellipsoidal, 3.5–6 × 3–5 µm), while M. hyalinus produces ovoid, 3.5–5 × 2–3.5 μm conidia [4]. In addition, currently, all known isolates of M. algicola was isolated from marine algae (listed above) in intertidal zones in China, while M. hyalinus was isolated from soil and dung in Europe and North America [44,45].
Microascus croci (J.F.H. Beyma) Sand.-Den., Gené and Guarro, in Sandoval-Denis, Gené, Sutton, Cano-Lira, de Hoog, Decock and Guarro, Persoonia 36: 17 (2015).
Examined isolates: CHINA, Shandong Province, Qingdao city, from Blidingia minima from a gravels beach, May 2014, M.M. Wang and W. Li (WL02042).
Microascus gennadii M.M. Wang, W. Li, and L. Cai sp. nov., Figure 5.
Fungal names: FN 571624.
Etymology: named after the ellipsoidal conidia of this species.
Typus: CHINA, unknown location, from intertidal sediment, May 2014, X.M. Bian and W. Li (HMAS352503, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25344 = WL02353).
Sexual morph is not observed. Asexual morph on PDA and OA conidiophores are simple, straight, septate, branched or rarely unbranched, and hyaline. Conidiogenous cells are solitary on aerial hyphae or in whorls of 2–3 on the apex of conidiophores, lageniform to ampulliform, straight or slightly curved, pale brown, and 9–14 × 2–3.5 µm. Conidia are in long chains, ellipsoidal, smooth to slightly rough, thick-walled, hyaline, and 4–8 × 3–6 µm.
Culture characteristics—colonies on PDA are 35–45 mm diam in 7 d at 25 °C, flat, felty to floccose, and pale grey near the centre, with a pale brown irregular margin; the reverse is pale brown near the centre and white near the margin. Colonies on OA are 35–40 mm diam in 7 d at 25 °C, margin regular, flat, and dark olive green, with a white regular margin; the reverse is dark olive green near the centre and white near the margin. Colonies on SNA are 30–40 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia, and pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre, with a white regular margin.
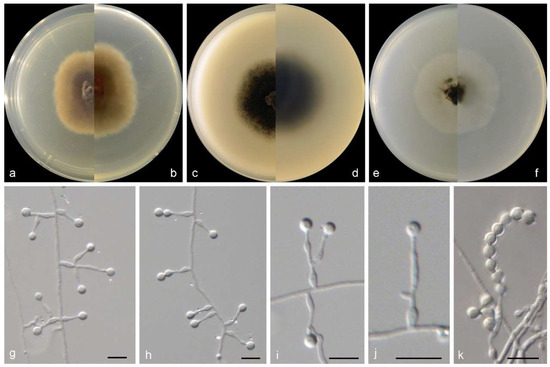
Figure 5.
Morphological characters of Microascus gennadii (from ex-type WL02353). (a–f): colonies on PDA, OA, and SNA after 7 d; (g–j): Conidiophores and conidiogenous cells; (k): Conidia in chains. Bars: (g–k) = 10 μm.
Other examined isolates: CHINA, unknown location, from intertidal sediment, May 2014, X.M. Bian and W. Li (WL06354); ibid. (WL06355).
Notes: phylogenetically, Microascus gennadii is closely related to M. murinus (Samson and Klopotek) Sand.-Den., Gené and Guarro (Figure 2), but differs by 241 bp in the four loci dataset. Morphologically, the two species are distinct in the shape and size of conidia (ellipsoidal, 4–8 × 3–6 µm in M. gennadii vs. cylindrical, 4–6 × 1.5–2 μm in M. murinus) [5].
Microascus croci (Samson and Klopotek) Sand.-Den., Gené and Guarro, in Sandoval-Denis, Gené, Sutton, Cano-Lira, de Hoog, Decock and Guarro, Persoonia 36: 21 (2015).
Examined isolates: CHINA, Shandong Province, Yantai city (120°42′07.50″ E, 37°48′41.22″ N), from the intertidal sediment of a gravel beach, June 2014, X.M. Bian and W. Li (WL03684); Qingdao city (120°11′42.05″ E, 36°3′45.58″ N), from the intertidal sediment of a gravel beach, August 2014, Y. Zheng, H.P. Yang and W. Li (WL05483).
Microascus restrictus Sand.-Den., Gené and Deanna A. Sutton, in Sandoval-Denis, Gené, Sutton, Cano-Lira, de Hoog, Decock and Guarro, Persoonia 36: 21 (2015).
Examined isolates: CHINA, Liaoning Province, Huludao city (120°47′33.34″ E, 40°36′14.56″ N), from the intertidal sediment of a sand beach, May 2014, X.M. Bian and W. Li (WL02392).
Microascus trigonosporus C.W. Emmons and B.O. Dodge, Mycologia 23(5): 317 (1931).
Examined isolates: CHINA, Shandong Province, Qingdao city, from Cladophora sp. from a sand beach, October 2010, Y.T. Peng, C.H. Feng and C.L. Li (WL00670); ibid., from Ulva linza from a sand beach, April 2014, M.M. Wang (WL01853); ibid., from Gracilaria textorii from sand beach, June 2014, M.M. Wang (WL01882); ibid., from the intertidal sediment of a sand beach, X.M. Bian and W. Li (WL02833).
Microascus verrucosus Sand.-Den., Gené and Cano, in Sandoval-Denis, Gené, Sutton, Cano-Lira, de Hoog, Decock and Guarro, Persoonia 36: 23 (2015).
Examined isolates: CHINA, Shandong Province, Dongying city (119°09′45.18″ E, 37°45′34.86″ N), from the intertidal sediment of a mudflat, January 2014, M.M. Wang (WL02828).
Scedosporium Sacc. ex Castell. and Chalm., Manual of Tropical Medicine (London): 1122 (1919).
Scedosporium boydii (Shear) Gilgado, Gené, Cano and Guarro, J. Clin. Microbiol. 46(2): 770 (2008).
Examined isolates: CHINA, Shandong Province, Qingdao city (120°19′06.25″ E, 36°03′34.13″ N), from the intertidal sediment of a sand beach, January 2014, X.M. Bian and W. Li (WL02794); Weihai city (122°10′13″E, 37°30′2″ N), from the intertidal sediment of a sand beach, November 2020, M.M. Wang and Y. Zheng (WL05602); Tianjin city (117°45′38″ E, 39°6′36.64″ N), from the intertidal sediment of a mudflat, April 2021, M.M. Wang and Y. Zheng (WL05951).
Scedosporium ellipsosporium M.M. Wang, W. Li and L. Cai sp. nov., Figure 6.
FungalNames: FN 571625.
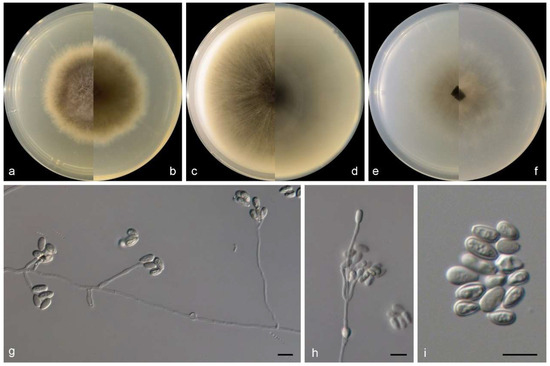
Figure 6.
Morphological characters of Scedosporium ellipsosporium (from ex-type WL02370). (a–f): colonies on PDA, OA, and SNA after 7 d; (g,h): Conidiophores and conidiogenous cells; (i): Conidia. Bars: (g–i) = 10 μm.
Etymology: referring to the shape of conidia, ellipsoidal.
Typus: CHINA, Guangdong Province, Shenzhen city (114°20′45.63″ E, 22°36′01.03″ N), from intertidal sediment of a gravel beach, May 2014, X.M. Bian and W. Li (HMAS352504, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25345 = WL02370).
Sexual morph is not observed. Asexual morph on PDA and SNA conidiophores are solitary, often reduced to a single conidiogenous cell arising laterally from undifferentiated hypha, or stalked, bearing two or three conidiogenous cells at the top. Conidiogenous cells are annellidic, lateral or terminal, hyaline, smooth- and thin-walled, cylindrical to flask-shaped, and 10–40 × 1.5–2.5 µm, with several, distinct annellations at the top with age. Conidia aseptate, solitary, arranged in slimy masses, hyaline, ellipsoidal, flat at the base, and 5–9 × 3–5 µm.
Culture characteristics—colonies on PDA are 40–55 mm diam in 7 d at 25 °C, flat, felty to floccose, and olive green near the centre, with a white regular margin; the reverse is olive green near the centre, and white near the margin. Colonies on OA are 50–65 mm diam in 7 d at 25 °C, flat, and olive green near the centre, with a white regular margin; the reverse is olive green near the centre, and white near the margin. Colonies on SNA are 30–40 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia, and pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre, with a white regular margin.
Other examined isolates: CHINA, Shandong Province, Qingdao city (120°19′09.12″ E, 36°03′38.64″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (WL02793).
Notes: phylogenetically, Scedosporium ellipsosporium is closely related to S. dehoogii Gilgado, Cano, Gené and Guarro (Figure 2), but differs by 42 bp in the ITS-tub2 sequences. Morphologically, the two species could be distinguished in the colour, shape, and size of conidia (hyaline, ellipsoidal, and 5–9 × 3–5 µm in S. ellipsosporium vs. subhyaline or slightly grey, and usually ovate, 5–8 × 5–6 μm in S. dehoogii) [46].
Scedosporium hainanense Zhi Y. Zhang, Y.F. Han and Z.Q. Liang, in Zhang, Shao, Li, Chen, Liang, Han, Huang and Liang, Microbiology Spectrum 9(2): e00867-21, 16 (2021).
Examined isolates: CHINA, Jiangsu Province, Yancheng city (119°52′32″ E, 34°27′27″ N), from intertidal sediment of a mudflat, April 2021, Z.Q. Zeng and C. Liu (WL05931).
Scedosporium shenzhenensis M.M. Wang, W. Li and L. Cai sp. nov., Figure 7.
FungalNames: FN 571626.
Etymology: named after the location of the type specimen, Shenzhen.
Typus: CHINA, Guangdong Province, Shenzhen city (113°56′59.08″ E, 22°31′18.51″ N), from intertidal sediment of a gravel beach, May 2014, X.M. Bian and W. Li (HMAS352505, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25346 = WL02375).
Sexual morph is not observed. Asexual morph on PDA and SNA conidiophores are solitary, often reduced to a single conidiogenous cell arising laterally from undifferentiated hypha, or stalked, bearing two or three conidiogenous cells at the top. Conidiogenous cells are annellidic, lateral or terminal, hyaline, smooth and thin walled, cylindrical to flask-shaped, and 8–30 × 1.5–2.5 µm, with several distinct annellations at the top with age. Conidia aseptate, solitary, arranged in slimy masses, hyaline, ellipsoidal, flat at the base, smooth to slightly rough, and 7–16 × 3.5–7 µm.
Culture characteristics—colonies on PDA are 40–55 mm diam in 7 d at 25 °C, flat, felty to floccose, and pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre and white near the margin. Colonies on OA are 50–65 mm diam in 7 d at 25 °C, flat and pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre with white near the margin. Colonies on SNA are 30–40 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia and are pale olive green near the centre, with a white regular margin; the reverse is pale olive green near the centre, with a white regular margin.
Other examined isolates: CHINA, Guangdong Province, Shenzhen city (113°56′59.08″ E, 22°31′18.51″ N), from intertidal sediment of a gravels beach, May 2014, X.M. Bian and W. Li (WL06356); ibid. (WL06357).
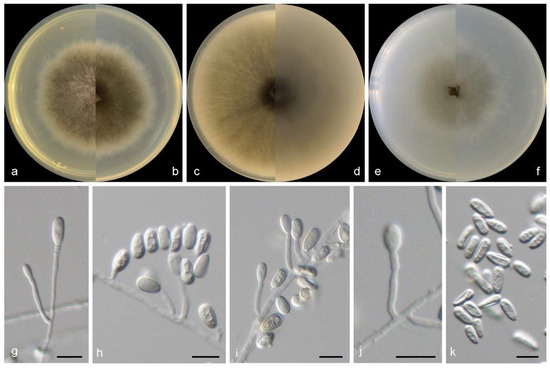
Figure 7.
Morphological characters of Scedosporium shenzhenensis (from ex-type WL02375). (a–f): colonies on PDA, OA, and SNA after 7 d; (g–j): Conidiophores and conidiogenous cells; (k): Conidia. Bars: (g–k) = 10 μm.
Notes: Phylogenetically, Scedosporium shenzhenensis formed a distinct basal clade in Scedosporium (Figure 2). Considering the typical Scedosporium morphology of our isolates (Figure 8), we tentatively placed this species in the genus Scedosporium. Morphologically, S. shenzhenensis is similar to S. aurantiacum Gilgado, Cano, Gené and Guarro, S. dehoogii, S. ellipsosporium (Arx and Fassat.) McGinnis, A.A. Padhye and Ajello and S. sphaerospermum in the cylindrical to flask-shaped conidiogenous cells, but differs in the shape and size of conidia (ellipsoidal, 7–16 × 3.5–7 µm in S. shenzhenensis vs. obovoid, 6–10 × 3–5 µm in S. aurantiacum, obovoid, 5–8 × 5–6 µm in S. dehoogii, ellipsoidal, 5–9 × 3–5 µm in S. ellipsosporium and globose to ellipsoidal, 6–8 × 4–5 µm in S. sphaerospermum) [46,47,48].
Scedosporium sphaerospermum M.M. Wang, W. Li and L. Cai sp. nov., Figure 8.
FungalNames: FN 571627.
Etymology: referring to the globose shape of conidia.
Typus: CHINA, Shandong Province, Qingdao city (120°19′09.12″ E, 36°03′38.64″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (HMAS352506, holotype designated here, dried culture on SNA; culture ex-type CGMCC3.25347 = WL02796).
Sexual morph is not observed. Asexual morph on PDA and SNA conidiophores are solitary, sometimes reduced to a single conidiogenous cell arising laterally from undifferentiated hyphae. Conidiogenous cells are annellidic, lateral or terminal, hyaline, smooth- and thin walled, cylindrical to flask-shaped, and 2–20 × 1.5–2.5 µm, with several, distinct annellations at the top with the age. Conidia aseptate, solitary, arranged in slimy masses, hyaline to pale brown, globose to ellipsoidal, sometimes elliptic to oblong obovate, flat at the base, and 6–8 × 4–5 µm.
Culture characteristics—colonies on PDA are 15–25 mm diam in 7 d at 25 °C, flat, felty to floccose, and pale grey near the centre, with a white regular margin; the reverse is yellowish brown near the centre and white near the margin. Colonies on OA are 20–35 mm diam in 7 d at 25 °C, flat, and pale grey near the centre, with a white regular margin; the reverse is a pale greyish yellow near the centre and white near the margin. Colonies on SNA are 10–20 mm diam in 7 d at 25 °C, flat, with scarce aerial mycelia, and white; the reverse is white.
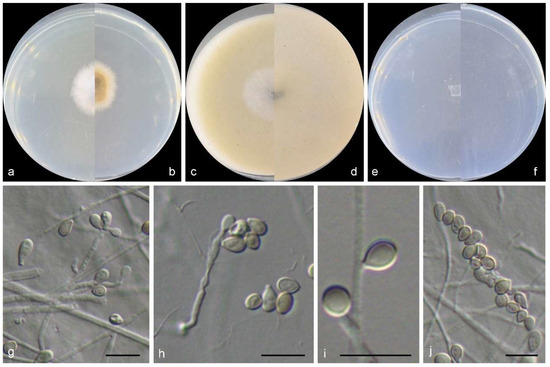
Figure 8.
Morphological characters of Scedosporium sphaerospermum (from ex-type WL02796). (a–f): colonies on PDA, OA, and SNA after 7d; (g–i): Conidiophores and conidiogenous cells; (j): Conidia. Bars: (g–j) = 10 μm.
Other examined isolates: CHINA, Shandong Province, Qingdao city (120°19′09.12″ E, 36°03′38.64″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (WL06360); ibid. (WL06361).
Notes: phylogenetically, S. sphaerospermum forms a sister clade to S. angustum and S. fusoideum (Arx) McGinnis, A.A. Padhye and Ajello in the genus Scedosporium (Figure 2), and differs from the latter species by 36 bp and 129 bp in the LSU-ITS-tef1-tub2 dataset, respectively. Morphologically, the three species could be distinguished by the characters of the sexual morph (absence in S. sphaerospermum, ascomata spherical, nonostiolate, dark brown, 100–150 µm, with allipsoidal or nearly cylindrical with rounded ends ascospores in S. angustum and ascomata submerged or semi-immersed, spherical or nearly so, non-ostiolate, dark brown, 50–100 µm, with broadly fusiform, and yellowish or straw-coloured ascospores in S. fusoideum), and shape and size of conidia (globose to ellipsoidal, 6–8 × 4–5 µm S. sphaerospermum vs. clavate or nearly cylindrical, 5–10 × 3–4.5 µm in S. angustum and clavate, 6–10 × 3.5–5 µm in S. fusoideum) [49].
Scopulariopsis Bainier, Bull. Soc. mycol. Fr. 23(2): 98 (1907).
Scopulariopsis brevicaulis (Sacc.) Bainier [as ‘brevicaule’], Bull. Soc. mycol. Fr. 23(2): 99 (1907).
Examined isolates: CHINA, Shandong Province, Qingdao city, from Ulva pertusa from a sand beach, October 2011, Y.T. Peng, C.H. Feng, and C.L. Li (WL00657); Fujian Province, Ningde city (120°03′39.98″ E, 26°52′51.89″ N), from intertidal sediment of a mudflat, June 2014, X.M. Bian and W. Li (WL03882).
Wardomyces F.T. Brooks and Hansf., Trans. Br. Mycol. Soc. 8(3): 137 (1923).
Wardomyces inflatus (Marchal) Hennebert, Trans. Br. Mycol. Soc. 51(5): 755 (1968).
Examined isolates: CHINA, Shandong Province, Weihai city, from intertidal sediment of a sand beach, June 2010, K.M. Sun and W. Li (WL00510); Liaoning Province, Huludao city (120°47′33.34″ E, 40°36′14.56″ N), from intertidal sediment of a sand beach, May 2014, X.M. Bian and W. Li (WL02318).
4. Discussion
A systemic survey of fungal resources in Chinese intertidal areas has been implemented by us since 2007, and more than 3000 fungal strains were successfully isolated from thousands of marine algae and sediment samples. Based on morphological and phylogenetic analyses, approximately 600 species have been identified from these fungal strains (unpublished data). Using brine shrimp, agricultural pests, and plant pathogens as targets, we have screened the bioactivities of 181 fungal strains and found more than half of them displayed marked insecticidal and antifungal activities [19]. These results are consistent with the notion that the intertidal zone is an ideal shelter for hosting numerous fungi that can produce diverse metabolic activities [19,50].
Most of the microascaceous fungi have been previously reported as saprobes in seawater and sediments, and on mangrove plants and marine algae [8,9,10]. They can also act as endophytes on marine algae and seaweeds or be isolated from the inner tissue of marine animals such as marine sponges [7,8,9,51] (Table 2). In this study, 18 microascaceous species (48 isolates) were retrieved from Chinese intertidal areas. According to the catalogue shown in Table 2, the number of marine microascaceous species increased from 22 to 33 under our efforts. The newly increased species include six new species (Gamisa sedimenticola, Microascus algicola, M. gennadii, Scedosporium ellipsosporium, S. shenzhenensis, and S. sphaerospermum) and five new records for the marine environment (M. murinus, M. restrictus, M. verrucosus, S. hainanense, and Wardomyces inflatus).
Although only a small proportion of microascaceous fungi were recovered (~3% of the total fungal species recovered), we observed a relatively wide distribution of these fungi across different geographic regions. This distribution ranges from the higher-latitude regions, such as the Liaoning and Hebei provinces, to the lower regions, such as Fujian and Guangdong provinces. Furthermore, diverse substrates were also found to host microascaceous fungi in this study, such as several marine algae and sediments from mudflats, sandy beaches, and gravel beaches. These findings are consistent with our previous investigation using a molecular approach [14] and research based on culturable methods [8,52,53,54,55], This also suggests that microascaceous fungi probably possess a wide geographical distribution, diverse habitats/hosts, and multiple ecological functions in the ocean.
Altogether, our results broaden the information related to the diversity, distribution, and habitat of microascaceous fungi in the marine environment. However, the potential ecological roles and bioactivities of these microascaceous fungi are currently unknown and need further investigation.

Table 2.
Records of Microascaceae in the marine environment.
Table 2.
Records of Microascaceae in the marine environment.
| Species | Country/Region/Location | Known Habitats/Hosts | Recorded Database/Reference |
|---|---|---|---|
| Acaulium acremonium | na | na | MF |
| Cephalotrichum microsporum | China; Europe | unidentified brown algae, green algae, and red algae | WoRMS; this study |
| Ce. nanum | China; Europe | Intertidal sediment | WoRMS; this study |
| Ce. purpureofuscum | China | Marine algae Sargassum canfusum | This study |
| Ce. stemonitis | Europe | na | MF and WoRMS |
| Gamsia sedimenticola * | China | Intertidal sediment | This study |
| Microascus algicola * | China | Unidentified brown algae; marine algae Ahnfeltiopsis flabelliformis, Colpomenia sinuosa, Grateloupia filicina | This study |
| M. croci# | China; Hawaiian, Line, and Phoenix Islands | Marine algae Blidingia minima; coastal sands | [52]; This study |
| M. gennadii * | China | Intertidal sediment | This study |
| M. murinus +# | China | Intertidal sediment | This study |
| M. paisii | na | na | MF |
| M. restrictus +# | China | Intertidal sediment | This study |
| M. senegalensis | Senegal | Mangrove soil | [53] |
| M. trigonosporus | China, Europe; Hawaiian, Line, and Phoenix Islands | Marine algae Cladophora sp., Ulva linza, Gracilaria textorii; intertidal sediment; coastal sand | MF and WoRMS; [52] |
| M. verrucosus +# | China | Intertidal sediment | This study |
| Petriella sordida | na | na | MF |
| Scedosporium boydii | China | Intertidal sediment | MF; this study |
| S. dehoogii | China | Coastal sediment | [8] |
| S. ellipsosporium * | China | Intertidal sediment | This study |
| S. hainanense + | China | Intertidal sediment | This study |
| S. marinum | India | Decaying woody stem of Suaeda monoica | [54] |
| S. shenzhenensis * | China | Intertidal sediment | This study |
| S. sphaerospermum * | China | Intertidal sediment | This study |
| Scopulariopsis brevicaulis | China, Europe, Croatia; Hawaiian, Line, and Phoenix Islands | Marine sponge Tethya aurantium; marine algae Ulva pertusa; intertidal sediment; coastal sands | MF and WoRMS; [52]; This study |
| Sc. Brumptii | Hawaiian, Line, and Phoenix Islands | Coastal sands | MF; [52] |
| Sc. Candida | Egypt | Salt marshes | MF; [55] |
| Sc. Coprophila | Hawaiian, Line, and Phoenix Islands | Coastal seawater and sands | [52] |
| Sc. Fusca | Europe | na | WoRMS |
| Sc. Halophilica | na | na | MF and WoRMS |
| Sc. Hibernica | na | na | MF |
| Wardomyces anomalus | na | na | MF |
| W. inflatus+ | China | Intertidal sediment | This study |
| Yunnania carbonaria | Hawaiian | Coastal seawater | [52] |
Notes: new species described in this study are marked as “*”, new records for the marine environment with “+”, and new records for China with “#”. MS = the Marine Fungi database (https://www.marinefungi.org/ (accessed on 30 October 2023)), WoRMS = the World Register of Marine Species database (https://www.marinespecies.org/ (accessed on 30 October 2023)), na = no data available.
Author Contributions
Conceptualization, M.-M.W., W.L. and L.C.; methodology, M.-M.W., S.-Y.Y., Q.L. and Y.-H.T.; software, M.-M.W.; investigation, M.-M.W., Y.Z. and H.-H.M.; resources, M.-M.W., Y.Z. and H.-H.M.; data curation, M.-M.W.; writing—original draft preparation, M.-M.W.; writing—review and editing, W.L. and L.C.; visualization, M.-M.W.; supervision, W.L. and L.C.; project administration, M.-M.W.; funding acquisition, W.L. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Fundamental Resources Investigation Program (Grant No. 2019FY100700), the National Natural Science Foundation of China (32100005), and the STU Scientific Research Initiation Grant (NTF23002, NTF23018T).
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences generated in this study were submitted to GenBank (https://www.ncbi.nlm.nih.gov, accessed on 25 December 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luttrell, E.S. Taxonomy of the Pyrenomycetes. Univ. Mo. Stud. 1951, 24, 1–121. [Google Scholar]
- Malloch, D. New concepts in the Microascaceae illustrated by two new species. Mycologia 1970, 62, 727–740. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhou, S.Y.; Eurwilaichitr, L.; Ingsriswang, S.; Raza, M.; Chen, Q.; Zhao, P.; Liu, F.; Cai, L. Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Divers 2021, 106, 29–126. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.; Saxena, R.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarro, J.; Cano-Lira, J.F.; Sutton, D.; Wiederhold, N.; de Hoog, G.; Abbott, S.; Decock, C.; Sigler, L.; Gené, J. Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Stud. Mycol 2016, 83, 193–233. [Google Scholar] [CrossRef]
- Tubaki, K. An undescribed halophilic species of Scopulariopsis. Trans Mycol Soc Jpn. 1973, 14, 367–369. [Google Scholar]
- Clipson, N.; Landy, E.; Otte, M. European register of marine species: A check-list of the marine species in Europe and a bibliography of guides to their identification. Collect. Patrim. Nat. 2001, 50, 15–19. [Google Scholar]
- Feng, C.H.; Li, C.L.; Peng, Y.T.; Sun, K.; Li, W. A preliminary report of hyphomycetes from sediment of southern Yellow Sea. Mycosystema 2013, 32, 35–41. [Google Scholar]
- Jones, E.B.G.; Suetrong, S.; Sakayaroj, J.; Bahkali, A.H.; Abdel-Wahab, M.A.; Boekhout, T.; Pang, K.-L. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 2015, 73, 1–72. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.M.; Wang, X.G.; Cheng, X.L.; Guo, J.J.; Bian, X.M.; Cai, L. Fungal communities in sediments of subtropical Chinese seas as estimated by DNA metabarcoding. Sci. Rep. 2016, 6, 26528. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.M.; Pan, H.Q.; Burgaud, G.; Liang, S.; Guo, J.; Luo, T.; Li, Z.; Zhang, S.; Cai, L. Highlighting patterns of fungal diversity and composition shaped by ocean currents using the East China Sea as a model. Mol. Ecol. 2018, 27, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Ma, Y.Y.; Cai, L.; Tedersoo, L.; Bahram, M.; Burgaud, G.; Long, X.; Zhang, S.; Li, W. Seasonal dynamics of mycoplankton in the Yellow Sea reflect the combined effect of riverine inputs and hydrographic conditions. Mol. Ecol. 2021, 30, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.M.; Bian, X.M.; Guo, J.; Cai, L. A high-level fungal diversity in the intertidal sediment of Chinese Seas presents the spatial variation of community composition. Front. Microbiol. 2016, 7, 2098. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.M.; Burgaud, G.; Yu, H.; Cai, L. Fungal community composition and potential depth-related driving factors impacting distribution pattern and trophic modes from epi- to abyssopelagic zones of the Western Pacific Ocean. Microb. Ecol. 2019, 78, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Wong Chin, J.M.; Puchooa, D.; Bahorun, T.; Jeewon, R. Antimicrobial properties of marine fungi from sponges and brown algae of Mauritius. Mycology 2021, 22, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.F.; Liu, X.; Xu, R.F.; Wei, M.Y.; Fang, Y.W.; Shao, C.L. Scopuquinolone B, a new monoterpenoid dihydroquinolin-2(1H)-one isolated from the coral-derived Scopulariopsis sp. Fungus. Nat. Prod. Res. 2018, 32, 773–776. [Google Scholar] [CrossRef]
- Kramer, A.; Beck, H.C.; Kumar, A.; Kristensen, L.P.; Imhoff, J.F.; Labes, A. Proteomic analysis of anti-cancerous Scopularide production by a marine Microascus brevicaulis strain and its UV mutant. PLoS ONE 2015, 10, e0140047. [Google Scholar] [CrossRef]
- Wang, X.F.; Ji, G.X.; Cun, J.F.; Xu, P.J.; Wang, X.W.; Ren, G.W.; Li, W. Screening of insecticidal and antifungal activities of the culturable fungi isolated from the intertidal zones of Qingdao, China. J. Fungi 2022, 8, 1240. [Google Scholar] [CrossRef]
- Zeng, C.K. Seaweeds in Yellow Sea and Bohai Sea of China; Science Press: Beijing, China, 2009; 453p. [Google Scholar]
- Zhang, Z.F.; Liu, F.; Zhou, X.; Liu, X.; Liu, S.; Cai, L. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 2017, 39, 1–31. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Venkatachalam, A.; Thirunavukkarasu, N.; Ravishankar, J.P.; Doble, M.; Geetha, V. Internal mycobiota of marine macroalgae from the Tamilnadu coast: Distribution, diversity and biotechnological potential. Bot. Mar. 2010, 53, 457–468. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.Y.; Cai, L. An optimized protocol of single spore isolation for fungi. Cryptogam. Mycol. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Nirenberg, H.I. Untersuchungen über die morphologische und biologische differenzierung in der Fusarium-Sektion Liseola. Mitteilungen Biol. Bundesanst. Land Forstwirtsch. 1976, 169, 1–117. [Google Scholar]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978. [Google Scholar]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to the Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Rehner, S.A.; Buckley, E.A. Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Env. Microbiol 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic ModelAveraging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nylander, J.A.A.; Wilgenbusch, J.C.; Warren, D.L.; Swofford, D.L. AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24, 581–583. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Morelet, M. Micromycètes du var et d’ailleurs (2me Note). Ann. Société Sci. Nat. D’archéologie Toulon Var. 1969, 21, 104–106. [Google Scholar]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Cano-Lira, J.; de Hoog, G.; Decock, C.; Wiederhold, N.; Guarro, J. Redefining Microascus, Scopulariopsis and allied genera. Persoonia 2016, 36, 1–36. [Google Scholar] [CrossRef]
- von Arx, J.A. The Genera of Fungi Sporulating in Pure Culture, 3rd ed.; V.J. Cramer: Vaduz, Liechtenstein, 1981; pp. 1–424. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.; Hardy, G.; Smith, D.; Summerell, B.; Cano-Lira, J.; Guarro, J.; Houbraken, J.; et al. Fungal Planet description sheets: 625-715. Persoonia 2017, 39, 270–467. [Google Scholar] [CrossRef]
- Malloch, D. Wardomyces aggregatus sp. nov. and its possible relationship to Gymnodochium fimicolum. Can. J. Bot. 1970, 48, 883–885. [Google Scholar] [CrossRef]
- Malloch, D.; Cain, R.F. The genus Kernia. Can. J. Bot. 1971, 49, 855–867. [Google Scholar] [CrossRef]
- Guarro, J.; Gené, J.; Stchigel, A.M.; Figueras, M.J. Atlas of soil ascomycetes. In CBS Biodiversity Series; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012. [Google Scholar]
- Gilgado, F.; Cano, J.; Gené, J.; Sutton, D.A.; Guarro, J. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J. Clin. Microbiol. 2008, 46, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Gilgado, F.; Cano, J.; Gené, J.; Guarro, J. Molecular phylogeny of the Pseudallescheria boydii species complex: Proposal of two new species. J. Clin. Microbiol. 2005, 43, 4930–4942. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, M.; Duan, S.Y.; Sorrell, T.C.; Meyer, W.; Chen, S.C.A. The genus Scedosporium and Pseudallescheria: Current challenges in laboratory diagnosis. Curr. Clin. Micro. Rpt. 2014, 1, 27–36. [Google Scholar] [CrossRef]
- von Arx, J.A. The genera Petriellidium and Pithoascus (Microascaceae). Persoonia 1973, 7, 367–375. [Google Scholar]
- Burgaud, G.; Edgcomb, V.P.; Hassett, B.T.; Kumar, A.; Li, W.; Mara, P.; Peng, X.; Philippe, A.; Phule, P.; Prado, S.; et al. Marine fungi. In The Marine Microbiome; Stal, L.J., Cretoiu, M.S., Eds.; Springer: Cham, Switzerland, 2022; Volume 2, pp. 243–295. [Google Scholar]
- Yu, Z.G.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W. Fungus populations in marine waters and coastal sands of the Hawaiian Line, and Phenix Islands. Pac. Sci. 1967, 21, 317–331. [Google Scholar]
- von Arx, J.A. Revision of Microascus with the description of a new species. Persoonia 1975, 8, 191–197. [Google Scholar]
- Jayawardena, R.S.; Hyde, K.D.; Wang, S.; Sun, Y.-R.; Suwannarach, N.; Sysouphanthong, P.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Abeywickrama, P.D.; Abreu, V.P.; et al. Fungal diversity notes 1512–1610: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 2022, 117, 1–272. [Google Scholar] [CrossRef]
- Abdel-Fattah, H.M.; Moubasher, A.H.; Abdel-Hafez, S.I. Studies on mycoflora of salt marshes in Egypt. I. Sugar Fungi. Mycopathologia 1977, 61, 19–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).