Abstract
Magnaporthe oryzae, a fungal pathogen that causes rice blast, which is the most destructive disease of rice worldwide, has the potential to perform both asexual and sexual reproduction. MAT loci, consisting of MAT genes, were deemed to determine the mating types of M. oryzae strains. However, investigation was rarely performed on the development and molecular mechanisms of the sexual reproduction of the fungus. In the present work, we analyzed the roles of two MAT loci and five individual MAT genes in the sex determination, sexual development and pathogenicity of M. oryzae. Both of the MAT1-1 and MAT1-2 loci are required for sex determination and the development of sexual structures. MAT1-1-1, MAT1-1-3 and MAT1-2-1 genes are crucial for the formation of perithecium. MAT1-1-2 impacts the generation of asci and ascospores, while MAT1-2-2 is dispensable for sexual development. A GFP fusion experiment indicated that the protein of MAT1-1-3 is distributed in the nucleus. However, all of the MAT loci or MAT genes are dispensable for vegetative growth, asexual reproduction, pathogenicity and pathogenicity-related developments of the fungus, suggesting that sexual reproduction is regulated relatively independently in the development of the fungus. The data and methods of this work may be helpful to further understand the life cycle and the variation of the fungus.
1. Introduction
Sexual reproduction is one of the key events in the evolution of life, as it provides more abundant genetic variation and progeny diversity, which helps organisms survive and adapt to changing environments [1]. Compared to asexual reproduction, sexual reproduction involves more complex processes, such as plasmogamy, karyogamy and meiosis [2].
Fungi are composed of organisms belonging to more than 120,000 species, ranging from yeasts to higher mushrooms, and among them, many economically important species are used in industrial production and the food industry or for their ability to cause destructive diseases in plants. Reproduction in fungal species involves sexual and asexual processes, and in many cases, the two processes coexist in one species, making fungi a good model for understanding sexual evolution. The sexual reproduction of fungal species includes two types, homothallism and heterothallism. Homothallic fungal species are capable of self-mating, whereas heterothallic ones require compatible partners with opposite mating types to complete the mating and sexual reproductive process.
The gene loci determining the mating types are designated as MAT loci, generally as MAT1-1 and MAT1-2 in the heterothallic species, which represent the opposite mating types [3]. A heterothallic strain only encounters strains with the opposite mating type, and then fertilization can occur [4]. Each of the MAT loci usually contains several genes. Although MAT genes are factors that promote sexual development and determine the reproductive mode [5], their biological functions remain largely undefined and vary greatly in different species [6,7].
Magnaporthe oryzae (syn. Pyricularia oryzae) causes rice blast, the most destructive disease of rice, and is ranked top of the list of the top 10 fungal pathogens [8]. The fungus has evolved diverse races and pathotypes that infect various rice cultivars and other gramineous crops and weeds, such as wheat, barley and ryegrass. M. oryzae is a heterothallic ascomycete and possesses the ability of both asexual and sexual reproduction [9]. In rice fields, the spread and host infection of the fungus is accomplished by asexual spores. While in the lab, when two proper strains of opposite mating types are co-cultured, sexual reproduction can occur, forming sexual structures, such as perithecia, asci and ascospores [10].
In the past three decades, the molecular basis of the pathogenicity of M. oryzae has been investigated intensively. Hundreds of genes involved in infection and asexual reproduction have been identified and functionally characterized [11,12,13]. To date, the sexual reproduction of the fungus has still not been directly found in its natural environment. Despite this, the population structures and the predominant races of M. oryzae in the field change rapidly, which makes rice blast disease difficult to efficiently control. Such a rapid variation of the fungus hints at the possible existence of natural sexual reproduction. Moreover, studies on population genomics in recent years support this speculation [14,15]. An investigation of the sexual mechanism of M. oryzae is thus significant for a better understanding of whether and how sexual reproduction contributes to genetic diversity and pathogenicity variation. However, the molecular mechanisms of sexual reproduction of the fungus remain largely unknown, and the functions of the MAT genes have not yet been characterized by direct molecular strategies.
In the present work, we investigated the functions of the MAT loci and MAT genes in sexual development and the related processes using gene knockout strategies in M. oryzae. To our knowledge, this is the first direct investigation of the sexual processes at the molecular level in the fungus. The resulting data and strains may be helpful to further understand the life cycle and the variation of the fungus.
2. Materials and Methods
2.1. Strains and Culture
M. oryzae strains Guy11 (MAT1-1), TH16 (MAT1-1), 2539 (MAT1-2), 70-15 (MAT1-2) and TH3 (MAT1-2) were used as wild-type strains and cultured using routine methods. Complete medium (CM) was used to culture the strains for vegetative growth and asexual processes.
2.2. Sexual Reproduction
The M. oryzae strains Guy11, TH16, 2539, 70-15 and TH3 were cross-cultured on oatmeal medium (OMA), pairwise according to opposite mating types, to induce sexual reproduction. The OMA cultures were first incubated at 28 °C with 12/12 h light–dark cycles for 7 days and then moved to 22 °C with full illumination for 15 days. The experiments were repeated 3 times, and 3 replicates were performed for each group.
2.3. MAT Loci Deletion and Mutant Recovery
MAT1-1 and MAT1-2 loci were deleted by homologous recombination strategies. MAT1-1 was deleted in Guy11 and TH16, whereas MAT1-2 was deleted in 70-15, 2539 and TH3. The up- and down-stream fragments of MAT1-1 and MAT1-2 were amplified using the DNA sample from Guy11 or 70-15 as a template and ligated into pKO-Hph [16] by Pst I/BamH I and EcoR I/Xho I digestion, respectively, to generate two knockout vectors, pKO-MAT1-1 and pKO-MAT1-2. Then, pKO-MAT1-1 and pKO-MAT1-2 were introduced into the proper M. oryzae strains via Agrobacterium tumefaciens-mediated transformation (AtMT) to induce the targeted replacement of MAT1-1 or MAT1-2 loci with a hygromycin resistance gene (Hph), generating the MAT loci deletion mutants Guy11Δmat1, 70-15Δmat2, 2539Δmat2 and TH3Δmat2.
For mutant recovery, the fragments covering the full length of the MAT loci were amplified and inserted into p1300-Bar (Li et al. 2014) by Pst I/BamH I digestion to generate the complementary vectors p1300-MAT1-1 and pl300-MAT1-2. The vectors were integrated into the corresponding mutants using AtMT to generate the MAT loci recovery strains Guy11Δmat1/MAT1, 70-15Δmat2/MAT2, 2539Δmat2/MAT2 and TH3Δmat2MAT2.
2.4. MAT Gene Deletion and Mutant Recovery
For MAT gene deletion, the up- and down-stream fragments of each MAT gene were amplified and inserted into pKO-Hph (Li et al. 2014) via Pst I/BamH I, EcoR I/Xho I, Hind III/BamH I, Sma I/Kpn I and Sma I/Xho I digestion to generate the gene replacement vectors pKO-MAT1-1-1, pKO-MAT1-1-2, pKO-MAT1-1-3, pKO-MAT1-2-1 and pKO-MAT1-2-2, respectively. The vectors were introduced into the proper M. oryzae strains via AtMT to induce the targeted replacement of the MAT genes with the Hph gene, generating the mutants Guy11Δ111, Guy11Δ112, Guy11Δ113, 70-15Δ121, 70-15Δ122, 2539Δ122 and TH3Δ122.
For mutant recovery, the fragments containing the full length of the individual MAT genes were amplified and inserted into p1300-Bar via Pst I/BamH I digestion to generate the complementary vectors p1300-MAT1-1-1, p1300-MAT1-1-2, p1300-MAT1-1-3, p1300-MAT1-2-1 and p1300-MAT1-2-2. The vectors were integrated into the corresponding mutant strains via AtMT, generating the recovery strains Guy11Δ111/111, Guy11Δ112/112, Guy11Δ113/113, 70-15Δ121/121, 70-15Δ122/122, 2539Δ122/122 and TH3Δ122/122.
Genomic PCR was used to identify and confirm the recombinant events by applying targeted genes, the Hph gene and up- and down-stream fragments.
2.5. RNA Extraction and qRT-PCR
The wild-type strains, Guy11, 70-15, TH3 and TH16, and the mutant strains, Guy11Δ111, Guy11Δ112, Guy11Δ113, 70-15Δ121 and 70-15Δ122, were incubated separately or pairwise according to opposite mating types on OMA. The RNA extraction and cDNA synthesis were performed using a reagent kit (TIANGEN, Beijing, China), following the manufacturer’s instructions. The RT-qPCR experiments were performed with the cDNA samples as templates by following the manual of the reagent kit (Roche, Basel, Switzerland). The relative transcription levels were calculated with the β-tubulin gene (MGG_00604) as a reference. For each experiment, at least three independent biological replicates were conducted and statistically analyzed.
All of the primers used for vector construction, the confirmation of mutants and recovery strains and qRT-PCR are listed in Table S1.
2.6. Microscopic Analysis
The sexual structures were observed under a light microscope Axio Imager A2 ( Zeiss, Jena, Germany) and a stereo microscope SZX2-ILLT (Olympus, Tokyo, Japan). The structures were also paraffin sectioned or stained with calcofluor white (50 μg/mL) and detected under a fluorescence microscope Axio Imager A2 (Zeiss, Jena, Germany) or a laser scanning confocal fluorescence microscope LSM880 (Zeiss, Jena, Germany). Cryoelectronic scanning electron microscopy Regulus 8100 (Hitachi, Tokyo, Japan) and transmission electron microscopy H7650 (Hitachi, Tokyo, Japan) were also used to analyze the ultrastructure of the sexual offspring.
2.7. Subcellular Distribution
To monitor the subcellular distribution of the MAT genes in M. oryzae, the coding sequences of the MAT genes were amplified and introduced into the vector p1300BMG-C [16] by Sma I/EcoR I digestion to generate pBMG-111, pBMG-112, pBMG-113, pBMG-121 and pBMG-122. The vectors were introduced into the wild-type strain 70-15 via AtMT, resulting in the fluorescent transformants 70-15PBMG-111, 70-15PBMG-112, 70-15PBMG-113, 70-15PBMG-121 and 70-15PBMG-122, which were selected and confirmed by genomic PCR and GFP fluorescence detection. The fluorescence of the strains was detected under a fluorescence microscope.
2.8. Assays for Asexual Reproduction, Carbon Utilization, Chemical Resistance and Pathogenicity-Related Developments
The experiments to assay vegetative growth, asexual reproduction, carbon utilization, chemical resistance, host inoculation and pathogenicity-related developments were all performed using typical methods as described previously [16].
3. Results
3.1. Both of the MAT Loci Are Indispensable for Sexual Reproduction
The functions of the MAT1-1 and MAT1-2 loci (abbr. MAT1 and MAT2) in the sexual development of M. oryzae were investigated via gene knockout strategies. Mutants lacking MAT1-1 were derived from Guy11, and those lacking MAT1-2 were derived from 70-15, 2539 and TH3. Then, the sexual development ability of the mutants was tested by crossing experiments. The combinations of the wild-type strains could generate asci and ascospores normally (Figure 1; Table 1). When the Δmat1 and Δmat2 strains were crossed with their corresponding wild-type strains, such as Guy11Δmat1 with TH3, 70-15Δmat2 with TH16, 2539Δmat2 with TH16 and TH3Δmat2 with Guy11, the sexual structure was hardly formed. In addition, the recovery strains Guy11Δmat1/MAT1, 70-15 Δmat2/MAT2, 2539Δmat2/MAT2 and TH3Δmat2/MAT2 regained the ability to form sexual structures when mated with the strains with the opposite mating type. Moreover, the morphology of the perithecia and asci that were formed by the recovery strains showed no difference compared to those by the wild-type combinations (Figure 1). The data indicated that the MAT loci were indispensable for perithecium formation and sexual development.
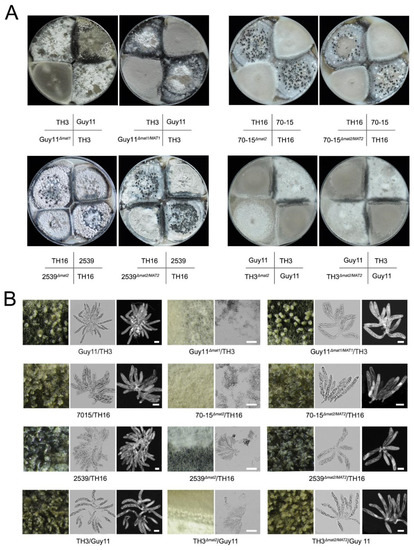
Figure 1.
Characterizing the function of MAT loci in sexual development by the gene deletion strategy. (A) Comparison of the development of perithecia of wild-type, MAT loci mutants and recovery strains by crossing with their partner strains on OMA plates; (B) the perithecia generated in different combinations were observed in 60× magnification under a stereomicroscope, and the asci were stained with calcofluor white and detected under a fluorescence microscope. Bars = 20 μm.

Table 1.
The numbers of perithecia formed in the cross of MAT loci mutants, the wild-type strains and complement strains on OMA plates (×104/plate).
3.2. MAT1-1-2 and MAT1-1-3 Genes Are Up-Regulated during Sexual Reproduction
To clarify the expression patterns of the MAT genes during the mating process, we cross-cultured the wild-type strains Guy11 and 70-15 on OMA for 20 days and investigated the relative transcription levels of each MAT gene at 5, 10, 15 and 20 dpc (days post-cultivation). As predicted, the transcripts of MAT1-1-1, MAT1-1-2 and MAT1-1-3 could be detected only in the Guy11 strain, while MAT1-2-1 and MAT1-2-2 only in 70-15 (Figure 2). In cross-cultured Guy11/70-15, the transcription levels of MAT1-1-2 and MAT1-1-3 were found to be significantly increased compared to those in the separately cultured Guy11 strain, suggesting that the MAT1-1-2 and MAT1-1-3 genes were up-regulated during sexual reproduction. Meanwhile, the transcription levels of all MAT genes tended to peak at 10–15 dpc, the key period for the formation of perithecium; then, along with the development of the sexual processes, the transcription of the genes reduced gradually.
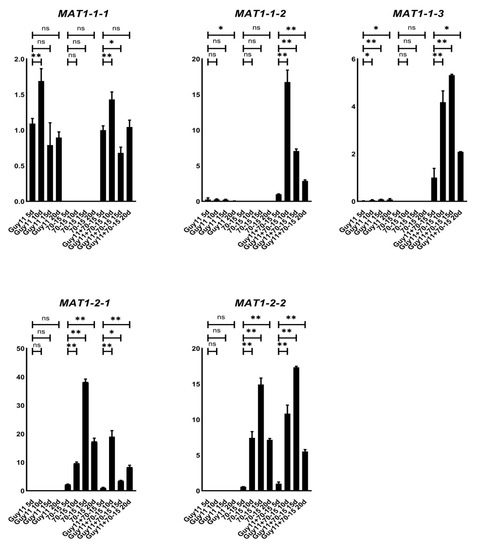
Figure 2.
Transcription levels of each individual MAT gene. The relative transcription levels of each individual MAT gene in wild-type strains (70-15 and Guy11) cultured alone and during the sexual process (70-15 crossed with Guy11) were tested using qRT-PCR at 5 10, 15 and 20 dpc (days post-cultivation). The expression levels in Guy11 strain cultured alone on OMA were used as control. Mean and standard error were calculated from three independent biological replicates. The difference significance for each gene in the different samples was calculated by comparing the genes in the cross-cultured samples (Guy11 + 70-15) at 5 dpc. Single stars indicate the significance at the 0.5 level and double stars indicate it at the 0.01 level.
3.3. Subcellular Distribution of the MAT Proteins
To elucidate the subcellular distribution of the MAT proteins, GFP-tagged versions of the MAT genes were constructed and introduced into M. oryzae strain 70-15, generating the fluorescent strains 70-15PBMG-112, 70-15PBMG-113 and 70-15PBMG-121. The 70-15PBMG-113 strain emitted fluorescence as a punctate pattern concentrated at the nucleic regions, indicating that MAT1-1-3 was distributed in the cell nucleus (Figure 3), corresponding with its potential as a transcription factor. However, the 70-15PBMG-112 and 70-15PBMG-121 strains emitted dispersive fluorescence in the hyphae, which suggested the cytoplasmic distribution of MAT1-1-2 and MAT1-2-1.
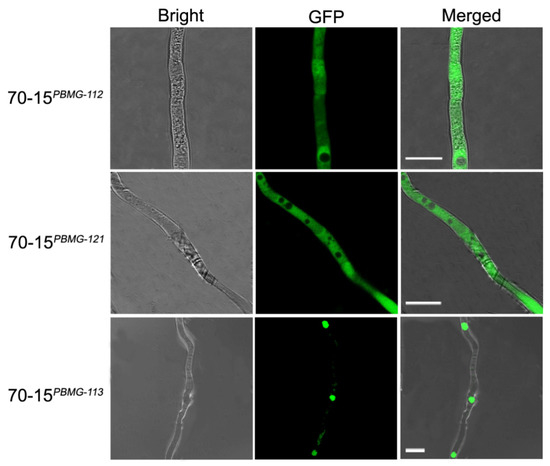
Figure 3.
Subcellular distribution of the MAT proteins in 70-15 were detected using the GFP fusion method. The GFP fluorescence in hyphae of 70-15PBMG-112, 70-15PBMG-121 and 70-15PBMG-113 was observed under a fluorescence microscope. MAT1-1-3 is distributed in the nucleus while MAT1-1-2 and MAT1-2-1 are cytosolically distributed. Bars = 10 μm.
3.4. MAT Genes Contribute Differently to the Formation of Sexual Structures
Although mutants lacking the whole MAT loci were unable to undergo sexual development, the roles of the individual genes in the MAT loci are undetermined. We thus generated mutants of each individual MAT gene. Each mutant, Guy11Δ111, Guy11Δ112, Guy11Δ113, 70-15Δ121, 70-15Δ122, 2539Δ122 and TH3Δ122, was mated with their corresponding wild-type strains (Figure 4; Table 2). The mutants of Guy11Δ111, Guy11Δ113 and 70-15Δ121 lost the ability to produce perithecium with their corresponding wild-type strains of opposite mating types, indicating the deletion of any of the three genes leads to infertility of the fungus. Meanwhile, the complement strains, Guy11Δ111/111, Guy11Δ113/113 and 70-15Δ121/121, fully recovered the ability to produce perithecium, confirming the vital roles of MAT1-1-1, MAT1-1-3 and MAT1-2-1 in perithecium formation. In contrast, the crossing of Guy11Δ112, 70-15Δ122, 2539Δ122 and TH3Δ122 with their corresponding wild-type strains exhibited no significant difference in sexual development compared with the combinations of the wild-type strains, indicating that MAT1-1-2 and MAT1-2-2 were dispensable for perithecium formation.
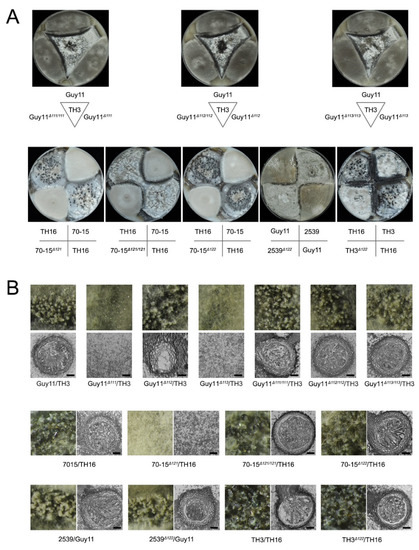
Figure 4.
Function of individual MAT genes in sexual reproduction. (A) Crossing experiments of the wild-type strains, mutants and recovery transformants on OMA plates; (B) the perithecia generated in different combinations were observed in 60× magnification under a stereomicroscope, and the inner structures were observed via paraffin section method. Bars = 20 μm.

Table 2.
The numbers of perithecia formed in the cross of MAT genes mutants, complement strains and the wild-type strains on OMA (×104/plate).
To further reveal the roles of the genes in sexual development, the structures of the perithecia, the asci and the ascospores were further investigated. The perithecia formed well in the cross of Guy11Δ112 and TH3; however, no asci and ascospores could be found in these perithecia, indicating that the lack of MAT1-1-2, though did not interfere with the development of perithecium, hindered the generation of asci and ascospores (Figure 4B). In contrast, the mutants 70-15Δ122, 2539Δ122, and TH3Δ122 developed asci and ascospores normally when crossed with the wild-type strains of the opposite mating type, indicating that MAT1-2-2 is dispensable for the development of asci and ascospores. Meanwhile, the complement strains Guy11Δ111/111, Guy11Δ112/112, Guy11Δ113/113 and 70-15Δ121/121 fully regained the ability to produce normal asci and ascospores.
3.5. Deleting Any of the Individual MAT Genes Down-Regulated the Others
To investigate whether the expression of the MAT genes was inter-affected between each other, we investigated the relative transcription levels of the MAT genes in the mutants. Guy11Δ111, Guy11Δ112, Guy11Δ113and Guy11 were crossed with 70-15, 70-15Δ121and 70-15Δ122, respectively, and the relative transcription levels of the genes were assessed using qPCR. All of the five MAT genes were highly expressed in the wide-type cross, Guy11/70-15, while they were significantly reduced in the crosses containing any of the mutants (Figure 5). For example, MAT1-1-1 was highly expressed in Guy11/70-15 and more down-regulated in the cross of Guy11Δ112/70-15, Guy11Δ113/70-15, Guy11/70-15Δ121and Guy11/70-15Δ122. The same situation was detected for MAT1-1-2 and MAT1-1-3. These results suggest that the expression of MAT1-1-1, MAT1-1-2 and MAT1-1-3 was severely interfered with by other MAT genes. MAT1-2-1 and MAT1-2-2 were also down-regulated by the deletion of the other MAT genes but in milder levels than that of MAT1-1-1, MAT1-1-2, and MAT1-1-3. Moreover, the inter-effects between MAT1-2-1 and MAT1-2-2 were the smallest ones. The results of the transcription support the fact that somehow MAT1-2-2 has less impact on sexual development than the other genes.
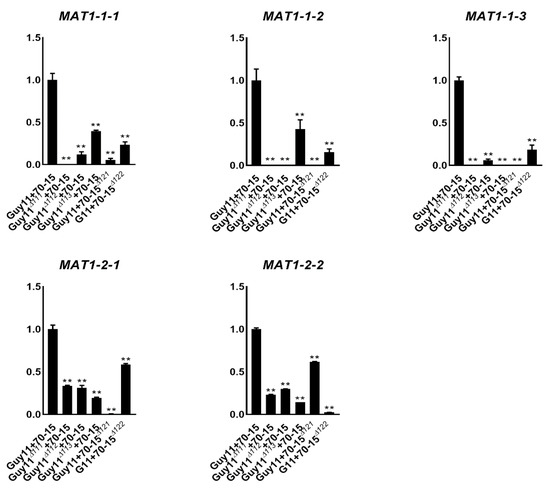
Figure 5.
Transcription levels of the individual MAT genes in the MAT mutants. Relative transcription levels of the individual MAT genes in the crossing of Guy11 and 70-15, Guy11∆111 and 70-15, Guy11∆112 and 70-15, Guy11∆113 and 70-15, Guy11 and 70-15∆121 and Guy11 and 70-15∆122. The transcription levels in the wild-type crossing (Guy11 + 70-15) were used as control for each MAT gene. Mean and standard error were calculated from three independent biological replicates. Single stars indicate significance at the 0.5 level and double stars indicate it at the 0.01 level.
3.6. The MAT Loci Are not Required in Vegetative Growth, Asexual Reproduction or Pathogenicity
To clarify whether the MAT loci and MAT genes are involved in vegetative growth, asexual development or pathogenicity of the fungus, the mutants of the MAT loci and individual MAT genes were compared with the wild-type strains using routine procedures. Incubated on CM for 7 days, no significant differences of the mutants were found in the colony morphology and growth rates compared to the wild-type strains and the complement strains (Figure 6A and Figure S1). The ability of the MAT loci mutants to produce asexual conidia was not influenced either (Figure 6B). The data indicate that the MAT loci and MAT genes are dispensable for vegetative growth and asexual conidiation of the fungus.
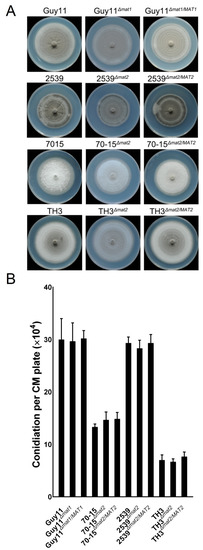
Figure 6.
Colonial morphology and conidiation of the MAT loci mutants. (A) Colonial morphology of the mutants Guy11Δmat1, 2539Δmat2, 70-15Δmat2 and TH3Δmat2, the wild-type strains Guy11, 2539, 70-15 and TH3, and the complement strains Guy11Δmat1/MAT1, 2539Δmat2/MAT2, 70-15Δmat2/MAT2 and TH3Δmat2/MAT2 cultured on CM for 7 days. (B) The conidiation ability of the strains was tested by counting the conidia harvested from 7-day colonies on CM. Mean and standard error were calculated from three independent biological replicates.
Inoculation was performed on rice seedlings and detached rice leaves with conidial suspensions of the wild-type strains Guy11 and 70-15, the mutants Guy11Δmat1 and 70-15Δmat2 and the complement strains Guy11Δmat1/MAT1 and 70-15Δmat2/MAT2. The rice seedlings and the leaves inoculated with any of the strains generated typical blast lesions without any significant difference in symptoms (Figure 7A), indicating that the deletion of the MAT loci has no impact on the pathogenicity of M. oryzae. To further determine whether the MAT loci a play role in infection-related development, the conidia of the mutants were allowed to germinate and form appressoria. The rates of conidial germination and the appressorium formation of the mutants were equivalent to those of the wild-type strains, and the appressorial morphology was unaltered (Figure 7B,C), indicating that the MAT loci were irrelevant to these infection-related developments.
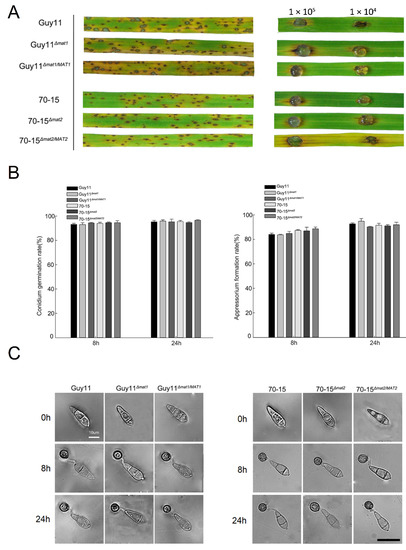
Figure 7.
The pathogenicity and infection-related morphology were unaltered in MAT loci mutants. (A) The 2-week-old rice seedlings (left) were spray inoculated with the conidial suspensions at 1 × 105 conidia/mL for Guy11 and Guy11-derived strains and 5 × 104 conidia/mL for 70-15 and 70-15-derived strains; the detached rice leaves (right) were drop inoculated with the conidial suspensions at 1 × 105 conidia/mL and 1 × 104 conidia/mL. The symptoms were observed at 7 days post-inoculation. (B) The conidia of the strains were incubated on hydrophobic membranes to allow gemination and appressorium formation. The conidial germination rates (left) and appressorium formation rates (right) were calculated and compared at 0, 8 and 24 hpi (hours post-incubation). Mean and standard error were calculated from three independent biological replicates. (C) the morphology of the conidia and appressoria of the strains at 0, 8 and 24 hpi.
The carbon utilization of the MAT gene mutants was tested on CM, minimal medium (MM), MM lacking a carbon source (MM-C) and MM-C supplemented with various carbon sources (MM-C + 1% Tween 80, MM-C + 50 mM CH3COONa or MM-C + 1% olive oil). On these media, no significant difference in radical growth and colonial morphology was found between the wild-type and mutant strains, indicating that carbon utilization was not affected by the deletion of the MAT genes (Figure 8A). Cell wall biogenesis and ROS elimination are vital metabolisms impacting fungal infection. To determine whether the MAT genes are involved in cell wall integrity or ROS elimination, the mutants and wild-type strains were incubated on CM containing Congo red, calcofluor white, H2O2 or methyl viologen. Compared with the wild-type strains, the mutant strains exhibited no significant difference, indicating that the MAT genes do not participate in cell wall integrity or ROSs elimination (Figure 8B).
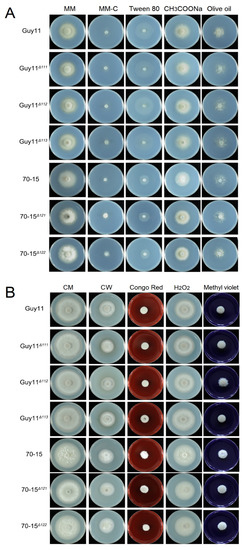
Figure 8.
The carbon utilization, cell wall biogenesis and ROSs endurance were unaffected in the MAT gene deletion mutants. (A) The strains were cultured on MM; MM-C; and MM-C supplemented with 1% Tween 80, 50 mM CH3COONa or 1% Olive oil at 28 °C for 7 days. (B) The strains were cultured on CM and CM containing 150 μM calcofluor white (CW), 200 μM Congo red, 0.1% hydrogen peroxide or 4 mM Methyl violet at 28 °C for 7 days.
4. Discussion
M. oryzae has the ability to undergo two modes of reproduction, asexual and sexual, but the latter maybe contribute more to the pathogenicity variation of the fungus and make the rice blast disease difficult to be controlled. Although the natural sexual process of this fungus has not yet been observed in rice fields [15], we found that the field strains have both mating types, and under laboratory conditions, some of these strains can undergo sexual reproduction (data not shown). The investigation of the mechanisms of the sexual cycle in M. oryzae may help us to fully understand the life cycle of the destructive pathogen.
Previous studies have investigated the functions of the MAT loci in the development of sexual structures in other fungal species. The MAT loci are essential for the formation of the sexual structure in Fusarium graminearum [17], Podospora anserine [10] and Botrytis cinereal [18]. As a heterothallic fungus, an M. oryzae strain is equipped with one allele of the MAT loci, which makes the situation and performance of the MAT genes more complicated and difficult to investigate in M. oryzae compared with the heterothallic fungal species. In the present work, we investigated the MAT1-1 and MAT1-2 loci in M. oryzae and revealed their importance in sexual development. This is the first direct evidence that proves the requirement of the MAT loci in sex determination and sexual reproduction in M. oryzae.
Although MAT loci were found to be associated with the proteins that regulate mate recognition, cell fusion and meiosis [10,19,20], the functions and even the numbers of the genes encoded in MAT loci vary greatly in different fungal species. MAT1-1-2 in F. graminearum and MAT1-1-5 in B. cinerea are essential in sexual reproduction [18,19,21,22]. In Sordaria macrospora, MAT1-1-2 is a crucial factor for fruiting body and ascospore development, whereas MAT1-1-1 and MAT1-1-3 deletion strains showed no difference in sexual reproduction compared to the wild-type strain [19]. MAT genes from different species usually have quite low sequence identities, which makes it difficult to deduce the functions of MAT genes in a species based on those of others. In the present work, we found that in M. oryzae, MAT1-1-1, MAT1-1-3 and MAT1-2-1 were essential for the development of perithecia, MAT1-1-2 impacted the formation of asci and ascospores, and MAT1-2-2 was dispensable for sexual development. The functional differences of MAT genes between closely related fungal species indicated the complexity of the regulation of fungal sexual reproduction. The nucleic sequences of MAT1-1-3 and MAT1-2-2 in M. oryzae are largely identical, while the functions of the two genes differ greatly. The deletion of the former leads to the vanishing of the perithecia, asci and ascospores, while the latter is dispensable for the formation of these structures. Thus, MAT1-2-2 is likely a redundant version of MAT1-1-3, which is perhaps the next topic worthy of investigation. In addition, the subcellular localization of the MAT proteins in M. oryzae was also found to be different; MAT1-1-3 was distributed in the nucleus, while MAT1-1-2 and MAT1-2-1 were found to be cytoplasmic. These results are partially the same as those for F. graminearum [6].
Sexual reproduction in fungi was regulated by complicated signal transduction networks, such as hormone receptor, G protein and MAPK pathways. Some of the pathways were also deemed as key regulators in the pathogenicity of M. oryzae [2,7,10]. In a comparison of the MAT loci mutants with the wild-types, however, we found that the MAT loci are not involved in vegetative growth, asexual reproduction or pathogenicity. It is maybe an evolutionary adaptation. In most natural environments and for the majority of its life cycle, M. oryzae finishes development and reproduction in asexual mode, while the sexual cycle perhaps exists in some specific regions [14,15]. The independence of the regulation of asexual and pathogenic development, with sex determination and sexual reproduction, ensures the highest possibility of survival and pathogenicity of the fungus in environments that are suitable or unsuitable for sexual reproduction. Despite that, how the MAT genes trigger or are triggered by the signal pathways, whether the MAT genes function cooperatively or competitively in regulation and whether the regulation networks for sexual reproduction and pathogenicity crosstalk in some way are questions that, to date, are still not fully understood, and more investigations are required in order to find answers.
In addition, the lack of proper methods is a barrier to the study of sexual mechanisms. In recent years, we established a series of related methods for the observation of sexual structures, such as chemical staining and fluorescence labeling [23,24,25]. The proper usage of these methods will be beneficial for the further investigation of the biological, cellular and molecular mechanisms of sexual reproduction in M. oryzae.
5. Conclusions
In this study, we characterized the roles of the two MAT loci and the individual MAT genes in M. oryzae via gene disruption strategies, provided the direct evidence for the involvement of the MAT loci and MAT genes in mating type determination and formation of sexual structures, and found the loci and genes are dispersible for pathogenicity and asexual development of the fungus.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7100858/s1, Figure S1 Colonial morphology of wild-type, MAT gene mutants and complement strains cultured on CM for 7 days; Table S1. The primers used in this work.
Author Contributions
Conceptualization, J.-y.W., G.-c.S. and F.-c.L.; methodology, J.-y.W. and X.-h.L.; validation, X.-x.F. and Z.Z.; formal analysis, X.-x.S. and J.-y.W.; investigation, X.-x.S., L.L. (Ling Li) and Z.-n.H.; resources, H.-p.Q., R.-y.C. and Y.-l.W.; data curation, J.-y.W. and S.-z.W.; writing—original draft preparation, X.-x.S., S.-z.W. and J.-y.W.; writing—review and editing, J.-y.W. and S.-z.W.; visualization, X.-m.Z. and L.L. (Lin Li); supervision, J.-y.W. and F.-c.L.; project administration, G.-c.S. and F.-c.L.; funding acquisition, J.-y.W., G.-c.S. and F.-c.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Zhejiang Province of China, grant number LZ20C140001; the National Natural Science Foundation of China (General Program), grant number 31470249; Science and Technology Program of Zhejiang Province of China, grant numbers 2019C02010 and 2021C02010; and Bio-health inner-cooperation plan in Zhejiang Academy of Agricultural Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nieuwenhuis, B.P.S.; James, T.Y. The frequency of sex in fungi. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Coelho, M.A.; David-Palma, M.; Priest, S.J.; Heitman, J. Genetic and genomic evolution of sexual reproduction: Echoes from LECA to the fungal kingdom. Curr. Opin. Genet. Dev. 2019, 10, 70–75. [Google Scholar] [CrossRef]
- Turgeon, B.G.; Yoder, O.C. Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet. Biol. 2000, 31, 1–5. [Google Scholar] [CrossRef]
- Seibel, C.; Tisch, D.; Kubicek, C.P.; Schmoll, M. ENVOY is a major determinant in regulation of sexual development in Hypocrea jecorina (Trichoderma reesei). Eukaryot. Cell 2012, 11, 885–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.-W.; Yun, S.-H.; Lee, T.; Turgeon, B.G. Altering sexual reproductive mode by interspecific exchange of MAT loci. Fungal Genet. Biol. 2011, 48, 714–724. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, R.; Ma, J.; Wu, Z.; Wang, G.; Wang, C.; Xu, J.R. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS ONE 2013, 8, e66980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-K.; Jo, S.-M.; Kim, G.-Y.; Kim, D.-W.; Kim, Y.-K.; Yun, S.-H. A large-scale functional analysis of putative target genes of mating-type loci provides insight into the regulation of sexual development of the cereal pathogen Fusarium graminearum. PLoS Genet. 2015, 11, e1005486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, R.; Kan, J.A.L.V.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.E. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Notteghem, J.L.; Silue, D. Distribution of the mating type alleles in Magnaporthe grisea populations pathogenic on rice. Phytopathology 1992, 82, 423–426. [Google Scholar] [CrossRef]
- Wilson, A.M.; Wilken, P.M.; Van Der Nest, M.A.; Wingfield, M.J.; Wingfield, B.D. It’s all in the genes: The regulatory pathways of sexual reproduction in filamentous ascomycetes. Genes 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, V.; Tong, L.; Nguyen, T.-S.; Debuchy, R.; Silar, P. PaPro1 and IDC4, Two genes controlling stationary phase, sexual development and cell degeneration in Podospora anserina. Fungi 2018, 4, 85. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Brown, D.; Ebbole, D.; Torto, T.; Oh, Y.; Deng, J.; Mitchell, T.; Dean, R. Gene Ontology annotation of the rice blast fungus, Magnaporthe oryzae. BMC Microbiol. 2009, 9, S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkhali, A.; Coppin, E.; Brun, S.; Peraza-Reyes, L.; Martin, T.; Dixelius, C.; Lazar, N.; Van Tilbeurgh, H.; Debuchy, R. A network of HMG-Box transcription factors regulates sexual cycle in the fungus Podospora anserina. PLoS Genet. 2013, 9, e1003642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, D.; Xu, P.; Shen, Y.; Li, C.; Adreit, H.; Milazzo, J.; Ravigne, V.; Bazin, E.; Notteghem, J.-L.; Fournier, E.; et al. Sex at the origin: An Asian population of the rice blast fungus Magnaporthe oryzae reproduces sexually. Mol. Ecol. 2012, 21, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, L.; Jia, Y.; Wang, Q.; Fukuta, Y.; Li, C. Characterization of field isolates of Magnaporthe oryzae with mating type, DNA fingerprinting, and pathogenicity assays. Plant Dis. 2016, 100, 298–303. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, Z.; Wang, Y.; Liu, M.; Jiang, H.; Chai, R.; Mao, X.; Qiu, H.; Liu, F.; et al. MoPex19, which is essential for maintenance of peroxisomal structure and woronin bodies, is required for metabolism and development in the rice blast fungus. PLoS ONE 2014, 9, e85252. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, T.; Lee, Y.W.; Yun, S.H.; Turgeon, B.G. Shifting fungal reproductive mode by manipulation of mating type genes: Obligatory heterothallism of Gibberella zeae. Mol. Microbiol. 2003, 50, 145–152. [Google Scholar] [CrossRef]
- Rodenburg, S.Y.A.; Terhem, R.B.; Veloso, J.; Stassen, J.H.M.; van Kan, J.A.L. Functional analysis of mating type genes and transcriptome analysis during fruiting body development of Botrytis cinerea. mBio 2018, 9, e01939-17. [Google Scholar] [CrossRef] [Green Version]
- Klix, V.; Nowrousian, M.; Ringelberg, C.; Loros, J.J.; Dunlap, J.C.; Pöggeler, S. Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot. Cell 2010, 9, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.V.-B.; An, Z.; Metzenberg, R.L.; Glass, N.L. Characterization of matA-2, matA-3 and ΔmatA mating-type mutants of Neurospora crassa. Genetics 1998, 148, 1069–1079. [Google Scholar] [CrossRef]
- Arnaise, S.; Debuchy, R.; Picard, M. What is a Bona Fide Mating-Type Gene? Internuclear complementation of mat mutants in Podospora anserina. Mol. Gen. Genet. 1997, 256, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Doughan, B.; Rollins, A. Characterization of MAT gene functions in the life cycle of Sclerotinia sclerotiorum reveals a lineage-specific MAT gene functioning in apothecium morphogenesis. Fungal Biol. 2016, 120, 1105–1117. [Google Scholar] [CrossRef]
- Gu, Z.; Li, L.; Wang, J.; Chai, R.; Wang, Y.; Zhang, Z.; Mao, X.; Qiu, H.; Sun, G. Observation of sexual structure of Magnaporthe oryzae via calcofluor white and nile red staining. Chin. J. Rice Sci. 2016, 30, 668–672. [Google Scholar]
- Guo, X.; Li, L.; Dong, B.; Wang, J.; Chai, R.; Zhang, Z.; Mao, X.; Qiu, H.; Hao, Z.; Wang, Y.; et al. Lighting the cellular structures of sexual generation in Magnaporthe oryzae with fluorescent proteins and fluorescent dyes. Chin. J. Cell Biol. 2018, 7, 1138–1145. [Google Scholar]
- Wang, J.; Guo, X.; Ling, L.; Qiu, H.; Zhen, Z.; Wang, Y.; Sun, G. Application of the fluorescent dye Bodipy in the study of lipid dynamics of the rice blast fungus Magnaporthe oryzae. Molecules 2018, 23, 1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).