Abstract
Invasive candidiasis remains one of the most prevalent systemic mycoses, and several studies have documented the presence of mixed yeast (MY) infections. Here, we describe the epidemiology, clinical, and microbiological characteristics of MY infections causing invasive candidiasis in a multicenter prospective study. Thirty-four centers from 14 countries participated. Samples were collected in each center between April to September 2018, and they were sent to a reference center to confirm identification by sequencing methods and to perform antifungal susceptibility testing, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST). A total of 6895 yeast cultures were identified and MY occurred in 150 cases (2.2%). Europe accounted for the highest number of centers, with an overall MY rate of 4.2% (118 out of 2840 yeast cultures). Of 122 MY cases, the most frequent combinations were Candida albicans/C. glabrata (42, 34.4%), C. albicans/C. parapsilosis (17, 14%), and C. glabrata/C. tropicalis (8, 6.5%). All Candida isolates were susceptible to amphotericin B, 6.4% were fluconazole-resistant, and two isolates (1.6%) were echinocandin-resistant. Accurate identification of the species involved in MY infections is essential to guide treatment decisions.
1. Introduction
Invasive candidiasis remains one of the most prevalent systemic mycoses [1,2,3]. The mortality associated with this infection is substantial, and it has been estimated to be between 10% to 47% [1,4]. Candida albicans is the most common species isolated, but surveillance studies have documented an increasing rate of non-albicans and frequently more resistant species, such as C. glabrata [3,5,6]. Furthermore, several studies have reported mixed yeast (MY) infections [7,8,9,10,11].
Although different microbiological media are available to detect MY cultures, some standard procedures may not be able to detect them and therefore may underestimate their burden. In one center, the detection of mixed fungemia increased from no cases to 2.8% since the introduction of chromogenic media [12]. The combination of susceptible and resistant species can complicate the clinical management, and therefore the detection of MY infections is important. We conducted a multicenter analysis to describe the epidemiology and clinical and microbiological characteristics of MY infections causing invasive candidiasis.
2. Materials and Methods
2.1. Study Design
We conducted a multicenter prospective study on invasive candidiasis caused by MY infections. The members of the ESCMID Fungal Infection Study Group (EFISG) and the Medical Mycology Study Group of the Spanish Society of Infectious Diseases and Clinical Microbiology (GEMICOMED-SEIMC) were invited to participate. In the study, 34 centers from 14 countries participated: Spain (11), France (4), Turkey (3), Iran (3), Greece (2), Italy (2), Poland (2), Germany (1), Austria (1), Czech Republic (1), India (1), Serbia (1), Thailand (1), and the United States (1). A case of MY infection was defined when 2 or more yeast species were isolated from a single culture of a normally sterile site. MY infections were detected at each participating center using a chromogenic medium. The isolates were collected prospectively between April to September 2018 and sent to the Mycology Reference Laboratory at the National Centre for Microbiology, Instituto de Salud Carlos III, Spain for further identification and susceptibility testing.
2.2. Data Collection
An electronic case report form (CRF) was designed using the platform clinicalsurveys.net (Questback, Cologne, Germany). All participants received the link and a personal password. Demographic, clinical, microbiological, and treatment information were recorded anonymously. For each participating center, number of primarily sterile specimens analyzed and number of sterile samples from which a yeast was isolated during the study period, were collected.
2.3. Molecular Identification
At the reference center (RC), isolates were cultured on 6.5% W/V Sabouraud Dextrose Agar (SDA) (Oxoid, Basingstoke, UK) and CHROMagar Candida medium (Oxoid, Basingstoke, UK) to visually confirm pure cultures. They were incubated at 30 °C for 24–48 h. Molecular identification was performed by polymerase chain reaction (PCR) amplifying and sequencing internal transcribed spacer regions (ITS) from the ribosomal DNA region, as previously described [13]. PCR amplicons were purified using Illustra ExoPro-Star 1-step technology (GE Healthcare Life Sciences, Buckinghamshire, UK), and subsequently sequenced by the Sanger method using a Big-Dye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). DNA sequences were analyzed with DNAStar Lasergene 12 software (DNAStar Inc., Madison, WI, USA) and compared with reference sequences from the GenBank database (https://www.ncbi.nlm.nih.gov/GenBank/). Additionally, we confirmed molecular identification using the InfoQuest FP software (Bio-Rad Laboratories, Madrid, Spain) with the in-house database of the Mycology Reference Laboratory of Spain.
2.4. Antifungal Susceptibility
Antifungal susceptibility testing was performed according the EUCAST method [14]. The following ranges of antifungals were tested: Amphotericin B (0.03–16 mg/L) (Sigma-Aldrich, Madrid, Spain), 5-flucytosine (0.25–64 mg/L) (Sigma-Aldrich, Madrid, Spain), fluconazole (0.25–64 mg/L) (Pfizer Inc, New York, NY, USA), isavuconazole (0.015–8 mg/L) (Basilea Pharmaceutica, Basel, Switzerland), itraconazole (0.015–8 mg/L) (Janssen Pharmaceutical, Madrid, Spain), posaconazole (0.015–8 mg/L) (Merck & Co., Inc., Rahway, NJ, USA), voriconazole (0.015–8 mg/L) (Pfizer Inc, New York, NY, USA), anidulafungin (0.008–4 mg/L) (Pfizer Inc, New York, NY, USA), caspofungin (0.03–16 mg/L) (Merck &Co., Inc., Rahway, NJ, USA), and micafungin (0.004–2 mg/L) (Astellas Pharma, Inc., Tokyo, Japan). Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains in all tests performed. The optical densities were read after 24 h. The minimal inhibitory concentrations (MIC) were defined as the lowest concentration that inhibited 90% (amphotericin B) and 50% (other antifungals) of growth. MIC values were interpreted according to EUCAST breakpoints(https://eucast.org/clinical_breakpoints/).
2.5. FKS Amplification and Sequencing
Hot spot regions (HS1 and HS2) of the FKS gene were amplified and sequenced in those isolates classified as resistant to echinocandins, as described previously [15]. DNA sequences were compared against reference sequences of C. tropicalis (GenBank number EU676168.2) and C. albicans (GenBank number XM_716336) downloaded from the GenBank database.
2.6. Data Analysis
Data were analyzed using SPSS Statistics 19 (SPSS Iberica, Madrid, Spain). The descriptive analysis used proportions and medians. A p value < 0.05 was considered significant. This study was approved by the ethical committee of the Instituto de Salud Carlos III, Madrid, Spain (Reference No. CEI PI 04_2018).
3. Results
3.1. Study Population and Clinical Characteristics
Between April and September 2018, a total of 359,686 sterile specimens were tested for yeast infections. Of these, 6895 (2%) tested positive for yeast cultures, and MY infections accounted for 150 cases (2.2%). In Europe, with 82% of the participating centers, a total of 266,579 sterile specimens were tested: 2840 (1.1%) tested positive for yeast cultures and MY infections accounted for 118 cases (4.2%). Nine centers did not report any MY cases during the study period, and one of them (from the United States) reported almost half (3157, 46%) of the total positive yeast cultures. Different rates by country were observed (Table 1). Higher rates of MY were found in Poland and France, with 6.4% and 5.6%, respectively. Austria, Serbia, Thailand, and the United States (represented by one center each) presented no cases. Among MY cases, the median age was 63 years (interquartile range: 39–73), and 53.3% were male. The patient’s information is summarized in Table 2. Frequent underlying conditions included major surgery (85, 56.6%), the use of a central vascular catheter (72, 48%), intensive care unit (ICU) stay (69, 46%), and hematological disease (53, 35.5%). A total of 95 (63.3%) cases had registered information in the CRF about antifungal administration, of which 5 (5.3%) cases received prophylaxis, 29 (30.5%) empiric treatment, and 61 targeted treatment. The overall mortality among MY cases was 29% (43 of 147 patients).

Table 1.
Number of centers and mixed yeast (MY) proportions per country.

Table 2.
Baseline characteristics and risk factors of 150 patients with MY infections.
3.2. Species Distribution and MY Combinations
In 5 cases of the 150 MY infections, the isolates could not be recovered at the RC despite several attempts. In 10 cases, only 1 isolate was sent to the RC, and in 13 cases, the same species was identified in both isolates (Figure S1). Therefore, 249 isolates of 122 MY cases were analyzed. Five patients (4.1%) had a yeast infection caused by three species. Table 3 shows the list of species combinations. The most frequent were C. albicans/C. glabrata (42, 34.4%), C. albicans/C. parapsilosis (17, 13.9%), and C. glabrata/C. tropicalis (8, 6.6%). We found a broad diversity of combinations, and unique combinations were found in 14% of cases (17 cases out of 122). Differences in MY distribution and combinations per country are shown in Figure 1. The molecular identification detected cryptic species, such as C. dubliniensis or C. orthopsilosis, in 6.5% (8) of the cases. These cases were also identified at the participating centers by Bruker Biotyper MS matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). In 11 (9%) cases, non-Candida species were identified.

Table 3.
Species combinations of 122 MY cases.
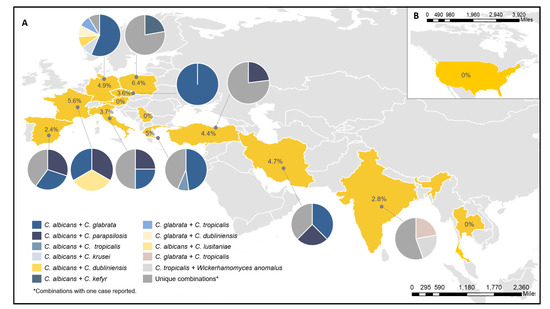
Figure 1.
Rates, numbers, and species combinations found per country. (A) MY cases in Europe and Asia. (B) MY cases in the United States. The percentages shown inside each country represented the rate of MY per positive yeast cultures in each country and pie charts represent the MY combinations for each country.
3.3. Antifungal Susceptibility Testing
Table 4 shows the geometric mean (GM), MIC ranges, MIC50, MIC90, and resistance or non-wild type (N-WT) of 245 study isolates. Four isolates did not grow after the identification. The results are shown for species with more than nine cases. According to EUCAST breakpoints, all Candida isolates were susceptible to amphotericin B. The MIC values of 5-flucytosine ranged from 0.120 mg/L to 64 mg/L. Fluconazole resistance was observed in 14 (6.4%) Candida isolates. Fluconazole-resistant C. tropicalis and C. albicans were observed in 18.2% (4 out of 22) and 4.9% (4 out of 82) of cases, respectively. As expected, C. krusei had elevated MICs to fluconazole (MIC90, 32 mg/L). The intrinsic diminished susceptibility of C. glabrata to fluconazole was also observed (MIC90, 4 mg/L), and one resistant isolate was detected (MIC50 64 mg/L). The remaining fluconazole-resistant strains were C. dubliniensis (2), C. inconspicua (2), and C. parapsilosis (1). Azoles were less active against C. krusei, C. tropicalis, and C. glabrata, showing higher MIC90 values for itraconazole (0.5 mg/L, 0.12 mg/L, and 0.5 mg/L), and posaconazole (0.25 mg/L, 0.12 mg/L, and 0.5 mg/L), compared to C. albicans with 0.03 mg/L for itraconazole and posaconazole. Among the 14 fluconazole-resistant cases, treatment information was available for 7. Of those, one received fluconazole, three received echinocandins, one received voriconazole, and in two cases, the antifungal drug was not recorded.

Table 4.
In vitro antifungal activities of the isolates analyzed.
Resistance to echinocandins was low, and two isolates were classified as resistant: One C. albicans and one C. tropicalis. Hot spot sequencing of the FKS1 gene showed a F641V mutation in the C. albicans isolate and a substitution of arginine for glycine in the seventh position of the HS1 FKS1 in the C. tropicalis. MIC values for anidulafungin and micafungin were 0.06 mg/L for the C. albicans isolate, and 0.125 mg/L and 2 mg/L for the C. tropicalis isolate. The resistant C. albicans was isolated from a patient who had received antifungal prophylaxis with anidulafungin. The C. tropicalis case had not recorded prophylactic treatment. Both strains were identified in combination with a C. parapsilosis.
4. Discussion
This study described the epidemiology and clinical and microbiological characteristics of mixed yeast (MY) infections in invasive candidiasis. In this study, we found that MY occurred in 2.2% of the yeast cultures analyzed from 14 countries. The rate of MY in Europe was 4.2%. Different proportions across countries were also observed (range 0% to 6.4%). In previous studies, MY infections accounted for 1.8% to 10.6% of cases [9,10,12,16,17]. The proportion of MY found in Spain (2.4%) was slightly higher than a previous study, which detected a rate of 1.8% [12]. Other studies carried out in tertiary care hospitals in Germany and Greece have detected rates of MY of 4.4% and 4.7% [17,18], consistent with the rates detected in this study (4.9% and 5%, respectively). In contrast, in Poland, a multicenter survey in patients with candidemia detected a lower proportion of MY infections (3.5% vs. 6.4% found in this study) [16]. Similarly, the incidence of MY infections reported in a retrospective study in Turkey was 3.7% [19], while the rate in this study was found to be 4.4%. In France, rates of MY cultures of 8.7%, and 7.5% in deep-seated samples were previously described [9], slightly higher than the 6.4% obtained in this study. Furthermore, in the United States, the transplant-associated infection surveillance network reported 10.6% of mixed infections in organ transplant recipients between 2001 and 2006 [8]. However, no MY cases were detected in the participating center from the United States. The underlying conditions found in MY cases were similar to the risk factors reported for invasive candidiasis [12]. These factors included major surgery, the presence of central venous catheter, and ICU stay [3,12,20].
The combination of C. albicans/C. glabrata was the most frequent (34.4%). This is not surprising, since these two species are the most frequently isolated in epidemiological studies [6]. Interestingly, a murine model of oropharyngeal candidiasis (OPC) showed that colonization by C. glabrata was increased by co-infection or a pre-established infection with C. albicans [21]. The hyphal wall adhesins Als1 and Als3 of C. albicans were important for the in vitro adhesion of C. glabrata and are possible for other species [21].
Antifungal therapy is a critical component in the clinical management. However, antifungal options are limited, and drug resistance is a growing concern [6,22]. The current guidelines of the Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommend the use of an echinocandin as first-line of treatment in invasive candidiasis [4,23]. In general, the echinocandins are active drugs against most Candida species [24]. In this study, the overall resistance to echinocandins was low (two cases, 1.6%). In C. glabrata, several studies have described an increasing level of echinocandin resistance and a rapid ability of resistance development during antifungal therapy [25,26], but we did not find resistance to echinocandins in this species. Therefore, resistance should be closely monitored. In this study, the two echinocandin resistant isolates harbored FKS1 mutations. In the C. tropicalis isolate, we found a substitution of arginine for a glycine in the seventh position. Different studies have also documented C. tropicalis isolates with echinocandin resistance and FKS mutations [6,27]. To our knowledge, this is the first time this mutation has been reported. In C. albicans, a F641V mutation was found in the FKS1. This mutation has already been associated with reduced susceptibility to caspofungin [28]. Interestingly, this isolate was found in a patient who received anidulafungin prophylaxis, but it was not possible to know if the patient was infected with an already resistant isolate or if the mechanism of resistance was developed during the antifungal treatment as recently investigated in other species [26].
The fluconazole-resistance rate was 6.4%, similar to the 6.9% found in a previous study in Spain [10]. In terms of patients, this rate represents 14 cases out of 122 cases (11.5%). Regarding species, the fluconazole-resistance in C. albicans (5.8%) was higher than those reported in monomicrobial candidemia cases in in Spain (0.9%), and Germany (0%) but was similar for C. tropicalis (18.2% vs. 22%) reported in Spain [10] and higher than those in Germany (12.7%) [17]. Moreover, MY combinations that included intrinsic or acquired azole-resistant strains and C. parapsilosis isolates might also represent a clinical concern due to the intrinsic reduced susceptibility to echinocandins of C. parapsilosis [29]. The overall mortality in MY infections was 29% (43 deaths of 147 cases). Other studies in patients with polymicrobial candidemia have documented mortality rates between 20% and 43% [12,20]. Although these studies did not find significant differences between cases with mixed infections and monomicrobial infections [12,20], the potential involvement of different susceptibility patterns is clinically relevant and require a rapid diagnosis.
This study has several limitations. First, as a multicentric analysis, differences in diagnostic practices might introduce variations in MY detection. Second, we estimated the proportion of MY cases over positive yeast cultures, which could lead to the underestimation of the rate of MY as one case can have several positive cultures. Differences between medical centers and the resulting patient selection may also have an influence in the results. Despite these limitations, this is the first multicentric study that has estimated the occurrence of mixed yeast infections, finding a global rate of 2.2% and 4.2% in Europe. C. albicans/C. glabrata was the most common combination, but a high diversity of combinations and distributions was identified. Resistance to echinocandins was present but rare and fluconazole resistance rates were variable compared with previous studies in monomicrobial infections. As different susceptibility patterns can be identified in MY infections, it is important to accurately identify the species involved and to perform susceptibility testing to support the clinical management of these infections.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/7/1/13/s1, Figure S1: Flow chart of the MY isolates analyzed.
Author Contributions
Conceptualization, C.L.-F., S.A.-A. and A.A.-I.; Formal analysis, N.M. and A.A.-I.; Resources, D.S.; Supervision, A.A.-I.; Validation, J.C.S.-D.; Writing–original draft, N.M.; Writing—review & editing, D.S., I.A., H.B., A.B., S.B., Y.C., C.C. (Carole Cassagne), C.C. (Carmen Castro), A.C., E.D., C.C. (Celia Cardozo), J.G.-R., J.G., P.H., M.H., T.J., S.K., G.L.C., M.C.M.-R., J.M., P.M., E.O., T.P., A.P.-A.B., J.P., E.R., M.R.-P.d.P., R.S., J.S., A.I.S.-B., R.T., L.T., L.V., T.W., I.Ż., H.Z., C.L.-F., S.A.-A. and A.A.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the ESCMID study group research grant and Gilead Sciences research grant.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by ethical committee of the Instituto de Salud Carlos III, Madrid, Spain (Reference No. CEI PI 04_2018) on the 28 February 2018.
Informed Consent Statement
Not applicable. There is no interventional aspect to this study.
Acknowledgments
MY Study Group: Fatemeh Ahangarkani (Antimicrobial Resistance Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran), Siriphan Boonsilp (Department of Clinical Pathology, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand), Athanasios Chatzimoschou (Infectious Diseases Unit, 3rd Department of Pediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Hippokration General Hospital, Thessaloniki, Greece), Pilar Escribano Martos (Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Universidad Complutense de Madrid, Madrid, Spain and Instituto de Investigación Sanitaria del Hospital Gregorio Marañón, Madrid, Spain.), Carolina Garcia-Vidal (Hospital Universitari Clínic, Barcelona, Spain.), Christophe Hennequin (Sorbonne Université, Inserm, Centre de Recherche Saint-Antoine, CRSA, AP-HP, Hôpital Saint-Antoine, Service de Parasitologie-Mycologie F-75012 Paris, France), Robert Krause (Section of Infectious Diseases and Tropical Medicine, Medical University of Graz, Auenbruggerplatz 15, 8036 Graz, Austria), Lucie Svobodova (Department of Microbiology, University Hospital Olomouc, Czech Republic), Deniz Turan (Istanbul Medeniyet University Goztepe Training and Research Hospital, Istanbul Turkey). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation/content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
S.A.A. has received speaker honoraria or travel grant from Astellas, Gilead, and Pfizer in the last five years outside the submitted work. A.A.-I. has received research grants or honoraria as a speaker or advisor from Astellas, Gilead Sciences, MSD, Pfizer, F2G, Amplyx and Scynexis in the last five years outside the submitted work. MH has received research funding from Gilead and Pfizer. ER has received research grants from Astellas, Gilead, Merck and Pfizer Inc.; he is a scientific advisor and member of speaker bureaus for Astellas, Gilead, Merck and Pfizer Inc. The rest of the authors none to declare.
References
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef]
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 2010, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e0101510. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997-2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Alexander, B.; Brumble, L.; Freifeld, A.; Hadley, S.; Herwaldt, L.; Kauffman, C.; Lyon, G.M.; et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl. Infect. Dis. 2016, 18, 921–931. [Google Scholar] [CrossRef]
- Cassagne, C.; Normand, A.C.; Bonzon, L.; L’Ollivier, C.; Gautier, M.; Jeddi, F.; Ranque, S.; Piarroux, R. Routine identification and mixed species detection in 6,192 clinical yeast isolates. Med. Mycol. 2016, 54, 256–265. [Google Scholar] [CrossRef]
- Guinea, J.; Zaragoza, Ó.; Escribano, P.; Martín-Mazuelos, E.; Pemán, J.; Sánchez-Reus, F.; Cuenca-Estrella, M. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob. Agents Chemother. 1990, 58, 315–322. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chu, W.-L.; Lin, C.-C.; Zhou, Z.-L.; Chen, P.-N.; Lo, H.-J.; Hospitals, T. Mixed yeast infections in Taiwan. Med. Mycol. 2018, 56, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Muñoz, P.; Guinea, J.; Rodríguez-Créixemes, T.; Bouza, E. Mixed Fungemia: Incidence, Risk Factors, and Mortality in a General Hospital. Clin. Infect. Dis. 2007, e109, 44. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplicication and Direct Sequiencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press, Inc.: Cambridge, MA, USA, 1990; Volume 2. [Google Scholar]
- Rodriguez-Tudela, J.; Arendrup, M.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.; Donnelly, J.; Dromer, F.; et al. EUCAST DEFINITIVE DOCUMENT EUCAST Definitive Document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST)*. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Bretagne, S.; Raoux, D.; Hoinard, D.; Dromer, F.; Dannaoui, E. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility testing. Antimicrob. Agents Chemother. 2008, 52, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, U.; Pajączkowska, M.; Fleischer, M.; Przondo-Mordarska, H.; Samet, A.; Piasecka-Pazik, D.; Komarnicka, J.; Sulik-Tyszka, B.; Swoboda-Kopeć, E.; Cieślik, J.; et al. Candidaemia in polish hospitals—A multicentre survey. Mycoses 2013, 56, 576–581. [Google Scholar] [CrossRef]
- Mohr, A.; Simon, M.; Joha, T.; Hanses, F.; Salzberger, B.; Hitzenbichler, F. Epidemiology of candidemia and impact of infectious disease consultation on survival and care. Infection 2020, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: A 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob. Agents Chemother. 2020, 64, 1–17. [Google Scholar] [CrossRef]
- Gülmez, D.; Alp, S.; Gursoy, G.; Ayaz, C.M.; Dogan, O.; Arikan-Akdagli, S.; Akova, M. Mixed fungaemia: An 18-year report from a tertiary-care university hospital and a systematic review. Clin. Microbiol. Infect. 2020, 26, 833–841. [Google Scholar] [CrossRef]
- Pulimood, S.; Ganesan, L.; Alangaden, G.; Chandrasekar, P. Polymicrobial candidemia. Diagn. Microbiol. Infect. Dis. 2002, 44, 353–357. [Google Scholar] [CrossRef]
- Tati, S.; Davidow, P.; McCall, A.; Hwang-Wong, E.; Rojas, I.G.; Cormack, B.; Edgerton, M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLOS Pathog. 2016, 12, e1005522. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Echinocandin Resistance, Susceptibility Testing and Prophylaxis: Implications for Patient Management. Drugs 2014, 74, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, C.; Guinea, J.; Valerio, M.; Escribano, P.; Bouza, E.; Muñoz, P. Infectious endocarditis caused by Candida glabrata: Evidence of in vivo development of echinocandin resistance. Rev. Esp. Quimioter 2019, 32, 395–397. [Google Scholar]
- Rivero-Menendez, O.; Navarro-Rodriguez, P.; Bernal-Martinez, L.; Martin-Cano, G.; Lopez-Perez, L.; Sanchez-Romero, I.; Perez-Ayala, A.; Capilla, J.; Zaragoza, O.; Alastruey-Izquierdo, A. Clinical and Laboratory Development of Echinocandin Resistance in Candida glabrata: Molecular Characterization. Front. Microbiol. 2019, 10, 1585. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjaeldgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013, 19, e343–e353. [Google Scholar] [CrossRef]
- Accoceberry, I.; Couzigou, C.; Fitton-Ouhabi, V.; Biteau, N.; Noël, T. Challenging SNP impact on caspofungin resistance by full-length FKS1 allele replacement in Candida lusitaniae. J. Antimicrob. Chemother. 2019, 74, 618–624. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).