Abstract
Rice bakanae disease (RBD) is a typical seed-borne fungal disease caused by Fusarium fujikuroi. Prochloraz is a sterol demethylation inhibitor, which is among the most important classes of active ingredients for the management of RBD. In 2022, the total resistance frequency of F. fujikuroi to prochloraz in Zhejiang Province was 62.67%. The fitness of the prochloraz-resistant population was lower than that of the susceptible population, but its pathogenicity was slightly stronger. The S312T and F511S double mutations of Ffcyp51b were detected in the resistant isolates. Loop-mediated isothermal amplification (LAMP) technology based on S312T was established to rapidly determine prochloraz resistance in F. fujikuroi. LAMP primer mismatch design was performed based on the cyp51b gene, and 100–300 bp sequences containing a mutation at codon 312 were amplified. In a 25 µL reaction tube, 1 pg/µL DNA of F. fujikuroi could be detected. The detection limit for the frequency of prochloraz resistance was 0.498% using this method. We performed LAMP detection on rice seedlings inoculated with prochloraz-sensitive and -resistant isolates and treated them with prochloraz. Prochloraz demonstrated good control in rice seedlings. A chromogenic reaction was observed in seedlings treated with prochloraz-resistant isolates, and the results were verified using electrophoresis. It has been demonstrated that LAMP technology based on the S312T genotype can quickly and specifically detect prochloraz-resistant isolates in rice seedlings.
1. Introduction
Fusarium fujikuroi causes rice bakanae disease (RBD) [1,2], but it can also infect other plants, including maize, soybean, and Lasia spinosa [3,4,5]. Among them, RBD is a typical seed-borne fungal disease that occurs in all major rice-producing areas [6]. It damages plants from the seedling stage to the heading stage, and mild occurrence in rice can cause yield losses between 5–50%. Seeds carrying the pathogenic fungus are the main source of RBD infection. When seeds germinate, F. fujikuroi can invade seedlings through the bud [7]. With the growth of fungal-bearing seedlings [8], mycelia gradually spread throughout the whole plant, and symptoms, such as internode elongation, weakened tillering ability, yellow leaf color, and abnormal root development, may occur [9,10]. RBD may also occur at the heading stage, and in severe cases, the ears turn brown and fail to set. The use of chemical agents to control RBD through seed treatment [11] is quick, economical, and effective and has been the most important technology for its management.
Prochloraz is a sterol demethylation inhibitor (DMI) [12] that was introduced in China at the end of the 20th century to replace carbendazim, for which fungi had developed serious resistance [13]. Thus far, prochloraz is still the main agent used to control RBD. In 1970, DMI compounds began to be applied to control agricultural diseases, and resistance to DMI fungicides in the field has been reported [14]. Wada observed a decrease in the field efficacy of prochloraz in Japan and reported for the first time that F. fujikuroi developed resistance to prochloraz in 1990 [15]. In 2002, Liu analyzed the sensitivity of F. fujikuroi isolated from Jiangsu Province to prochloraz. The EC50 of six isolates was 10-times higher than that of sensitive isolates (0.005 μg/mL was used as the standard for sensitive isolates) [16]. According to previous studies, the resistance mechanism of Fusarium spp. and other pathogens to DMIs includes the cyp51 gene point mutation, cyp51 gene overexpression, and the involvement of efflux actions by different transporters [17,18,19]. Zhang showed that the S312T point mutation of Ffcyp51b was the main mechanism by which F. fujikuroi resists prochloraz [20]. Gao’s experiment also demonstrated this mutation [21]. Li found that two point mutations, S312T and F511S, in Ffcyp51b had a synergistic effect on prochloraz resistance in F. fujikuroi from Japan [22]. However, this double mutation has not been reported in China. Therefore, resistance assessment is important for RBD control.
Traditional fungicide resistance detection methods are mainly based on the isolation and culture of pathogenic fungi on fungicide-amended plates and identification of resistant isolates according to the inhibitory effects on growth or spore germination [23]. This method requires a long test cycle and many human resources. As an important alternative, polymerase chain reaction (PCR) has been developed to detect fungicide-resistant isolate genotypes. However, cPCR, mPCR, and qRT-PCR detection methods require specially equipped laboratories (equipped with thermal cyclers and real-time PCR machines) and specialized technical expertise [24]. Loop-mediated isothermal amplification (LAMP) [25] is a new nucleic acid amplification technique developed in Japan. LAMP may become a novel alternative to PCR because of its rapidness, high specificity, and low cost [26,27]. It has very broad application prospects. LAMP has been used to detect fungicide resistance in plant pathogenic fungi, including the F200Y mutation for carbendazim-resistant Sclerotinia sclerotiorum [28], the E198A mutant genotype [27,29,30], and the cytb gene G143A mutation in Colletotrichum acutatum and C. gloeosporioides [31]. In this study, we seek to understand the evolution of resistance to prochloraz in China and clarify the new mutation genotype associated with prochloraz-resistant in F. fujikuroi since 2018. Meanwhile, rapid detection using LAMP was further established based on the S312T point mutation in cyp51b to provide technical support for resistance detection, management and the accurate and efficient control of RBD.
2. Materials and Methods
2.1. Resistance Monitoring and Sensitivity Determination of Fusarium fujikuroi to Prochloraz
The F. fujikuroi isolates used in this study were collected from Hangzhou, Jinhua, Ningbo, Shaoxing, and other cities in Zhejiang Province in 2018 and 2022. Potato dextrose agar medium (200 g potatoes, 20 g glucose, 15 g agar, and deionized water up to 1 L, PDA) was used for separation, preservation, and the fungicide sensitivity assay. Prochloraz (99.5%) was obtained from Tianfeng Biotech Corporation (Jinhua, China) [20].
In order to determine the resistance frequency of 75 strains of Fusarium fujikuroi to prochloraz, we used the differential dose method. Prochloraz is dissolved in methanol as stock solution firstly and then added to the melted PDA. Fresh plugs (5 mm in diameter) were cut from the growing edge of a mycelial culture and placed on PDA plates (90 mm in diameter) amended with 1 and 5 μg/mL prochloraz, at which concentrations the mycelial growth of the sensitive isolates was completely inhibited, while the resistant isolates continued to grow [21]. The sensitivity to prochloraz of each isolate was determined by the mycelial growth assay. The fresh plugs were placed on PDA plates amended with 1.6, 0.4, 0.1, 0.025, and 0.00625 μg/mL prochloraz. The diameter of each colony was measured perpendicularly after 7 days of incubation at 25 °C, and the median effective concentration (EC50) value for each isolate was calculated by linear regression of the inhibition percent of growth relative to the control versus the log10 transformation for each concentration [32,33].
2.2. Characterization of Prochloraz-Resistant Isolates and Prochloraz-Sensitive Isolates
2.2.1. Mycelial Growth Rate
The test isolates were cultured on the blank PDA medium in the dark at 25 °C for 5 days. Mycelial plugs (5 mm in diameter) were taken from the edge of the colony, transferred to the non-inoculated PDA medium, and cultured in the dark at 25 °C for 7 days. The colony diameter was measured, and the mycelial growth rate was calculated. Each isolate was repeated three times.
2.2.2. In Vitro Conidial Production and Germination
The resistant and sensitive isolates were used to inoculate PDA plates [23]. After 5 days of culture at 25 °C, mycelial plugs (5 mm in diameter) were taken from the edge of the colony, placed in 100 mL of 3% mung bean soup medium, and shaken at 175 rpm for 3 days at 25 °C. The spores were collected, and the number of conidia was observed by microscopic observation with a hemacytometer. Conidial germination was assessed by plating 100 μL of a conidial suspension (1 × 106 conidia/mL) on 1.5% water agar plates and incubating at 25 °C. After 4, 8, and 12 h, 100 spores were observed under a microscope. The number of germinated spores was counted, and the spore germination rate was calculated. Each isolate comprised three technical replicates, and the experiment was performed three times.
2.2.3. Pathogenicity on Rice Seedlings
Rice seeds were soaked in 70% alcohol for 1 min and in 3% NaClO for 3 min and then rinsed three times with sterile water. The conidial suspension was prepared as described above and cultured at 25 °C for 2–3 d. The germinated seeds were placed in 10 mL of conidial solution (1 × 106 conidia/mL) and shaken at 90 rpm for 12 h at 25 °C. The same treated seeds were placed in sterile water as the control. The seeds were then transplanted into the nutrient solution and cultured at 28 °C under a 12-h light and 12-h dark cycle. Seedling height was measured after 15 days. Each isolate contained 50 rice seedlings, and the overgrowth rate of the rice seedlings was calculated as follows [20]:
Overgrowth rate = (Height of treated − Height of control)/Height of control × 100%.
2.3. Cloning and Sequencing of Ffcyp51b in Resistant and Sensitive Isolates
Based on the gene sequence of Ffcyp51b (FFUJ_01179), primers (BF: 5′ AGGTGTGTGGGGTCTCTCTCT-3′; BR: 5′-AAGCAGCACAGTCGTCATGG-3′) were designed to amplify the 1.80-kbp DNA fragment of the prochloraz-resistant and -sensitive F. fujikuroi isolates. The total DNA of each isolate was extracted using a fungal genomic DNA Rapid Extraction Kit (B518229-0100, Sangon Biotech, Shanghai, China), and the DNA concentration was determined using BioDrop μLite (BioDrop, Cambridge, UK). All DNA were stored at −20 °C at Zhejiang A&F University until use to avoid repeated freezing and thawing. The PCR products were directly sequenced, and the results were aligned using Bioedit 7.2.5 software.
2.4. Primer Design
The point mutation sequence of the Cyp51b gene of F. fujikuroi JH1 was used as a template, and a mismatched base was introduced at position 312 using online primer design software Primer Explore V5 [34]. LAMP primers with two external primers (F3 and B3) and two internal primers (FIP and BIP) were designed. The optimal primers were selected according to the ΔG values at the 3′ terminal of F3/B3 and F2/B2, and the ΔG values at the 5′ terminal of F1c and B1c were less than −4 Kcal/mol [35].
2.5. PCR Amplification and Sequencing
The 25 μL LAMP reaction system (Table 1) consisted of 0.5 μL Bst DNA (8 U·μL−1), 2.5 μL 10× Thermo Pol, 4 μL Mg2+ (25 mM), 2.5 μL dNTP (10 mM), 2 μL FIP/BIP (20 μM), 0.75 μL F3/B3 (10 μM), 3 μL betine (5 M), 1 μL HNB (3.75 mM), 1 μL sample DNA, and 5 μL ddH2O. LAMP amplification was performed at 63 °C for 90 min and stored at 4 °C [36].

Table 1.
LAMP reflection system.
2.6. Detection of LAMP Specificity
To investigate the specificity of the LAMP reaction, genomic DNA from fungal isolates or mutants was used (Table 2). After 90 min, the reaction was verified using gel electrophoresis with 1% agarose.

Table 2.
Isolates used for primer design and LAMP-specific detection in this study.
2.7. Detection of LAMP Sensitivity
The genomic DNA of F. fujikuroi resistant isolate JH-1 was diluted into seven concentrations in a 10-fold gradient: 0.0001, 0.001, 0.01, 0.1, 1, 10, and 100 ng/μL. LAMP amplification was performed using 1 μL DNA template and ddH2O as a negative control. The experiment was repeated three times.
2.8. Detection Limit of Resistance Frequency in Different Sensitive: Resistant Isolate Ratios
JS16 and JH1 were selected and cultured in conditional medium (CM) at 26 °C in the dark for 5 days. Spore suspensions were prepared and quantified to 106 mL−1. DNA was extracted, and the volume ratios of DNA of the two isolates were as follows for JS16:JH1: 10:1, 20:1, 100:1, 200:1, and 400:1. The LAMP test was performed. After 90 min of reaction, 1% agarose was used for gel electrophoresis verification.
2.9. Detection of Resistant Isolates from Rice Seeds and Seedlings
To evaluate the feasibility of LAMP technology in detecting the mutant genotype of resistant isolate S312T, resistant isolate JH1 and sensitive isolate JS16 were used to inoculate sterilized seeds. Before inoculation, the two isolates were used to inoculate seeds impregnated with 10 μg/mL prochloraz, sterilized in a water bath at 60 °C for 15 min, and pre-germinated at 30 °C for 48 h. Rice seeds with uniform endosperms were placed in a 20 mL conidia suspension (105 mL−1) at 25 °C and shaken at 80 rpm for 20 h. The same treated seeds were placed in sterile water as the control. The seeds were then transplanted into nutrient solution and cultured at 25 °C and 75% humidity in a light incubator (12 h light: 12 h dark) for 15 days to determine the seedling height. Each isolate was replicated in 20 rice seedlings. The average height of the 20 rice seedlings was calculated, and the growth rate of diseased plants was calculated. Three pathogenicity tests were performed on each isolate. After inoculation, two seeds were selected from each treatment for DNA extraction. After the seeds were transplanted and grown into seedlings, two seedlings were selected from each treatment to extract DNA by cutting their internodes. The DNA was detected by LAMP, together with the previous DNA. After 90 min of reaction, gel electrophoresis was performed with 1% agarose.
3. Results
3.1. Resistance of F. fujikuroi to Prochloraz
Among the 75 tested isolates, 17 had high resistance and 30 had low resistance. The resistance frequency was 22.67 and 40%, respectively, and the total resistance frequency was 62.67% (Figure 1). In 2022, the resistance frequencies of Hangzhou, Shaoxing, Jinhua, Taizhou, and Ningbo were 50, 28.6, 64.3, 66.7, and 87.5%, respectively. Five resistant isolates (F1, F4, F54, F68, and F72), five sensitive isolates (F5, F8, F9, F10, and F25), and nine bakanae isolates collected in 2018 were selected to determine the EC50 (Table 2).
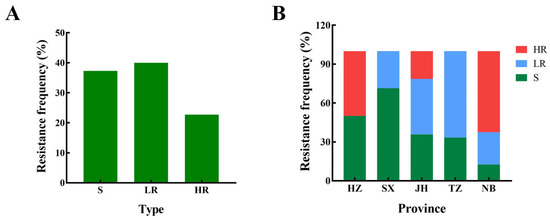
Figure 1.
Resistance frequency of Fusarium fujikuroi to prochloraz in Zhejiang province (A) and major regions (B) of Zhejiang province in 2022. HZ: Hangzhou, SX: Shaoxing, JH: Jinhua, TZ: Taizhou, NB: Ningbo. R: prochloraz resistance, S: prochloraz sensitive, HR: high-level resistance, LR: low-level resistance.
3.2. Characterization of Prochloraz-Resistant Isolates
Four resistant isolates (F1, F4, F68, and F72) and four sensitive isolates (F9, F10, F25, and F59) were randomly selected to determine the fitness of F. fujikuroi to prochloraz. There was a significant difference in the mycelial growth rate between sensitive and resistant isolates. The average mycelial growth rate of the sensitive isolates was 10.19 mm/d, which was higher than that of the resistant isolate (8.27 mm/d). In terms of sporulation, the average sporulation of prochloraz-resistant isolates was 6.74 × 106 spores/mL, which was significantly lower than that of sensitive isolates. The average spore germination rate of resistant isolates at 4, 8, and 12 h (5.22, 30.50, and 80.75%, respectively) was significantly lower than that of sensitive isolates (9.78, 44.50, and 90.89%, respectively). However, resistant isolates were significantly more aggressive than sensitive isolates (Table 3 and Table 4).

Table 3.
Biological characteristics of wild-type prochloraz-sensitive isolates and -resistant mutants of Fusarium fujikuroi.

Table 4.
Comparison of pathogenicity to rice seedlings between wild-type prochloraz-sensitive isolates and -resistant mutants of Fusarium fujikuroi.
3.3. Sequence Analysis of Ffcyp51 in F. fujikuroi
The Ffcyp51b gene of prochloraz-resistant and -sensitive F. fujikuroi isolates was amplified and sequenced (Figure 2). The 511th amino acid F (TTC) was replaced by S (TCC) between Pro-S and Pro-R. In addition to F511S, a previously reported S (TCT) to T (ACT) mutation at amino acid 312 in the prochloraz-resistant isolate was detected at FfCYP51B in Pro-R.
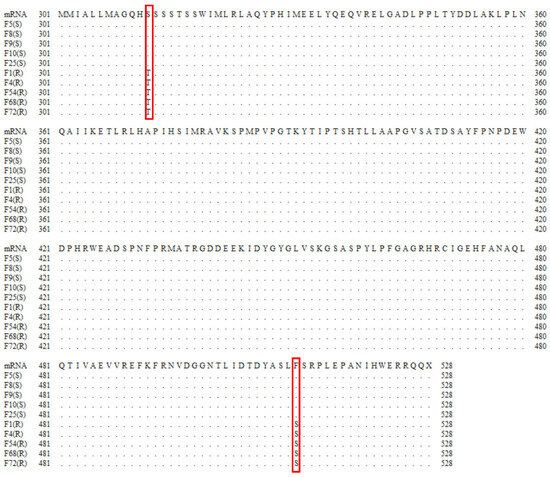
Figure 2.
The amino acid changes of FfCYP51B were related to the resistance of Fusarium fujikuroi to prochloraz. Partial FfCYP51B amino acid sequences of prochloraz-sensitive (S) and -resistant (R) isolates are displayed. The red box represents the position of the amino acid substitution (codons 312 and 511).
3.4. Primer Design
The cyp51b (GenBank accession: CP023101.1) containing point mutation sequence of prochloraz wild mutant JH1 was used as a template. Using the online software Primer Explore V5 (https://primerexplorer.jp/e/v5_manual/, accessed on 15 December 2019), a set of specific amplification detection primers (Table 5 and Figure 3), including the outer primer F3/B3 and the inner primer FIP/BIP, were screened according to the LAMP primer design principle. The primers were synthesized by GenScript Corporation, centrifuged at 8000 rpm for 15 s, dissolved in sterile water, and stored at 4 °C.

Table 5.
Primers used in the LAMP system for detection of prochloraz-resistant Fusarium fujikuroi with S312T mutation.
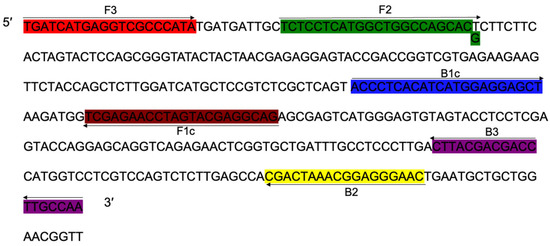
Figure 3.
Schematic illustration of loop-mediated isothermal amplification (LAMP) primers used to detect the S312T mutant genotype of Fusarium fujikuroi with resistance to prochloraz.
3.5. LAMP Specificity
Chromogenic reactions occurred in both resistant wild-type and site-specific mutant isolates, but not for sensitive isolates and U. virens, an important pathogen on rice. The results were consistent with those of electrophoresis (Figure 4a,b), indicating that the established LAMP system had good specificity in detecting prochloraz-resistant isolates (Cyp51b gene type 312 codon TCT). For isolates of 2022, chromogenic reactions also only occurred for resistant isolates with S312T mutation in FfCYP51B (Figure S1).
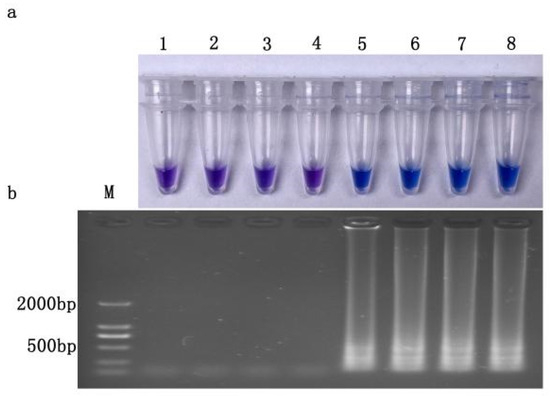
Figure 4.
Specificity of LAMP assay for prochloraz-resistant Fusarium fujikuroi with S312T mutation (a) Hydroxynaphthol blue-visualized color change or (b) gel electrophoresis. M, DL2000Maker; 1, ddH2O; 2, JS16(prochloraz-sensitive); 3, Ustilaginoidea virens; 4, SX-30JS−16Cyp51b; (prochloraz-sensitive); prochloraz-resistant: 5, JH1; 6, SX23; 7, SX24; 8, JS16JH−1Cyp51b.
3.6. LAMP Sensitivity
The genomic DNA of resistant isolate JH1 in gradient dilution was detected, and the results are shown in Figure 5. Bands were amplified, and a chromogenic reaction occurred when the DNA concentration was higher than 0.001 ng/μL. The results indicated that the detection limit of this LAMP technology was 0.001 ng/μL.
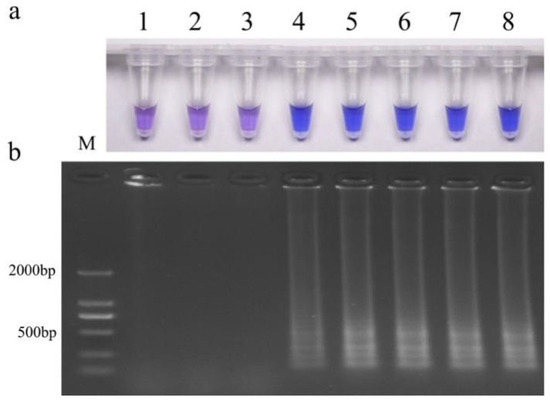
Figure 5.
Results of LAMP detection under different DNA template concentrations. (a) HNB color change; (b) gel electrophoresis. M, DNA marker; 1, ddH2O; 2, 0.0001 ng/μL; 3, 0.001 ng/μL; 4, 0.01 ng/μL; 5, 0.1 ng/μL; 6, 1 ng/μL; 7, 10 ng/μL; 8, 100 ng/μL.
3.7. Detection Limit of Resistance Frequency
Upon observing our blank control, sensitive isolate control and when S (FFJS-16S):R (FFJH-1) R) = 400:1, there was no positive reaction. All reactions occurred in R (JH1) (S:R = 10:1; S:R = 20:1; S:R = 100:1; S:R = 200:1), and gel electrophoresis verified that the reaction was not a false positive (Figure 6); that is, the detection limit of resistance frequency by LAMP was 0.498%.
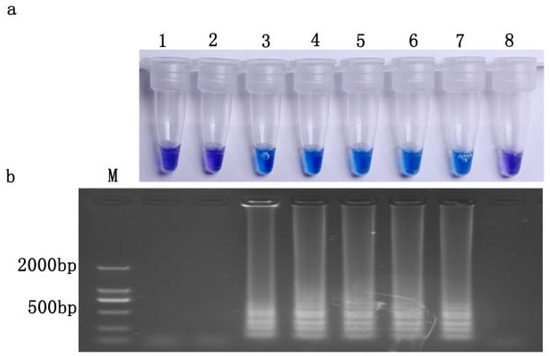
Figure 6.
Proportion of resistant isolates detected by LAMP. (a) HNB color change; (b) gel electrophoresis. M, DL2000Maker; 1, ddH2O; 2, S(JS16); 3, R(JH1); 4, S:R = 10:1; 5, S:R = 20:1; 6, S:R = 100:1; 7, S:R = 200:1; 8, S:R = 400:1. S: Isolates sensitive to prochloraz; R: Resistant to prochloraz.
3.8. Detection of Prochloraz-Resistant Isolates from Seeds and Seedlings
In this study, the sensitive and resistant isolates were used to inoculate rice seeds at the same time for LAMP detection(Figure 7). The seeds inoculated with sensitive isolates showed a negative reaction, while the seeds inoculated with resistant isolates showed a positive reaction. These results were further confirmed using electrophoresis [37].
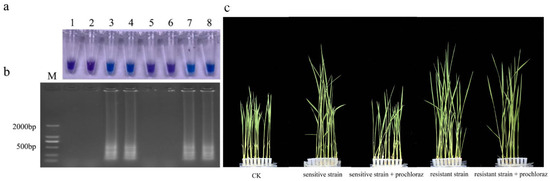
Figure 7.
LAMP detection of prochloraz-resistant Fusarium fujikuroi with S312T mutation from rice seeds and seedlings. (a) Hydroxynaphthol blue-visualized color change; (b) gel electrophoresis. M, DL2000 Maker; 1–2, Sensitive isolate seeds; 3–4, Resistant isolate seeds; 5–6, Sensitive isolate seedlings + 10 μg/mL prochloraz; 7–8, Resistant isolate seedling + 10 μg/mL prochloraz; (c) CK; Sensitive isolate seedlings; Sensitive isolate seedlings + 10 μg/mL prochloraz; Resistant isolate seedling; Resistant isolate seedling + 10 μg/mL prochloraz.
Rice seedlings inoculated with both sensitive and resistant isolates showed spindling. There was no significant difference in pathogenicity between the sensitive and resistant isolates. Rice seedlings treated with prochloraz after inoculation with sensitive isolates were consistent with the control, while rice seedlings inoculated with resistant isolates still showed spindling. Prochloraz had a good control effect on malignant seedling disease in rice, but the resistance isolate had no obvious effect. The LAMP test showed positive results for seedlings inoculated with resistant isolates. Therefore, this LAMP system can detect resistant isolates in rice seeds and seedlings.
4. Discussion
Fusarium fujikuroi is the main pathogen causing RBD. In China, prochloraz has been widely used as a seed treatment fungicide to control RBD for several years, and the frequency of prochloraz resistance has gradually increased. Several studies have reported the resistance of F. fujikuroi to prochloraz and its related resistance mechanism [20,38,39]. In the current study, 75 isolates of bakanae pathogens were collected from Zhejiang Province, and their resistance to prochloraz was determined using the discriminatory dose method. The total resistance frequency was 62.67%, including 30 high-resistance isolates and 17 low-resistance isolates. Previous studies have shown that pathogen resistance to fungicides is always accompanied by fitness costs [40,41]. In this study, prochloraz-sensitive isolates were stronger than the resistant isolates in mycelial growth, sporulation, and spore germination, but the pathogenicity was slightly weaker, which may be related to the ability of the isolates to produce toxins.
Findings from a previous study indicated that the resistance mechanism of F. fujikuroi to prochloraz is due to the S312T point mutation of Ffcyp51b and the overexpression of Ffcyp51a and Ffcyp51b. In recent years, an amino acid mutation at the 511th position of the Ffcyp51b gene sequence has been demonstrated, and the S312T and F511S mutations on Ffcyp51b have a synergistic effect on prochloraz resistance of pathogens causing bakanae disease [22]. We amplified the cyp51b sequence of prochloraz-resistant isolates isolated in Zhejiang Province in 2022 and found that it was consistent with that found in Japan. There were double mutations of S312T and F511S at the same time, but the sensitivity of our prochloraz-resistant isolates was 10–20 times higher than that previously found. Therefore, the relationship between this newly discovered amino acid site mutation and the resistance of F. fujikuroi to prochloraz remains to be further studied.
LAMP is a novel nucleic acid amplification method with high specificity, sensitivity, and simple operation. At present, LAMP has been successfully used to detect bacterial, viral [42,43], and fungal pathogens [44]. The DNA intercalation dye was HNB, and the color change from purple to sky blue indicated a positive reaction, while purple indicated a negative reaction. The reaction times and temperatures were consistent with those in previous studies. By adding HNB (0.15 μM) to the LAMP test mixture, the test results can be displayed. The reaction mixture change from purple to sky blue can be seen with the naked eye, and the test results can be directly and quickly judged without amplification. In addition, the addition of HNB dye did not reduce the sensitivity or specificity of the reaction system.
RBD is a monocyclic disease, which is mainly transmitted through germ-bearing seeds, and seeds with fungi are the main source of initial infection. The main stage of infection is the process of seed soaking and bud promotion. Previous studies on the isolation and identification of Asian rice seeds showed that the fungus-carrying rate ranged from 3 to 92%, and there were great differences among regions. The pathogenic fungi attach to the seed surface in the form of conidia or latent mycelium to overwinter [45]. Therefore, it is of great significance to detect pathogenic fungi at the seed stage. Through the established visual detection methods, seed health can be monitored quickly and accurately, and the seed health status can be effectively evaluated to minimize the threat of pathogenic fungi.
Chemical treatment is the primary method for preventing and controlling RBD [46]. Failure to treat seeds with chemical agents can result in contamination of healthy seeds when soaked with infected ones. The pathogen can then invade rice seedlings through new buds and gradually spread to the whole plant as it grows. Prochloraz is a DMI, which has an obvious control effect on diseases caused by ascomycetes and ascocarps in many crops [47]. Since its introduction in China at the end of the 20th century, prochloraz has become the main chemical control for RBD. Due to the long-term use of a single agent and low concentration of seeds, the pathogen is prone to develop resistance. Studies have shown that resistance to prochloraz and phenamacril has developed to different degrees. If rice seeds with a prochloraz-resistant isolate are treated with prochloraz, RBD would not be effectively controlled.
The traditional detection methods of seed fungi include the water agar method and the isolation and culture method [48]. These detection methods are time-consuming and can only be identified through morphological and biological characteristics combined with the relevant literature, easily resulting in misidentification of similar species. Modern molecular biology technologies include common PCR detection based on target gene sequences and real-time fluorescence quantitative PCR. However, the common characteristics of these technologies are a long cycle time, expensive instruments, and the inability to meet the needs of grassroot detection [49].
The point mutation in the 312th codon of the cyp51b gene results in the alteration of the S312T amino acid in F. fujikuroi, resulting in high prochloraz resistance. In this study, we developed a LAMP assay to detect the genotype of the prochloraz-resistant S312T mutant of F. fujikuroi. Before seed treatment, the seeds were tested, providing technical support for the detection of the S312T genotype of the prochloraz-resistant F. fujikuroi mutant before sowing. Based on our results, this LAMP detection method can accurately detect rice seeds and seedlings simulated with different sensitive isolates, providing technical support for the next step in detecting whether rice seeds contain prochloraz-resistant fungi before sowing. For further study, the S312T-LAMP test should be used to collect RBD samples from different regions to avoid the inefficiency of fungicides due to drug resistance. Evolutionary patterns of genotypes in resistant isolates should also be studied to develop new control strategies using combinations of different fungicides to control RBD.
5. Conclusions
In this study, we found that the S312T + F511S double mutations are responsible for prochloraz resistance in F. fujikuroi after previous report of S312T single mutation. Based on this resistance molecular mechanism, a LAMP system was established to detect the mutation site (Cyp51b 312 codon TCT-ACT) in F. fujikuroi for prochloraz resistance. This method can rapidly detect prochloraz resistance on rice seeds and seedlings to provide support for the scientific control of RBD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10080560/s1, Figure S1: Detection of isolates collected in 2022.
Author Contributions
C.G., D.D. and C.M. conceived the study. C.G., Q.Z. and C.Z. performed computational analyses and interpreted the data. C.G., Q.Z. and C.Z. prepared the figures and tables. C.Z., C.G. and D.D. wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Key Research and Development Project of Zhejiang Province, China (No. 2015C02), Development Plan of Science and Technology for Hangzhou (202203A07), and National Natural Science Foundation of China (No. 32160522).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.ncbi.nlm.nih.gov/ (accessed on 10 December 2019).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Sultana, S.; Kitajima, M.; Kobayashi, H.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; Suga, H. A natural variation of fumonisin gene cluster associated with fumonisin production difference in Fusarium fujikuroi. Toxins 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kitajima, M.; Nagumo, R.; Tsukiboshi, T.; Uegaki, R.; Nakajima, T.; Kushiro, M.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; et al. A single nucleotide polymorphism in the translation elongation factor 1α gene correlates with the ability to produce fumonisin in Japanese Fusarium fujikuroi. Fungal Biol. 2014, 118, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Pedrozo, M.R.; Fenoglio, M.J.; Little, C. First report of seedborne Fusarium fujikuroi and its potential to cause pre- and post-emergent damping-off on soybean (Glycine max) in the United States. Plant Dis. 2015, 22, 2–4. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, D.; Hu, X.; Wen, R. First report of leaf spot on Lasia spinosa caused by Fusarium fujikuroi in China. Plant Dis. 2020, 104, 2525. [Google Scholar] [CrossRef]
- Nur, I.M.Z.; Razak, A.; Salleh, B. Bakanae disease of rice in Malaysia and Indonesia: Etiology of the causal agent based on morphological, physiological and pathogenicity characteristics. J. Plant Prot. Res. 2008, 48, 475–485. [Google Scholar]
- Nicolli, C.P.; Haidukowski, M.; Susca, A.; Gomes, L.B.; Logrieco, A.; Stea, G.; Del Ponte, E.M.; Moretti, A.; Pfenning, L.H. Fusarium fujikuroi species complex in Brazilian rice: Unveiling increased phylogenetic diversity and toxigenic potential. Int. J. Food Microbiol. 2020, 330, 108667. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.; Huang, K.J.; Chen, S.Y.; Kuo, Y.F. Detecting bakanae disease in rice seedlings by machine vision. Comput. Electron. Agric. 2016, 121, 404–411. [Google Scholar] [CrossRef]
- Piombo, E.; Bosio, P.; Acquadro, A.; Abbruscato, P.; Spadaro, D. Different phenotypes, similar genomes: Three newly sequenced Fusarium fujikuroi strains induce different symptoms in rice depending on temperature. Phytopathology 2020, 110, 656–665. [Google Scholar] [CrossRef]
- Matic, S.; Gullino, M.L.; Spadaro, D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front. Biosci. 2017, 9, 333–344. [Google Scholar]
- Saito, H.; Sasaki, M.; Nonaka, Y.; Tanaka, J.; Tokunaga, T.; Kato, A.; Thuy, T.T.T.; Vang, L.V.; Tuong, L.M.; Kanematsu, S.; et al. Spray application of nonpathogenic Fusaria onto rice flowers controls bakanae disease (caused by Fusarium fujikuroi) in the next plant generation. Appl. Environ. Microb. 2021, 87, e01959–20. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Hass, U.; Dalgaard, M.; Andersen, H.R.; Bonefeld-Jørgensen, E.; Christiansen, S.; Laier, P.; Poulsen, M.E. Prochloraz: An imidazole fungicide with multiple mechanisms of action. Int. J. Androl. 2006, 9, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.P.; Li, J.S.; Wang, J.X.; Wu, L.Y.; Wang, Y.F.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Effects of the dinitroaniline fungicide fluazinam on Fusarium fujikuroi and rice. Pestic. Biochem. Physiol. 2018, 152, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.A.; McDonald, B.A.; Brunner, P.C. Mutations in the CYP51 gene reduce DMI sensitivity in Parastagonospora nodorum populations in Europe and China. Pest Manag. Sci. 2017, 73, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, Y.H.; Hong, S.K.; Lee, Y.K.; Nam, Y.J.; Lee, J.G.; Han, S. Monitoring for the resistance to prochloraz of Fusarium species causing bakanae disease in Korea. Korean J. Mycol. 2015, 43, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, Z.Y.; Zhou, B.H.; Liu, R.; Lu, J. Detection of resistance of Fusarium moniliforme to rice seed soaking agents in some rice areas of Jiangsu Province. Jiangsu J. Agri. 2002, 3, 190–192. [Google Scholar]
- Fan, J.; Urban, M.; Parker, J.E.; Brewer, H.C.; Kelly, S.L.; Hammond-Kosack, K.E.; Fraaije, B.A.; Liu, X.; Cools, H.J. Characterization of the sterol 14α-demethylases of Fusarium graminearum identifies a novel genus-specific CYP51 function. New Phytol. 2013, 198, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.; Yun, Y.; Yin, Y.; Ma, Z. A sterol C-14 reductase encoded by FgERG24B is responsible for the intrinsic resistance of Fusarium graminearum to amine fungicides. Microbiology 2011, 157, 1665–1675. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Loza-Reyes, E.; Atkins, S.L.; Fraaije, B.A. The CYP51C gene, a reliable marker to resolve interspecific phylogenetic relationships within the Fusarium species complex and a novel target for species-specific PCR. Int. J. Food Microbiol. 2010, 144, 301–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, C.X.; Zhai, X.Y.; Jamieson, P.A.; Zhang, C.Q. Mutation in cyp51b and overexpression of cyp51a and cyp51b confer multiple resistant to DMIs fungicide prochloraz in Fusarium fujikuroi. Pest Manag. Sci. 2021, 77, 824–833. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Q.; Yuan, K.; Li, Y.; Shi, M.; Miao, J.; Liu, X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022, 187, 105189. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ebihara, A.; Sakahara, Y.; Matsumoto, S.; Ueno, R.; Bao, W.; Kimura, M.; Fuji, S.I.; Shimizu, M.; Kageyama, K.; et al. Synergistic effect of amino acid substitutions in CYP51B for prochloraz resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2023, 189, 105291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Mao, C.; Wu, J.; Zhang, C. Activity of a SDHI fungicide penflufen and the characterization of natural-resistance in Fusarium fujikuroi. Pestic. Biochem. Physiol. 2021, 179, 104960. [Google Scholar] [CrossRef] [PubMed]
- Buddhachat, K.; Sripairoj, N.; Ritbamrung, O.; Inthima, P.; Ratanasut, K.; Boonsrangsom, T.; Rungrat, T.; Pongcharoen, P.; Sujipuli, K. RPA-assisted Cas12a system for detecting pathogenic Xanthomonas oryzae, a causative agent for bacterial leaf blight disease in rice. Rice Sci. 2022, 29, 340–352. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hu, X.R.; Zhang, C.Q. Molecular detection of QoI resistance in Colletotrichum gloeosporioides causing strawberry anthracnose based on Loop-mediated isothermal amplification assay. Plant Dis. 2019, 103, 1319–1325. [Google Scholar] [CrossRef]
- Hou, Y.P.; Mao, X.W.; Lin, S.P.; Song, X.S.; Duan, Y.B.; Wang, J.X.; Zhou, M.G. Activity of a novel succinate dehydrogenase inhibitor fungicide pyraziflumid against Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 145, 22–28. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, Y.; Li, T.; Zhao, D.; Cao, J.; Shi, Y.; Wang, J.; Zhou, M. Development of a rapid and high-throughput molecular method for detecting the F200Y mutant genotype in benzimidazole-resistant isolates of Fusarium asiaticum. Pest Manag. Sci. 2016, 72, 2128–2135. [Google Scholar] [CrossRef]
- Chen, S.; Schnabel, G.; Yuan, H.; Luo, C. LAMP detection of the genetic element ‘Mona’ associated with DMI resistance in Monilinia fructicola. Pest Manag. Sci. 2019, 75, 779–786. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Lee, S.; Oliveira, M.S.; Peres, N.A. Development of high-throughput snp genotyping assays for rapid detection of strawberry Colletotrichum species and the G143A mutation. Phytopathology 2018, 108, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yan, L.; Liang, W.; Yang, Q. Paralogous Cyp51s mediate the differential sensitivity of Fusarium oxysporum to sterol demethylation inhibitors. Pest Manag. Sci. 2019, 75, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Van Diepeningen, A.D.; Curfs-Breuker, I.; De Hoog, G.S.; Meis, J.F. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J. Antimicrob. Chemother. 2015, 70, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Denschlag, C.; Vogel, R.F.; Niessen, L. Hyd5 gene-based detection of the major gushing-inducing Fusarium spp. in a loop-mediated isothermal amplification (LAMP) assay. Int. J. Food Microbiol. 2012, 156, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Duan, X.; Peng, Y.; Rui, Y. Rapid detection of a novel B1-β-lactamase gene, blaAFM-1 using a loop-mediated isothermal amplification (LAMP) assay. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.Y.; Zhang, C.Q. Establishment of a rapid detection method for rice blast fungus based on one-step loop-mediated isothermal amplification (LAMP). Plant Dis. 2019, 103, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.F.; Tomlinson, J.; Hodgetts, J.; Spadaro, D.; Gullino, M.L.; Boonham, N. Development of loop-mediated isothermal amplification assays for the detection of seedborne fungal pathogens Fusarium fujikuroi and Magnaporthe oryzae in Rice Seed. Plant Dis. 2018, 102, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Waqas Younas, M.; Yang, J.; Zhu, H.; Miao, J.; Gu, B.; Liu, X. Characterization of prochloraz resistance in Fusarium fujikuroi from Heilongjiang province in China. Plant Dis. 2022, 106, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Myeong, U.K.; Hyo, S.; Kang, J.; Lee, Y.H.; Kim, H.T. Detection for the resistance of Fusarium spp. isolated from rice seeds to prochloraz and cross-resistance to other fungicides inhibiting sterol biosynthesis. Korean J. Pestic. Sci. 2008, 12, 276–281. [Google Scholar]
- Forcelini, B.B.; Rebello, C.S.; Wang, N.Y.; Peres, N.A. Fitness, competitive ability, and mutation stability of isolates of Colletotrichum acutatum from strawberry resistant to QoI fungicides. Phytopathology 2018, 108, 462–468. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Fraaije, B.A. Fitness penalties in the evolution of fungicide resistance. Phytopathology 2018, 56, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, B.W.; Ateka, E.M.; Miano, D.W.; Fuentes, S.; Perez, A.; Low, J.W.; Kreuze, J.F. Loop-mediated isothermal amplification assays for on-site detection of the main sweet potato infecting viruses. J. Virol. Methods 2021, 298, 114301. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Yaqinuddin, A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Dai, D.J.; Wang, H.D.; Zhang, C.Q. One-step loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Fusarium fujikuroi in bakanae disease through NRPS31, an important gene in the gibberellic acid bio-synthesis. Sci. Rep. 2019, 9, 3726. [Google Scholar] [CrossRef] [PubMed]
- Amira, M.B.; Faize, M.; Karlsson, M.; Dubey, M.; Frąc, M.; Panek, J.; Fumanal, B.; Gousset-Dupont, A.; Julien, J.L.; Chaar, H.; et al. Fungal x-intrinsic protein aquaporin from Trichoderma atroviride: Structural and functional considerations. Biomolecules 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Shen, X.; Li, Z.; Wang, J.; Li, X.; Xu, Z.; Shen, Y.; Lei, Y.; Huang, X.; Wang, X.; et al. Antibody generation and rapid immunochromatography using time-resolved fluorescence microspheres for propiconazole: Fungicide abused as growth regulator in vegetable. Foods 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In vitro and in field response of different fungicides against aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hu, S.; Wu, Y.; Wu, C.; Yang, Y.; Gu, B.; Du, H. A sers platform for rapid detection of drug resistance of non-Candida albicans using Fe3O4@PEI and triangular silver nanoplates. Int. J. Nanomed. 2022, 17, 3531–3541. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Z.; Gao, J.; Tian, E.; Yu, W.; Li, H.; Zhang, J.; Xie, R.; Zhao, X.; Chen, A. Rapid and visual identification of Chlorophyllum molybdites with loop-mediated isothermal amplification method. Front. Microbiol. 2021, 12, 638315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).