Abstract
Diaporthe (anamorph: Phomopsis) species are endophytes or fungal pathogens for many different plant species. Soybean (Glycine max) can be infected by many different Diaporthe species; among them, D. caulivora and D. longicolla are responsible for the most significant damages. Diaporthe goulteri is a species that was only recently described and has so far been found on sunflower (Helianthus annuus) in Australia and an unknown host in Thailand. Here, we report isolation of D. goulteri from soybean in southern Germany, molecular species identification, and additional morphological description. We also show that D. goulteri can infect soybean and describe the symptoms we observed, both on the plant where the isolate came from and following artificial inoculation.
1. Introduction
Because of increased demand for soybean (Glycine max) as a source for oil and protein, its cultivation is increasing in Germany like in other countries. As soybean cultivation area is on the rise, it is expected that the incidence of soybean pathogens will increase. Species of the fungal genus Diaporthe are important pathogens for soybean in all major soybean cultivation areas in North and South America, Asia, and Europe [1,2,3,4,5,6]. Diaporthe longicolla was identified as a seed decay pathogen on German soybean seeds together with D. caulivora, D. eres, and D. novem [7]. Since globally many more Diaporthe spp. are reported as soybean pathogens [8], the occurrence of more Diaporthe spp. on soybean in Germany was expected.
Genus Diaporthe is large and complex, containing more than one hundred species. The phylogeny of the genus has been revised based on sequence comparisons [9,10,11] and studies concerned with updating the molecular phylogeny of Diaporthe regularly discover new species belonging to the genus. Diaporthe goulteri is among those species discovered relatively recently: Thompson et al. [12] isolated the species from a sunflower (Helianthus annuus) seed, described it, and named it. Apart from this, there are scant reports about the species; there are some isolates from China, Taiwan, and Thailand with corresponding sequences deposited in GenBank, but we found only one publication [13] that provides additional information about the species: Bundhun et al. [13] reported the isolation of D. goulteri from “a dead branch of an unknown host” in Thailand and described its sexual morph.
Here, we report isolation of D. goulteri from soybean in southern Germany. We provide evidence that soybean is a host plant for D. goulteri and expand on the species description.
2. Materials and Methods
2.1. Sampling and Isolation
Soybean plants showing signs of Diaporthe infection, i.e., pycnidia or perithecia showing up as black dots on the stem were collected from fields shortly before harvest. Seeds, pods, and stem pieces were disinfected with 3% sodium hypochlorite solution for 1 min, rinsed three times with sterile water, dried on filter paper, and placed on Petri plates with potato dextrose agar (PDA). These plates were incubated at room temperature for one week. After seven days, cultures, which were tentatively identified as Diaporthe sp. based on culture morphology, were subcultured by transferring an agar plug with a small piece of the cultures to fresh PDA plates that were also incubated at room temperature.
2.2. Species Identification
2.2.1. Species Identification from Pure Cultures Using Culture Morphology and Microscopy as Well as Sequencing and Phylogenetic Analysis
Approximately 20 or 40 days-old cultures were evaluated morphologically. This way, it was decided for which cultures molecular species identification should be carried out. The data also now contribute to the species description. The cultures on PDA were observed from the top and from the bottom (front and back side of the plates). Conidiomata on the plates and pycnidia on soybean stems were observed using a Stemi 2000 binocular loupe (Carl Zeiss, Oberkochen, Germany) and conidia using a Primo Star microscope (Carl Zeiss). The images were taken using an AxioCam HRC color camera (Carl Zeiss) and evaluated with AxioVision software, Release 4.8.3 Special Edition 1 (Carl Zeiss, Oberkochen, Germany).
For molecular species identification, genomic DNA was extracted from 7 days-old cultures using the protocol published by [14]. ITS, TEF1, and TUB genes were amplified using the primer pairs ITS1-F/ITS4 [15], EF1-728F/EF1-986R [16], and Bt-2a/Bt-2b [17] under the same conditions as described by [7] and sequenced. The sequences were then searched against NCBI GenBank using BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&BLAST_SPEC=&LINK_LOC=blasttab&LAST_PAGE=blastn accessed on 10 October 2024).
For the isolate (DPC_HOH36) with high sequence similarity to D. goulteri, we downloaded highly similar sequences from GenBank and performed phylogenetic analysis. For downloading, we chose >90 percent identity as a criterion and cutoff. The sequences were aligned using ClustalW [18] and the alignments were edited using BioEdit version 7.0.5.3 [19]. For the three-gene phylogeny, the alignments were concatenated using a text editor. Maximum likelihood phylogenetic analysis using the Tamura–Nei model [20] was performed using MEGA X version 10.0.5 [21]. Initial trees were obtained automatically by applying the maximum parsimony method.
2.2.2. Direct Species Identification from Infected Plant Parts Using qPCR
For qPCR detection, pieces of leaves, stems, pods, or seeds were surface disinfected as described in Section 2.1 and used for DNA isolation. Samples of roughly 100 mg were put into 2-mL micro screw tubes (Sarstedt, Nümbrecht, Germany) with two steel balls (4.50 mm in diameter, Niro, Sturm Präzision GmbH, Oberndorf am Neckar, Germany). The tubes with the samples were frozen in liquid nitrogen for 3 min and homogenized for 20 s at 4.5 m/s using a FastPrep®-24 homogenizer (MP Biomedicals GmbH, Santa Ana, CA, USA). DNA extraction was performed using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions.
Field samples were tested for the presence of D. caulivora, D. eres, D. longicolla, or D. novem. The reactions for the quadruplex TaqMan qPCR to detect these species were prepared as previously described [22].
The 20-μL reactions used here for detection of D. goulteri in plant samples consisted of 10 μL ProbeMasterMix (2×) No-ROX (Genaxxon bioscience GmbH, Ulm, Germany), 4 pmol of each forward and reverse primers (DPCG-F: 5′-cttacactcacaaaactcgc-3′; DPCG-R: 5′-gctcgattcaccgggttg-3′), 1 pmol probe (DPCG-P: 5′-6-FAM-ccagagcaaacaccaccgacgc-BMN-Q535-3′), and 2 μL template DNA. Design and testing of this primer and probe combination was performed analogous to what was described previously [22]. The reaction mixes were incubated for 3 min at 95 °C and then subjected to 40 cycles of 95 °C for 15 s and 60 °C for 45 s. Reactions were run in technical duplicates on a CFX96 Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) using FrameStar1 96-Well Skirted PCR Plates (4titude, Brooks Automation, Chelmsford, MA, USA).
2.3. Testing for Pathogenicity on Soybean
Inoculations for the pathogenicity test were performed using the toothpick method [23,24]. To prepare toothpicks for inoculation, autoclaved toothpicks were placed on PDA plates together with agar plugs overgrown with mycelium of the Diaporthe isolate. The plates were incubated at room temperature for 20 days. Toothpicks overgrown with mycelium were inserted into the stem in the middle of the internode between cotyledons and the first trifoliate of three weeks old healthy soybean plants (cv. Shouna) at an angle of 90 degrees. Control plants were identically treated with sterile toothpicks. Six plants each were used for inoculation and control plants were kept in the greenhouse at 16 h light / 8 h dark (24 °C/22 °C); air humidity was kept high using a vaporizer (Condair, Norderstedt, Germany). Symptom development was observed weekly for up to two months.
The inoculated plants were tested for infection with D. goulteri by two different methods. One was re-isolation, and the other was detection using qPCR with a newly designed primer–probe combination for species-specific detection based on the TEF sequence of D. goulteri, as described in Section 2.2.2.
For re-isolation, the stem of an infected plant was cut close (1 cm removed) to the inoculation site. After surface disinfection (as described in Section 2.1), 2 to 3 mm long and 1 mm thick pieces of the center part of these sections of the stems were placed on PDA plates. The developing fungal colonies were determined as identical to the inoculum based on culture morphology to fulfil Koch’s postulate.
For qPCR detection, pieces of the center part of the above sections of the stems of the infected plant close to the inoculation site (treated as for re-isolation) and identical stem pieces from a control plant were used for DNA extraction. DNA extraction and qPCR were performed as described in Section 2.2.2.
3. Results
3.1. D. goulteri Found on a Soybean Plant Collected in a Field Close to Tübingen, Germany
As part of a survey monitoring the incidence of Diaporthe spp. in Germany, soybean plants showing typical Diaporthe symptoms were collected from fields close to Tübingen on 2 October 2023. Using the qPCR assay developed by [22], plants infected with D. caulivora, D. eres, D. longicolla, or D. novem were identified (78% of all sampled plants); the highest incidence was found for D. caulivora. From some plants, especially those that tested negative in the qPCR assay, parts were also used for gaining Diaporthe spp. isolates (see Section 2.1). This way, we gained five new isolates of Diaporthe spp.; the other samples either yielded nothing or fungi from different genera. One isolate (designated DPC_HOH36) recovered from a seed of a plant collected from the following coordinates (48.525801, 9.096385) showed high similarity in all three tested genes to sequences annotated with D. goulteri in the GenBank database. This, together with morphological observations, allowed for the identification of isolate DPC_HOH36 as D. goulteri.
To refine this finding, similar sequences were downloaded from GenBank. Because of the chosen cutoff, the download was restricted to sequences annotated with either D. goulteri or D. ambigua. To broaden the phylogenetic analysis we also included sequences representing the four Diaporthe spp. reported previously on soybean in Germany (D. caulivora, D. eres, D. longicolla, and D. novem) [7] as outgroups (Figure 1).
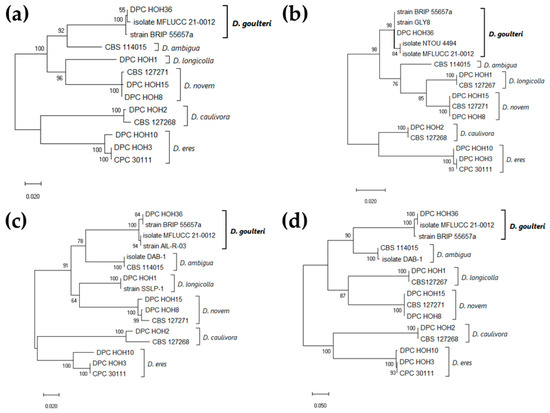
Figure 1.
Maximum Likelihood phylogeny identifying isolate DPC_HOH36 as Diaporthe goulteri. Trees with the highest log likelihood (lL) are shown. Trees are drawn to scale, with branch lengths indicating the number of substitutions per site. Numbers next to the branches are bootstrapping percentages. (a) Combined phylogeny based on 13 concatenated ITS, TUB, and TEF sequences, 1183 positions in final dataset, lL −4658.69. (b) Phylogeny based on 16 ITS sequences, 394 positions in final dataset, lL −1253.80. (c) Phylogeny based on 16 TUB sequences, 493 positions in final dataset, lL −1581.92. (d) Phylogeny based on 15 TEF sequences, 324 positions in final dataset, lL −1799.90.
For all three genes, and also in the analysis combining the three genes, our sequences clustered with the sequences annotated as D. goulteri in GenBank. The ITS sequence of DPC_HOH36 is identical to that of strain BRIP 55657a, the isolate corresponding to the first description of D. goulteri [12] (Figure 1b). The same is true for the TUB sequence (Figure 1c), while the TEF sequence of DPC_HOH36 is identical to that of isolate MFLUCC 21-0012 [13] (Figure 1d). Additional sequences annotated with D. goulteri were found and are represented in the phylogenies; however, no publications were found related to the GenBank records. Altogether, the analysis of our sequence data clearly allowed for the identification of isolate DPC_HOH36 as D. goulteri.
Isolates and corresponding GenBank accession numbers used for the phylogenies are detailed in Table 1.

Table 1.
Strains or isolates of Diaporthe spp. and GenBank accession numbers of the ITS (internal transcribed spacer), TUB (β-tubulin), and TEF (translation elongation factor 1-α) sequences used for the phylogenies in Figure 1.
3.2. Morphological Description of D. goulteri Isolate DPC_HOH36
Growth of D. goulteri isolate DPC_HOH36 on PDA was fast compared to other Diaporthe spp. (D. longicolla, D. caulivora, D. eres, and D. novem) and after 5 days the entire plate was covered with mycelium of white-yellowish-light green color. The back side of the plates was white and after longer incubation (40 days) it became light brown with scattered dark spots (Figure 2b).
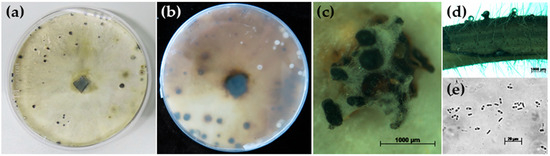
Figure 2.
Morphological characteristics of Diaporthe goulteri isolate DPC_HOH36. (a,b) Colony front and back after 40 days on PDA. (c) Conidiomata on PDA. (d) Pycnidia on soybean stem after three weeks in culture (on water agar). (e) α-conidia.
After approximately 40 to 50 days, dark brown pycnidia appeared (Figure 2a,c) that contained numerous α-conidia (Figure 2e). The α-conidia were fusiform to cylinder-shaped, biguttulate, hyaline, and measured 5.19 to 8.06 × 1.92 to 2.88 µm. Perithecia and β-conidia were not observed. The morphological characteristics of isolate DPC_HOH36 were similar to those described for D. goulteri strain BRIP 55657a [12]. Thus, the morphology corroborated the identification of isolate DPC_HOH36 as D. goulteri.
3.3. D. goulteri Can Infect Soybean in the Laboratory Test
We used an established method for isolating fungi growing inside plant tissue to produce our isolates (see Section 2.1). Therefore, our isolate should be either an endophyte or a pathogen. To further establish whether D. goulteri can infect soybean, which parts of the plant are affected, and to observe symptoms caused by D. goulteri, we performed a pathogenicity test, as described in Section 2.3.
Dark discoloration of the plant tissue extending roughly 1 cm from where the inoculated toothpicks had been inserted was observed at the soybean stem (Figure 3c). When the stems were cracked open, this discoloration, indicating necrosis, could also be found in the center of the stem, where it extended to roughly 2 cm from the inoculation site (Figure 3d). In contrast, control plants that had been stuck with sterile toothpicks showed a tan discoloration that extended less than 2 mm from the insertion site.
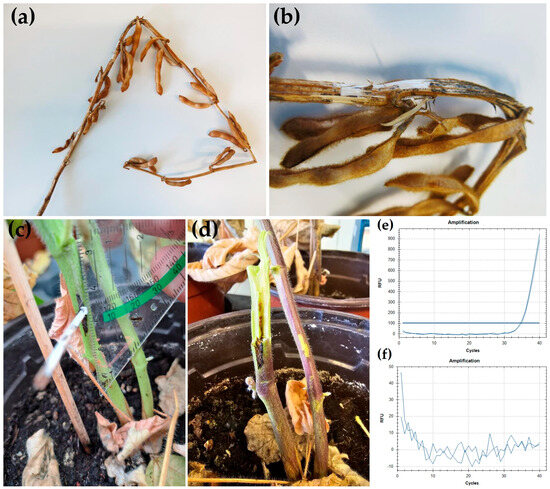
Figure 3.
Diaporthe goulteri on soybean. (a) Soybean plant from which D. goulteri isolate DPC_HOH36 was recovered (without rootstock). (b) Close-up from (a) where the stem is broken open. (c) Stem of the soybean plant inoculated with D. goulteri isolate DPC_HOH36 using the toothpick method 30 days after inoculation. (d) Discoloration of central tissue close to the inoculation site two months after the inoculation. (e,f) Amplification curves of qPCR assays on DNA sample from plant inoculated with DPC_HOH36 and DNA sample from control plant, respectively.
Based on culture morphology, fungal mycelium originating from re-isolation from the discolored plant parts was identified as D. goulteri. Using qPCR, we also could detect D. goulteri in the stem of inoculated plant close to the infection site (Figure 3e). No D. goulteri was detected on any sample taken from control plants (Figure 3f). These observations strongly indicate that D. goulteri can infect soybean plants.
4. Discussion
Strain DPC_HOH36 was isolated from a soybean plant in a field close to Tübingen, Germany. BLAST comparisons of the TUB, TEF, and ITS sequences showed high similarity to published sequences of D. goulteri. This similarity can also be seen in phylogenies built based on these sequences. These results strongly suggest that our isolate DPC-HOH36 is D. goulteri.
Thompson et al. [12] described D. goulteri colonies growing on PDA and OMA (oatmeal agar) with relatively fast-growing mycelium that was first white before dark spots appeared. On PDA, we made the same observation. Droplets containing conidia formed on OMA were quite colorful—pale yellow, orange, or ochre and reddish, or as the authors described it, “sienna colored”. It is probably due to the medium that we could not observe these colors on our PDA plates. The form of the conidia they observed was exactly as seen by us, and with 6 to 9 × 2 to 3 µm, the dimensions they observed also encompass what we measured. The conidiomata observed by [12] on OMA and on sterilized wheat straw are slightly different from what we observed on PDA and on sterilized soybean stems. Nevertheless, morphological observations also indicate that isolate DPC_HOH36 is the same species. Our description of the species should be seen as an extension of the existing descriptions.
Thompson et al. [12] isolated D. goulteri from a sunflower seed. No further tests regarding the pathogenicity of D. goulteri on sunflower or on association to any other plant species were performed. The species from which other researchers isolated D. goulteri are either unidentified or not mentioned [13]. Therefore, the host range of D. goulteri remains largely unknown. Our pathogenicity test on soybean is the first demonstration of infection of a plant by D. goulteri, so it can be stated that G. max definitely is a host for D. goulteri.
We performed surface disinfection of the plant material before isolation of Diaporthe spp. Using this procedure, no growth of fungi only randomly associated (i.e., spores sticking to the surface) with the sampled soybean plants should occur. Therefore, isolating D. goulteri from a symptomatic soybean plant means that the fungus was growing inside the plant, which fulfils the first of Koch’s postulates and indicates that it is either an endophyte or a pathogen. Together with that, inoculation, infection and detection of the fungus in the inoculated soybean tissue demonstrated that D. goulteri can infect and grow in soybean. This constitutes the first demonstration of the ability of D. goulteri to infect plants. More findings of D. goulteri in the field together with observations of symptoms and damages will be necessary to establish whether D. goulteri acts as a pathogen and how severe damages caused by the species may be.
Author Contributions
Conceptualization, B.H. and T.I.L.; methodology, B.H.; validation, B.H. and T.I.L.; formal analysis, T.I.L.; investigation, B.H., M.G.G. and T.I.L.; resources, T.I.L.; writing—original draft preparation, B.H. and T.I.L.; writing—review and editing, B.H. and T.I.L.; visualization, B.H., M.G.G. and T.I.L.; project administration, T.I.L.; funding acquisition, T.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Food and Agriculture, grant number 2815EPS082 (SoySound) to T.I.L. The APC was funded by the University of Hohenheim.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences underlying our analyses were submitted to GenBank, accession numbers are given in the manuscripts. All other data are contained within the manuscript.
Acknowledgments
We thank Heike Popovitsch for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pioli, R.N.; Morandi, E.N.; Bisaro, V. First report of Soybean Stem Canker caused by Diaporthe phaseolorum var. caulivora in Argentina. Plant Dis. 2001, 85, 95. [Google Scholar] [CrossRef] [PubMed]
- Costamilan, L.M.; Yorinori, J.T.; Almeida, A.M.; Seixas, C.D.S.; Binneck, E.; Araújo, M.R.; Carbonari, J.A. First report of Diaporthe phaseolorum var. caulivora infecting soybean plants in Brazil. Trop. Plant Pathol. 2008, 33, 381–385. [Google Scholar] [CrossRef]
- Santos, J.M.; Vrandečić, K.; Ćosić, J.; Duvnjak, T.; Phillips, A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 2011, 27, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, K.; Zheng, S.; Yang, J.; Chen, C.; Zheng, X.; Wang, Y.; Ye, W. Diaporthe diversity and pathogenicity revealed from a broad survey of Soybean Stem Blight in China. Plant Dis. 2022, 106, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Petrović, K.; Riccioni, L.; Vidić, M.; Đorđević, V.; Balešević-Tubić, S.; Đukić, V.; Miladinov, Z. First report of Diaporthe novem, D. foeniculina, and D. rudis associated with soybean seed decay in Serbia. Plant Dis. 2016, 100, 2324. [Google Scholar] [CrossRef]
- Petrović, K.; Skaltsas, D.; Castlebury, L.A.; Kontz, B.; Allen, T.W.; Chilvers, M.I.; Gregory, N.; Kelly, H.M.; Koehler, A.M.; Kleczewski, N.M.; et al. Diaporthe seed decay of soybean [Glycine max (L.) Merr.] is endemic in the United States, but new fungi are involved. Plant Dis. 2021, 105, 1621–1629. [Google Scholar] [CrossRef]
- Hosseini, B.; El-Hasan, A.; Link, T.; Voegele, R.T. Analysis of the species spectrum of the Diaporthe/Phomopsis complex in European soybean seeds. Mycol. Prog. 2020, 19, 455–469. [Google Scholar] [CrossRef]
- Hosseini, B.; Voegele, R.T.; Link, T.I. Diagnosis of soybean diseases caused by fungal and oomycete pathogens: Existing methods and new developments. J. Fungi 2023, 9, 587. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef]
- Santos, L.; Alves, A.; Alves, R. Evaluating multi-locus phylogenies for species boundaries determination in the genus Diaporthe. PeerJ 2017, 5, e3120. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Tan, Y.P.; Shivas, R.G.; Neate, S.; Morin, L.; Bissett, A.; Aitken, E. Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia. Persoonia 2015, 35, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, D.; Senanayake, I.; Jayawardena, R.S.; Camporesi, E.; Huang, Y.; Dong, Z.; Hyde, K.D.; To-Anun, C.; Cheewangkoon, R. First reports of the sexual morphs of Diaporthe forlicesenica nom. nov. and Diaporthe goulteri (Diaporthaceae, Diaporthales) revealed by molecular phylogenetics. Phytotaxa 2021, 516, 1–27. [Google Scholar] [CrossRef]
- Liu, D.; Coloe, S.; Baird, R.; Pedersen, J. Rapid mini-preparation of fungal DNA for PCR. J. Clin. Microbiol. 2000, 38, 471. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, N., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetic Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hosseini, B.; Voegele, R.T.; Link, T.I. Establishment of a quadruplex real-time PCR assay to distinguish the fungal pathogens Diaporthe longicolla, D. caulivora, D. eres, and D. novem on soybean. PLoS ONE 2021, 16, e0257225. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Petrović, K.; Kontz, B.J.; Bradley, C.A.; Chilvers, M.I.; Mueller, D.S.; Smith, D.L.; Wise, K.A.; Mathew, F.M. Inoculation method impacts symptom development associated with Diaporthe aspalathi, D. caulivora, and D. longicolla on soybean (Glycine max). Plant Dis. 2019, 103, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Li, Z.; Buck, J.W. Development of southern stem canker disease on soybean seedlings in the greenhouse using a modified toothpick inoculation assay. Crop Protect. 2017, 100, 57–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).