Evaluation of the Interest in and Tolerance of a Topical Emollient in the Management of Canine Nasal Hyperkeratosis: An Open-Label, Prospective, Uncontrolled Pilot Study †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment Protocol
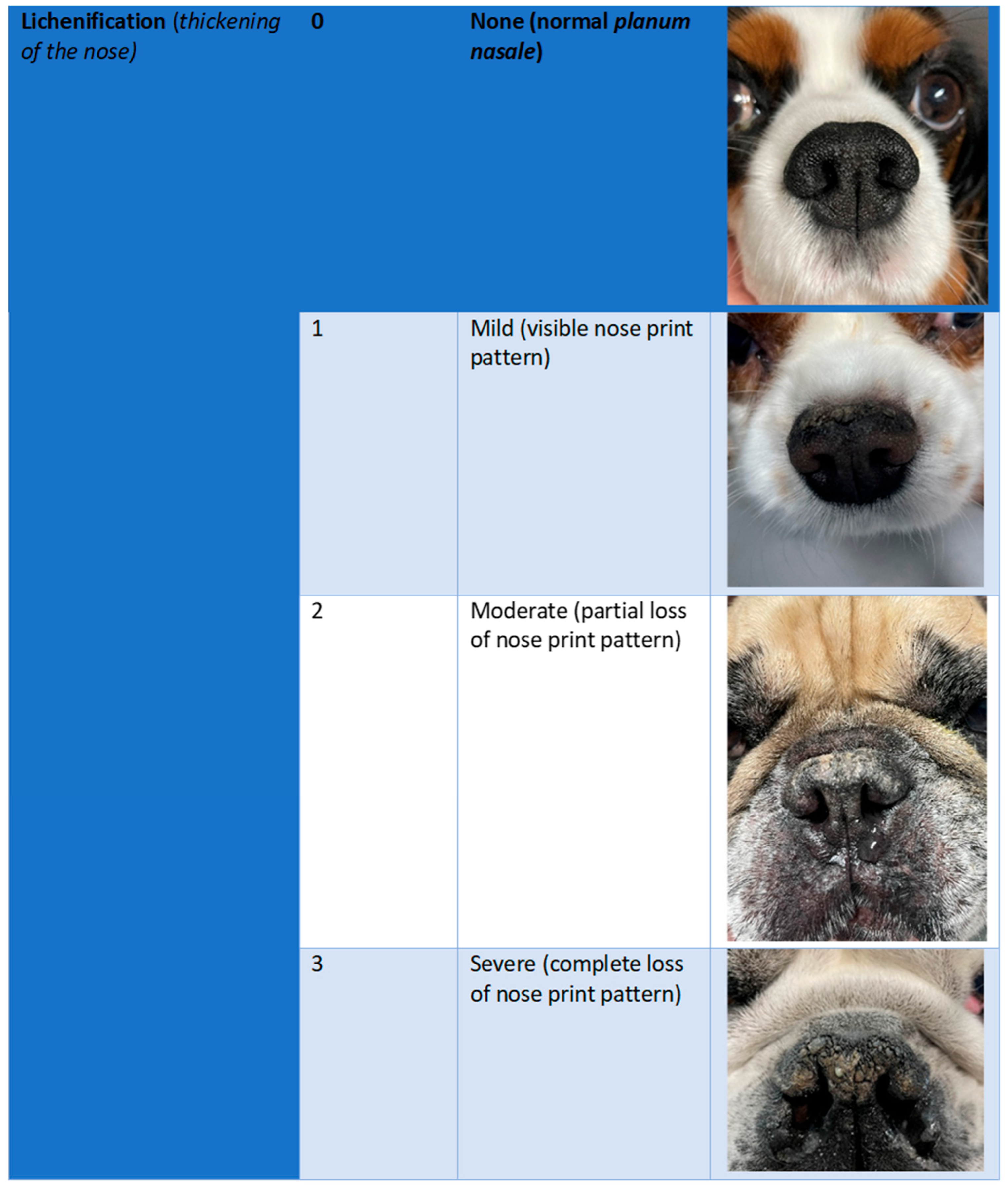
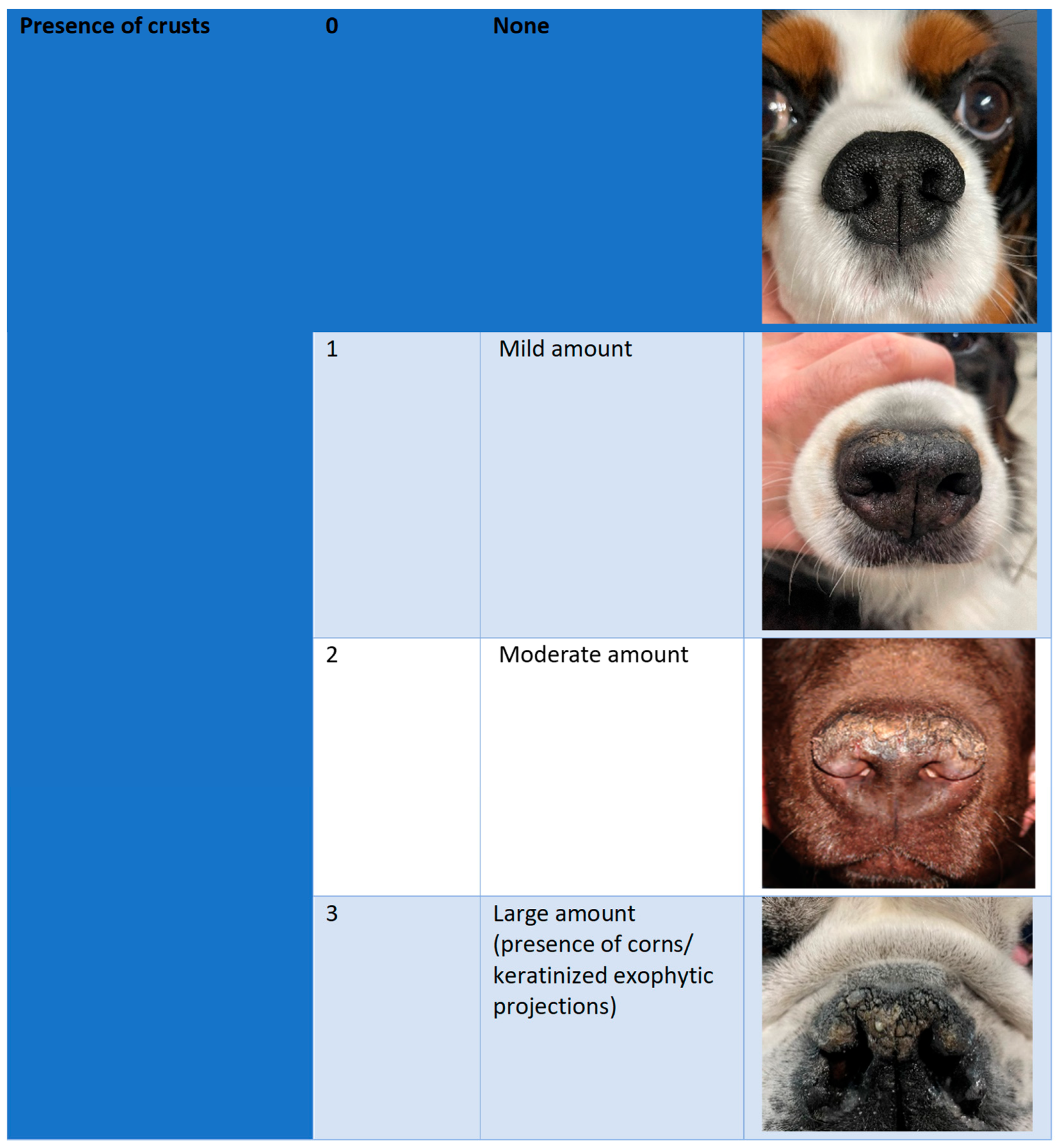
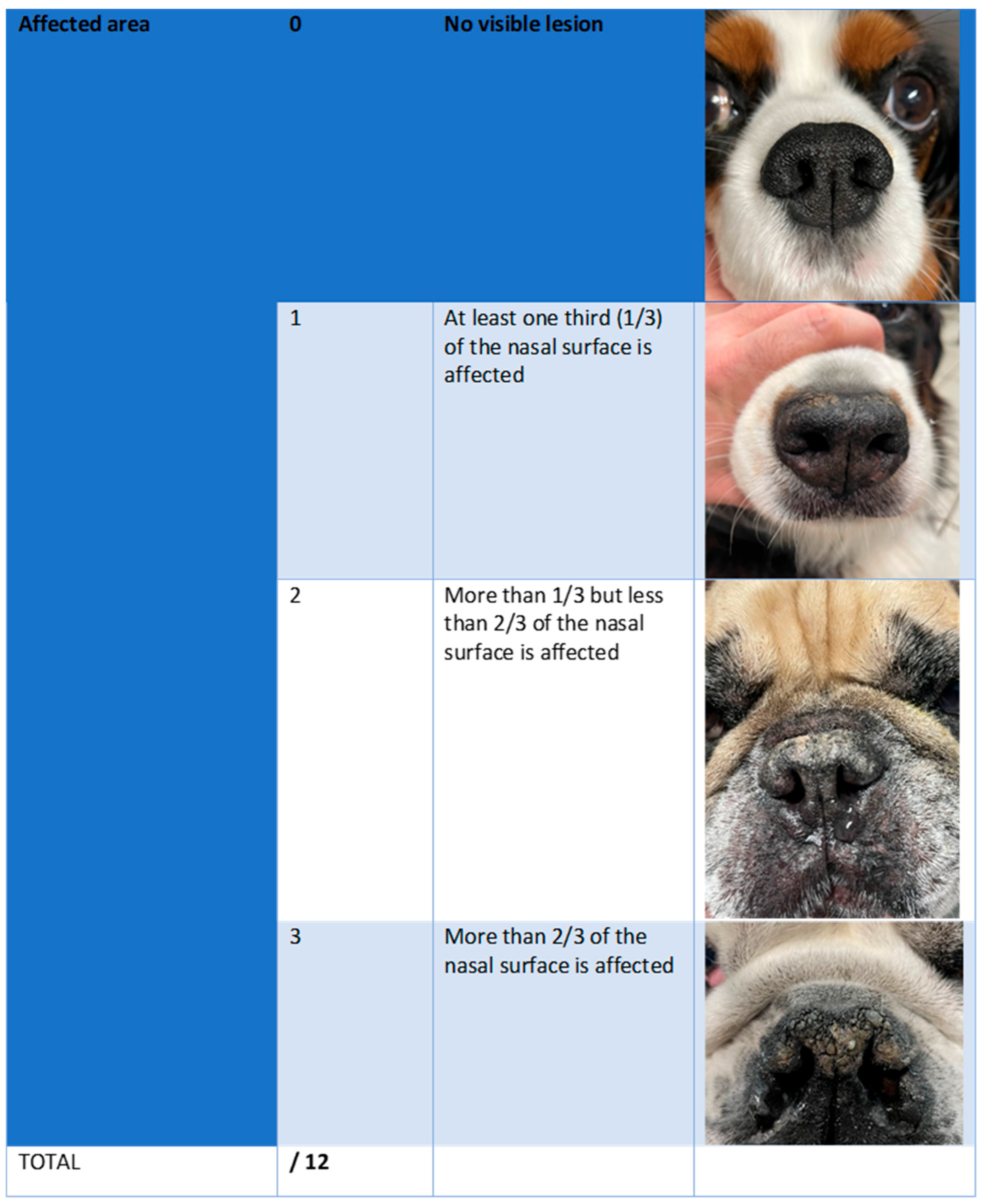
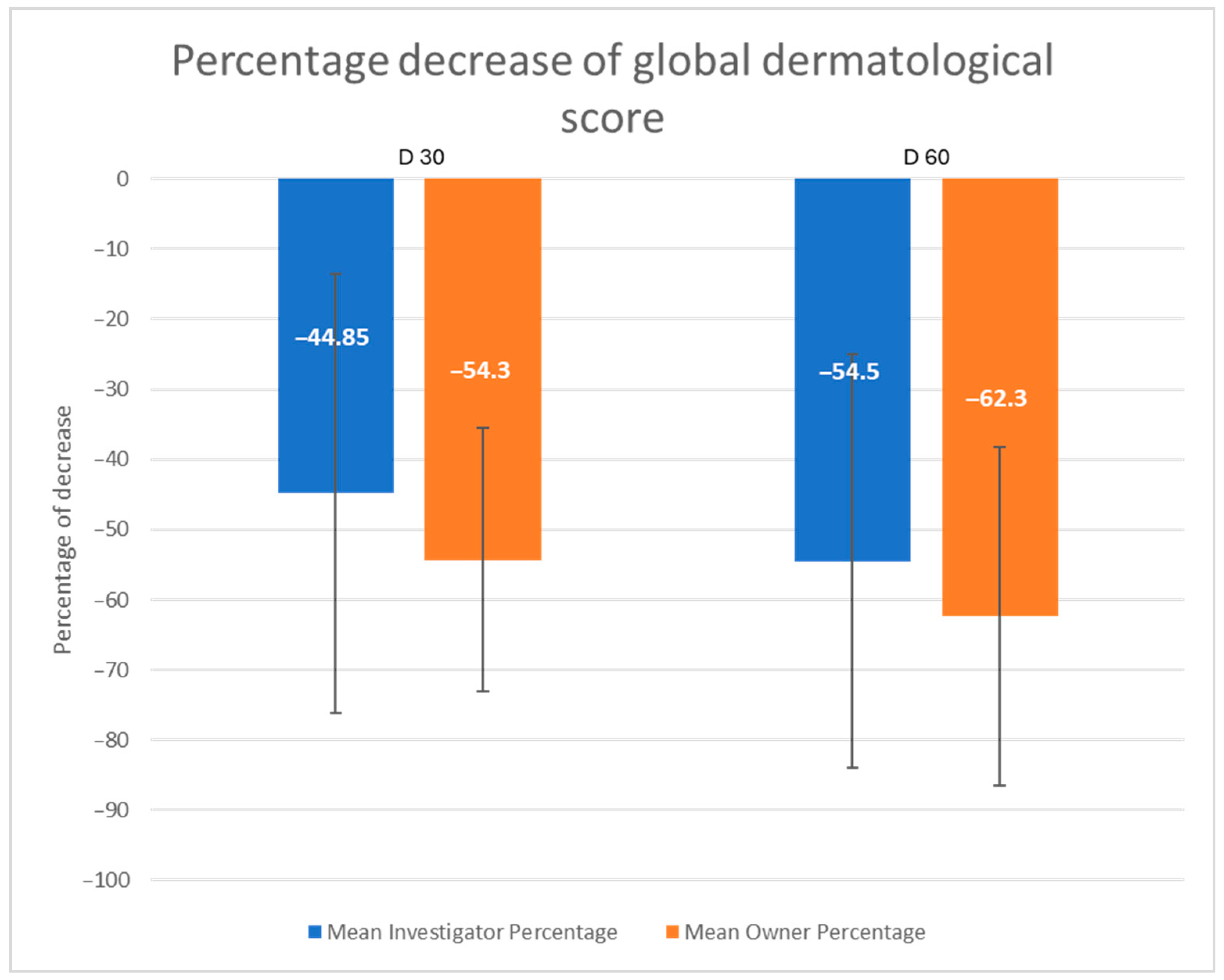
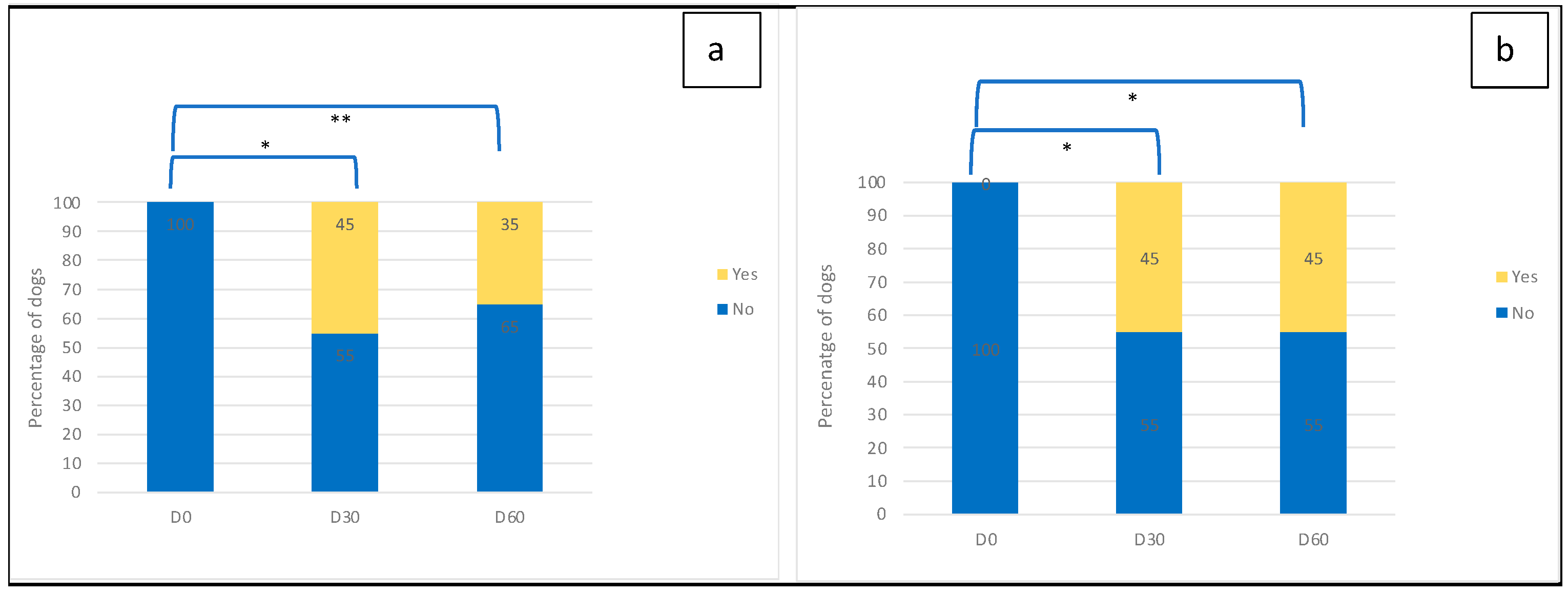
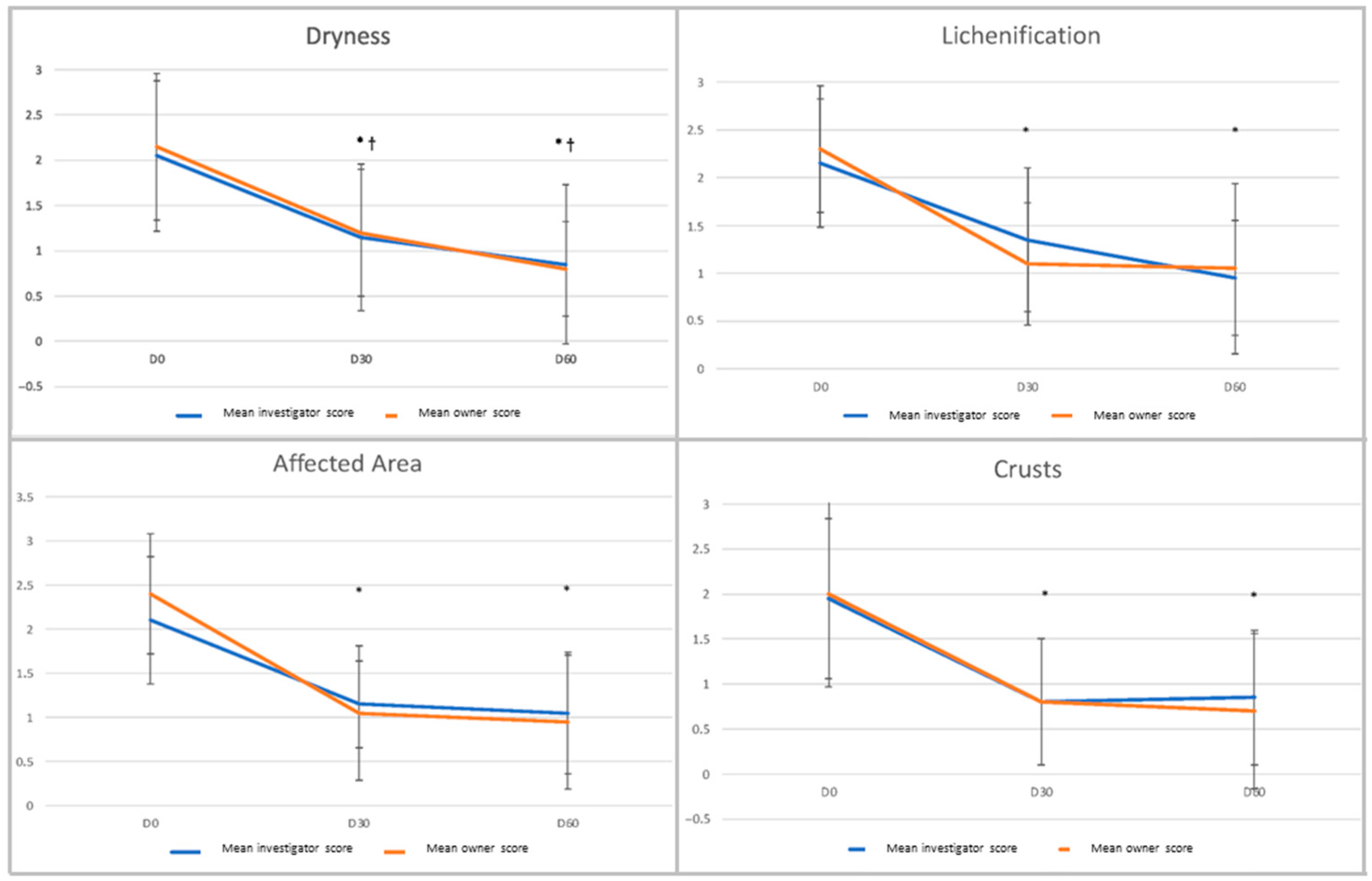
2.3. Efficacy Outcomes
2.4. Safety Evaluation
2.5. Statistical Methods
3. Results
3.1. Animals
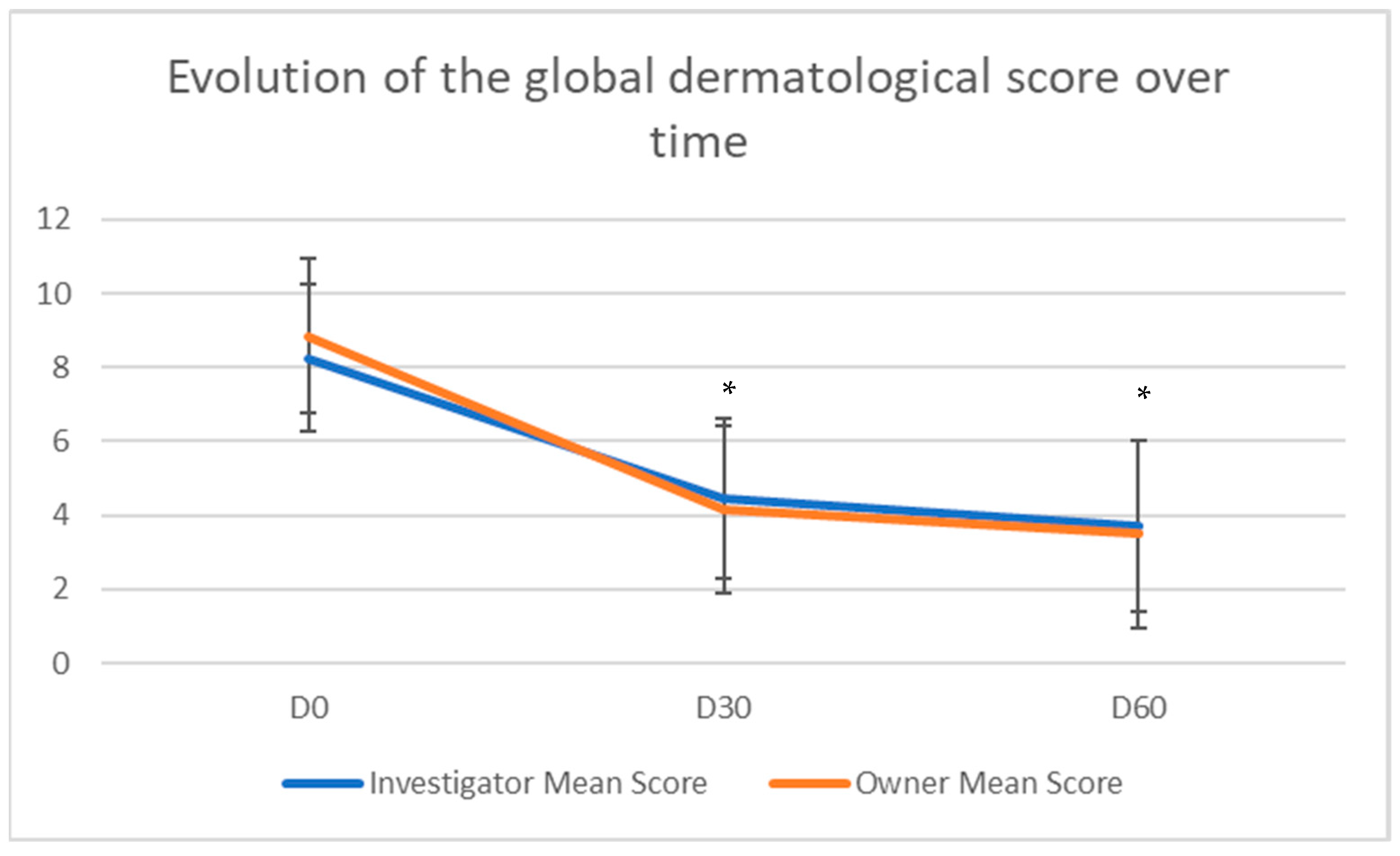
3.2. Outcome
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDS | Global Dermatological Score |
| NHK | Nasal Hyperkeratosis |
| VAS | Visual Analogue Scale |
References
- Scott, D.W.; Miller, W.H.; Griffin, C.E. Small Animal Dermatology, 6th ed.; DiBernadino, C., Kersey, R., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2001. [Google Scholar]
- Berger, D. Nasal Planum Disease in Dogs. Clinician’s Brief 2018, 15–20. Available online: https://www.cliniciansbrief.com/article/nasal-planum-disease-dogs (accessed on 12 June 2024).
- Mauldin, E.A.; Elias, P.M. Ichthyosis and hereditary cornification disorders in dogs. Vet. Dermatol. 2021, 32, 567-e154. [Google Scholar] [CrossRef] [PubMed]
- Pagé, N.; Paradis, M.; Lapointe, J.M.; Dunstan, R.W. Case report: Hereditary nasal parakeratosis in Labrador Retrievers. Vet. Dermatol. 2003, 14, 103–110. [Google Scholar] [CrossRef]
- Peters, J.; Scott, D.W.; Erb, H.N.; Miller, W.H. Hereditary nasal parakeratosis in Labrador retrievers: 11 New cases and a retrospective study on the presence of accumulations of serum (‘serum lakes’) in the epidermis of parakeratotic dermatoses and inflamed nasal plana of dogs. Vet. Dermatol. 2003, 14, 197–203. [Google Scholar] [CrossRef]
- Bauer, A.; Nimmo, J.; Newman, R.; Brunner, M.; Welle, M.M.; Jagannathan, V.; Leeb, T. A splice site variant in the SUV39H2 gene in Greyhounds with nasal. Anim. Genet. 2018, 49, 137–140. [Google Scholar] [CrossRef]
- Bannoehr, J.; Balmer, P.; Stoffel, M.H.; Jagannathan, V.; Gaschen, V.; Kühni, K.; Sayar, B.; Drögemüller, M.; Howald, D.; Wiener, D.J.; et al. Abnormal keratinocyte differentiation in the nasal planum of Labrador Retrievers with hereditary nasal parakeratosis (HNPK). PLoS ONE 2020, 15, e0225901. [Google Scholar] [CrossRef]
- Jagannathan, V.; Bannoehr, J.; Plattet, P.; Hauswirth, R.; Drögemüller, C.; Drögemüller, M.; Wiener, D.J.; Doherr, M.; Owczarek-Lipska, M.; Galichet, A.; et al. A Mutation in the SUV39H2 Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation. PLoS Genet. 2013, 9, e1003848. [Google Scholar] [CrossRef] [PubMed]
- Cikota, R.; Åberg, L.; Karlstam, E.; Shokrai, A.; Åhman, S. Nasal hyperkeratosis in Griffon breeds: Clinical, histopathological features and the prevalence in the Swedish population compared to a control group and other brachycephalic breeds. Vet. Rec. Open 2021, 8, e10. [Google Scholar] [CrossRef]
- Catarino, M.; Combarros-Garcia, D.; Mimouni, P.; Pressanti, C.; Cadiergues, M.C. Control of canine idiopathic nasal hyperkeratosis with a natural skin restorative balm: A randomized double-blind placebo-controlled study. Vet. Dermatol. 2018, 29, 134-e53. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Granger, C.; Trullàs, C.; Jesús-Silva, A.; Krutmann, J. Urea in Dermatology: A Review of its Emollient, Moisturizing, Keratolytic, Skin Barrier Enhancing and Antimicrobial Properties. Dermatol. Ther. 2021, 11, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.; Heldreth, B. Safety Assessment of Butyrospermum Parkii (Shea)-Derived Ingredients as Used in Cosmetics: Status Tentative Report for Public Comment. CIR Safety Report. 2016. Available online: https://www.cir-safety.org/sites/default/files/shea092016tent.pdf (accessed on 12 June 2024).
- Panzuti, P.; Vidémont, E.; Fantini, O.; Fardouet, L.; Noël, G.; Cappelle, J.; Pin, D. A moisturizer formulated with glycerol and propylene glycol accelerates the recovery of skin barrier function after experimental disruption in dogs. Vet. Dermatol. 2020, 31, 344-e89. [Google Scholar] [CrossRef]
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, H.O.; Woo, S.M.; Lee, D.H. Use of Dexpanthenol for Atopic Dermatitis—Benefits and Recommendations Based on Current Evidence. J. Clin. Med. 2022, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.; Skazik, C.; Marquardt, Y.; Czaja, K.; Sebastian, K.; Kurschat, P.; Gan, L.; Denecke, B.; Ekanayake-Bohlig, S.; Wilhelm, K.-P.; et al. Dexpanthenol Modulates Gene Expression in Skin Wound Healing in vivo. Ski. Pharmacol. Physiol. 2012, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- George, M.; Joseph, L.; Ramaswamy. Anti-allergic, anti-pruritic, and anti-inflammatory activities of Centella asiatica extracts. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 554–559. [Google Scholar] [CrossRef]
- Paradis, M. Case Report: Footpad Hyperkeratosis in a Family of Dogues de Bordeaux. Vet. Dermatol. 1992, 3, 75–78. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report of the safety assessment of urea. Int. J. Toxicol. 2005, 24, 1–56. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito Dv Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Gill, L.J.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38 (Suppl. S3), 6S–22S. [Google Scholar] [CrossRef]
- Burnett, C.L.; Fiume, M.M.; Bergfeld, W.F.; Belsito Dv Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Plant-Derived Fatty Acid Oils. Int. J. Toxicol. 2017, 36 (Suppl. S3), 51S–129S. [Google Scholar] [CrossRef]
- Bergfeld, W.F.; Donald, F.A.C.P.; Belsito, V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Panthenol, Pantothenic Acid, and Derivatives as Used in Cosmetics [Internet]. Available online: http://www.cir-safety.org/ingredients (accessed on 12 June 2024).
- Becker, L.C.; Bergfeld, W.F.; Belsito Dv Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final report of the safety assessment of allantoin and its related complexes. Int. J. Toxicol. 2010, 29 (Suppl. S3), 84S–97S. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W. Final report of the safety assessment of Acacia Catechu Gum, Acacia Concinna Fruit Extract, Acacia Dealbata Leaf Extract, Acacia Dealbata Leaf Wax, Acacia Decurrens Extract, Acacia Farnesiana Extract, Acacia Farnesiana Flower Wax, Acacia Farnesiana Gum, Acacia Senegal Extract, Acacia Senegal Gum, and Acacia Senegal Gum Extract. Vol. Int. J. Toxicol. 2005, 24, 75–118. [Google Scholar]
- Reeder, M.J. Allergic Contact Dermatitis to Fragrances. Dermatol. Clin. 2020, 38, 371–377. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viaud, S.; Pariente, S.; Jahier, B.; Navarro, C.; Destaing, C.; Gard, C. Evaluation of the Interest in and Tolerance of a Topical Emollient in the Management of Canine Nasal Hyperkeratosis: An Open-Label, Prospective, Uncontrolled Pilot Study. Vet. Sci. 2025, 12, 792. https://doi.org/10.3390/vetsci12090792
Viaud S, Pariente S, Jahier B, Navarro C, Destaing C, Gard C. Evaluation of the Interest in and Tolerance of a Topical Emollient in the Management of Canine Nasal Hyperkeratosis: An Open-Label, Prospective, Uncontrolled Pilot Study. Veterinary Sciences. 2025; 12(9):792. https://doi.org/10.3390/vetsci12090792
Chicago/Turabian StyleViaud, Sébastien, Sarah Pariente, Bruno Jahier, Christelle Navarro, Cécile Destaing, and Carole Gard. 2025. "Evaluation of the Interest in and Tolerance of a Topical Emollient in the Management of Canine Nasal Hyperkeratosis: An Open-Label, Prospective, Uncontrolled Pilot Study" Veterinary Sciences 12, no. 9: 792. https://doi.org/10.3390/vetsci12090792
APA StyleViaud, S., Pariente, S., Jahier, B., Navarro, C., Destaing, C., & Gard, C. (2025). Evaluation of the Interest in and Tolerance of a Topical Emollient in the Management of Canine Nasal Hyperkeratosis: An Open-Label, Prospective, Uncontrolled Pilot Study. Veterinary Sciences, 12(9), 792. https://doi.org/10.3390/vetsci12090792




