A Non-Invasive Diagnostic Platform for Canine Leishmaniasis Using VOC Analysis and Distributed Veterinary Infrastructure
Simple Summary
Abstract
1. Introduction
2. Related Works
2.1. Traditional Diagnostic Techniques for Canine Leishmaniasis
2.2. Non-Invasive Diagnostics and VOC Analysis
2.3. Artificial Intelligence and Machine Learning in Veterinary Diagnostics
2.4. Veterinary Health Platforms and Distributed Systems
3. Methodology
4. AI and Machine Learning Models
4.1. Feature Selection
4.2. Model Training
4.3. Results and Analysis
5. Data Analysis and Dataset Description
5.1. Dataset Composition
5.2. Pre-Processing and Feature Engineering
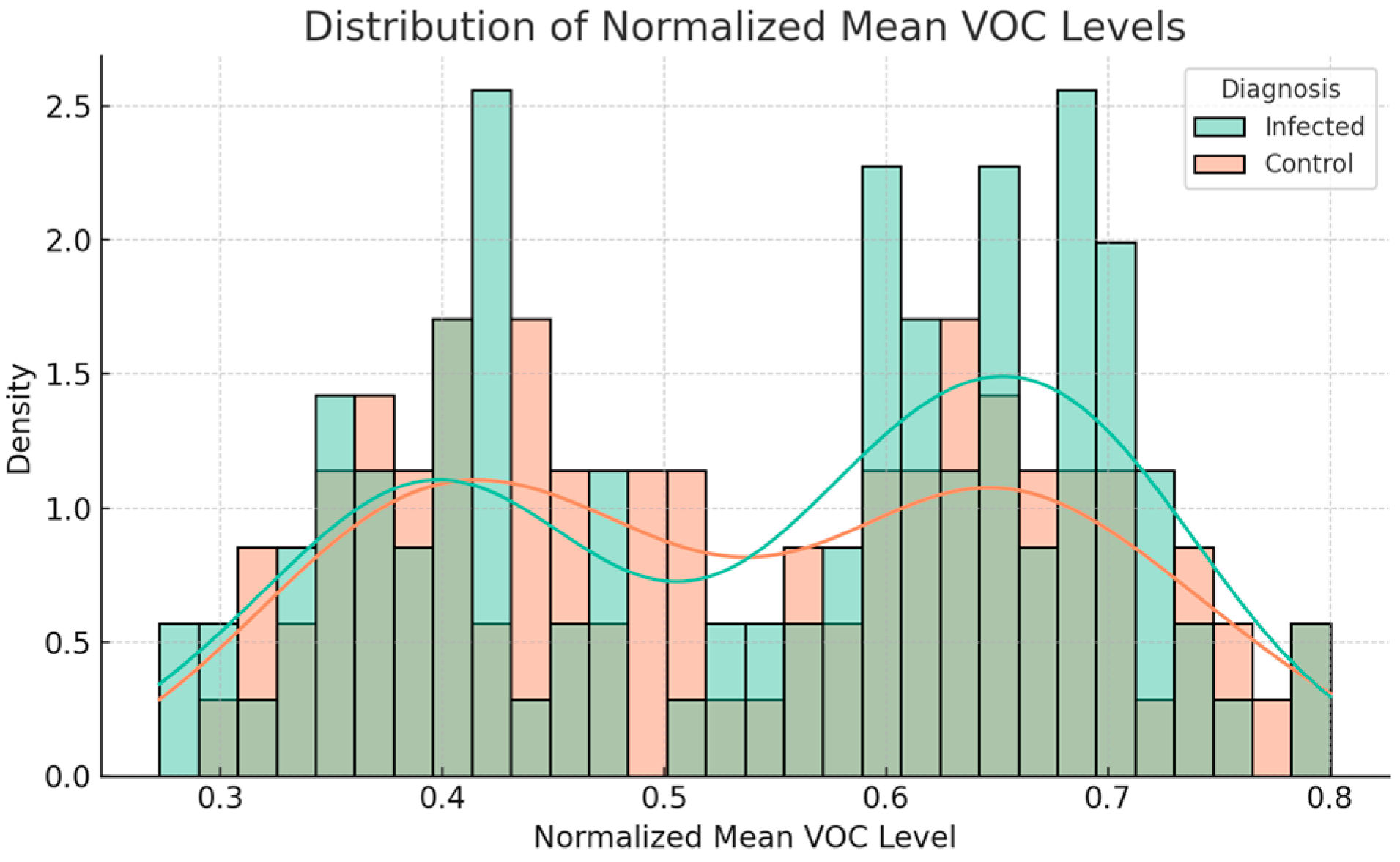
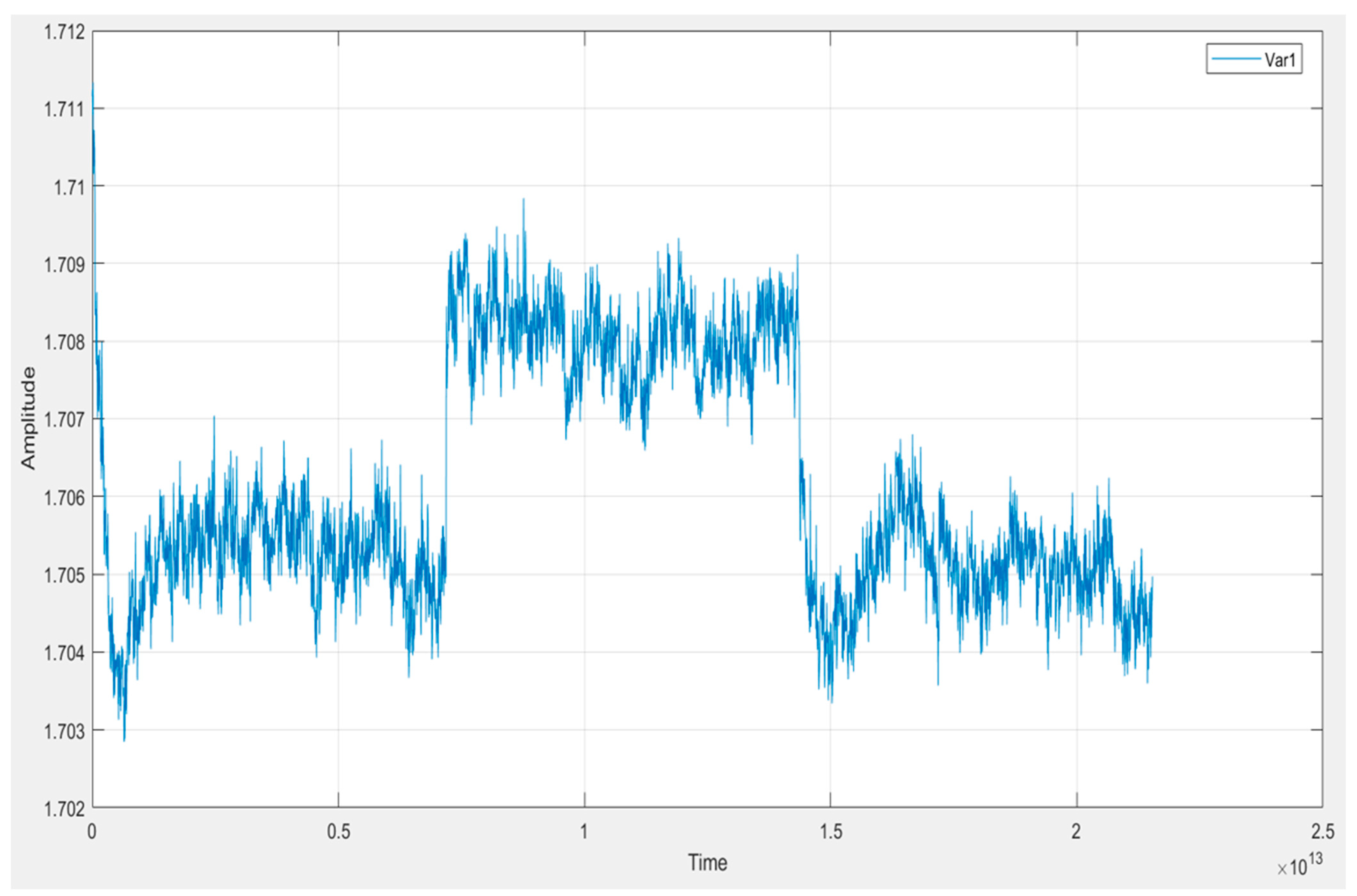
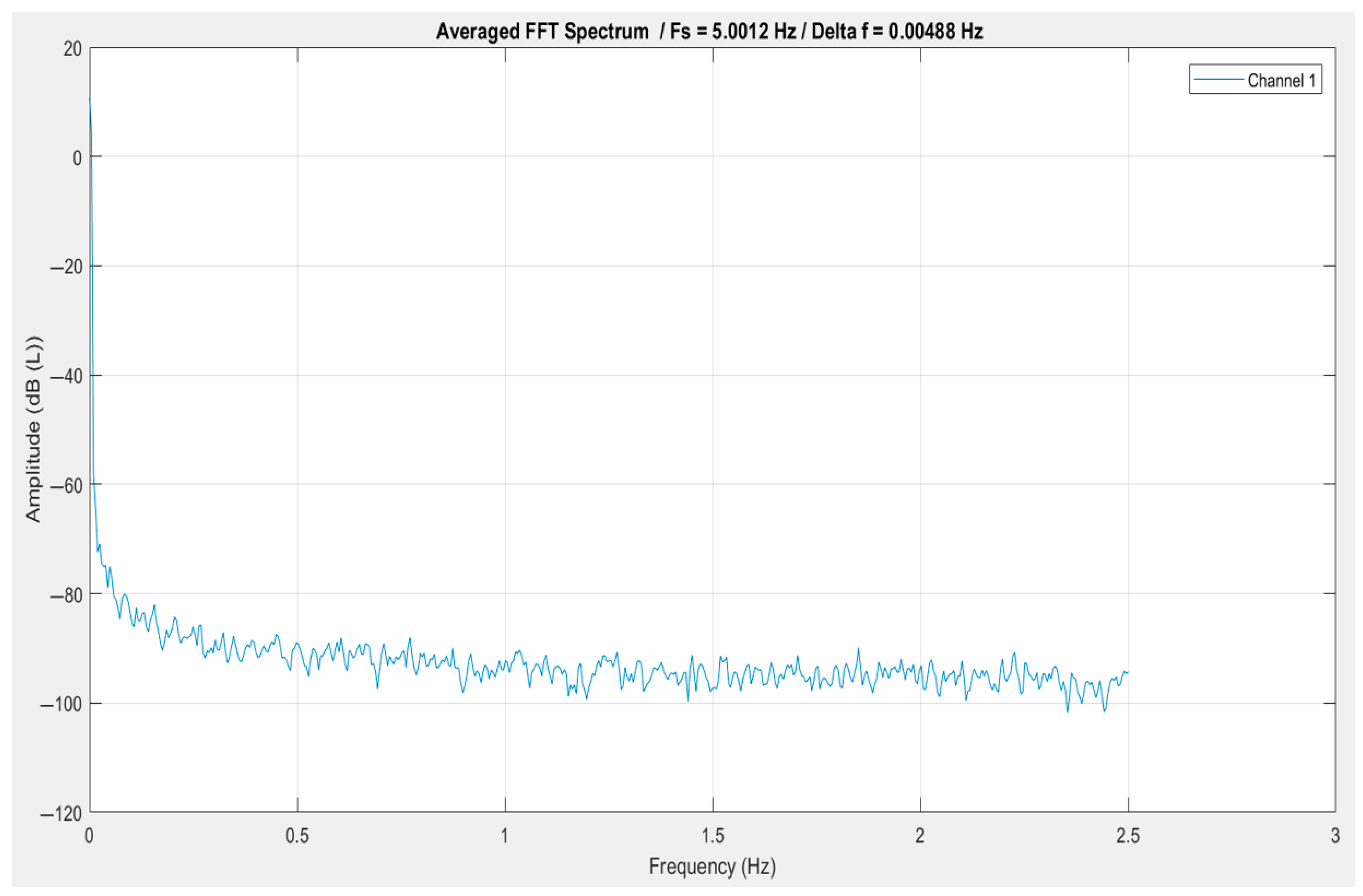
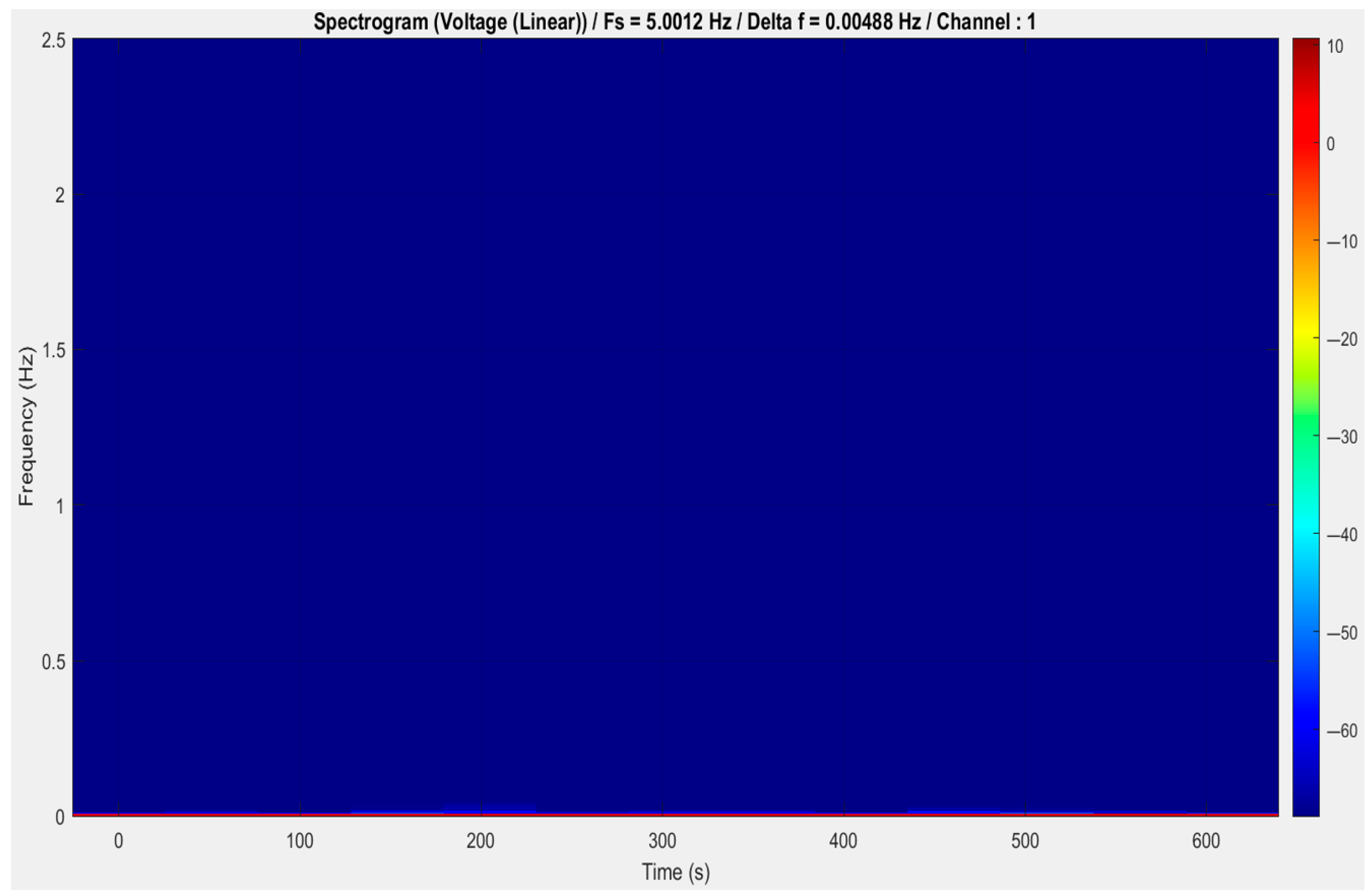
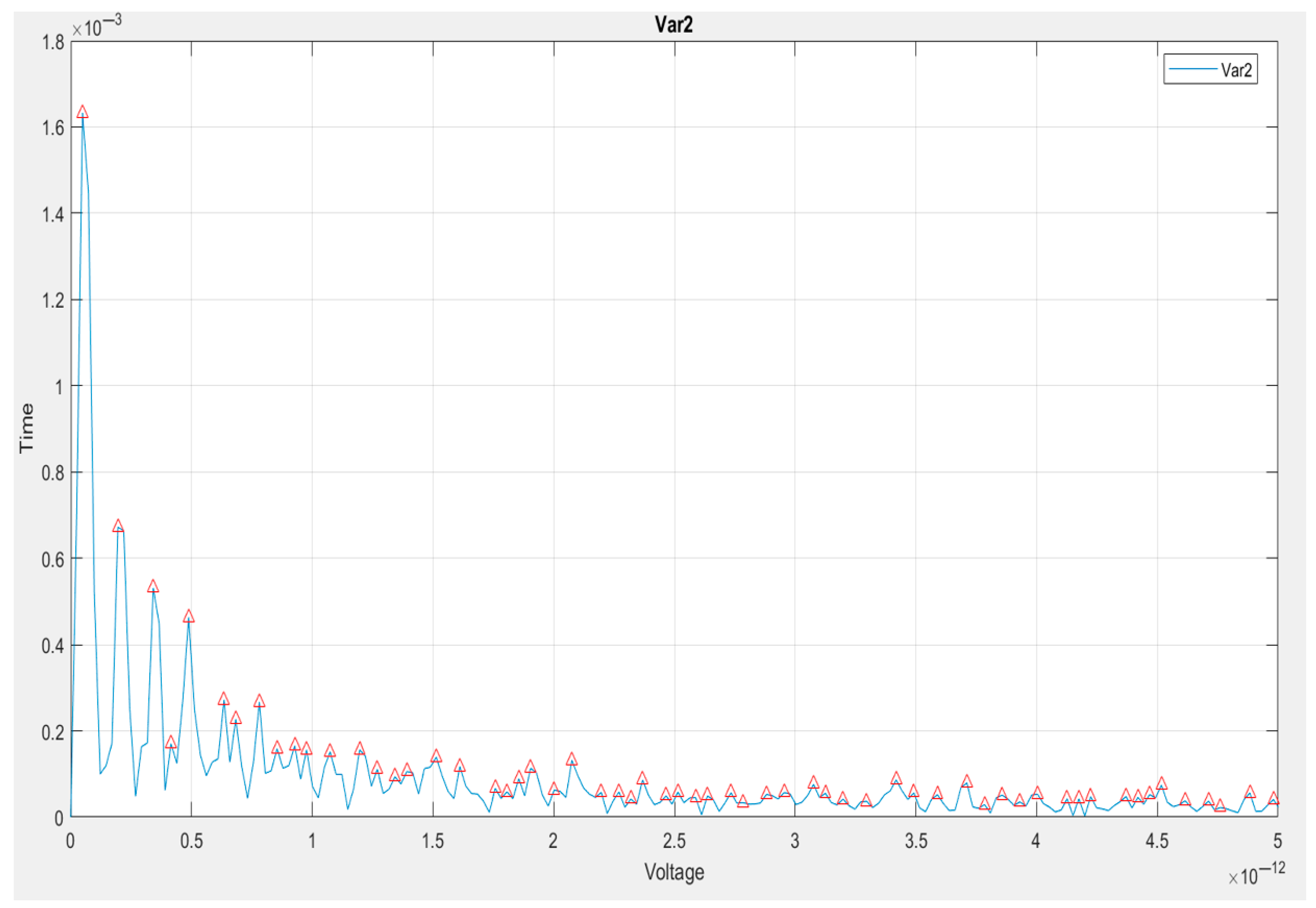
5.3. Exploratory Data Analysis (EDA)
5.4. Interpretation of Key Signal Features
5.5. Data Quality and Limitations
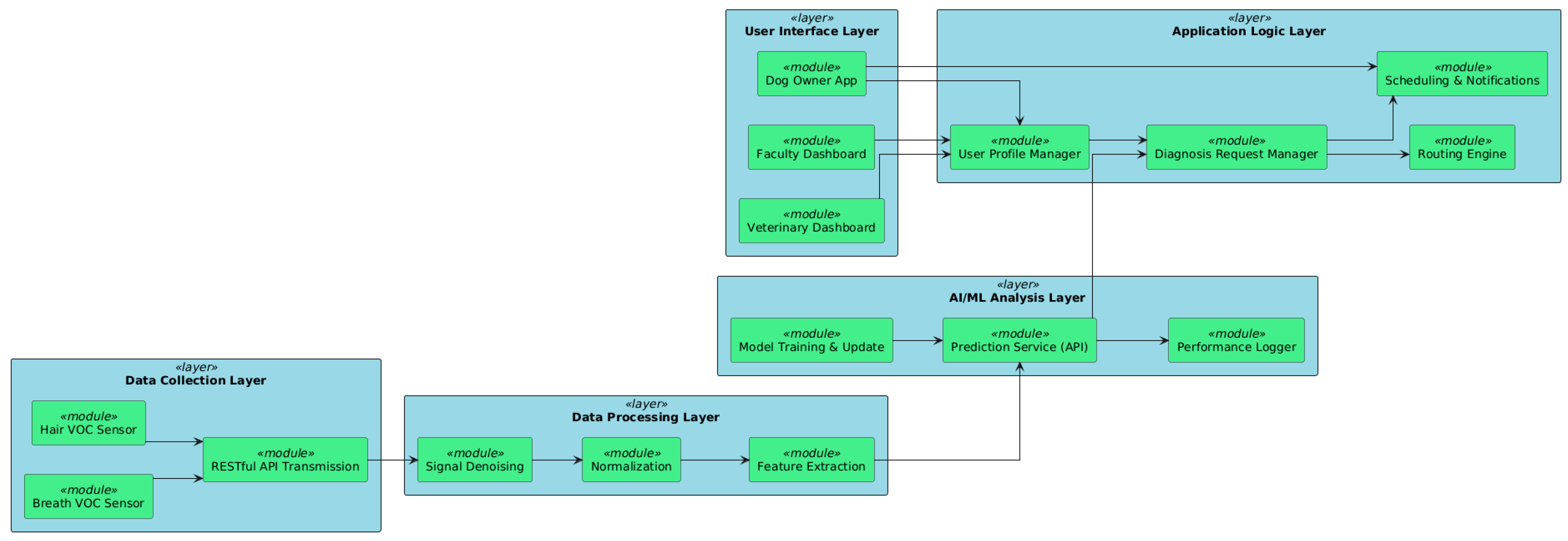
6. Software Architecture and System Design
6.1. Multi-Tiered System Architecture
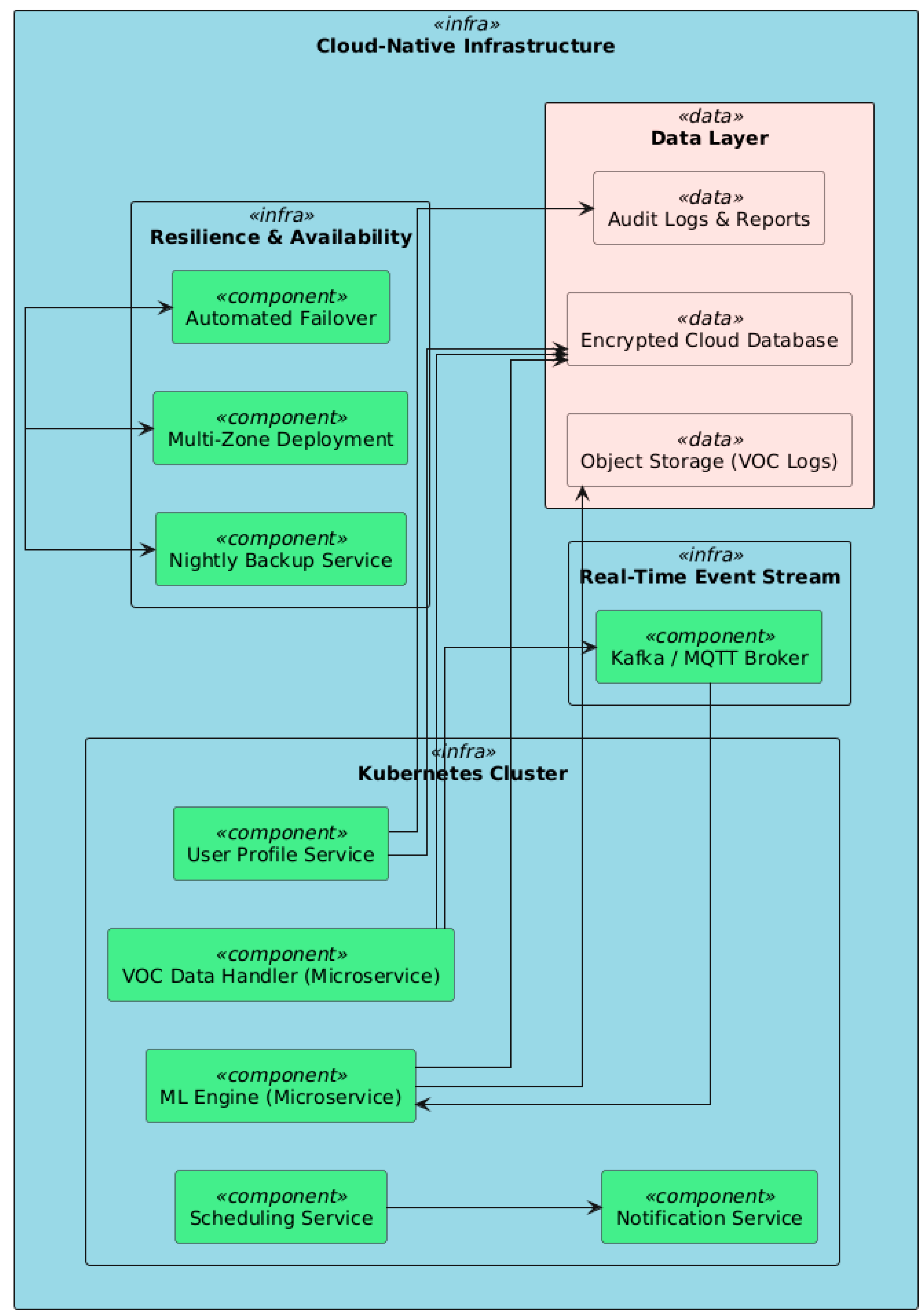
6.2. Cloud Infrastructure
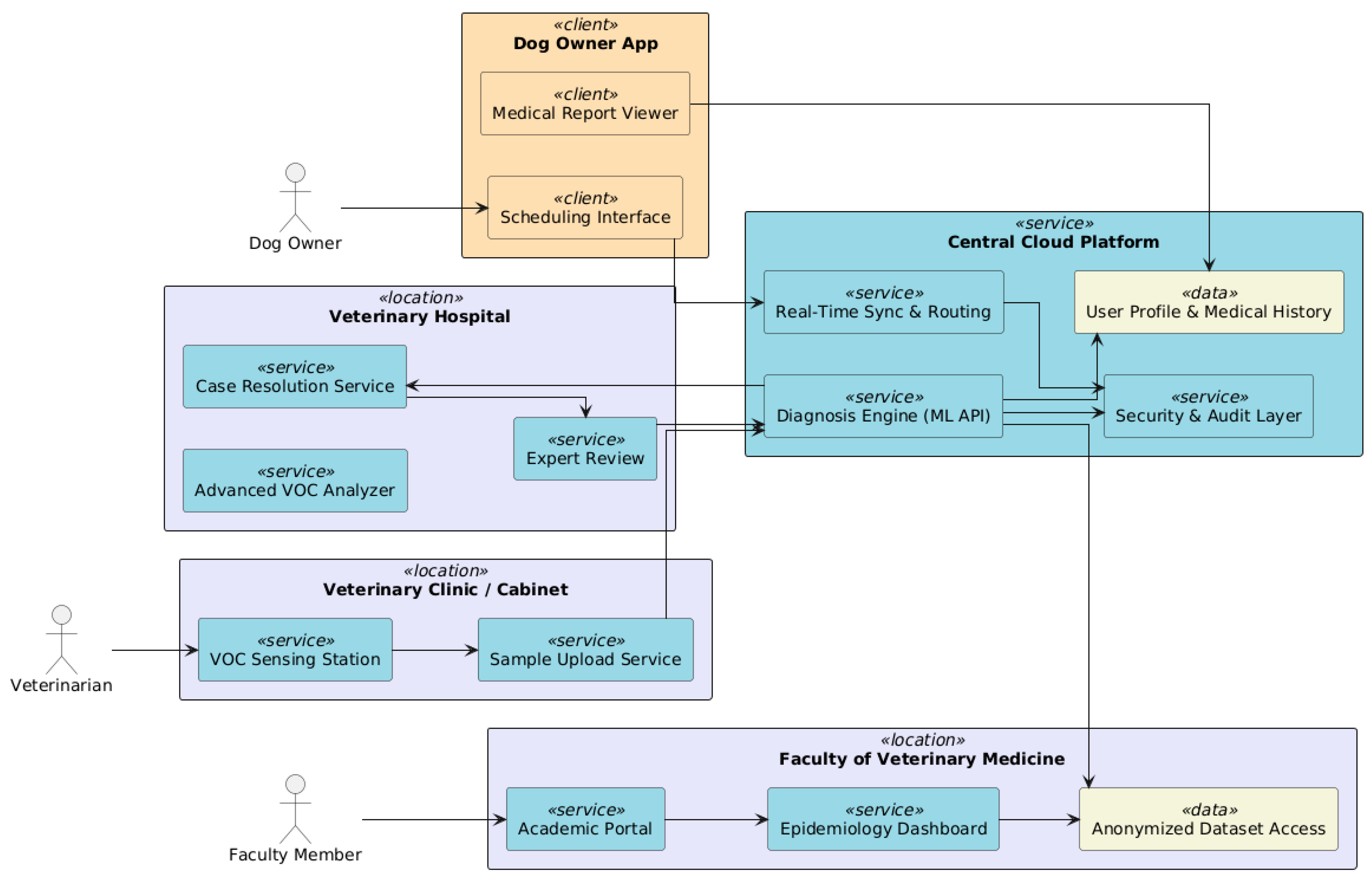
6.3. Geographic Integration
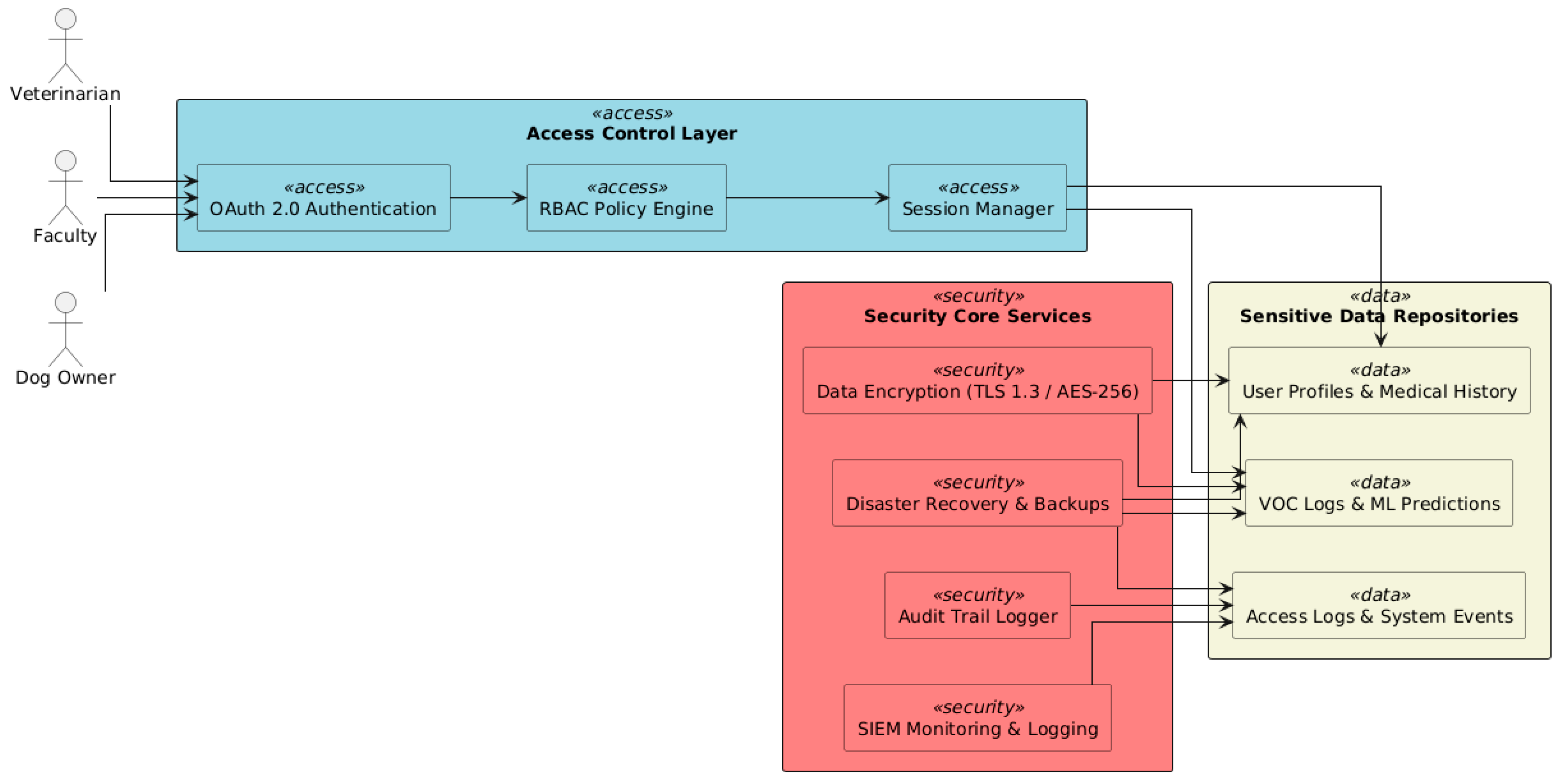
6.4. Security and Privacy
7. Interfaces with End-User
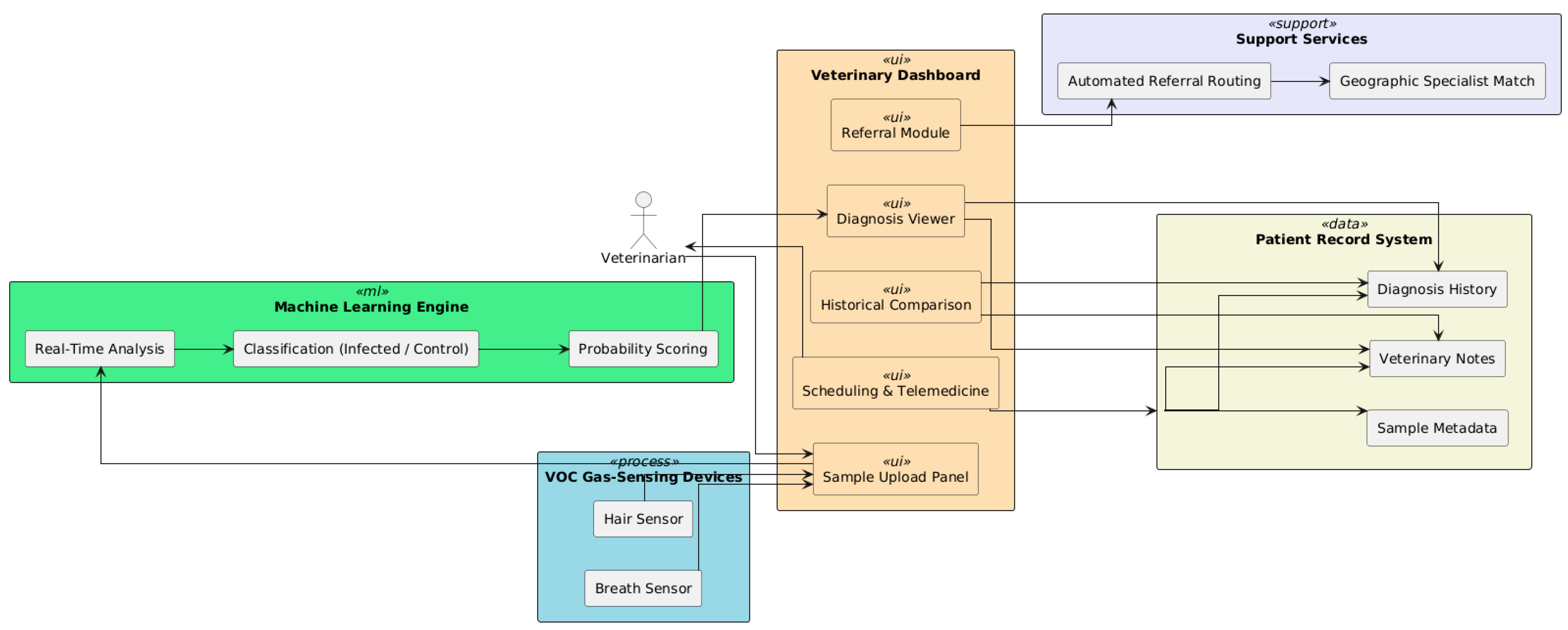
7.1. Veterinary Dashboard
7.2. Academia and Researchers
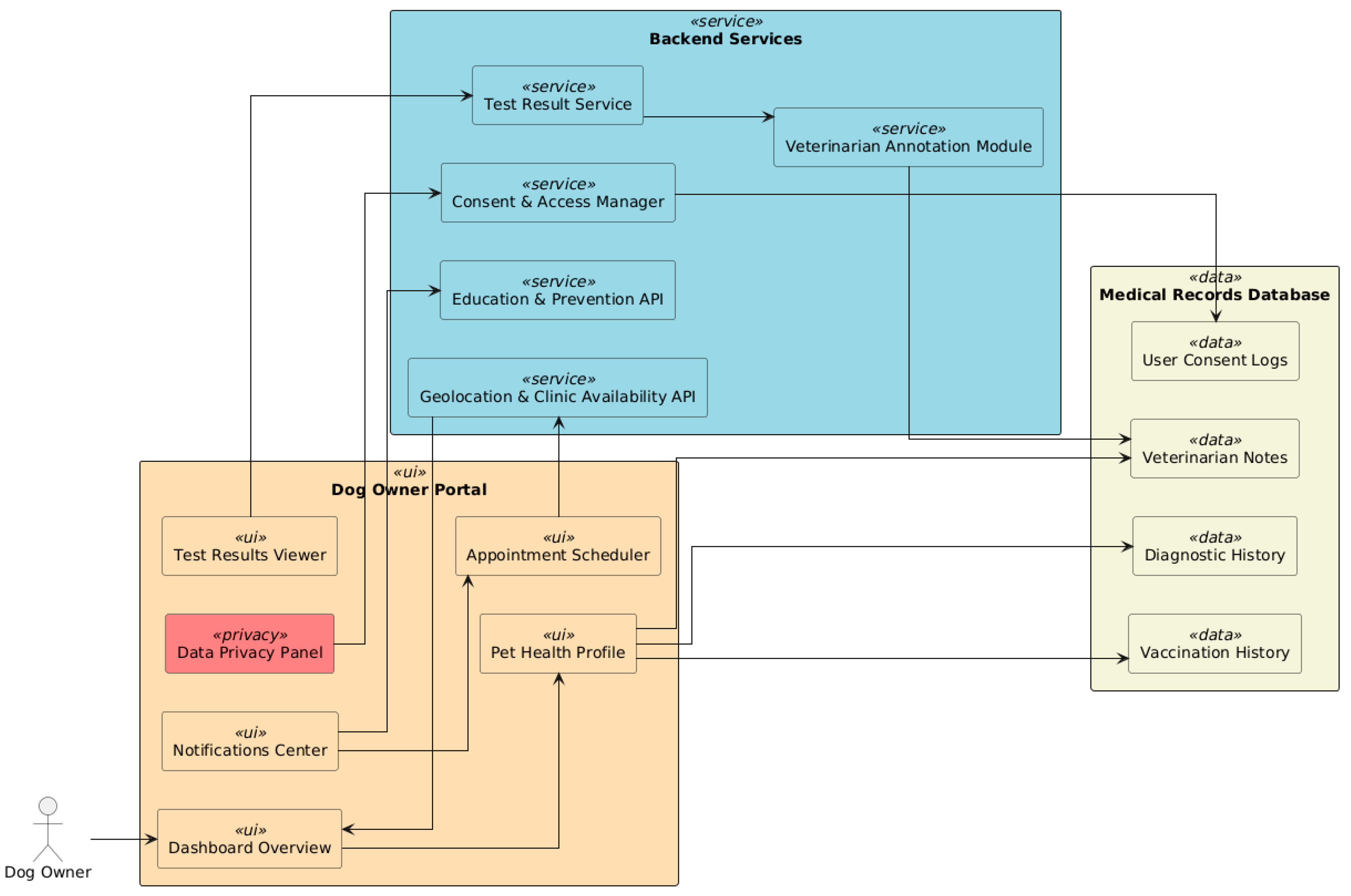
7.3. Dog Owner Portal
8. Implementation Steps
8.1. Deployment Overview
8.2. Testing the Platform Using the VOCs from 72 Dogs
8.3. Notes and Lessons Learned
9. Case Study and Discussions
9.1. Technical and Operational Challenges
9.2. Scalability and Generalizability to Other Conditions
9.3. Ethical Aspects and Trust in the User
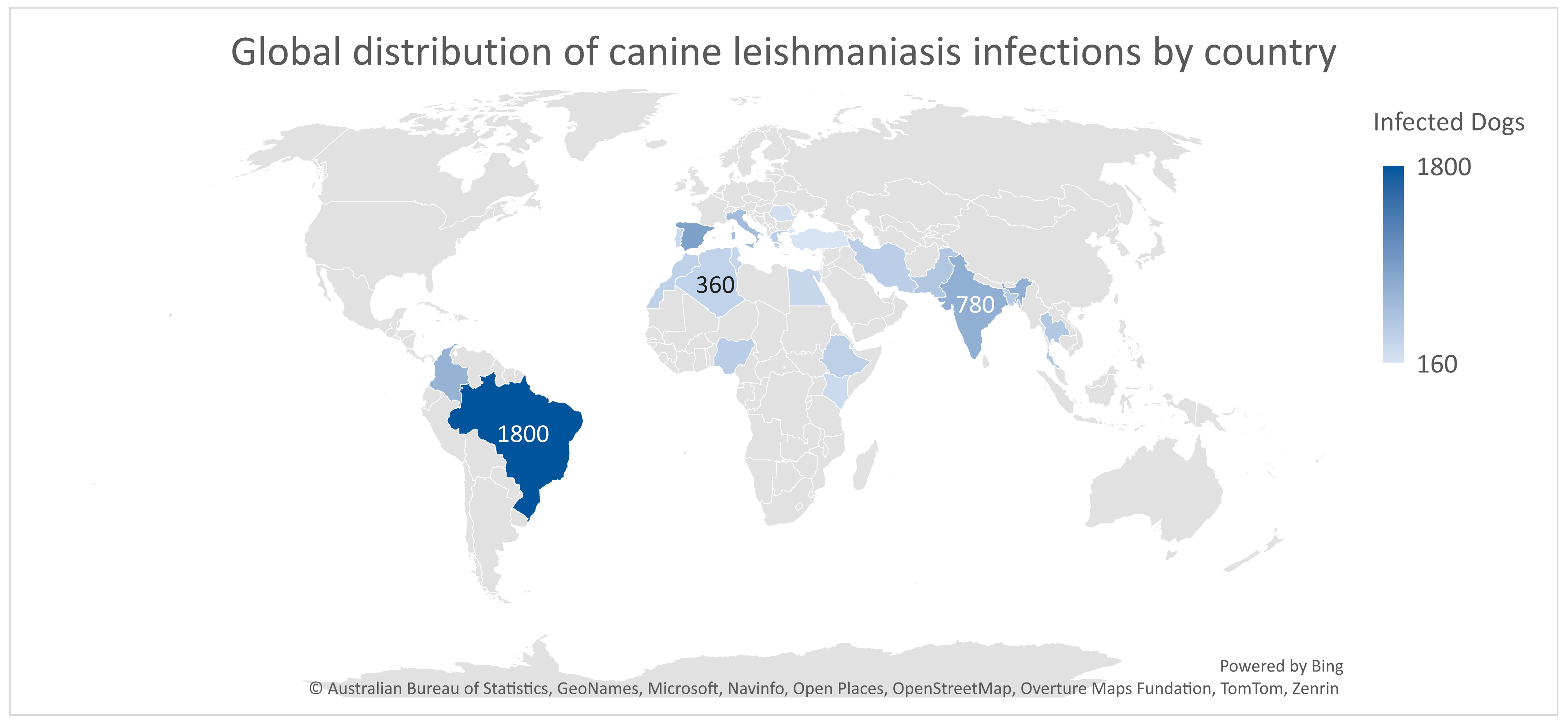
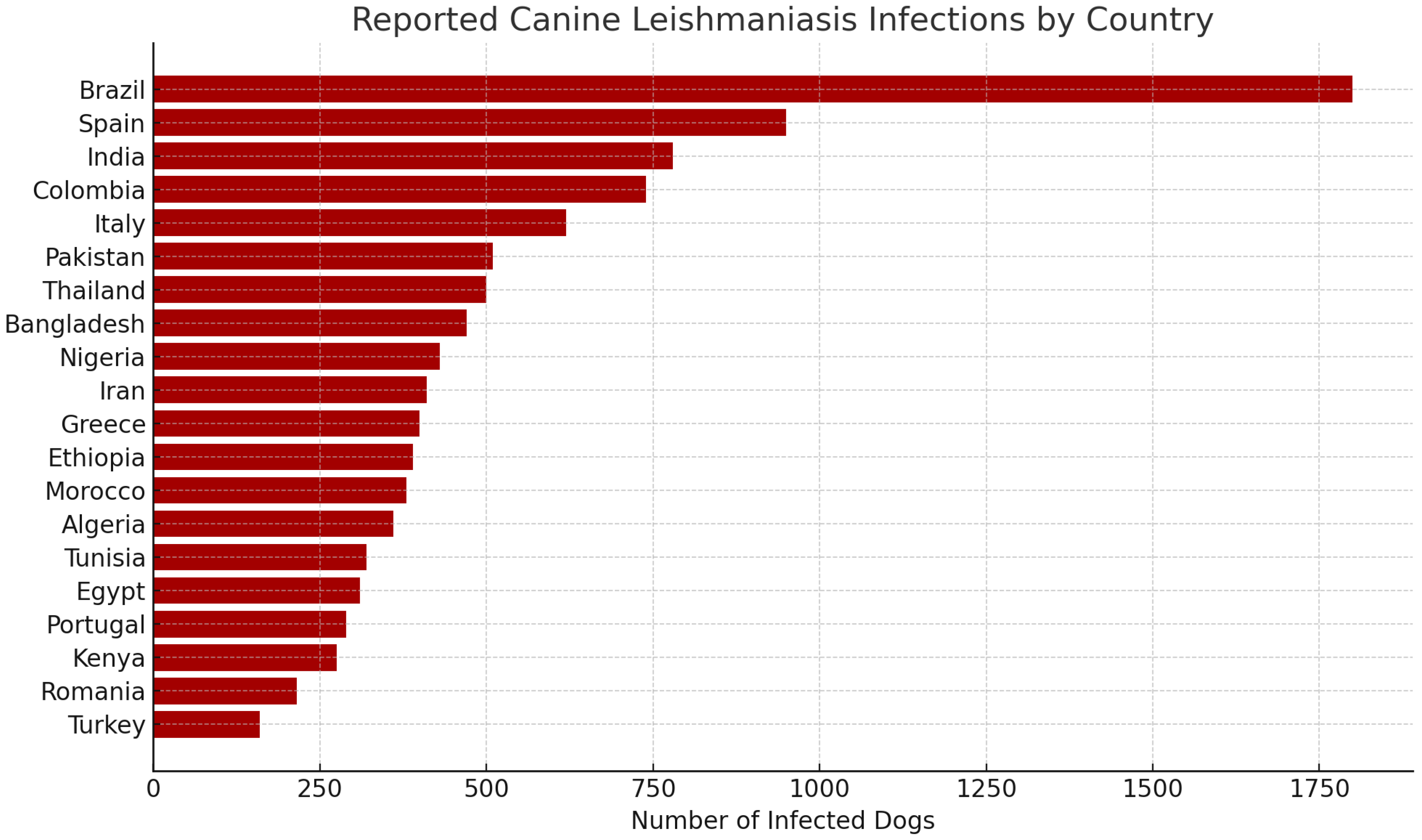
9.4. Geographic and Epidemiological Distribution
Infection Burden by Country
10. Conclusions
11. Patents and Copyrights
- Marius Iulian, Mihailescu. Veterinary Enhanced Telemetry & Analysis for Lab-based Knowledge (VETALK), Medical Veterinary Cloud-based Platform for Chemical Compounds Analysis from Signal Processing, Part 1—Software Architecture and System Design. Registration Certificate no.: 1685/28.04.2025, filled on 20 April 2025 and issued on 28 April 2025 by Romanian Office for Copyright (ORDA).
- Marius Iulian, Mihailescu. Veterinary Enhanced Telemetry & Analysis for Lab-based Knowledge (VETALK), Medical Veterinary Cloud-based Platform for Chemical Compounds Analysis from Signal Processing, Part 2—Graphic User Interfaces. Registration Certificate no.: 1684/28.04.2025, filled on 20 April 2025 and issued on 28 April 2025 by Romanian Office for Copyright (ORDA).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sousa, E.S.S.; Sousa, M.E.S.; Pereira, M.D.C.A.; Negreiros, R.A.M.; Eloy, L.R.C.; Brasil, A.W.L.; Clementino, I.J.; Azevedo, S.S.; Lucena, R.B. Data Platform for Animal Mortality Information System (DATASIMA): Monitoring Companion Animal’s Euthanasia Causes in City of João Pessoa, Brazil. Vet. Sci. 2025, 12, 28. [Google Scholar] [CrossRef]
- Staniek, M.E.; Sedda, L.; Gibson, T.D.; de Souza, C.F.; Costa, E.M.; Dillon, R.J.; Hamilton, J.G.C.; Werneck, G.L. eNose analysis of volatile chemicals from dogs naturally infected with Leishmania infantum in Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magalhães-Junior, J.T.; Mesquita, P.R.R.; Oliveira, W.F.d.S.; Oliveira, F.S.; Franke, C.R.; Rodrigues, F.d.M.; de Andrade, J.B.; Barrouin-Melo, S.M. Identification of biomarkers in the hair of dogs: New diagnostic possibilities in the study and control of visceral Leishmaniasis. Anal. Bioanal. Chem. 2014, 406, 6691–6700. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Maia, C.; Khan, N.; Infantino, M.; Del Riccio, M. Leishmaniasis in Humans and Animals: A One Health Approach for Surveillance, Prevention and Control in a Changing World. Trop. Med. Infect. Dis. 2024, 9, 258. [Google Scholar] [CrossRef]
- de Freitas, N.D.A.; Freire, L.J.M.; Silva, S.R.; do Nascimento, N.G.; Cordeiro-Estrela, P. Spatial Analysis and Socio-Environmental Determinants of Canine Visceral Leishmaniasis in an Urban Area in Northeastern Brazil. Trop. Med. Infect. Dis. 2025, 10, 6. [Google Scholar] [CrossRef]
- Santos, I.C.S.; Avelar, D.M.; Miranda, L.F.C.; de Mello, C.X.; Keidel, L.; Pimentel, M.I.F.; Ventura, L.S.; Fagundes, A.; Santos, F.N.; Oliveira, L.F.A.; et al. Standardization and Evaluation of the LAMP Technique for the Diagnosis of Canine Visceral Leishmaniasis in Conjunctival Swab Samples Using DNA Extracted by a Silica Column and Boiling. Trop. Med. Infect. Dis. 2024, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Mendes Júnior, A.A.V.; Figueiredo, F.B.; Ferreira, L.C.; Keidel, L.; Ornellas, R.O.; Almeida, A.B.; Santos, F.N.; Miranda, L.d.F.C.; Marcelino, A.P.; Pereira, S.A.; et al. Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis. Animals 2025, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Capistrano Costa, N.T.; de Souza Pereira, A.M.; Silva, C.C.; Souza, E.d.O.; de Oliveira, B.C.; Ferreira, L.F.G.R.; Hernandes, M.Z.; Pereira, V.R.A. Exploring Bioinformatics Solutions for Improved Leishmaniasis Diagnostic Tools: A Review. Molecules 2024, 29, 5259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gopalan, S.; Naidu, R. Advancements in nanotechnological approaches to volatile organic compound detection and separation. Curr. Opin. Environ. Sci. Health 2024, 37, 100528. [Google Scholar] [CrossRef]
- Zare, M.; Akbarialiabad, H.; Parsaei, H.; Asgari, Q.; Alinejad, A.; Bahreini, M.S.; Hosseini, S.H.; Ghofrani-Jahromi, M.; Shahriarirad, R.; Amirmoezzi, Y.; et al. A machine learning-based system for detecting Leishmaniasis in microscopic images. BMC Infect. Dis. 2022, 22, 48. [Google Scholar] [CrossRef]
- Hong-Geller, E.; Adikari, S. Volatile Organic Compound and Metabolite Signatures as Pathogen Identifiers and Biomarkers of Infectious Disease; Open Access Peer-Reviewed Chapter; IntechOpen: London, UK, 2017; Submitted. [Google Scholar] [CrossRef][Green Version]
- Digiaro, S.; Recchia, A.; Colella, A.; Cucciniello, S.; Greco, B.; Buonfrate, D.; Paradies, P. Treatment of Canine Leishmaniasis with Meglumine Antimoniate: A Clinical Study of Tolerability and Efficacy. Animals 2024, 14, 2244. [Google Scholar] [CrossRef]
- Checa, R.; Sánchez-Vizcaíno, F.; Miró, G.; Pinchbeck, G.; Jones, H.; Noble, P.J.; Radford, A.D. Updating the epidemiology of canine leishmaniosis in the United Kingdom through the use of electronic health data. Vet. Parasitol. 2025, 333, 110350. [Google Scholar] [CrossRef]
- Bia, T.; Sanchez, C.; Zait, H.; Kouidri, M.; Mabrouk, S.K.; Nieto, J.; Ammar, S.S.M.; Moreno, J.; Ahlem, B.N. Diagnosis and prevalence of canine Leishmaniasis in the Atlas shepherd dog. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100787. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Canine leishmaniasis in the Americas: Etiology, distribution, and clinical and zoonotic importance. Parasites Vectors 2024, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath volatile organic compounds (VOCs) as biomarkers for the diagnosis of pathological conditions: A review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical Application of Volatile Organic Compound Analysis for Detecting Infectious Diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Khashayar, P.; Mousazadeh, M.; Daneshpour, M.; Rostami, M.; Goodlett, D.R.; Manji, K.; Fardindoost, S.; Akbari, M.; Hoorfar, M. Volatile organic compounds (VOCs) detection for the identification of bacterial infections in clinical wound samples. Talanta 2025, 292, 127991. [Google Scholar] [CrossRef]
- Suschinel, R.; Jaimes-Mogollón, A.L.; Sim, S.F.; Ting, W.; Cáceres-Tarazona, J.M.; Alvarez-Valdez, E.; Rosero-Moreano, M.; Diouani, M.F.; Chouihi, E.; Brebu, M.; et al. Identification of putative volatile biomarkers of canine Leishmaniasis in dog’s breath and hair employing a novel algorithm for automated chromatographic peak detection and matching. Anal. Bioanal. Chem. 2025, 417, 771–783. [Google Scholar] [CrossRef]
- Sousa-Paula, L.C.; Silva, L.G.D.; Sales, K.G.S.; Dantas-Torres, F. Failure of the dog culling strategy in controlling human visceral Leishmaniasis in Brazil: A screening coverage issue? PLoS Negl. Trop. Dis. 2019, 13, e0007553. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, M.; Cho, G.; Oh, H.; Jeong, J.; Lee, Y.; Seo, H.; Yu, D.; Bae, H.; Hyun, S.H.; et al. A Two-stage AI Framework to Detect and Classify White Blood Cells for Supporting Diseases Diagnosis in Veterinary Medicine. In Proceedings of the 2024 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Lisbon, Portugal, 3–6 December 2024; pp. 4436–4443. [Google Scholar] [CrossRef]
- Mihali, S.I.; Niță, Ș.L. Credit Card Fraud Detection based on Random Forest Model. In Proceedings of the 2024 International Conference on Development and Application Systems (DAS), Suceava, Romania, 23–25 May 2024; pp. 111–114. [Google Scholar] [CrossRef]
- Mihali, S.I.; Niță, Ș.L. Cybersecurity of Online Financial Systems using Machine Learning Techniques. In Proceedings of the 2024 16th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Iasi, Romania, 27–28 June 2024; pp. 1–6. [Google Scholar] [CrossRef]
- EVetPractice Cloud-Based System. Available online: https://www.nectarvet.com (accessed on 21 April 2025).
- VetCompass Cloud-Based System. Available online: https://www.vetcompass.org/ (accessed on 21 April 2025).
- Himanshu; Chopra, T. Analysis of Cloud Service Providers and Computing Services in Modem IT Infrastructure. In Proceedings of the 2025 2nd International Conference on Computational Intelligence, Communication Technology and Networking (CICTN), Ghaziabad, India, 6–7 February 2025; pp. 474–479. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Robles, C.R.; Espino, S.I.; Matignas, K.G.; Abisado, M.; Palenzuela, R. Comparative Analysis of Current Infrastructure of Cloud Computing Unveiling the Trends of Industry 5.0. In Proceedings of the 2024 IEEE 7th International Conference on Computer and Communication Engineering Technology (CCET), Beijing, China, 16–18 August 2024; pp. 200–204. [Google Scholar] [CrossRef]
- Lupşe, C.; Fărcaş, F.; Nagy, J.; Truşcă, M. Building and Managing Cloud Infrastructure with OpenStack. In Proceedings of the 2024 International Conference on Advanced Scientific Computing (ICASC), Cluj-Napoca, Romania, 23–25 October 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Ungurean, I.; Gaitan, N.C. Integrating Cloud and Edge/Fog Computing in Advanced Metering Infrastructure. In Proceedings of the 2024 International Symposium on Sensing and Instrumentation in 5G and IoT Era (ISSI), Lagoa, Portugal, 29–30 August 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Galij, S.; Pawlak, G.; Grzyb, S. Modeling Data Sovereignty in Public Cloud—A Comparison of Existing Solutions. Appl. Sci. 2024, 14, 10803. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Zhang, Y.; Chen, Y.; Sui, H. Positioning and application practice of personal cloud service infrastructure in the computing power network. In Proceedings of the 2024 Sixth International Conference on Next Generation Data-driven Networks (NGDN), Shenyang, China, 26–28 April 2024; pp. 88–95. [Google Scholar] [CrossRef]
- Goel, P.K.; Gulati, S.; Singh, A.; Tyagi, A.; Komal, K.; Mahur, L.S. Energy-Efficient Block-Chain Solutions for Edge and Cloud Computing Infrastructures. In Proceedings of the 2024 2nd International Conference on Disruptive Technologies (ICDT), Greater Noida, India, 15–16 March 2024; pp. 852–856. [Google Scholar] [CrossRef]
- Guerrero-Osuna, H.A.; García-Vázquez, F.; Ibarra-Delgado, S.; Mata-Romero, M.E.; Nava-Pintor, J.A.; Ornelas-Vargas, G.; Castañeda-Miranda, R.; Rodríguez-Abdalá, V.I.; Solís-Sánchez, L.O. Developing a Cloud and IoT-Integrated Remote Laboratory to Enhance Education 4.0: An Approach for FPGA-Based Motor Control. Appl. Sci. 2024, 14, 10115. [Google Scholar] [CrossRef]
- Mei, Z.; Zeng, J.; Zhang, C.; Yao, S.; Zhang, S.; Wang, H.; Li, H.; Shi, J. Efficient and Verifiable Range Query Scheme for Encrypted Geographical Information in Untrusted Cloud Environments. ISPRS Int. J. Geo-Inf. 2024, 13, 281. [Google Scholar] [CrossRef]
- Ruess, S.; Paulus, G.; Lang, S. Automated Derivation of Vine Objects and Ecosystem Structures Using UAS-Based Data Acquisition, 3D Point Cloud Analysis, and OBIA. Appl. Sci. 2024, 14, 3264. [Google Scholar] [CrossRef]
- Augustyn, D.R.; Wyciślik, Ł.; Sojka, M. Tuning a Kubernetes Horizontal Pod Autoscaler for Meeting Performance and Load Demands in Cloud Deployments. Appl. Sci. 2024, 14, 646. [Google Scholar] [CrossRef]
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.d.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.d.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailescu, M.I.; Simion, V.E.; Ursachi, A.; Somaudon, V.; Jaimes-Mogollón, A.L.; Acevedo, C.M.D.; Cuastumal, C.; Lixandru, L.-M.; Llauradó, X.; Bari, N.E.; et al. A Non-Invasive Diagnostic Platform for Canine Leishmaniasis Using VOC Analysis and Distributed Veterinary Infrastructure. Vet. Sci. 2025, 12, 732. https://doi.org/10.3390/vetsci12080732
Mihailescu MI, Simion VE, Ursachi A, Somaudon V, Jaimes-Mogollón AL, Acevedo CMD, Cuastumal C, Lixandru L-M, Llauradó X, Bari NE, et al. A Non-Invasive Diagnostic Platform for Canine Leishmaniasis Using VOC Analysis and Distributed Veterinary Infrastructure. Veterinary Sciences. 2025; 12(8):732. https://doi.org/10.3390/vetsci12080732
Chicago/Turabian StyleMihailescu, Marius Iulian, Violeta Elena Simion, Alexandra Ursachi, Varanya Somaudon, Aylen Lisset Jaimes-Mogollón, Cristhian Manuel Durán Acevedo, Carlos Cuastumal, Laura-Madalina Lixandru, Xavier Llauradó, Nezha El Bari, and et al. 2025. "A Non-Invasive Diagnostic Platform for Canine Leishmaniasis Using VOC Analysis and Distributed Veterinary Infrastructure" Veterinary Sciences 12, no. 8: 732. https://doi.org/10.3390/vetsci12080732
APA StyleMihailescu, M. I., Simion, V. E., Ursachi, A., Somaudon, V., Jaimes-Mogollón, A. L., Acevedo, C. M. D., Cuastumal, C., Lixandru, L.-M., Llauradó, X., Bari, N. E., Bouchikhi, B., Laouini, D., Diouani, M. F., Bessou, A. B. E., Messaoudi, N., Zeroual, F., & Marascu, V. (2025). A Non-Invasive Diagnostic Platform for Canine Leishmaniasis Using VOC Analysis and Distributed Veterinary Infrastructure. Veterinary Sciences, 12(8), 732. https://doi.org/10.3390/vetsci12080732











