Simple Summary
Bovine respiratory disease (BRD) is a major health concern in feedlot cattle, and macrolide antibiotics are commonly used to treat it. However, the rise in antimicrobial resistance threatens their effectiveness, making it crucial for veterinarians to have fast and accurate tools to guide treatment decisions. Traditional diagnostic methods are often slow and require specialized equipment, limiting their practicality in feedlot settings. This study explored a rapid molecular technique called recombinase polymerase amplification (RPA) to detect three key macrolide resistance genes—msrE, mphE, and erm42—directly from nasal swabs taken from calves shortly after arrival at the feedlot. The performance of RPA was compared to conventional bacterial culture and susceptibility testing and polymerase chain reaction. While RPA showed high sensitivity (95%), its specificity was lower (58%), meaning it detected resistance genes when other methods did not. This was likely due to the presence of these genes in non-BRD bacteria. Overall, RPA offers a promising, rapid alternative for identifying macrolide resistance, but further refinement is needed to improve its accuracy and expand its usefulness to detect a broader range of BRD pathogens and resistance mechanisms in commercial feedlot environments.
Abstract
Macrolides are crucial for the management and treatment of bovine respiratory disease (BRD). However, antimicrobial resistance (AMR) threatens the efficacy of these and other antimicrobials. We developed real-time recombinase polymerase amplification (RPA) assays targeting three clinically relevant macrolide antimicrobial resistance genes (ARGs)—msrE-mphE and erm42—in ≤30 min using extracted DNA. A set of 199 deep nasopharyngeal swabs (DNPS) collected from feedlot calves near the time of arrival were selected based on bacterial culture (BC) results for Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni and antimicrobial susceptibility testing (AST) for tulathromycin, tilmicosin, tildipirosin, or gamithromycin. Samples were also tested for the same targets using RPA and polymerase chain reaction (PCR). In samples that were culture-positive for one or more macrolide-resistant BRD-associated bacteria (n = 101), msrE-mphE and/or erm42 were detected in 95% of cases using RPA. The remaining 98 samples were either culture-negative, or the recovered bacteria were macrolide-susceptible: 43% of these were RPA-positive for at least one macrolide ARG. Together with BC-AST and PCR, Bayesian latent class modelling estimated the clinical sensitivity of RPA for macrolide ARGs to be 95% and specificity to be 58%, with moderate agreement between RPA and BC-AST (κ = 0.52) or PCR (κ = 0.55). The estimated sensitivity of the RPA multiplex assay for the targeted macrolide ARGs was very good, although estimated specificity was limited. However, Sanger sequencing confirmed RPA detection of msrE-mphE in BC-AST/PCR-negative samples (n = 23), reflecting the presence of this locus in non-target bacteria, as well as potential ARG variants among BRD bacteria. These findings support the potential of RPA for rapid ARG detection from extracted DNA. Continued assay optimization and evaluation for detection of respiratory bacteria and ARGs will further enhance its diagnostic utility.
1. Introduction
Bovine respiratory disease (BRD) is a multifactorial syndrome that is the primary cause of morbidity and mortality in fall-placed, North American feedlot cattle [,,]. An economically important disease, BRD imposes a significant financial burden on producers due to the cost of antimicrobial therapy and metaphylaxis, as well as the losses associated with treatment failure, chronic illness, poor performance, and mortality [,].
Respiratory infections associated with BRD are often polymicrobial in nature and involve a complex of bacteria and viruses []. Bacterial agents including members of the Pasteurellaceae family (Mannheimia haemolytica, Pasteurella multocida, Histophilus somni) and Mycoplasmopsis bovis are commensal to the bovine upper respiratory tract, but frequently contribute to BRD. Viral agents, such as bovine respiratory syncytial virus, bovine herpesvirus type 1, bovine parainfluenza-3 virus, bovine coronavirus are also implicated [,]. Numerous host and management risk factors, including fall placement of young, lighter-weight calves purchased at auction, contribute to stress and immunosuppression, colonization of the lower respiratory tract, and the development of disease [,,]. Owing to its complex nature, the prevention and treatment of BRD in cattle is challenging []. Antimicrobial use remains central to BRD management, where tetracyclines, macrolides, and phenicols are commonly used for disease control and treatment [,,].
As members of the category II (high importance) medically important antimicrobials (MIAs) defined by Health Canada, macrolides are used in both human and veterinary medicine []. Tulathromycin (TUL), tilmicosin (TIL), and tildipirosin (TILD) are approved for parenteral use in Canadian cattle to manage and treat clinical BRD [,]. In a survey of 36 western Canadian feedlots, macrolides accounted for 41% of the antimicrobials administered for BRD metaphylaxis between 2008–2012 []. However, as with any antimicrobial drug (AMD), widespread use of macrolides increases selective pressure for antimicrobial resistance (AMR), threatening their long-term effectiveness [].
Resistance of BRD-associated Pasteurellaceae to macrolides is frequently related to the presence of the antimicrobial resistance genes (ARGs) msrE, mphE, and erm42 [,,]. These macrolide ARGs confer resistance via several mechanisms: erm42 encodes rRNA methylases, which methylate 23S rRNA to mask the antimicrobial binding site of the 50S ribosomal subunit [,,]; msrE encodes for a macrolide efflux pump; and mphE encodes for a macrolide-inactivating phosphotransferase [,].
Horizontal transfer of ARGs on mobile genetic elements (MGEs), such as integrative and conjugative elements (ICEs), further complicates the management of BRD []. These transmissible elements can integrate into bacterial genomes and carry ARGs as well as genes involved in virulence, stress response, or heavy metal resistance []. Recent studies have shown that BRD-associated Pasteurellaceae can harbor ARGs within ICEs [,,] and disseminate ARGs via conjugation [,].
Coupled with increasing pressure to minimize antimicrobial use in agriculture, the Canadian Action Plan on Antimicrobial Resistance has recommended that the use of MIAs in livestock production be reduced []. To achieve this, beef producers need rapid, accurate diagnostic tools to develop therapies that optimize drug efficacy while minimizing selection for AMR. Recombinase polymerase amplification (RPA) is a rapid, sensitive, DNA-based detection system that requires minimal sample preparation and very little specialized equipment []. In contrast to standard diagnostic methods like bacterial culture (BC) and PCR, RPA-mediated DNA amplification occurs typically between 37–39 °C [,]. With a time to result of approximately 30 min using extracted DNA, RPA presents an appealing complement to PCR and the industry standard of culture and antimicrobial susceptibility testing (AST) []. Since its introduction in 2006, RPA has been employed in various assays to detect infectious agents in human and veterinary medicine [,,,,,].
For RPA tests to be useful for informing antimicrobial use, end-users require estimates of test validity including clinical sensitivity and specificity. Assessing the validity of a diagnostic test has traditionally relied on comparison of the new assay to an existing reference test assumed to be a “gold standard”. Bayesian latent class modelling is a method commonly used to estimate the validity of a diagnostic test when a gold standard test is unavailable [,]. Models are used to determine a latent, unknown variable, such as true disease status, which cannot be measured directly. A priori information from the measured outcomes of two or more imperfect diagnostic tests, applied to two or more populations, are used to estimate the sensitivity and specificity of each test, to determine its diagnostic accuracy, or to estimate true disease prevalence.
Herein, a real-time RPA assay was developed and validated for detection of multiple macrolide resistance genes and was used along with previously developed RPA assays to detect BRD-associated bacteria [] and ICE variants [] in DNA extracted from nasal swabs obtained from feedlot cattle. The RPA results were compared to quantitative, real-time PCR (qPCR), culture and AST, to estimate the clinical sensitivity and specificity of each test using Bayesian latent class models (BLCMs).
2. Materials and Methods
2.1. Standardized Bacterial Culture for RPA Assay Development
Strains of Mannheimia haemolytica known to contain msrE, mphE, and/or erm42 (BioProject Numbers: PRJN1088094, PRJNA181191) based on whole-genome sequencing were used as positive controls in this study (Table 1) [,]. Bacterial cultures were grown on Columbia blood agar supplemented with 5% defibrinated sheep blood (Thermo Fisher Scientific, Mississauga, ON, Canada) and incubated at 37 °C for 24 h.

Table 1.
List of bacterial strains and whole-genome sequence data used in this study to develop and validate a recombinase polymerase amplification assay for the detection of macrolide resistance genes.
2.2. Bacterial DNA Extraction and Preparation of Stock DNA
To generate positive control template DNA, genomic DNA was extracted from M. haemolytica cultured cells (~10 µL) resuspended in 200 µL of phosphate-buffered saline (PBS), using the DNeasy Blood and Tissue kit (Qiagen, Toronto, ON, Canada) as per manufacturer instructions.
DNA concentration was measured using a Qubit fluorometer and the Qubit dsDNA BR (Broad-Range) Assay Kit (Thermo Fisher Scientific Inc., Ottawa, ON, Canada). DNA from M. haemolytica strains was standardized to 2.0 ng/µL, then diluted to a stock of 20,000–50,000 genome copies per microlitre (gc/µL) and stored at −20 °C for future testing. To estimate copy numbers, the reference genome size of M. haemolytica (2.6 Mbp) was used as per Conrad et al. [].
2.3. Conventional RPA Assay Design: msrE, mphE, erm42
Macrolide resistance genes were identified in whole-genome sequences of M. haemolytica, P. multocida, and H. somni using the Pathogen Detection Reference Gene Catalog (National Database of Antibiotic Resistant Organisms, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Bethesda, MD, United States), as well as from our laboratory (Table 1). Three clinically relevant macrolide ARGs (erm42, msrE, and mphE) [,,] were shown to be conserved and were selected as targets for development of RPA assays.
Primer sets for RPA were designed for erm42, msrE, and mphE using the Geneious Prime bioinformatics software platform (version 2020.0.4; Biomatters, Inc., San Diego, CA, USA). Further genomic analysis revealed that mphE was consistently located within an operon downstream from msrE in the genomes of M. haemolytica, H. somni, and P. multocida []. For this operon, primers were designed (Table 2) to span conserved regions of each of the two genes, as well as the intergenic region.

Table 2.
Summary of primer and probe sets used to identify bacterial pathogens, integrative and conjugative elements, and macrolide resistance genes, in DNA from deep nasopharyngeal swabs collected from feedlot calves.
The macrolide ARG targets, msrE-mphE and erm42 were each detected in a singleplex assay using conventional RPA, with the TwistAmp® Basic Kit (TwistDx Limited, Maidenhead, UK). A reaction volume of 50 μL was prepared following the conventional reaction protocol []. The mastermix was composed of 29.5 μL rehydration buffer (TwistDx Limited, Maidenhead, UK), 11.2 μL nuclease-free water, and 2.4 μL of each forward and reverse primer (final concentration for msrE-mphE and erm42 primers: 480 nM) per reaction. The mixture was vortexed and 45.5 μL aliquots were dispensed into 0.2 mL tubes in a strip (eight tubes/strip) containing a lyophilized enzyme pellet (TwistDx Limited, Maidenhead, UK). Genomic DNA of M. haemolytica was added at 500 genome copies per reaction (2 µL), followed by 2.5 µL of magnesium acetate (MgAc). Tubes were sealed, vortexed, and briefly spun down using a mini-centrifuge to collect droplets, then placed into the T16-ISO machine (Axxin Ltd., Fairfield, Victoria, Australia) at 39 °C for 30 min. After 4 min, the tubes were removed, manually vortexed, briefly centrifuged again, and returned to the T16 machine for an additional 26 min.
Post-amplification products were purified using a QIAquick PCR Purification Kit (Qiagen, Toronto, ON, Canada) and subjected to electrophoresis on a 2% (w/v) agarose gel with ethidium bromide at 150 V for 55 min. A fluorescence imager (BioRad, Mississauga, ON, Canada) was used for visualization.
2.4. Real-Time RPA Assay Design: msrE-mphE, erm42
Based on the top-performing conventional RPA primer sets, three real-time RPA assays were developed: two singleplex assays for msrE-mphE and erm42, and one multiplex assay targeting both loci. The singleplex assays were designed to search for potential secondary primer products, which might impede the multiplex reaction, and to determine the expected limit of detection (LOD) and strength of target amplification of each assay without potential competitive inhibition.
A fluorescence probe was designed for each locus (Table 2) using Geneious Prime v2020.0.4 (Biomatters Ltd., Newark, NJ, USA), with probes sized at 48 and 46 nucleotides (fluorescein amidite (FAM) for msrE-mphE; dichloro-diphenyl-fluorescein (SIMA) for erm42; LGC Biosearch Technologies, Novato, CA, USA). The singleplex reactions used the TwistAmp® exo Kit (TwistDx Limited, Maidenhead, UK), with a reaction mix containing 29.5 µL of rehydration buffer, 11.2 µL nuclease-free water, 2.1 µL of forward and reverse primers (420 nM), and 0.6 µL corresponding probe. The reaction was run on a T16-ISO machine (Axxin Ltd., Eaglemont, VIC, Australia) at 39 °C for 26 min, including four min of initial reaction time, followed by a manual vortex and centrifugation step, then the remaining 22 min of the reaction. A positive identification was defined for either assay as a fluorescence signal threshold of ≥400 mV for at least 60 s. This algorithm was selected to resolve initial false-positive readings in the ‘no template’ control (NTC) of the msrE-mphE singleplex assay, which occurred at the original fluorescence threshold (≥200 mV) [] when “background” fluorescence in the NTC exceeded the threshold, at times to a maximum of ~390 mV. The new algorithm threshold of 400 mV was selected to resolve these false-positive readings with minimal intervention, while also minimizing misclassification of low-positive samples.
To allow for simultaneous detection of both macrolide resistance loci, a multiplex assay was configured using a 45:55 ratio of msrE-mphE to erm42 primers, as initial tests run at a 50:50 ratio showed competitive inhibition of erm42. Using the T16-ISO Desktop Application, we also adjusted the fluorescence channel settings to 20% for FAM and 50% for hexachloro-fluorescein (HEX)/SIMA to improve erm42 detection. Assay validation was completed by running reactions with 29.5 µL rehydration buffer, 10.63 µL nuclease-free water, 210 nM msrE-mphE primers, 260 nM erm42 primers, and aliquots of each probe—73.34 nM (erm42) and 60 nM (msrE-mphE), using the same reaction parameters as for the singleplex assay. The multiplex assay consistently detected both gene targets in the known erm42-msrE-mphE-positive M. haemolytica MH44 strain. The known msrE-mphE-positive, erm42-negative MH007 strain [] was used to ensure assay specificity for erm42.
2.5. Limit of Detection Testing
Singleplex and multiplex RPA assays underwent LOD testing using serially diluted M. haemolytica DNA (104, 103, 500, 400, 200, 102, and 50 genome copies (gc) per reaction). Each dilution was tested up to 30 times (except for the 104 gc/reaction—three iterations, and 500 gc/reaction—six iterations) to determine the LOD in ≥95% of the runs. The LOD for each singleplex and multiplex assay was calculated by Probit regression analysis using SPSS (IBM SPSS Statistics for Windows, Version 26.0, IBM Corp, Armonk, NY, USA), with a step limit of 0.1 and a maximum of 999 iterations. By using a defined and stringent approach to determine the lowest concentration at which msrE-mphE and erm42 could be reliably detected, the LOD of each assay was considered equivalent to the limit of quantitation.
2.6. Field Validation of the Multiplex RPA Macrolide ARG Assay
2.6.1. Sample Population
Deep nasopharyngeal swab (DNPS) samples were obtained from feedlot calves in 2020 as previously described []. Sampling procedures for this project were approved by an Animal Care Committee (Animal Use Protocol: 20190069; University of Saskatchewan, Saskatoon, SK, Canada) and are described in detail elsewhere []. Briefly, high BRD-risk calves were purchased at auction and housed at the Livestock and Forage Centre of Excellence (LFCE) feedlot (University of Saskatchewan, Clavet, SK, Canada) in eight pens of 100 calves per pen. Swab samples were collected from calves at arrival and after 13 and 36 days on feed (DOF). Three nasal swabs were collected per calf, alternating nostrils, and swab heads were cut and placed into a vial containing 3 mL of liquid Amies transport medium (GMP, University of Saskatchewan, Saskatoon, SK, Canada), as previously described [,]. Samples were transported to the laboratory at the Western College of Veterinary Medicine (University of Saskatchewan, Saskatoon, SK, Canada) and processed within one hour of sampling []. Samples were vortexed for one min, and 300 µL of the raw sample suspension was submitted to Prairie Diagnostic Services Inc. (PDS, Saskatoon, SK, Canada) for bacterial culture and AST. The remaining aliquot was saved at 4 °C for subsequent analyses.
2.6.2. Bacterial Culture and Antimicrobial Susceptibility Testing
Samples were processed as previously described with quality assurance and control procedures [] for the isolation and identification of M. haemolytica, P. multocida, and H. somni. A 10 µL aliquot of each sample suspension was plated onto Columbia blood agar supplemented with 5% sheep blood (Thermo Fisher Scientific, Mississauga, ON, Canada) to isolate M. haemolytica and P. multocida and incubated at 35 °C for 42 h. For H. somni, samples were cultured on chocolate agar and incubated at 35 °C for 48 h, in a 5% CO2 atmosphere. The identity of colonies exhibiting morphology consistent with target pathogens was confirmed using a MALDI-TOF MS Microflex LT instrument (Bruker Corporation, Billerica, Massachusetts, USA) and MALDI Biotyper Microflex LT Compass software (version 1.4).
As was consistent with current surveillance initiatives [], recent research studies [], and protocols for diagnostic submissions by the regional laboratory [], a single, confirmed isolate of each target bacterial species was selected randomly from each sample plate and underwent AST using the Sensititre platform and Bovine BOPO7F AST plate (ThermoFisher Scientific, Waltham, MA, USA). Minimum inhibitory concentration (MIC) breakpoints for M. haemolytica, P. multocida, and H. somni were used according to the Clinical and Laboratory Standards Institute (CLSI) guidelines as previously described in detail with quality assurance and control processes [].
2.6.3. DNA Extraction
Prior to DNA extraction, bovine DNA was depleted using the HostZEROTM Microbial DNA Kit (Zymo Research, Irvine, CA, USA). Sample suspensions were centrifuged at 6000× g for 5 min and the supernatant was discarded, leaving ~100 µL with the pellet. Host depletion solution (500 µL; Zymo Research, Irvine, CA, USA) was added, mixed, and the resulting homogeneous suspension was rotated end-to-end at room temperature for 15 min. Tubes were centrifuged at 10,000× g for 5 min and the supernatant was removed. Microbial selection buffer (100 µL; Zymo Research, Irvine, CA, USA) and microbial selection enzyme (0.5 µL; Zymo Research, Irvine, CA, USA) were added, and the pellet was resuspended by vortexing for 5–10 s. Tubes were centrifuged (≤1500× g) and incubated for 30 min at 37 °C. Following incubation, 10 µL of proteinase K was added to each sample. The tubes were vortexed for 10 s, centrifuged (1500× g), and then incubated at 55 °C for 10 min. Finally, DNA/RNA ShieldTM (200 µL; Zymo Research, Irvine, CA, USA) was added to each tube, which was vortexed for 10 s and incubated at room temperature for 5 min to complete depletion of host DNA prior to extraction. Bacterial DNA was then extracted using the DNeasy Blood and Tissue kit (Qiagen, Toronto, ON, Canada) and eluted into 100 µL of elution buffer (Buffer AE, Qiagen, Toronto, ON, Canada).
2.6.4. Inclusion Criteria for Field Samples: Macrolide Resistance
DNA was extracted from 199 DNPS collected from feedlot calves in the fall of 2020 and selected for RPA screening of msrE, mphE, erm42, BRD-associated bacteria, and ICEs (Table 2). The DNA from the 199 DNPS was strategically selected from all available samples to optimize the power of subsequent analyses by targeting a mix of samples with and without evidence of macrolide-resistant bacteria. Inclusion criteria were based on bacterial culture results and phenotypic resistance to TUL, TIL, TILD, and/or gamithromycin (GAM). Samples were divided into two groups: (i) samples with detected “phenotypic-macrolide-resistance-positive” (MacRes+) bacteria (n = 101), where any of M. haemolytica, P. multocida, and/or H. somni were isolated and exhibited phenotypic macrolide resistance, and (ii) samples where no “phenotypic-macrolide-resistance-negative” (MacRes-) bacteria were detected (n = 98). The resistance-negative group included 68 samples from which one or more BRD-associated bacteria were isolated, but were susceptible to macrolides (TUL, TIL, TILD, GAM), and 30 samples from which no BRD pathogens were cultured. Among the BRD-associated bacteria isolated from the MacRes+ samples, the most common resistance pattern was to GAM and TUL (GAM-TUL) in M. haemolytica isolates (Table 3).

Table 3.
Phenotypic resistance patterns of macrolide-resistant, bovine-respiratory-disease-associated bacterial pathogens isolated from deep nasopharyngeal swabs collected from fall-placed calves (n = 101).
Resistance to other drugs among MacRes- samples included a single M. haemolytica isolate resistant to ampicillin (AMP), and nine P. multocida isolates that were resistant to spectinomycin (SPECT) and tetracycline (TET) (Table S1). The six H. somni isolates recovered from the macrolide-susceptible samples were pansusceptible.
2.7. Experimental Testing
Field samples selected using BC-AST (Section 2.6.2) data, were then tested using RPA and qPCR (Figure 1).
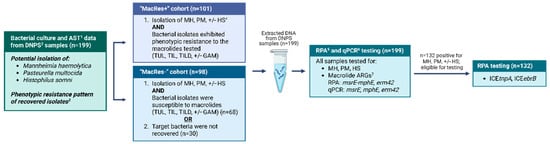
Figure 1.
Flowchart of the diagnostic testing strategy used for bacterial culture, antimicrobial susceptibility testing, recombinase polymerase amplification, and quantitative polymerase chain reaction testing of deep nasopharyngeal swabs collected from calves for the detection of bacteria commonly associated with bovine respiratory disease and antimicrobial resistance targets of interest (n = 199). 1 AST: antimicrobial susceptibility testing; 2 DNPS: deep nasopharyngeal swab. 3 Possible phenotypic resistance to antimicrobial drugs included ampicillin, gamithromycin (GAM), spectinomycin, tetracycline, tilmicosin (TIL), tildipirosin (TILD), and tulathromycin (TUL). 4 MH: Mannheimia haemolytica, PM: Pasteurella multocida, HS: Histophilus somni. 5 RPA: recombinase polymerase amplification; 6 qPCR: quantitative polymerase chain reaction; 7 ARGs: antimicrobial resistance genes, in this case referring to macrolide ARGs msrE, mphE, and erm42. Only samples that tested positive for M. haemolytica, P. multocida, or H. somni using RPA were tested for mobile genetic element variants ICEtnpA and ICEebrB; Created in BioRender. L, S. (2025) https://www.biorender.com/ accessed on 10 November 2025.
2.7.1. RPA Testing
Reaction setup for RPA testing of DNA from the 199 field samples was analogous to that of the multiplex assay development protocol, with the exception that the volume of extracted template DNA was increased to 10 µL per reaction and nuclease-free water was reduced to 2.63 µL. Additional RPA targets followed the testing protocols established by Conrad et al. [,] for bacterial BRD pathogens (P. multocida, H. somni, and M. haemolytica serotypes 1 and 6) [,] and ICE complex variants (ICEtnpA: tetH_tnpA, ICEebrB: tetH_ebrB; Table 2). Samples were further tested for ICE variants only if at least one BRD bacterium of interest was identified by RPA.
2.7.2. Quantitative, Real-Time PCR Testing
Following RPA, the remaining volume of each of the same 199 extracted DNA samples was tested for the presence of macrolide resistance genes (msrE, mphE, erm42) and key bacterial pathogens (M. haemolytica, P. multocida, H. somni) by the Nebraska Veterinary Diagnostic Centre (NVDC; Lincoln, NE, USA), using multiplex, qPCR assays on the Rotor-Gene Q (RGQ) platform (Qiagen, Hilden, Germany) and protocols outlined by Loy et al. [] and Dutta et al. [].
For the detection of macrolide ARGs, DNA extracted from the 199 DNPS samples was tested at NVDC using the RGQ platform and a validated, four-plex qPCR assay targeting msrE, mphE, and erm42 individually, as well as tetR []. For each 25 µL reaction, the PCR mastermix comprised 12.5 µL of 2× QuantiFast multiplex PCR master mix (Qiagen, Hilden, Germany), including 1 µL of each, respective primer-probe mix (forward primer [10 µM], reverse primer [10 µM], probe [10 µM] for the four targets, 4 µL total), 6.5 µL of nuclease-free water, and 2 µL of genomic template DNA. The thermocycling conditions for this assay consisted of 5 min at 95 °C, followed by 45 cycles at 95 °C for 15 s, and 59 °C for 40 s for both the primer annealing and extension steps [].
A mean Ct threshold of 31.8 for detecting msrE, mphE, and erm42 was reported for the qPCR assay using receiver operating characteristic (ROC) curve analysis. The reported sensitivity and specificity of the qPCR assay were 43% and 83%, respectively [].
Additionally, samples were tested for M. haemolytica (all serotypes), P. multocida, and H. somni using the RGQ platform. For each 25 µL reaction, PCR mastermix was prepared with 10 µL of 2× QuantiFast multiplex PCR master mix (Qiagen, Hilden, Germany), 1.0 µL of primer-probe mix for each target (10 µM, 5.0 µL total), and 5.0 µL of extracted template DNA. The thermocycling conditions included an initial denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 40 s for both the primer annealing and extension steps []. A Ct value > 33.4 (M. haemolytica, P. multocida), or >39.8 (H. somni) was designated as negative, or “not detected”, while a value below this threshold was considered positive for the detection of each target bacterium [] (Supplementary Material).
2.7.3. Sanger Sequencing and Selected Long-Read Metagenomic Next-Generation Sequencing
A subset of 23 samples tested for macrolide ARGs were submitted to the National Research Council (NRC) of Canada (Saskatoon, SK, Canada) for Sanger sequencing (Table 4). The samples were of specific interest because they were presumptive RPA false positives as they were macrolide-resistance-negative by BC-AST, negative for the msrE and mphE by PCR, and positive for msrE-mphE using RPA. Another subset of samples (n = 8) that were RPA positive erm42, were tested as these results also differed from BC-AST, and qPCR testing (Table 4).

Table 4.
Sanger sequencing of purported false-positive recombinase polymerase amplification DNA products for the alignment of primer and probe sets associated with detection of macrolide resistance genes msrE-mphE and erm42, compared to the results of antimicrobial susceptibility testing and polymerase chain reaction testing (n = 31).
For sequencing, the designated RPA primers for each target were prepared at 5 pmole/µL (forward and reverse), and the RPA post-amplification samples were normalized to 10 ng/µL. Samples were considered Sanger-sequencing-positive for macrolide ARGs if the primers and/or probe annealed at the correct genetic location in silico using Geneious Prime software (version 2020.0.4), and negative if no primer or probe sequence information could be deduced.
The same subset of 23 suspected RPA false-positive samples submitted for Sanger sequencing for msrE and mphE were also tested using a previously described protocol for long-read metagenomic next-generation sequencing (mNGS), where ARGs associated with identified bacterial were reported []. The objective of this work was to potentially identify the bacterial source of ARGs.
A BLAST search for the amplified RPA msrE-mphE target sequence was also completed using the Discontiguous Megablast program (Geneious Prime v2020.0.4) and core nucleotide database (core_nt) framework (NCBI) to align the amplicon with curated bacterial genomes (Table 2). A high, 2000-hit maximum was selected to search for these ARGs in other, non-BRD-related bacteria common to the bovine nasopharyngeal microbiome, which could account for msrE-mphE in samples where resistance had not been detected in Pasteurellaceae.
Conversely, any database hits resulting in detection of genetic sequences closely resembling, but different than msrE-mphE could account for RPA false positives or variants.
2.8. Statistical Analysis
Bayesian latent class models were used in the absence of a gold standard reference test to assess clinical test performance of the RPA assays relative to bacterial culture and phenotypic AST, and qPCR for detecting macrolide resistance and BRD-associated bacteria. Three-test and three population models were developed for both macrolide resistance (BC-AST, RPA, qPCR) and specific BRD bacteria (BC, RPA, qPCR). Samples collected at each time point were expected to have varied prevalence and therefore comprised the three populations in the BLCM: 1DOF (n = 97), 13DOF (n = 77), and 36DOF (n = 25). Assumed conditional dependence between RPA and qPCR tests was modeled as covariance. Models were developed and run using JAGS software v4.3.0 [] and the ‘runjags’ package [] in R v4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) [].
For BRD-bacterial-pathogen models, uninformative priors (beta (1, 1)) were used for the sensitivity of each test, the specificity of RPA, and the prevalence in each population. A beta (100, 1.5) distribution was used to model the prior for the clinical specificity of bacterial culture for the detection of each bacterial target based on subsequent confirmation using whole-genome sequencing []. The prior for the specificity of PCR was based on values reported by Loy et al. [] and modeled with a beta (31.4, 10.2) distribution for M. haemolytica, a beta (24.8, 7.8) distribution for P. multocida, and a beta (100, 5.6) distribution for H. somni [].
The BLCM for macrolide resistance used uninformative priors modeled with beta [] distributions for all parameters, due to a lack of comparable prior information for RPA, BC-AST, and qPCR. Convergence was assessed for all models, and deemed satisfactory if the evaluated model diagnostics, including potential scale reduction factor (PSRF < 1.05), effective sample size (>1000), and Monte Carlo standard errors as a percent of standard deviation (<5%), as well as visual inspection of trace and autocorrelation plots were satisfactory. Model parameters were estimated following 100,000 iterations of each of three Monte Carlo Markov chains, after a burn-in phase of 5000 iterations per chain. Estimates for test sensitivity and specificity were reported as the median of the posterior distribution along with 95% credible intervals (95% CrI). Of note for the BLCM estimating the performance of M. haemolytica detection, the RPA assay reported M. haemolytica serotypes 1 and 6 specifically, while bacterial culture and qPCR reported all serotypes.
Further agreement beyond that expected by chance between RPA, BC-AST, and qPCR for the detection of macrolide resistance or BRD-associated bacterial pathogens (RPA M. haemolytica assay serotypes 1, 6) in the DNPS was assessed using kappa (Stata/IC 15.1, StataCorp LLC, College Station, TX, USA). Kappa (κ) was interpreted as described by Dohoo, Martin, and Stryhn (κ ≤ 0.00—poor, 0.01–0.20—slight, 0.21–0.40—fair, 0.41–0.60—moderate, 0.61–0.80—substantial, 0.81–1.00—near perfect) [] (pp. 97–99). Finally, odds ratios (Stata/IC 15.1) were calculated using two-by-two tables to describe the odds of detecting one or both macrolide ARG targets, given the presence of ICEs, in samples that were also positive or negative for M. haemolytica, P. multocida, and/or H. somni.
3. Results
3.1. Validation and Limit of Detection of RPA Assays for Macrolide Resistance Targets
The LOD for the singleplex RPA assay was 220 gc/reaction (95% CI (166, 371)) for the msrE-mphE assay, and 122 gc/reaction (95% CI (83, 412)) for the erm42 assay. Once validated, the assays were combined at a 45 (msrE-mphE)-to-55 (erm42) primer and probe ratio, and validation of the multiplex macrolide resistance assay was completed using diluted genomic DNA from M. haemolytica MH44. The LODs of the multiplex assay were 168 gc/reaction (95% CI (130, 322)) for msrE-mphE and 185 gc/reaction (95% CI (144, 337)) for erm42.
3.2. Detection of Antimicrobial Resistance Determinants and Bacterial BRD Pathogens in DNPS
3.2.1. Antimicrobial Resistance Gene Detection
The RPA assay detected msrE-mphE in 65% of samples, erm42 in 1% of samples, and both gene targets in 4% of samples (Supplementary Table S3). From DNPS where at least one M. haemolytica, P. multocida, or H. somni isolate was resistant to macrolides (MacRes+, n = 101), msrE and/or mphE were detected in 95% of samples using RPA and 84% using PCR. Additionally, 41% of samples for RPA and 4–6% of samples for PCR were positive for msrE and/or mphE where target Pasteurellaceae bacteria not resistant to macrolides were identified by BC-AST (Table 5). Similarly, RPA identified msrE-mphE in 47% of samples where no target Pasteurellaceae bacteria were cultured. However, neither msrE nor mphE were identified by PCR in any of these samples (Table 5).

Table 5.
Summary of the reported phenotypic macrolide resistance compared to macrolide resistance genes detected by recombinase polymerase amplification and real-time polymerase chain reaction in DNA extracted from deep nasopharyngeal swabs from fall-placed calves (n = 199).
When parsed for the detection of msrE and/or mphE by RPA or PCR alone (Table 6), both msrE and mphE were typically detected or not detected as a pair by both tests. However, 3% of samples contained mphE without msrE as per PCR, where the same samples were positive for the msrE-mphE operon using RPA (Table 6). Detection of erm42 using RPA was uncommon (4.5%) and no samples were positive for erm42 using PCR (Table 5).

Table 6.
Summary of macrolide resistance genes detected by recombinase polymerase amplification and real-time polymerase chain reaction in DNA extracted from deep nasopharyngeal swabs from fall-placed calves (n = 199).
Based on the alignment of several partial bacterial sequences (Table 1 and Supplementary Materials—Figure S1, Geneious Prime v2020.0.4), the RPA and PCR primer/probe annealing sites for msrE and mphE differed. RPA primers targeted a highly conserved region of the msrE-mphE operon, and the previously validated PCR primers [] annealed to a conserved region of msrE upstream. The PCR primer/probe annealing sites for mphE were downstream from the RPA reverse primer, and single nucleotide polymorphisms were observed in this region for one M. haemolytica and one H. somni isolate (Figure S1).
3.2.2. BRD Pathogen Detection
Detection of BRD-associated bacteria varied across testing methods; RPA identified at least one bacterial pathogen of interest in 66% of samples. M. haemolytica of any serotype was identified in 73% of samples by culture and 74% of samples by qPCR, while RPA specifically detected serotypes 1 and 6 in 33% of samples (Table 7).

Table 7.
Frequency of detection of bovine-respiratory-disease-associated bacterial pathogens by recombinase polymerase amplification, bacterial culture, and quantitative polymerase chain reaction (n = 199).
3.2.3. ICE Detection
Samples positive for one or more of M. haemolytica, P. multocida, and/or H. somni using RPA (66%) were further tested for variants ICEtnpA and ICEebrB (Table 8). Of these 132 samples, 38% possessed one or both variants. ICEtnpA was detected most frequently, in 70% (35/50) of ICE-positive samples, and 6% (3/50) of samples were found to carry both ICE variants.

Table 8.
Prevalence of integrative and conjugative element (ICE) variants and bovine-respiratory-disease (BRD) bacteria detected by recombinase polymerase amplification in samples positive for at least one species of Mannheimia haemolytica, Pasteurella multocida, or Histophilus somni (n = 132 *).
Of the 132 samples positive for BRD organisms and tested for ICE, 94 were positive for msrE-mphE and/or erm42 by RPA. Thirty-nine of these 94 ARG positive samples (41%) also harbored at least one ICE variant; msrE-mphE and/or erm42 were detected by RPA in 78% (39/50) of samples with at least one detected ICE variant. Twenty-seven of the 50 ICE positive samples (54%) also had M. haemolytica, P. multocida, and/or H. somni isolates classified as phenotypically resistant to macrolides.
There was no significant association between the detection of either ICE variant by RPA in samples and the likelihood of identifying phenotypic macrolide resistance in the same samples (OR 1.21 (95% CI (0.63, 2.36)), p = 0.57) (n = 199). However, there was a significant association between detection of at least one ICE variant and detection of msrE-mphE and/or erm42 by RPA (OR 7.44 (95% CI (3.37, 16.4)), p = 0.001) when only samples positive for M. haemolytica, P. multocida, or H. somni were considered (n = 199).
All samples ineligible for RPA testing for ICE and ARGs, as they were RPA negative for M. haemolytica, P. multocida, and H. somni, were considered negative in this serial testing strategy. The association between the detection of ICE and ARGs of interest was no longer significant if msrE-mphE and/or erm42 detected by RPA were considered in all samples, regardless of the presence or absence of BRD pathogens as detected by RPA (OR 1.90 (95% CI (0.89, 4.10)), p = 0.10) (n = 199).
3.3. Diagnostic Performance Comparison
The clinical sensitivity of the real-time, multiplex RPA assay for msrE-mphE and erm42 was estimated to be 95% and specificity was estimated to be 58% for all samples tested (Table 9). Sensitivity of culture was very similar to RPA and while the median sensitivity of PCR was slightly lower than RPA, the difference was not significant. However, the clinical specificity of both culture and qPCR were significantly higher (>95%) than that of RPA.

Table 9.
Clinical sensitivity and specificity of recombinase polymerase amplification, bacterial culture and antimicrobial susceptibility testing, and polymerase chain reaction for detection of macrolide antimicrobial resistance genes in deep nasopharyngeal swabs obtained from feedlot calves, based on Bayesian latent class models (n = 199).
The same diagnostic testing strategy used for detection of ICEs was then applied to the detection of macrolide resistance genes by both RPA and qPCR in these samples, to investigate the effect on RPA specificity. Briefly, only samples positive for at least one BRD pathogen by RPA were tested for ARG by RPA, and similarly, only samples positive for at least one BRD pathogen using qPCR were tested for ARGs by qPCR. Samples that were not tested were considered negative for ARGs potentially associated with BRD pathogens. While this strategy improved the median specificity of RPA relative to the other tests, the increase was not significant (Table 9). Under this testing strategy, the sensitivity of RPA was significantly lower and was also significantly lower than BC-AST and qPCR (Table 9).
The differences in detection of macrolide resistance when only samples that were positive on RPA for at least one of the BRD organisms were tested (Table 9) as compared to when all samples were tested was summarized in Figure 2.
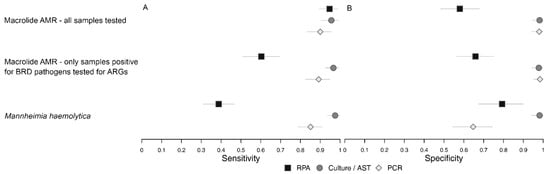
Figure 2.
Plot of Bayesian latent class model estimates of sensitivity (A) and specificity (B) for models comparing detection of msrE-mphE +/− erm42 by recombinase polymerase amplification (RPA), macrolide resistance classified by antimicrobial susceptibility testing (AST), and detection of msrE OR mphE +/− erm42 by polymerase chain reaction (PCR) when all samples were tested and when only samples positive for bovine respiratory disease bacteria were tested, as well as the model comparing detection of M. haemolytica by RPA (serotypes 1 and 6 only), culture (all serotypes) and PCR (all serotypes).
The test performance metrics from M. haemolytica were also included for context (Table 10), as most of the phenotypic macrolide resistance was described in this species (Table 3).

Table 10.
Clinical sensitivity and specificity of recombinase polymerase amplification (RPA), bacterial culture, and polymerase chain reaction (PCR) for the detection of bovine-respiratory-disease bacteria in deep nasopharyngeal swabs obtained from feedlot calves, based on Bayesian latent class analysis (n = 199).
The qPCR test allowed for detection of either or both of msrE and mphE (“OR”). When the interpretation of the assay was changed so that both msrE and mphE (“AND”) had to be detected for the sample to be considered positive, the sensitivity of qPCR dropped slightly, and the RPA estimates were not impacted (Table 9).
Finally, the impact of how intermediate MICs were considered in the analysis was evaluated (Table 9). There was no observed difference in any of the estimates, except for a small but non-significant decrease in the specificity of culture and AST when intermediate MICs were considered together with resistant as “nonsusceptible”.
The results of Sanger sequencing were compared to those of bacterial culture, RPA, and PCR for an associated set of potential RPA false positives (n = 23; RPA-positive, and BC/AST- and qPCR-negative), to assess the lower RPA specificity for macrolide resistance genes (Table 4). Sanger sequencing for msrE-mphE did not confirm any false positives or false negatives for RPA. In contrast, the sequence quality of the eight samples tested for erm42 was poor, and four false positives and 1 false negative were identified in these samples (Table 4 and Table S2).
The potential false positive samples subject to Sanger sequencing were also tested by long-read metagenomic sequencing, where msrE and/or mphE were identified in four samples. Of these, mphE was associated with a target BRD bacteria, P. multocida, in only one sample. In the remaining three samples, msrE and/or mphE genes were identified within reads of other bacterial species: Moraxella bovoculi (sample 2), Citrobacter freundii (sample 3), Acinetobacter towneri (sample 3), and E. coli (sample 3 and 4).
3.4. BRD Pathogen Sensitivity and Specificity Analysis
A similar Bayesian model was employed to estimate the clinical sensitivity and specificity of RPA, bacterial culture, and qPCR for the identification of the BRD pathogens of interest. RPA was insensitive to the choice of informative priors. Bayesian analysis of M. haemolytica, P. multocida, and H. somni across all three test types demonstrated highly variable sensitivities and specificities (Table 10), even though culture was highly specific for all three targets. For M. haemolytica, bacterial culture had the highest sensitivity. However, for both P. multocida and H. somni the sensitivity of qPCR was higher than for either culture or RPA. A two-test model for culture and qPCR for M. haemolytica generated almost identical specificity for both tests as compared to the three-test model that included RPA which only considered serotypes 1 and 6.
3.5. Agreement Among Assays
The agreement of RPA and AST or qPCR for the detection of macrolide resistance was moderate between RPA and both reference tests, and near perfect between AST and PCR (Table 11). The agreement between assays in the present study for the detection of organisms was fair between AST and qPCR and slight or poor for all other comparisons involving RPA (Table 11). The agreement for comparisons of qPCR and culture to RPA for identification of organisms tended to be lower for P. multocida and H. somni, where the assays evaluated the same target than for M. haemolytica where RPA considered only serotypes 1 and 6.

Table 11.
Inter-test agreement (kappa, κ) between recombinase polymerase amplification, bacterial culture, antimicrobial susceptibility testing, and/or quantitative polymerase chain reaction for detection of bovine-respiratory-disease-associated bacteria and macrolide antimicrobial resistance genes in deep nasopharyngeal swab samples obtained from feedlot calves (n = 199).
4. Discussion
Bovine respiratory disease remains the foremost reason for injectable antimicrobial use in Canadian feedlot cattle []. Macrolides are important for the management of both human and animal disease, and laboratory testing has been recommended to better inform antimicrobial use for BRD [,]. However, current diagnostic tools used to detect BRD bacterial pathogens and their antimicrobial resistance determinants are time-consuming and require specialized equipment. This two-part study developed and validated a novel, multiplex RPA assay targeting three clinically relevant macrolide resistance genes frequently associated with BRD bacterial pathogens. Development was supported by a curated isolate collection and DNA samples from DNPS collected from fall-placed calves that had been cultured and tested for antimicrobial susceptibility [].
The assay enabled simultaneous and real-time detection of ARGs associated with macrolide resistance. The genetic linkage between msrE and mphE as an operon [,,], supported by the sequencing data from isolates used in development, informed the design of the multiplex assay for three macrolide ARG targets (msrE, mphE, erm42). A combined assay for msrE-mphE was necessary for simultaneous analysis of all three genes, as the T16-ISO RPA machine was limited to two fluorescence channels. Nearly all samples (95%) where M. haemolytica, P. multocida, or H. somni isolates were phenotypically resistant to one of the macrolides were also positive for one or both RPA-targeted macrolide ARGs. In addition, the RPA targets for macrolide ARGs were detected in ~40% of samples where either the detected isolates were not phenotypically resistant to macrolides tested or no BRD-associated bacteria were detected.
Bayesian latent class modelling (BLCM) was used to estimate the diagnostic performance in the absence of a gold standard. RPA, AST, and qPCR all demonstrated good clinical sensitivity for macrolide ARGs and phenotypic macrolide resistance. However, RPA had lower specificity than AST and qPCR. In addition, both clinical sensitivity and specificity varied across all tests for identification of M. haemolytica (RPA: serotypes 1 and 6; culture/qPCR: all serotypes), P. multocida, and H. somni. The exceptions were the estimates for culture specificity, which were high (>97%) for all three bacterial targets.
BLCM validity relied on three assumptions: conditional independence of tests, differing prevalence of targets across populations, and consistent test performance across populations [,]. Culture and AST were considered independent from RPA and qPCR, which shared DNA extraction and some amplification methods. In the present study, samples collected at different times were expected to have different frequencies of both macrolide resistance and organisms of interest. Recent studies using the same calf data described varying prevalence of phenotypic macrolide resistance and Pasteurellaceae bacteria in cohorts of calves over time []. Capik et al. also found that isolation of BRD-related Pasteurellaceae from nasopharyngeal swabs varied over time in some calves treated for BRD []. Similarly, Holman et al. (2017) reported a significant shift over time in the recovery of P. multocida and M. bovis [].
Although low RPA specificity suggested false positives, Sanger sequencing confirmed msrE-mphE in many suspect samples. Long-read metagenomic sequencing identified these genes in non-BRD bacteria, including E. coli, Moraxella bovoculi, Citrobacter freundii, and Acinetobacter towneri in three out of four suspect false-positive samples, as well as in P. multocida in one suspect RPA false-positive sample. This is consistent with conserved distribution of these ARGs across species such as Acinetobacter spp., Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Moraxella osloensis, and Citrobacter freundii, among others. These non-BRD-related species are common to humans, cattle, other animals, and the environment. Thus, RPA likely detected ARGs missed by BC-AST in part due to limited species testing.
Despite frequent use as a gold standard test, bacterial culture is imperfect and has limited sensitivity []. Further, phenotypic resistance cannot be confirmed without organism recovery. However, in the present study, bacterial recovery rates from DNPS from healthy, fall-placed calves were good, particularly for M. haemolytica, where most phenotypic macrolide resistance was identified. Overall M. haemolytica recovery rates ranged from 33% at 1DOF to 75% at 36 DOF [], and compared well or were improved relative to similar reports [,,], likely due to optimized sample handling. Pooled nasal swabs and prompt culture likely also improved recovery, as supported by studies emphasizing storage below 8 °C and culture within 24 h [,].
The culture recovery rates were reflected by very high estimated sensitivities from the BLCMs in the present analysis for both M. haemolytica and macrolide phenotypic resistance. Estimates of clinical sensitivity were lower for culture of both P. multocida and H. somni. Compared to M. haemolytica and P. multocida, H. somni has previously been reported to be challenging to culture []. In the present study, failure to culture target organisms and subsequently detect macrolide resistance could have affected RPA specificity estimates. Another potential factor could be that AST was completed on a single, purified colony per species per sample, a standard practice in diagnostic testing and field studies [,,]. While necessary to manage logistics and cost, this approach also reflects how culture and AST are typically used to guide clinical decisions. However, testing only one colony presumes that all colonies of the same species on a plate share the same resistance profile [,]. This assumption could cause less prevalent, resistant subpopulations to be overlooked, particularly since the number of colonies needed to fully capture AMR diversity in a DNPS sample is not well defined [,,]. In this study, although AST could have missed the detection of rare, resistant isolates of target bacteria [,], the high estimated sensitivity of BC-AST for detecting macrolide resistance suggested that the impact of this testing strategy was limited.
Despite the potential challenges associated with the sensitivity of culture and AST, the estimated low specificity of RPA for detecting macrolide ARGs was derived from a three-test Bayesian model that also included qPCR data. As a result, any potential limitations to the sensitivity of BC-AST would have been expected to impact the estimated specificity of qPCR and RPA in a similar manner. However, the qPCR specificity estimated from the same three-test BLCM was almost identical to that of culture, and significantly higher than the specificity of RPA. Both RPA and PCR interrogate DNA extracted from an entire sample. Therefore, the ARGs detected could have also been present in BRD-associated bacteria beyond the cultured species, such as Bibersteinia trehalosi, or in other microbiota commonly present in the bovine respiratory tract and the environment, such as Moraxella spp. or Acinetobacter spp. [,,]. Interestingly, in the present study, the msrE-mphE operon was identified more frequently by RPA than msrE and/or mphE by qPCR in samples where no target Pasteurellaceae organisms were cultured (40% RPA versus 0% qPCR) and where macrolide-susceptible Pasteurellaceae were detected (41% RPA versus 4–6% qPCR).
Differences in RPA and qPCR assay performance might also reflect variations in primer design and assay thresholds. For example, the RPA primers targeted a highly conserved region of the msrE-mphE operon across Pasteurellaceae and other bacteria, whereas the validated PCR primers [], specifically for mphE, annealed to a region more prone to single nucleotide polymorphisms in certain, rare gene variants of M. haemolytica and H. somni. This could be reflected in the median sensitivity of the qPCR assay, which was determined using the same DNA, but was estimated to be slightly lower than the sensitivity of RPA and AST.
These findings highlight a key diagnostic challenge: neither RPA nor qPCR can directly link detected ARGs to specific bacterial species of interest when applied to whole-sample DNA. To explore whether restricting interpretation of results could improve clinical relevance, macrolide ARG results were considered only in samples where the corresponding assay (RPA or qPCR) also detected M. haemolytica, P. multocida, or H. somni. This adjustment modestly increased the specificity of RPA but at the cost of a marked reduction in sensitivity. While these results supported the possibility that RPA detected macrolide ARGs in non-BRD-related bacteria, they also underscore the need for diagnostic tools that can resolve whether ARGs are associated with clinically relevant pathogens versus non-target microbiota.
Recognizing that some uncertainty remains in explaining why RPA detected msrE-mphE in samples missed by both BC-AST and qPCR, the Sanger sequencing data supporting these RPA-positive results was compelling. A possible contributing factor not evaluated in the present study was the physical configuration of the RPA reaction strip tube, which could allow microdroplet dispersal and cross-contamination between tubes in the T16-ISO instrument []. Although the tube containing the NTC was closed following the addition of the reaction mastermix, the other tubes typically remain open unless lids are cut and individually closed, creating a separate potential for contamination. While no identified publications have directly addressed this issue, Crannell et al. reported a similar, isolated issue during the development of an RPA-lateral flow assay for detection of Cryptosporidium spp. [].
Contrary to the detection of msrE-mphE, the infrequent detection of erm42 by RPA was less reliable. Sanger sequencing confirmed only a subset of results, with four of eight RPA-positive samples identified as false positive and one false negative. The low prevalence of erm42 within the samples from this investigation made it difficult to evaluate assay performance, but was not surprising, as it was consistent with previous metagenomic and whole-genome sequencing studies of calf DNPS that identified abundant msrE and mphE, but no erm42 [,]. Some other studies using PCR and whole-genome sequencing have identified a higher frequency of erm42 as well as other ARGs harbored by Pasteurellaceae in both DNPS collected at feedlot arrival and pneumonic lung samples from BRD mortalities [,,].
The interpretation of ARGs detected in this study was supported by parallel detection of ICEs by RPA. ICEs are known to harbor and transmit ARGs horizontally between members of the BRD bacterial complex, as well as bacteria unrelated to BRD []. In the present study, detection of at least one ICE variant was associated with the detection of msrE-mphE, but only within samples that were RPA-positive for one of the BRD bacteria. Previous investigations have reported macrolide resistance genes, along with other ARGs within ICE variants in BRD-associated bacteria [,,,,,,,]. However, DNA-target-based test methods like RPA and qPCR cannot link ICEs or ARGs to specific bacteria within a sample, and testing is limited to known ARG targets. In this study, five samples contained at least one of the key BRD-associated bacterial targets that were phenotypically resistant to macrolides, but msrE-mphE or erm42 were not detected by RPA. In this case, it is possible that resistance might instead have been facilitated by genes that were recently discovered or are yet to be identified. For example, Dhindwal et al. identified estT, a previously undescribed macrolide esterase that hydrolyzes and inactivates macrolide antimicrobials commonly used in veterinary medicine (e.g., tylosin, tilmicosin, tildipirosin) [,,]. As more ARGs are detected, additional RPA targets will be needed to maximize testing efficiency.
During assay design, the study could have also been limited by the need to alter the fluorescence threshold for detection of msrE-mphE and erm42. While previous studies by Conrad et al. used a fluorescence threshold of ≥100–200 mV for ≥60 s for detection of BRD-related bacterial targets and ICEs using RPA, the real-time assay for macrolide ARG detection required a more stringent fluorescence threshold (≥400 mV for ≥60 s) to resolve low-level, false-positive signals [,]. While raising the fluorescence threshold to mitigate false positives could have impacted the proportion of false-negative samples, the high sensitivity of the RPA assay (95%) suggests that this would have a negligible impact.
The low clinical sensitivity of RPA for M. haemolytica, P. multocida, and H. somni detection resulted in a high risk of false-negative samples. Samples that tested negative for all BRD organisms were not tested for ICEs and would not be tested for ARGs either if the serial testing strategy was applied, resulting in a missed opportunity for downstream information on AMR determinants. For detection of P. multocida (29%) and H. somni (48%) a previous study by Conrad et al. found the sensitivity of RPA to be similarly low relative to culture []. This finding of low sensitivity for detection of BRD bacteria likely explained the substantial drop in sensitivity for msrE-mphE/erm42 detection when a serial clinical testing strategy was applied to the RPA results. It also further limits the future use of serial testing to target clinical decisions on ARG and ICE detection in samples positive for BRD bacteria.
However, for M. haemolytica, the interpretation of the BLCM was limited as the RPA assay targeted serotypes 1 and 6, while AST and qPCR were not specific to any serotype. M. haemolytica serotypes 1 and 6 have been identified most frequently in cattle with BRD []. Serotype 2 is more common in healthy cattle [,]. This target mismatch would not be expected to affect the estimated specificity of RPA relative to culture and qPCR. It could, however, explain in part the clinical sensitivity of RPA, which was estimated to be <40%; samples with target DNA from serotype 2 M. haemolytica would not be detected. This focus on targeting serotypes most often associated with BRD was intentional, but would fail to detect all M. haemolytica, thus impacting RPA assay sensitivity.
5. Conclusions
Despite certain limitations, RPA offers advantages of speed for individual assays, relative ease of use, and minimal equipment requirements, making it suitable for use in a dedicated workspace with DNA extraction capacity. The high clinical sensitivity of the msrE-mphE RPA assay supports its use as a rapid screening tool to rule out macrolide resistance in nasal swabs from high-risk cattle. The interpretation of a positive test result, however, might be limited by the broad range of bacteria not associated with BRD where these genes have been detected. The results of the Sanger sequencing support the detection of the targeted msrE-mphE by RPA, but not always in the targeted organisms.
Large-scale application would be constrained by current equipment configurations as, like all targeted assays, RPA cannot capture resistance mediated by ribosomal mutations, or novel or untargeted ARGs [,,]. As more genes and mechanisms of resistance are identified, expansion of assay panels to include emerging determinants, such as estT, will be essential. Additional studies applied to greater numbers of samples from different geographic areas will provide additional context around the generalizability of these tests and the estimates of their performance. Further work is also needed to evaluate the economics and practicality of multiplexing several RPA assays per sample in commercial feedlot settings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci12111079/s1. Table S1: Phenotypic resistance patterns of bacterial bovine respiratory disease pathogens Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolated from deep nasopharyngeal swabs, which were susceptible to macrolide antimicrobials (n = 68). Table S2: Results of Sanger sequencing of recombinase polymerase amplification DNA products for the alignment of associated primer and probe sets for msrE-mphE and erm42, compared to different combinations of results of antimicrobial susceptibility testing and polymerase chain reaction (n = 48). Table S3: Summary of macrolide resistance genes detected by recombinase polymerase amplification and real-time polymerase chain reaction in DNA extracted from deep nasopharyngeal swabs (n = 199). Table S4: Select list of bacterial species identified by metagenomic sequencing to contain macrolide resistance genes msrE and/or mphE (preliminary findings). Figure S1: Alignment of recombinase polymerase amplification and polymerase chain reaction primers and probes for detection of macrolide resistance genes msrE and/or mphE.
Author Contributions
Conceptualization, T.F. and C.L.W.; methodology, T.F., C.C.C., R.Z., C.L.W. and T.A.M.; software, L.M.; validation, T.F., L.M. and C.L.W.; formal analysis, T.F., L.M. and C.L.W.; investigation, T.F. and C.C.C.; resources, C.L.W., R.Z. and T.A.M.; data curation, T.F., L.M. and C.L.W.; writing—original draft preparation, T.F., L.M. and C.L.W.; writing—review and editing, T.F., L.M., C.C.C., R.Z., S.J.G.O., C.L.W. and T.A.M.; visualization, T.F.; supervision, C.L.W. and T.A.M.; project administration, L.M., S.J.G.O. and C.L.W.; funding acquisition, T.F., R.Z., S.J.G.O., C.L.W. and T.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Beef Cattle Research Council (ANH.18.19), Beef Farmers of Ontario (20-04 WaldnerFunk), and the “Genomic ASSETS (Antimicrobial Stewardship Systems from Evidence-based Treatment Strategies) for Livestock” project funded by Genome Canada with support from Genome Prairie, Genome Alberta, Saskatchewan Agriculture Development Fund, and the University of Saskatchewan.
Institutional Review Board Statement
The animal research protocol for this project was approved by the University of Saskatchewan Animal Care Committee (AUP 20190069, 25 May 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to gratefully acknowledge Stacey Lacoste for providing her laboratory expertise and guidance as well as the many hours invested in sample handling and testing, and also Curtis Claassen and Christina Yevtushenko for completing RPA testing for bacterial BRD pathogens and ICE complexes. Thank you to Jennifer Abi Younes and Jayce Fossen for their efforts in sample collection, Jennifer Abi Younes for providing information on bacterial culture protocols, and to Janet Hill, Champika Fernando, and Darien Deschner for kindly providing access to their strains of M. haemolytica for RPA assay validation. Thank you to Karen Gesy for aiding in sample handling, and for providing her valued expertise. We acknowledge and appreciate the great contribution of the Prairie Diagnostic Services Inc. bacteriology team for their assistance with bacterial culture and antimicrobial susceptibility testing. Finally, the authors acknowledge the contributions of Brian Loy and Duan Loy of the Nebraska Veterinary Diagnostic Centre for facilitating sample testing and providing their expertise on PCR analysis.
Conflicts of Interest
The authors declare that this study was conducted in the absence of any financial or commercial relationships that could be construed as a potential conflict of interest.
References
- Andrés-Lasheras, S.; Jelinski, M.; Zaheer, R.; McAllister, T.A. Bovine Respiratory Disease: Conventional to Culture-Independent Approaches to Studying Antimicrobial Resistance in North America. Antibiotics 2022, 11, 487. [Google Scholar] [CrossRef]
- Klima, C.L.; Holman, D.B.; Cook, S.R.; Conrad, C.C.; Ralston, B.J.; Allan, N.; Anholt, R.M.; Niu, Y.D.; Stanford, K.; Hannon, S.J.; et al. Multidrug Resistance in Pasteurellaceae Associated With Bovine Respiratory Disease Mortalities in North America From 2011 to 2016. Front. Microbiol. 2020, 11, 606438. [Google Scholar] [CrossRef]
- Hilton, W.M. BRD in 2014: Where Have We Been, Where Are We Now, and Where Do We Want to Go? Anim. Health Res. Rev. 2014, 15, 120–122. [Google Scholar] [CrossRef]
- Booker, C.W. Bovine Respiratory Disease Treatment Failure: Definition and Impact. Anim. Health Res. Rev. 2020, 21, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Confer, A.W. Laboratory Test Descriptions for Bovine Respiratory Disease Diagnosis and Their Strengths and Weaknesses: Gold Standards for Diagnosis, Do They Exist? Can. Vet. J. 2012, 53, 754–761. [Google Scholar] [PubMed]
- Panciera, R.J.; Confer, A.W. Pathogenesis and Pathology of Bovine Pneumonia. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Erickson, N.; Gow, S.; West, K.; Lacoste, S.; Godson, D. Infection of Calves with In-Vivo Passaged Bovine Parainfluenza-3 Virus, Alone or in Combination with Bovine Respiratory Syncytial Virus and Bovine Coronavirus. Can. J. Vet. Res. 2020, 84, 163–171. [Google Scholar]
- Brault, S.A.; Hannon, S.J.; Gow, S.P.; Warr, B.N.; Withell, J.; Song, J.; Williams, C.M.; Otto, S.J.G.; Booker, C.W.; Morley, P.S. Antimicrobial Use on 36 Beef Feedlots in Western Canada: 2008–2012. Front. Vet. Sci. 2019, 6, 329. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Preventive Measures? Can. Vet. J. 2010, 51, 1351–1359. [Google Scholar]
- Ives, S.E.; Richeson, J.T. Use of Antimicrobial Metaphylaxis for the Control of Bovine Respiratory Disease in High-Risk Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Health Canada Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html (accessed on 31 January 2025).
- Snyder, E.R.; Alvarez-Narvaez, S.; Credille, B.C. Genetic Characterization of Susceptible and Multi-Drug Resistant Mannheimia haemolytica Isolated from High-Risk Stocker Calves Prior to and after Antimicrobial Metaphylaxis. Vet. Microbiol. 2019, 235, 110–117. [Google Scholar] [CrossRef]
- Stanford, K.; Zaheer, R.; Klima, C.; McAllister, T.; Peters, D.; Niu, Y.D.; Ralston, B. Antimicrobial Resistance in Members of the Bacterial Bovine Respiratory Disease Complex Isolated from Lung Tissue of Cattle Mortalities Managed with or without the Use of Antimicrobials. Microorganisms 2020, 8, 288. [Google Scholar] [CrossRef]
- Douthwaite, S.; Poehlsgaard, J. Macrolide Antibiotic Interaction and Resistance on the Bacterial Ribosome. Curr. Opin. Investig. Drugs 2003, 4, 140–148. [Google Scholar]
- Kadlec, K.; Michael, G.B.; Sweeney, M.T.; Brzuszkiewicz, E.; Liesegang, H.; Daniel, R.; Watts, J.L.; Schwarz, S. Molecular Basis of Macrolide, Triamilide, and Lincosamide Resistance in Pasteurella multocida from Bovine Respiratory Disease. Antimicrob. Agents Chemother. 2011, 55, 2475–2477. [Google Scholar] [CrossRef]
- Skinner, R.; Cundliffe, E.; Schmidt, F.J. Site of Action of a Ribosomal RNA Methylase Responsible for Resistance to Erythromycin and Other Antibiotics. J. Biol. Chem. 1983, 258, 12702–12706. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Update on Macrolide-Lincosamide-Streptogramin, Ketolide, and Oxazolidinone Resistance Genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef]
- de Assis, J.C.S.; Gonçalves, O.S.; Fernandes, A.S.; de Queiroz, M.V.; Bazzolli, D.M.S.; Santana, M.F. Genomic Analysis Reveals the Role of Integrative and Conjugative Elements in Plant Pathogenic Bacteria. Mob. DNA 2022, 13, 19. [Google Scholar] [CrossRef]
- Farghaly, M.; Hynes, M.F.; Nazari, M.; Checkley, S.; Liljebjelke, K. Examination of the Horizontal Gene Transfer Dynamics of an Integrative and Conjugative Element Encoding Multidrug Resistance in Histophilus somni. Can. J. Microbiol. 2023, 69, 123–135. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Alhamami, T.; Venter, H.; Veltman, T.; Carr, M.; Mollinger, J.; Trott, D.J.; Djordjevic, S.P. Identification and Evolution of ICE-PmuST394: A Novel Integrative Conjugative Element in Pasteurella multocida ST394. J. Antimicrob. Chemother. 2024, 79, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Klima, C.L.; Zaheer, R.; Cook, S.R.; Booker, C.W.; Hendrick, S.; Alexander, T.W.; McAllister, T.A. Pathogens of Bovine Respiratory Disease in North American Feedlots Conferring Multidrug Resistance via Integrative Conjugative Elements. J. Clin. Microbiol. 2014, 52, 438–448. [Google Scholar] [CrossRef]
- Bhatt, K.; Timsit, E.; Rawlyk, N.; Potter, A.; Liljebjelke, K. Integrative Conjugative Element ICEHs1 Encodes for Antimicrobial Resistance and Metal Tolerance in Histophilus somni. Front. Vet. Sci. 2018, 5, 153. [Google Scholar] [CrossRef]
- Volkov, S. WHO List of Medically Important Antimicrobials a Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024; ISBN 9789240084612.
- Lobato, I.M.; O’Sullivan, C.K. Recombinase Polymerase Amplification: Basics, Applications and Recent Advances. TrAC-Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.C.; Daher, R.K.; Stanford, K.; Amoako, K.K.; Boissinot, M.; Bergeron, M.G.; Alexander, T.; Cook, S.; Ralston, B.; Zaheer, R.; et al. A Sensitive and Accurate Recombinase Polymerase Amplification Assay for Detection of the Primary Bacterial Pathogens Causing Bovine Respiratory Disease. Front. Vet. Sci. 2020, 7, 208. [Google Scholar] [CrossRef]
- Nelson, M.M.; Waldron, C.L.; Bracht, J.R. Rapid Molecular Detection of Macrolide Resistance. BMC Infect. Dis. 2019, 19, 144. [Google Scholar] [CrossRef]
- Conrad, C.C.; Funk, T.; Andrés-Lasheras, S.; Yevtushenko, C.; Claassen, C.; Otto, S.J.G.; Waldner, C.; Zaheer, R.; McAllister, T.A. Improving the Detection of Integrative Conjugative Elements in Bovine Nasopharyngeal Swabs Using Multiplex Recombinase Polymerase Amplification. J. Microbiol. Methods 2024, 221, 106943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, H.; Wang, H. Use of a Recombinase Polymerase Amplification Commercial Kit for Rapid Visual Detection of Pasteurella multocida. BMC Vet. Res. 2019, 15, 154. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, G.; Wang, P.; Niu, X.; Liu, Q.; Zhang, S.; Gao, W.; Li, Y. Simultaneous Detection of Bovine Viral Diarrhea Virus (BVDV) and Bovine Herpesvirus 1 (BoHV-1) Using Recombinase Polymerase Amplification. Sci. Rep. 2024, 14, 10169. [Google Scholar] [CrossRef] [PubMed]
- Kostoulas, P.; Leontides, L.; Enøe, C.; Billinis, C.; Florou, M.; Sofia, M. Bayesian Estimation of Sensitivity and Specificity of Serum ELISA and Faecal Culture for Diagnosis of Paratuberculosis in Greek Dairy Sheep and Goats. Prev. Vet. Med. 2006, 76, 56–73. [Google Scholar] [CrossRef]
- Lewis, F.I.; Torgerson, P.R. A tutorial in estimating the prevalence of disease in humans and animals in the absence of a gold standard diagnostic. Emerg. Themes Epidemiol. 2012, 9, 9. [Google Scholar] [CrossRef]
- Deschner, D.; Voordouw, M.J.; Fernando, C.; Campbell, J.; Waldner, C.L.; Hill, J.E. Identification of Genetic Markers of Resistance to Macrolide Class Antibiotics in Mannheimia haemolytica Isolates from a Saskatchewan Feedlot. Appl. Environ. Microbiol. 2024, 90, e00502-24. [Google Scholar] [CrossRef]
- Klima, C.L.; Cook, S.R.; Zaheer, R.; Laing, C.; Gannon, V.P.; Xu, Y.; Rasmussen, J.; Potter, A.; Hendrick, S.; Alexander, T.W.; et al. Comparative Genomic Analysis of Mannheimia haemolytica from Bovine Sources. PLoS ONE 2016, 11, e0149520. [Google Scholar] [CrossRef]
- Beker, M.; Rose, S.; Lykkebo, C.A.; Douthwaite, S. Integrative and Conjugative Elements (ICEs) in Pasteurellaceae Species and Their Detection by Multiplex PCR. Front. Microbiol. 2018, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.B.; Kadlec, K.; Sweeney, M.T.; Brzuszkiewicz, E.; Liesegang, H.; Daniel, R.; Murray, R.W.; Watts, J.L.; Schwarz, S. ICEPmu1, an Integrative Conjugative Element (ICE) of Pasteurella multocida: Structure and Transfer. J. Antimicrob. Chemother. 2012, 67, 91–100. [Google Scholar] [CrossRef]
- Andrés-Lasheras, S.; Ha, R.; Zaheer, R.; Lee, C.; Booker, C.W.; Dorin, C.; Van Donkersgoed, J.; Deardon, R.; Gow, S.; Hannon, S.J.; et al. Prevalence and Risk Factors Associated With Antimicrobial Resistance in Bacteria Related to Bovine Respiratory Disease—A Broad Cross-Sectional Study of Beef Cattle at Entry Into Canadian Feedlots. Front. Vet. Sci. 2021, 8, 692646. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, S.; Gao, Y.; Zhang, P.; Mao, D.; Luo, Y. Macrolides Mediate Transcriptional Activation of the Msr(E)-Mph(E) Operon through Histone-like Nucleoid-Structuring Protein (HNS) and CAMP Receptor Protein (CRP). J. Antimicrob. Chemother. 2022, 77, 391–399. [Google Scholar] [CrossRef]
- Younes, J.A.; Ramsay, D.E.; Lacoste, S.; Deschner, D.; Hill, J.E.; Campbell, J.; Waldner, C.L. Changes in the Phenotypic Susceptibility of Mannheimia haemolytica Isolates to Macrolide Antimicrobials during the Early Feeding Period Following Metaphylactic Tulathromycin Use in Western Canadian Feedlot Calves. Can. Vet. J. 2022, 63, 920–928. [Google Scholar] [PubMed]
- Abi Younes, J.N.; Campbell, J.R.; Otto, S.J.G.; Gow, S.P.; Woolums, A.R.; Jelinski, M.; Lacoste, S.; Waldner, C.L. Variation in Pen-Level Prevalence of BRD Bacterial Pathogens and Antimicrobial Resistance Following Feedlot Arrival in Beef Calves. Antibiotics 2024, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.N.; Herman, E.K.; Abi Younes, J.; Ramsay, D.E.; Erikson, N.; Stothard, P.; Links, M.G.; Otto, S.J.G.; Waldner, C. Evaluating the Potential of Third Generation Metagenomic Sequencing for the Detection of BRD Pathogens and Genetic Determinants of Antimicrobial Resistance in Chronically Ill Feedlot Cattle. BMC Vet. Res. 2022, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Gow, S.; Bergen, R.; Booker, C.; Butters, A.; Dorin, C.; Dimmers, G.; Erickson, N.; Hannon, S.; Hendrick, S.; Ramsay, D.; et al. National Surveillance of Antimicrobial Use and Antimicrobial Resistance in Canadian Feedlots. In Proceedings of the AABP 54th Annual Conference; American Association of Bovine Practitioners, Salt Lake City, UT, USA, 7–9 October 2021; Volume 54. Available online: https://bovine-ojs-tamu.tdl.org/AABP/article/view/8291 (accessed on 1 October 2025).
- Klima, C.L.; Alexander, T.W.; Read, R.R.; Gow, S.P.; Booker, C.W.; Hannon, S.; Sheedy, C.; McAllister, T.A.; Selinger, L.B. Genetic Characterization and Antimicrobial Susceptibility of Mannheimia haemolytica Isolated from the Nasopharynx of Feedlot Cattle. Vet. Microbiol. 2011, 149, 390–398. [Google Scholar] [CrossRef]
- Loy, J.D.; Leger, L.; Workman, A.M.; Clawson, M.L.; Bulut, E.; Wang, B. Development of a Multiplex Real-Time PCR Assay Using Two Thermocycling Platforms for Detection of Major Bacterial Pathogens Associated with Bovine Respiratory Disease Complex from Clinical Samples. J. Vet. Diagn. Investig. 2018, 30, 837–847. [Google Scholar] [CrossRef]
- Dutta, E.; Loy, J.D.; Deal, C.A.; Wynn, E.L.; Clawson, M.L.; Clarke, J.; Wang, B. Development of a Multiplex Real-Time PCR Assay for Predicting Macrolide and Tetracycline Resistance Associated with Bacterial Pathogens of Bovine Respiratory Disease. Pathogens 2021, 10, 64. [Google Scholar] [CrossRef]
- Herman, E.K.; Lacoste, S.R.; Freeman, C.N.; Otto, S.J.G.; McCarthy, E.L.; Links, M.G.; Stothard, P.; Waldner, C.L. Bacterial Enrichment Prior to Third-Generation Metagenomic Sequencing Improves Detection of BRD Pathogens and Genetic Determinants of Antimicrobial Resistance in Feedlot Cattle. Front. Microbiol. 2024, 15, 1386319. [Google Scholar] [CrossRef]
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), Vienna, Austria, 20–22 March 2003; Hornik, K., Leisch, F., Zeileis, A., Eds.; [Google Scholar]
- Denwood, M.J. Runjags: An R Package Providing Interface Utilities, Model Templates, Parallel Computing Methods and Additional Distributions for MCMC Models in JAGS. J. Stat. Softw. 2016, 71, 1–25. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Waldner, C. (University of Saskatchewan, Saskatoon, SK, Canada). Personal Communication, 2025.
- Dohoo, I.R.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER, Inc.: Charlottetown, PE, Canada, 2014; ISBN 9780919013605. [Google Scholar]
- Canadian Feedlot AMU/AMR Surveillance Program Injectable Antimicrobial Use (AMU) in Canadian Feedlot Cattle 2019–2022 (Veterinarians). Available online: https://cfaasp.ca/resources/cfaasp-resources/Injectable-Antimicrobial-Use-AMU-In-2022-in-Canadian-Feedlot-Cattle-2019-2022-Veterinarians (accessed on 27 March 2025).
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically Important Antibiotics: Criteria and Approaches for Measuring and Reducing Their Use in Food Animal Agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef]
- Kostoulas, P.; Nielsen, S.S.; Branscum, A.J.; Johnson, W.O.; Dendukuri, N.; Dhand, N.K.; Toft, N.; Gardner, I.A. STARD-BLCM: Standards for the Reporting of Diagnostic Accuracy Studies That Use Bayesian Latent Class Models. Prev. Vet. Med. 2017, 138, 37–47. [Google Scholar] [CrossRef]
- Gardner, I.A.; Colling, A.; Greiner, M. Design, Statistical Analysis and Reporting Standards for Test Accuracy Studies for Infectious Diseases in Animals: Progress, Challenges and Recommendations. Prev. Vet. Med. 2019, 162, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Capik, S.F.; White, B.J.; Lubbers, B.V.; Apley, M.D.; DeDonder, K.D.; Larson, R.L.; Harhay, G.P.; Chitko-McKown, C.G.; Harhay, D.M.; Kalbfleisch, T.S.; et al. Comparison of the Diagnostic Performance of Bacterial Culture of Nasopharyngeal Swab and Bronchoalveolar Lavage Fluid Samples Obtained from Calves with Bovine Respiratory Disease. Am. J. Vet. Res. 2017, 78, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Timsit, E.; Amat, S.; Abbott, D.W.; Buret, A.G.; Alexander, T.W. The Nasopharyngeal Microbiota of Beef Cattle before and after Transport to a Feedlot. BMC Microbiol. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Jamsen, K.; Arayawichanont, A.; Simpson, J.A.; White, L.J.; Lee, S.J.; Wuthiekanun, V.; Chantratita, N.; Cheng, A.; Day, N.P.J.; et al. Defining the True Sensitivity of Culture for the Diagnosis of Melioidosis Using Bayesian Latent Class Models. PLoS ONE 2010, 5, e12485. [Google Scholar] [CrossRef]
- Noyes, N.R.; Benedict, K.M.; Gow, S.P.; Booker, C.W.; Hannon, S.J.; Mcallister, T.A.; Morley, P.S. Mannheimia haemolytica in Feedlot Cattle: Prevalence of Recovery and Associations with Antimicrobial Use, Resistance, and Health Outcomes. J. Vet. Intern. Med. 2015, 29, 705–713. [Google Scholar] [CrossRef]
- Garzon, A.; Hoyos-Jaramillo, A.; Hustad, S.; Byrne, B.A.; Fritz, H.M.; Lehenbauer, T.W.; Aly, S.; Pereira, R. In Vitro Evaluation of the Effect of Transport Medium, Temperature, and Time on the Recovery of Mannheimia haemolytica and Pasteurella multocida. JDS Commun. 2023, 4, 214–218. [Google Scholar] [CrossRef]
- Van Driessche, L.; De Neve, C.; Haesebrouck, F.; Van Leenen, K.; Boyen, F.; Pardon, B. Storage Time and Temperature Affect the Isolation Rate of Mannheimia haemolytica and Pasteurella multocida from Bovine Bronchoalveolar Lavage Samples. BMC Vet. Res. 2020, 16, 238. [Google Scholar] [CrossRef]
- O’Toole, D.; Sondgeroth, K.S. Histophilosis as a Natural Disease. Curr. Top. Microbiol. Immunol. 2015, 351, 15–48. [Google Scholar] [CrossRef]
- Capik, S.F.; White, B.J.; Lubbers, B.V.; Apley, M.D.; Mosier, D.A.; Larson, R.L.; Murray, R.W. Characterization of Mannheimia haemolytica in Beef Calves via Nasopharyngeal Culture and Pulsed-Field Gel Electrophoresis. J. Vet. Diagn. Investig. 2015, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.F.; Wills, R.W.; Scott, M.A.; Thompson, A.C.; Singer, R.S.; Loy, J.D.; Karisch, B.B.; Epperson, W.B.; Woolums, A.R. Assessment of Diversity of Antimicrobial Resistance Phenotypes and Genotypes of Mannheimia haemolytica Isolates From Bovine Nasopharyngeal Swabs. Front. Vet. Sci. 2022, 9, 883389. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. North. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Capik, S.F.; Kegley, B.; Richeson, J.T.; Powell, J.G.; Zhao, J. Bovine Respiratory Microbiota of Feedlot Cattle and Its Association with Disease. Vet. Res. 2022, 53, 4. [Google Scholar] [CrossRef]
- McMullen, C.; Orsel, K.; Alexander, T.W.; van der Meer, F.; Plastow, G.; Timsit, E. Evolution of the Nasopharyngeal Bacterial Microbiota of Beef Calves from Spring Processing to 40 Days after Feedlot Arrival. Vet. Microbiol. 2018, 225, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Delphia, R.E.; Glidden, N.; Long, E.; Ellis, A.; Hoffman, S.; Mosier, K.; Ulloa, N.; Cheng, J.J.; Davidson, J.L.; Mohan, S.; et al. Nasal Pathobiont Abundance Is a Moderate Feedlot-Dependent Indicator of Bovine Respiratory Disease in Beef Cattle. Anim. Microbiome 2025, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Zaheer, R.; McAllister, T.A. Emerging Variants of the Integrative and Conjugant Element ICEMh1 in Livestock Pathogens: Structural Insights, Potential Host Range, and Implications for Bacterial Fitness and Antimicrobial Therapy. Front. Microbiol. 2019, 10, 2608. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J.; Von Stetten, F. Review: A Comprehensive Summary of a Decade Development of the Recombinase Polymerase Amplification. Analyst 2019, 144, 31–67. [Google Scholar] [CrossRef]
- Crannell, Z.A.; Castellanos-Gonzalez, A.; Irani, A.; Rohrman, B.; White, A.C.; Richards-Kortum, R. Nucleic Acid Test to Diagnose Cryptosporidiosis: Lab Assessment in Animal and Patient Specimens. Anal. Chem. 2014, 86, 2565–2571. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Klima, C.L.; Stanford, K.; Alexander, T.; Topp, E.; Read, R.R.; McAllister, T.A. Effect of Subtherapeutic vs. Therapeutic Administration of Macrolides on Antimicrobial Resistance in Mannheimia haemolytica and Enterococci Isolated from Beef Cattle. Front. Microbiol. 2013, 4, 133. [Google Scholar] [CrossRef]
- Eidam, C.; Poehlein, A.; Leimbach, A.; Michael, G.B.; Kadlec, K.; Liesegang, H.; Daniel, R.; Sweeney, M.T.; Murray, R.W.; Watts, J.L.; et al. Analysis and Comparative Genomics of ICEMh1, a Novel Integrative and Conjugative Element (ICE) of Mannheimia haemolytica. J. Antimicrob. Chemother. 2015, 70, 93–97. [Google Scholar] [CrossRef]
- Michael, G.B.; Kadlec, K.; Sweeney, M.T.; Brzuszkiewicz, E.; Liesegang, H.; Daniel, R.; Murray, R.W.; Watts, J.L.; Schwarz, S. ICEPmu1, an Integrative Conjugative Element (ICE) of Pasteurella multocida: Analysis of the Regions That Comprise 12 Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 84–90. [Google Scholar] [CrossRef]
- Dhindwal, P.; Thompson, C.; Kos, D.; Planedin, K.; Jain, R.; Jelinski, M.; Ruzzini, A. A Neglected and Emerging Antimicrobial Resistance Gene Encodes for a Serine-Dependent Macrolide Esterase. Proc. Natl. Acad. Sci. USA 2023, 120, e2219827120. [Google Scholar] [CrossRef]
- Dhindwal, P.; Myziuk, I.; Ruzzini, A. Macrolide Esterases: Current Threats and Opportunities. Trends Microbiol. 2023, 31, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lv, H.; Wang, T.; Tao, H.; Zhong, Y.; Zhou, Y.; Tang, Y.; Xie, F.; Zhuang, G.; Xu, C.; et al. The Global Distribution of the Macrolide Esterase EstX from the Alpha/Beta Hydrolase Superfamily. Commun. Biol. 2024, 7, 781. [Google Scholar] [CrossRef]
- Klima, C.L.; Alexander, T.W.; Hendrick, S.; McAllister, T.A. Characterization of Mannheimia haemolytica Isolated from Feedlot Cattle That Were Healthy or Treated for Bovine Respiratory Disease. Can. J. Vet. Res. 2014, 78, 38–45. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).