Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects
Abstract
Simple Summary
Abstract
1. Introduction
2. Oxidative Stress
2.1. Reactive Species
2.2. Endogenous Antioxidants
2.3. Imbalance between Free Radicals and Antioxidants
2.4. Biomarkers of Oxidative Stress
3. Factors for Oxidative Stress and Inflammation in Poultry: Secret Killers
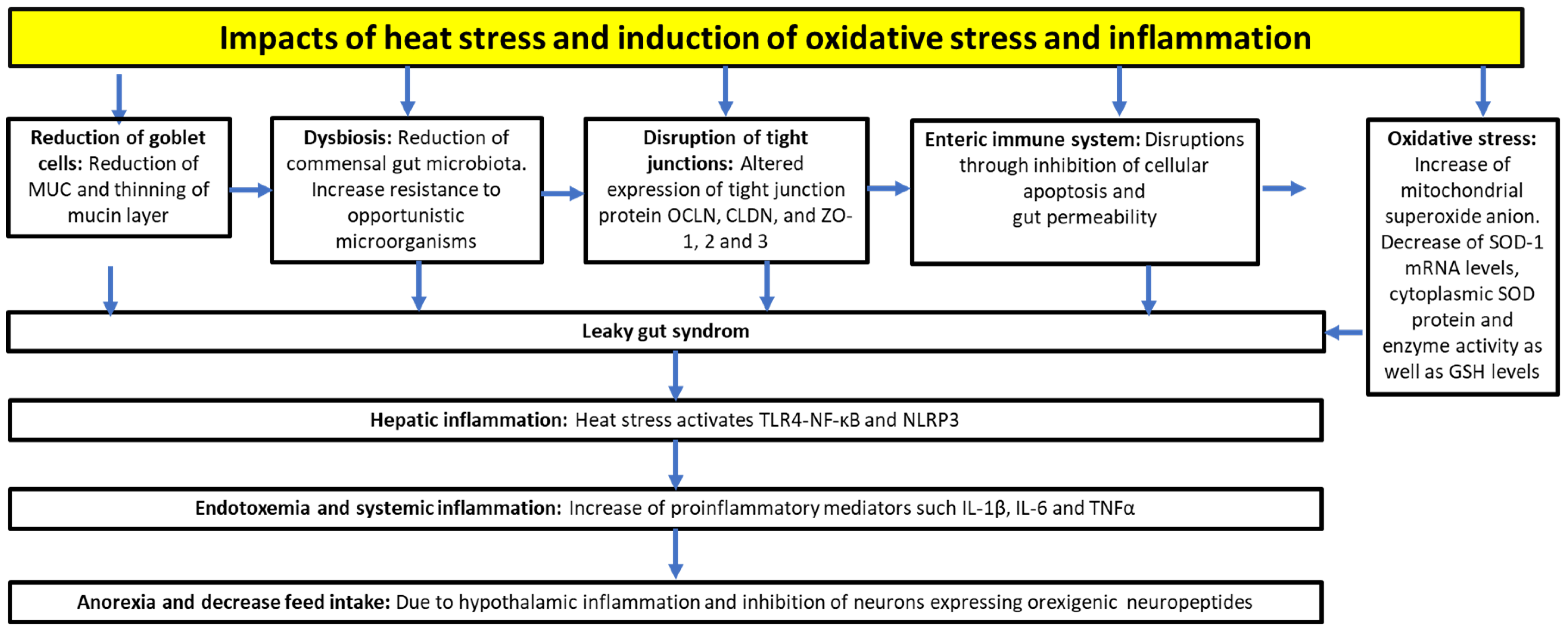
3.1. Heat Stress
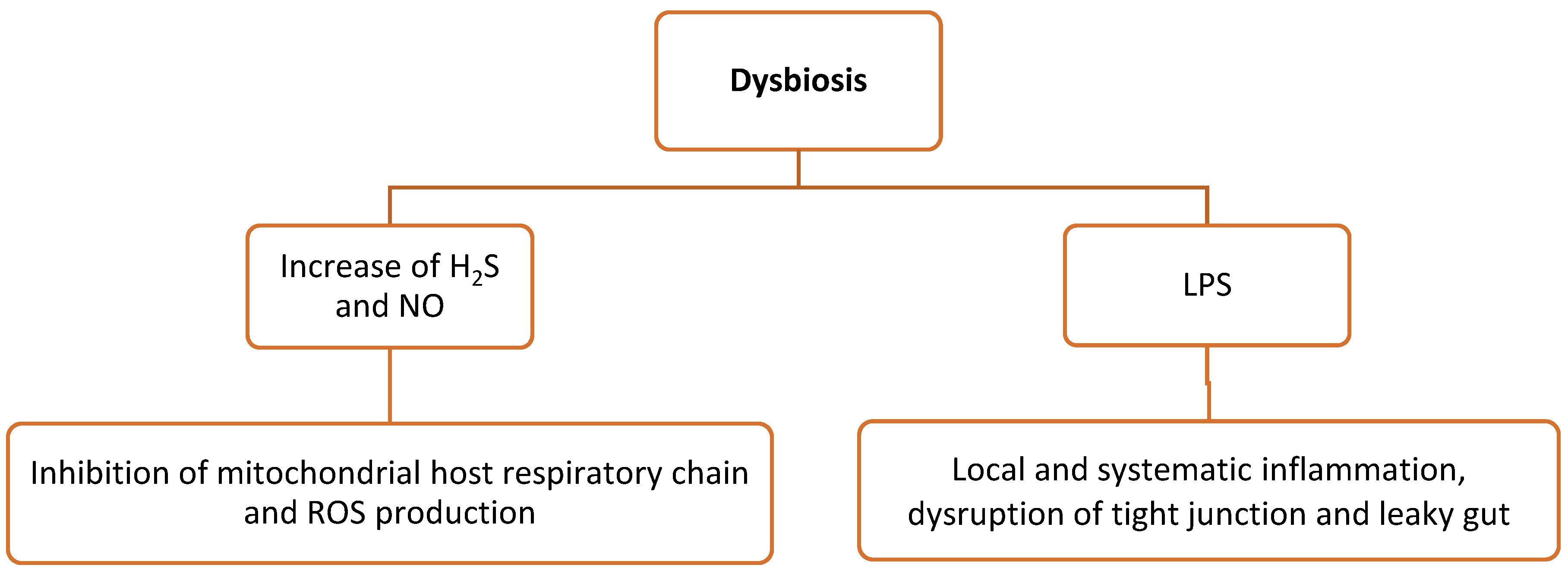
3.2. Dysbiosis
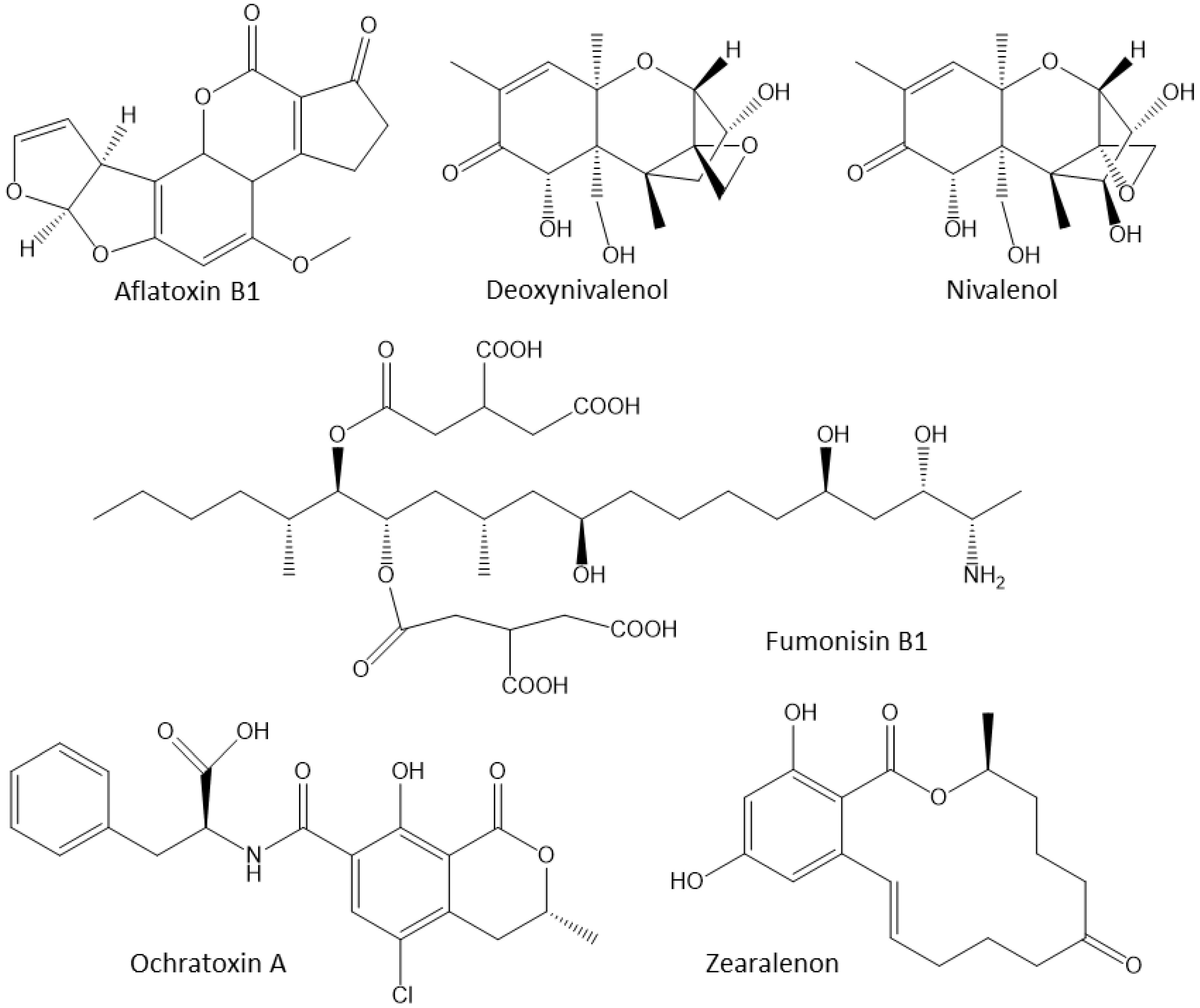
3.3. Mycotoxins
3.4. Diet-Mediated Oxidative Stress
4. Anti-Inflammatory Plants and Their Active Components
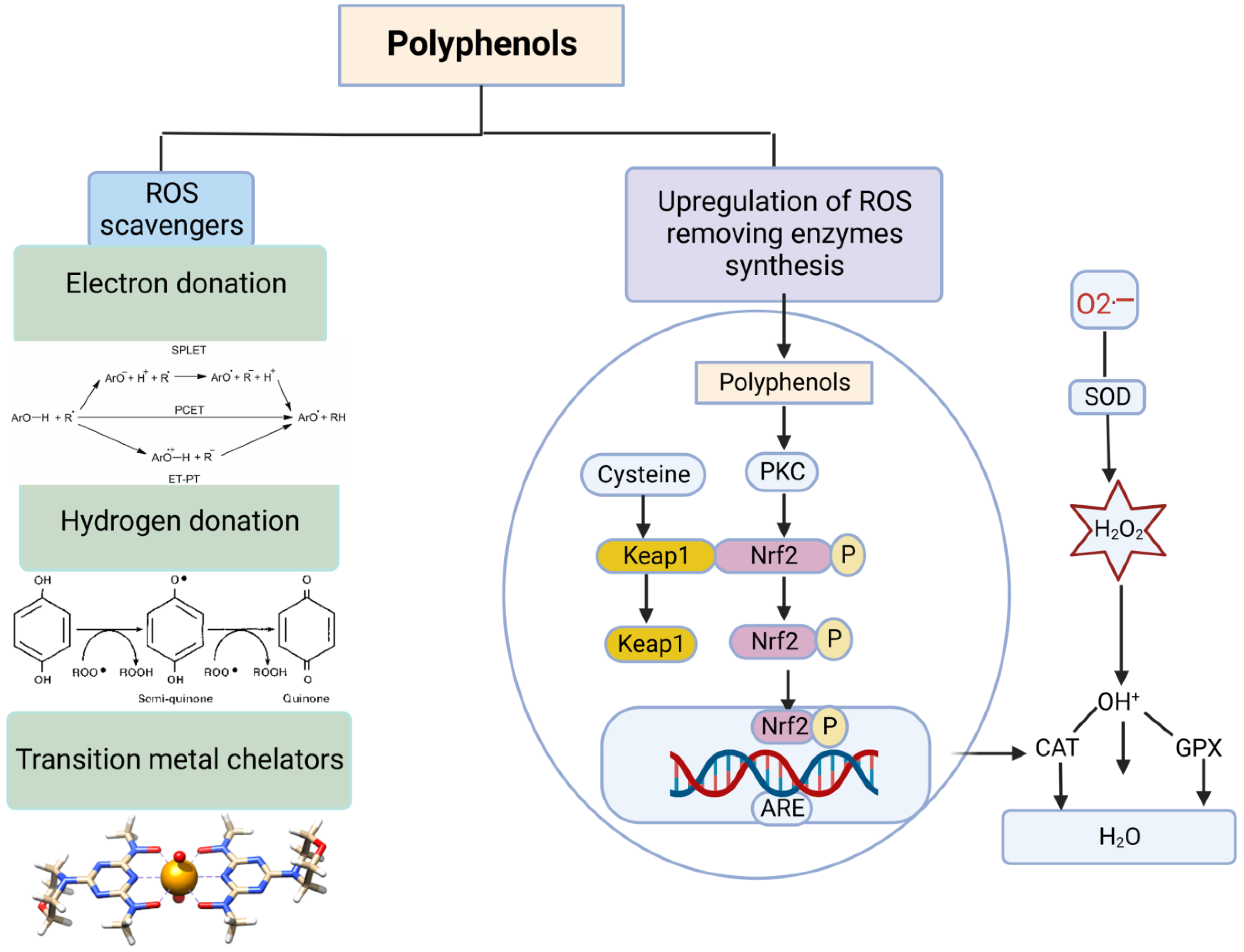
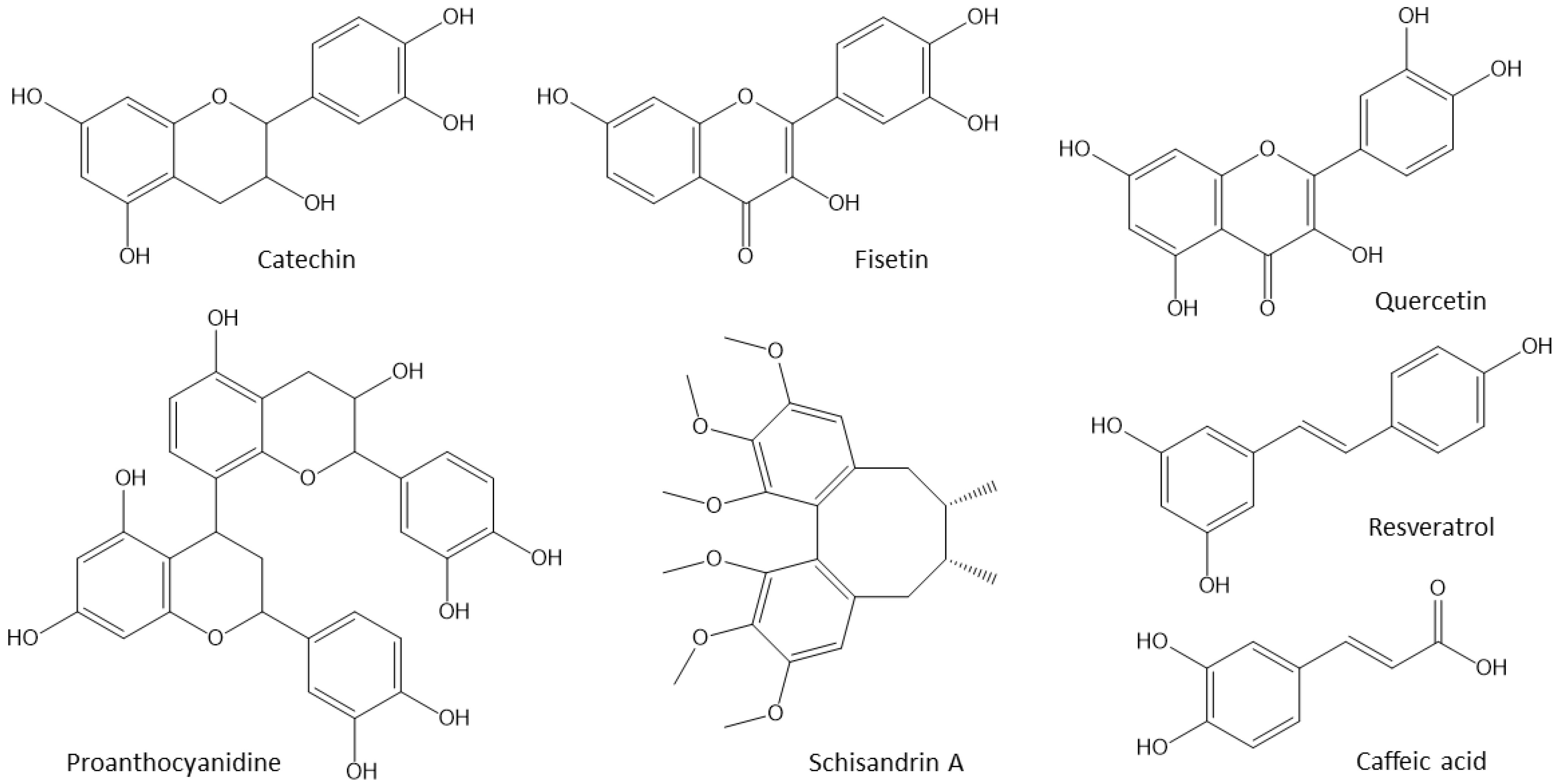
4.1. Polyphenols
| Antioxidant | Dose | Main Findings | Reference |
|---|---|---|---|
| Cinnamon bark essential oil | Commercial broilers supplemented with 300 mg/kg |
| [147] |
| Condensed tannins from grape seed extract | Commercial broilers supplemented with 125, 250, 500, 1000 and 2000 mg/kg for 42 days |
| [148] |
| Eucalytus leaves extract | Layers supplemented with 0.8 g/kg. Birds suffered from acute ethanol-induced oxidative damage conditions |
| [149] |
| Resveratrol from Polygonum cuspidatum | Heat-stressed broilers supplemented 350 and 500 mg/kg for seven days (from 28 to 42 days old) |
| [150] |
| Resveratrol | Heat-stressed commercial broiler supplemented with 0.2, 0.4 and 0.6 g/kg |
| [151] |
| Salix spp. | Heat-stressed commercial broilers supplemented with 0.025% and 0.05% in their diet |
| [152] |
| Turmeric rhizome extract | Commercial broilers supplemented with 0.1–0.3 g/kg |
| [153] |
| Grape Proanthocyanidins | Commercial broilers supplemented with 7.5, 15 and 30 mg/kg for 42 days |
| [154] |
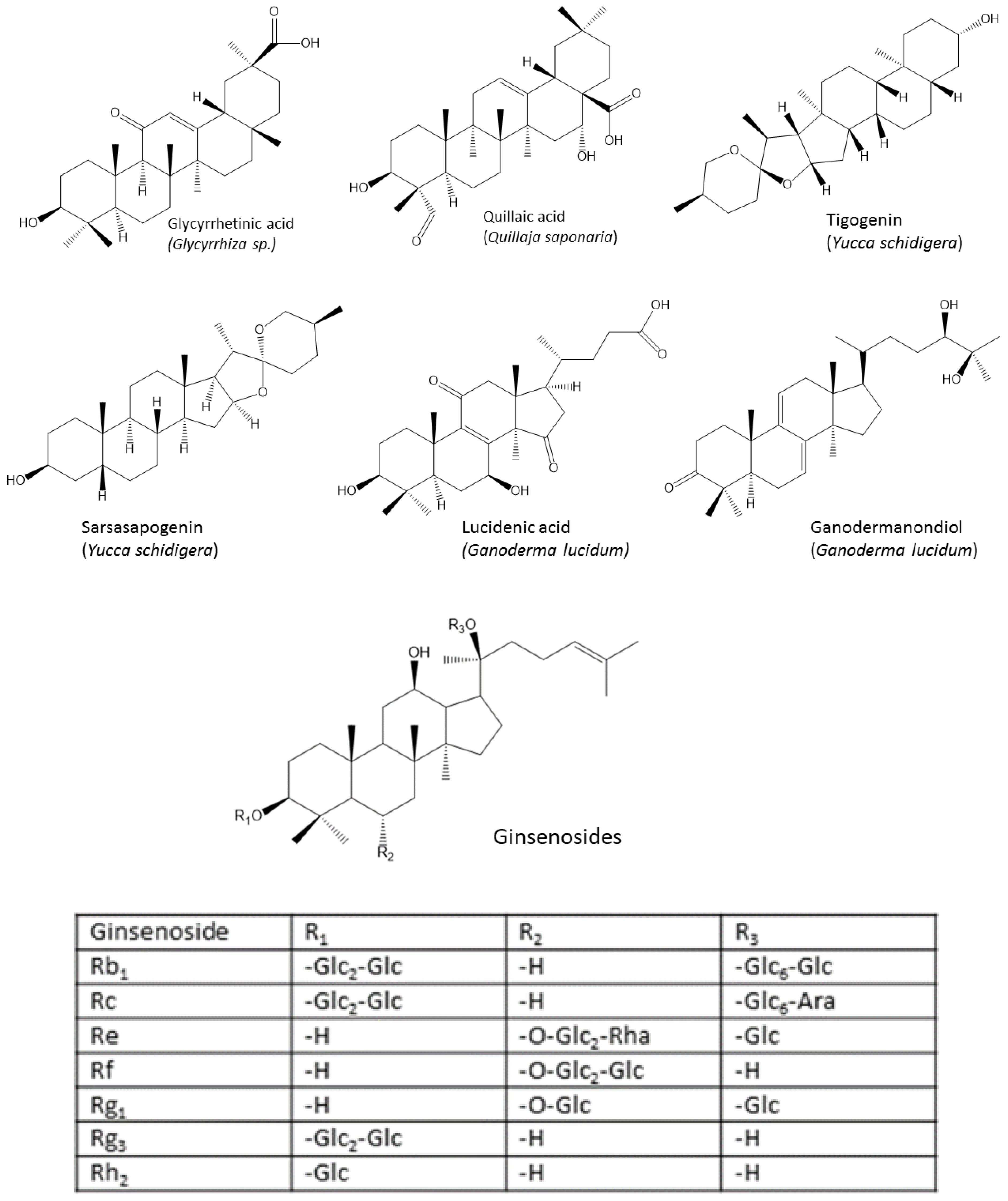
4.2. Triterpenes
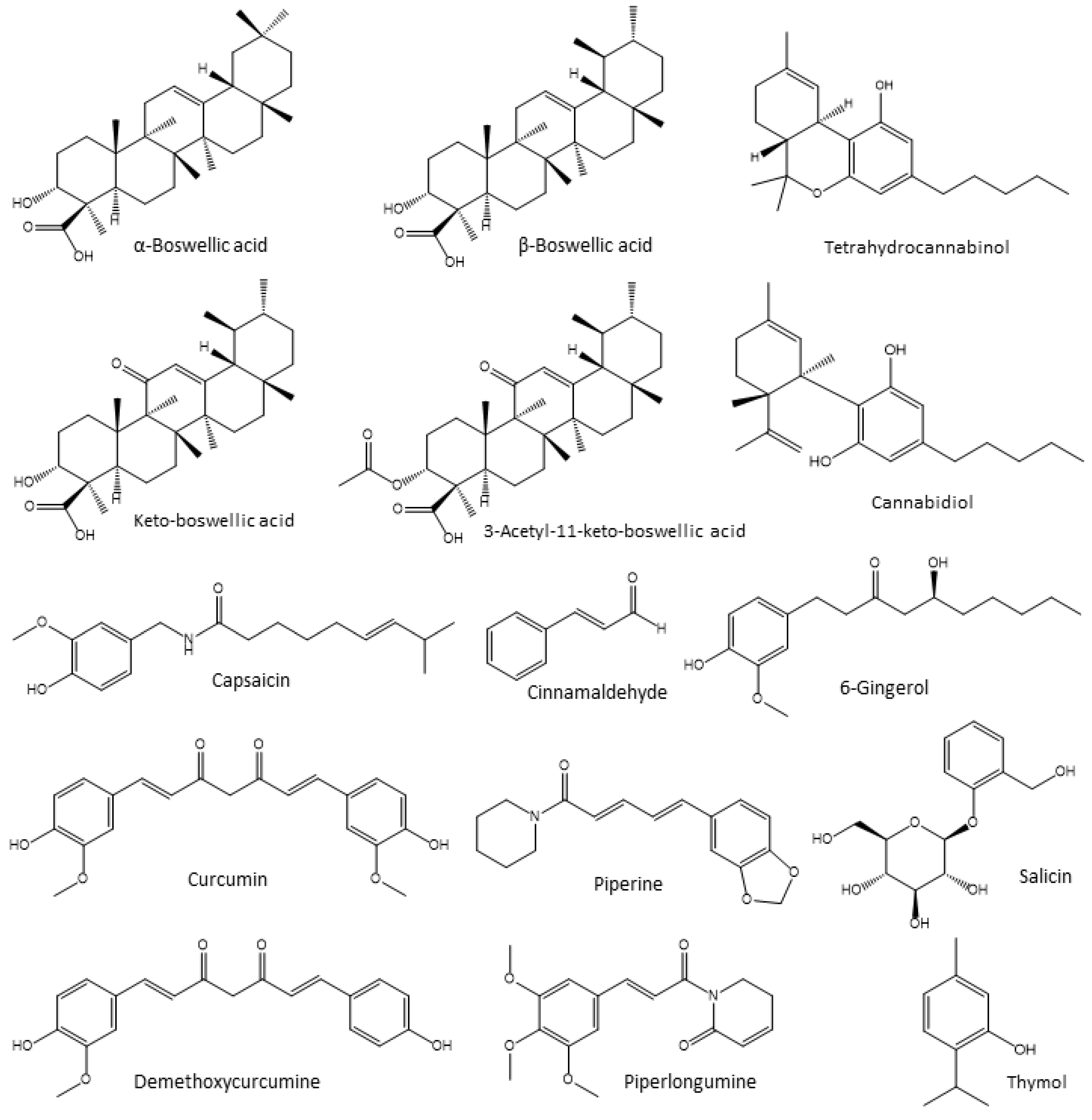
4.3. Anti-Inflammatory and Antioxidant Phytogenic Feed Additives Used in Poultry
4.3.1. Boswellia Extracts
4.3.2. Cannabis
4.3.3. Capsaicin
4.3.4. Cinnamaldehyde
4.3.5. Curcumin
4.3.6. Ginger Extracts
4.3.7. Piperamides
4.3.8. Salix Extracts
4.3.9. Thyme
5. Challenges and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M.E. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Miller, W.L. Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Maechler, P. Mitochondrial signal transduction in pancreatic β-cells. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-Imetabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Li, J.-M.; Shah, A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1014–R1030. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative Stress, Polyunsaturated Fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010, 72, 365–369. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mahesh, G.; Anil Kumar, K.; Reddanna, P. Overview on the discovery and development of anti-inflammatory drugs: Should the focus be on synthesis or degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86 (Suppl. S14), E140–E148. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic Compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Achilonu, M.C.; Umesiobi, D.O. Bioactive phytochemicals: Bioactivity, sources, preparations, and/or modifications via silver tetrafluoroborate mediation. J. Chem. 2015, 2015, 629085. [Google Scholar]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Ferro, E.; Goitre, L.; Retta, S.F.; Trabalzini, L. The Interplay between ROS and Ras GTPases: Physiological and pathological implications. J. Signal. Transduct. 2012, 2012, 365769. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as antioxidants for extending food shelf-life and in the prevention of health diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schröder, K. Nox Family NADPH Oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Handbook of Antioxidants for Food Preservation, 1st ed.; Woodhead Publishing: Hamilton, UK, 2015. [Google Scholar]
- Halliwell, B. Dietary Polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Hayes, J.D.; Strange, R.C. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic. Res. 1995, 22, 193–207. [Google Scholar] [CrossRef]
- Pickett, C.B.; Lu, A.Y. Glutathione S-transferases: Gene structure, regulation, and biological function. Annu. Rev. Biochem. 1989, 58, 743–764. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Retsky, K.L.; Freeman, M.W.; Frei, B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. anti- rather than prooxidant activity of vitamin c in the presence of transition metal ions. J. Biol. Chem. 1993, 268, 1304–1309. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Kaplowitz, N.; Fernández-Checa, J.C. Mitochondrial glutathione: Features, Regulation and role in disease. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3317–3328. [Google Scholar] [CrossRef]
- Matés, J.M.; Sánchez-Jiménez, F. Antioxidant enzymes and their implications in pathophysiologic processes. Front. Biosci. 1999, 4, D339–D345. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Q.; Jia, L.; Li, M.; Wang, X. Pinocembrin attenuates 6-ohda-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell. Mol. Neurobiol. 2015, 35, 323–333. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Farley, N.; Pedraza-Alva, G.; Serrano-Gomez, D.; Nagaleekar, V.; Aronshtam, A.; Krahl, T.; Thornton, T.; Rincón, M. P38 mitogen-activated protein kinase mediates the fas-induced mitochondrial death pathway in CD8+ T cells. Mol. Cell. Biol. 2006, 26, 2118–2129. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2016, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Blackwell, T.R.; Holden, E.P.; Christman, B.W.; Christman, J.W. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J. Immunol. 1996, 157, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-KappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Cai, D.; Khor, S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-KappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase Beta (IKKbeta)/NF-KappaB pathway. J. Biol. Chem. 2011, 286, 32324–32332. [Google Scholar] [CrossRef]
- Jones, S.V.; Kounatidis, I. Nuclear Factor-kappa B and alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Collins, A.R. Measuring oxidative damage to dna and its repair with the comet assay. Biochim. Biophys. Acta 2014, 1840, 794–800. [Google Scholar] [CrossRef]
- Tellez-Isaias, G.; Eisenreich, W.; Shehata, A.A. Nutraceuticals to mitigate the secret killers in animals. Vet. Sci. 2022, 9, 435. [Google Scholar] [CrossRef]
- Bickler, S.W.; Prieto, J.M.; Cauvi, D.M.; De Cos, V.; Nasamran, C.; Ameh, E.; Amin, S.; Nicholson, S.; Din, H.; Mocumbi, A.O.; et al. Differential Expression of nuclear genes encoding mitochondrial proteins from urban and rural populations in Morocco. Cell Stress Chaperones 2020, 25, 847–856. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Li, S.; Sun, X.; Wang, Y.; Shao, Y.; Hou, Z.; Xing, M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor dependent pathways in chicken immune organs. Oncotarget 2017, 8, 40327–40344. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.V. The human gutome: Nutrigenomics of the host-microbiome interactions. OMICS 2011, 15, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Xu, X.; Miwa, H. Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The microbiota-gut-brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef]
- Stecher, B. The Roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef]
- Lara, L.; Rostagno, M. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- ICAR-National Institute of Abiotic Stress Management; Pawar, S.S.; Basavaraj, S.; Dhansing, L.V.; Pandurang, K.N.; Sahebrao, K.A.; Vitthal, N.A.; Pandit, B.M.; Kumar, B.S. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016, 4, 332–341. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Mount, L.E. Heat transfer between animal and environment. Proc. Nutr. Soc. 1978, 37, 21–27. [Google Scholar] [CrossRef]
- Lasiewski, R. Physiological responses to heat stress in the poorwill. Am. J. Physiol.-Leg. Content 1969, 217, 1504–1509. [Google Scholar] [CrossRef]
- Ortega, A.D.S.V.; Szabó, C. Adverse effects of heat stress on the intestinal integrity and function of pigs and the mitigation capacity of dietary antioxidants: A review. Animals 2021, 11, 1135. [Google Scholar] [CrossRef]
- Yi, H.; Xiong, Y.; Wu, Q.; Wang, M.; Liu, S.; Jiang, Z.; Wang, L. Effects of Dietary supplementation with L-arginine on the intestinal barrier function in finishing pigs with heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1134–1143. [Google Scholar] [CrossRef]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.-R. The effect of heat stress on intestinal integrity and salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef]
- Mercer, E.H.; Singh, A.P. Endosymbiosis and cellular tolerance in the hawaiian soft coral sarcothelia edmondsoni verrill. Adv. Exp. Med. Biol. 1975, 64, 69–76. [Google Scholar]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Yu, Q.; Tang, C.; Xun, S.; Yajima, T.; Takeda, K.; Yoshikai, Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J. Immunol. 2006, 176, 6180–6185. [Google Scholar] [CrossRef]
- von Meyenburg, C.; Hrupka, B.H.; Arsenijevic, D.; Schwartz, G.J.; Landmann, R.; Langhans, W. Role for CD14, TLR2, and TLR4 in bacterial product-induced anorexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Altan, Ö.; Pabuçcuoğlu, A.; Altan, A.; Konyalioğlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B. Stress and its impact on farm animals. Front. Biosci. 2012, E4, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Tse, J.K.Y. Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447. [Google Scholar] [CrossRef]
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota–mitochondria inter-talk: Consequence for microbiota–host interaction. Pathog. Dis. 2016, 74, ftv096. [Google Scholar] [CrossRef]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr. Opin. Microbiol. 2020, 53, 78–89. [Google Scholar] [CrossRef]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across CACO-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin Improves intestinal barrier function: Modulation of Intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, 438–445. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef]
- Gilani, S.; Chrystal, P.V.; Barekatain, R. Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 2021, 7, 801–811. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Dickson, M.J.; Abuajamieh, M.; Snider, D.B.; Fernandez, M.V.S.; Johnson, J.S.; Keating, A.F.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; et al. Intentionally Induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 2017, 100, 4113–4127. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Fui, S.X.; Miao, C.H.; Feng, D.Y. Effects of different mycotoxin adsorbents on performance, meat characteristics and blood profiles of avian broilers fed mold contaminated corn. Asian Australas. J. Anim. Sci. 2005, 19, 72–79. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine: Effect of deoxynivalenol on gut health. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, J.; Borzemska, W.; Goliński, P.; Karpińska, E.; Szeleszczuk, P.; Celeda, T. The effect of ochratoxin A on egg quality, development of embryos and the level of toxinin eggs and tissues of hens and chicks. J. Anim. Feed Sci. 1994, 3, 309–316. [Google Scholar] [CrossRef]
- Longobardi, C.; Andretta, E.; Romano, V.; Lauritano, C.; Avantaggiato, G.; Schiavone, A.; Jarriyawattanachaikul, W.; Florio, S.; Ciarcia, R.; Damiano, S. Effects of some new antioxidants on apoptosis and ROS production in AFB1 treated chickens. Med. Sci. Forum 2020, 2, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W. Aflatoxin B1 Impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes: AFB1 on apoptosis and Nrf2 pathway in PBHs. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Wang, W.-J.; Xu, Z.-L.; Yu, C.; Xu, X.-H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017, 88, 1561–1568. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Fan, Y.; Zhao, L.; Wei, H.; Ji, C.; Zhang, J. Molecular mechanisms of lipoic acid protection against aflatoxin b₁-induced liver oxidative damage and inflammatory responses in broilers. Toxins 2015, 7, 5435–5447. [Google Scholar] [CrossRef]
- Maurya, B.K.; Trigun, S.K. Fisetin modulates antioxidant enzymes and inflammatory factors to inhibit aflatoxin-B1 induced hepatocellular carcinoma in rats. Oxidative Med. Cell. Longev. 2016, 2016, 1972793. [Google Scholar] [CrossRef]
- da Silva, E.O.; Bracarense, A.P.F.L.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Mousa, S.A.; Abdel-Raheem, S.M.; Abdel-Raheem, H.A.; Sadeek, A.L.S. Effect of dietary fat sources and antioxidant types on growth performance and carcass quality of japanese quails. Int. J. Poult. Sci. 2017, 16, 443–450. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Abo El-Maaty, H.M. The effects of different oil sources on performance, digestive enzymes, carcass traits, biochemical, immunological, antioxidant, and morphometric responses of broiler chicks. Front. Vet. Sci. 2020, 7, 181. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and omega-6 Fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573. [Google Scholar] [CrossRef]
- Labuza, L.R.; Dugan, J.R. Kinetics of lipid oxidation in foods critical reviews in food science and nutrition. Crit. Rev. Food Sci. Nutr. 1971, 2, 355–405. [Google Scholar]
- St. Angelo, A.J. (Ed.) Lipid Oxidation in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 500, ISBN 978-0-8412-2461-2. [Google Scholar]
- Goicoechea, E.; Brandon, M.H.; Blokland, M.D. Guillén Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem. Toxicol. 2011, 49, 115–124. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Volatile compounds generated in corn oil stored at room temperature. presence of toxic compounds. Eur. J. Lipid Sci. Technol. 2014, 116, 395–406. [Google Scholar] [CrossRef]
- Grigorakis, K.; Giogios, I.; Vasilaki, A.; Nengas, I. Effect of the fish oil, oxidation status and of heat treatment temperature on the volatile compounds of the produced fish feeds animal feed science and technology. Anim. Feed Sci. Technol. 2010, 158, 73–84. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Zribi, A.; Mansour, A.B.; Bouaziz, M. Effect of deep-frying on 3-MCPD esters and glycidyl esters contents and quality control of refined olive pomace blended with refined palm oil. Eur. Food Res. Technol. 2017, 243, 1219–1227. [Google Scholar] [CrossRef]
- Takahashi, K.; Akiba, Y. Effect of oxidized fat on perfor mance and some physiological responses in broiler chickens. Jpn. Poult. Sci. 1999, 36, 304–310. [Google Scholar] [CrossRef]
- Anjum, M.I.; Mirza, I.H.; Khan, A.G.; Azim, A. Effect of fresh versus oxidized soybean oil on growth performance, or gans weights and meat quality of broiler chicks. Pakistan Vet. J. 2004, 24, 173–178. [Google Scholar]
- Tavárez, M.A.; Boler, D.D.; Bess, K.N.; Zhao, J.; Yan, F.; Dilger, A.C.; McKeith, F.K.; Killefer, J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011, 90, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Boler, D.D.; Fernández-Dueñas, D.M.; Kutzler, L.W.; Zhao, J.; Harrell, R.J.; Campion, D.R.; McKeith, F.K.; Killefer, J.; Dilger, A.C. Effects of oxidized corn oil and a synthetic antioxidant blend on performance, oxidative status of tissues, and fresh meat quality in finishing barrows. J. Anim. Sci. 2012, 90, 5159–5169. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ashida, H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim. Biophys. Acta 1998, 1393, 349–361. [Google Scholar] [CrossRef]
- Engberg, R.M.; Lauridsen, C.; Jensen, S.K.; Jakobsen, K. Inclusion of oxidized vegetable oil in broiler diets. its influence on nutrient balance and on the antioxidative status of broilers. Poult. Sci. 1996, 75, 1003–1011. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, H.J.; Kim, I.H. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest. Sci. 2014, 160, 82–88. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary Inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Tellez-Isaias, G.; Latorre, J.D. Editorial: Alternatives to antimicrobial growth promoters and their impact in gut microbiota, health and disease: Volume II. Front. Vet. Sci. 2022, 9, 857583. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ros-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox Biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by Tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef]
- Singhal, S.S.; Awasthi, S.; Pandya, U.; Piper, J.T.; Saini, M.K.; Cheng, J.Z.; Awasthi, Y.C. The effect of curcumin on glutathione-linked enzymes in K562 human leukemia cells. Toxicol. Lett. 1999, 109, 87–95. [Google Scholar] [CrossRef]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J. Am. Coll. Nutr. 2009, 28, 492–499. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Budzyńska, A.; Różalska, B.; Sadowska, B. The immunomodulatory role of plant polyphenols. Postepy Hig. Med. Dosw. 2012, 66, 637–646. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidants in poultry nutrition and reproduction: An update. Antioxidants 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Naveed, M.; Arain, M.A.; Arif, M.; Abd El-Hack, M.E.; Alagawany, M.; Siyal, F.A.; Soomro, R.N.; Sun, C. Quercetin: Nutritional and beneficial effects in poultry. World’s Poult. Sci. J. 2017, 73, 355–364. [Google Scholar] [CrossRef]
- Silvestro, S.; Bramanti, P.; Mazzon, E. Role of quercetin in depressive-like behaviors: Findings from animal models. Appl. Sci. 2021, 11, 7116. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.-H.; Liu, M.; Zhang, N.-Y.; Khalil, M.M.; Gu, C.-Q.; Qi, D.-S.; Sun, L.-H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018, 148, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S. Different essential oils in diets of broiler chickens: 2. gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Farahat, M.H.; Abdallah, F.M.; Ali, H.A.; Hernandez-Santana, A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal 2017, 11, 771–777. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Li, W.; Miao, J.; Chen, N.; Shao, X.; Cao, Y. Polyphenols in Eucalyptus leaves improved the egg and meat qualities and protected against ethanol-induced oxidative damage in laying hens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 214–223. [Google Scholar] [CrossRef]
- He, S.; Li, S.; Arowolo, M.A.; Yu, Q.; Chen, F.; Hu, R.; He, J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019, 90, 401–411. [Google Scholar] [CrossRef]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Soica, C.; Tabuc, C.; Olteanu, M.; Predescu, C.; Rotar, M.C.; Criste, R.D. Use of a hydroalcoholic extract of Salix alba L. bark powder in diets of broilers exposed to high heat stress. S. Afr. J. Anim. Sci. 2019, 49, 942–954. [Google Scholar] [CrossRef]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of wenchang broiler chickens. Ital. J. Anim. Sci. 2015, 14, 3870. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Jiang, X.R.; Qi, G.H. Effects of dietary grape proanthocyanidins on the growth performance, jejunum morphology and plasma biochemical indices of broiler chicks. Animal 2017, 11, 762–770. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Afonso, C.L.; Miller, P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013, 41, 447–453. [Google Scholar] [CrossRef]
- Bafundo, K.W.; Johnson, A.B.; Mathis, G.F. The effects of a combination of quillaja saponaria and yucca schidigera on eimeria spp. in broiler chickens. Avian Dis. 2020, 64, 300–304. [Google Scholar] [CrossRef]
- Li, T.; Yu, H.; Song, Y.; Zhang, R.; Ge, M. Protective effects of ganoderma triterpenoids on cadmium-induced oxidative stress and inflammatory injury in chicken livers. J. Trace Elem. Med. Biol. 2019, 52, 118–125. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: Evidence from studies with heat-stressed Caco-2 cells, c. elegans and growing broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef]
- Lai, M.M.C.; Zhang, H.A.; Kitts, D.D. Ginseng prong added to broiler diets reduces lipid peroxidation in refrigerated and frozen stored poultry meats. Molecules 2021, 26, 4033. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, G.-D.; Choi, I.-H. Effects of dietary supplementation of red ginseng marc and α-tocopherol on the growth performance and meat quality of broiler chicken: Effects of red ginseng marc and α-tocopherol. J. Sci. Food Agric. 2014, 94, 1816–1821. [Google Scholar] [CrossRef]
- Song, Z.; Xie, K.; Zhang, Y.; Xie, Q.; He, X.; Zhang, H. Effects of dietary ginsenoside rg1 supplementation on growth performance, gut health, and serum immunity in broiler chickens. Front. Nutr. 2021, 8, 705279. [Google Scholar] [CrossRef]
- Chung, T.; Choi, I. Growth performance and fatty acid profiles of broilers given diets supplemented with fermented red ginseng marc powder combined with Red Koji. Rev. Bras. Cienc. Avic. 2016, 18, 733–738. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Wang, W.; Duan, T.; Yin, N.; Guo, T.; Guo, H.; Liu, N.; An, X.; Qi, J. Effects of Taraxacum Mongolicum Hand.-Mazz. (Dandelion) on growth performance, expression of genes coding for tight junction protein and mucin, microbiota composition and short chain fatty acids in ileum of broiler chickens. BMC Vet. Res. 2022, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Yener, Y.; Yalçin, S.; Çolpan, İ. Effects of dietary supplementation of red ginseng root powder on performance, immune system, cecal microbial population and some blood parameters in broilers. Ank. Üniversitesi Vet. Fakültesi Derg. 2020, 68, 137–145. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Sivakumar, N.; Asadi, H.; Chang-Chien, A.; Qoronfleh, M.W.; Ojcius, D.M.; Essa, M.M. Effects of frankincense compounds on infection, inflammation, and oral health. Molecules 2022, 27, 4174. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Attia, A.I.; Reda, F.M.; Abd El-Hack, M.E.; Ismail, I.E. Impacts of dietary supplementation of boswellia serrata on growth, nutrients digestibility, immunity, antioxidant status, carcase traits and caecum microbiota of broilers. Ital. J. Anim. Sci. 2021, 20, 205–214. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.M.; Kiczorowska, B.; Samolińska, W.; Kowalczuk-Vasilev, E.; Kowalczyk-Pecka, D. The effect of boswellia serrata resin diet supplementation on production, hematological, biochemical and immunological parameters in broiler chickens. Animal 2017, 11, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Dalmonte, T.; Lupini, C.; Andreani, G.; Salaroli, R.; Quaglia, G.; Zannoni, A.; Scozzoli, M.; Forni, M.; Isani, G. Influence of dietary supplementation with boswellia serrata and salix alba on performance and blood biochemistry in free-range leghorn laying hens. Vet. Sci. 2022, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Szkopek, D.; Kinsner, M.; Fotschki, B.; Juśkiewicz, J.; Banach, J. Cannabis-derived cannabidiol and nanoselenium improve gut barrier function and affect bacterial enzyme activity in chickens subjected to C. perfringens challenge. Vet. Res. 2020, 51, 141. [Google Scholar] [CrossRef]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis glandular trichomes: A cellular metabolite factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Vispute, M.M.; Sharma, D.; Mandal, A.B.; Rokade, J.J.; Tyagi, P.K.; Yadav, A.S. Effect of dietary supplementation of hemp (Cannabis Sativa) and dill seed (Anethum Graveolens) on performance, serum biochemicals and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 525–533. [Google Scholar] [CrossRef]
- Zamljen, T.; Jakopič, J.; Hudina, M.; Veberič, R.; Slatnar, A. Influence of intra and inter species variation in chilies (Capsicum spp.) on metabolite composition of three fruit segments. Sci. Rep. 2021, 11, 4932. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, H.-W.; Lee, M.-K.; Kim, H.-J.; Kim, J.-B.; Choe, J.-S.; Lee, Y.-M.; Jang, H.-H. Antioxidant and Anti-inflammatory activities in relation to the flavonoids composition of pepper (Capsicum Annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Pérez-González, A.; Prejanò, M.; Russo, N.; Marino, T.; Galano, A. Capsaicin, a powerful •OH-inactivating ligand. Antioxidants 2020, 9, 1247. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, Y.; Tang, D.; Yang, H.; Liu, X. Structural and gelation properties of five polyphenols-modified pork myofibrillar protein exposed to hydroxyl radicals. LWT 2022, 156, 113073. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Pettigrew, J.E. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J. Anim. Sci. 2012, 90, 2774–2783. [Google Scholar] [CrossRef]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Meza-Ríos, A.; Zuñiga-Ramos, L.; Dominguez-Rosales, A.; Vazquez-Del Mercado, M.; Sanchez-Orozco, L.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin induces a protective effect on gastric mucosa along with decreased expression of inflammatory molecules in a gastritis model. J. Funct. Foods 2019, 59, 345–351. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, J.; He, T.F.; Liu, H.S.; Piao, X.S. Effects of natural capsicum extract on growth performance, nutrient utilization, antioxidant status, immune function, and meat quality in broilers. Poult. Sci. 2021, 100, 101301. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: From mechanism to clinical implications. Biosci. Rep. 2017, 37, BSR20170286. [Google Scholar] [CrossRef]
- Liu, J.G.; Xia, W.G.; Chen, W.; Abouelezz, K.F.M.; Ruan, D.; Wang, S.; Zhang, Y.N.; Huang, X.B.; Li, K.C.; Zheng, C.T.; et al. Effects of capsaicin on laying performance, follicle development, and ovarian antioxidant capacity in aged laying ducks. Poult. Sci. 2021, 100, 100901. [Google Scholar] [CrossRef]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A natural feed additive for poultry health and production—A review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid.-Based Complementary Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Pannee, C.; Wacharee, L.; Chandhanee, I. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 Cells. J. Adv. Pharm. Technol. Res. 2014, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Kim, D.K.; Bravo, D.M.; Lee, S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 2011, 5 (Suppl. S4), S34. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lee, K.W.; Park, M.S.; Bravo, D.; Lillehoj, E.P. Cinnamaldehyde enhances in vitro parameters of immunity and reduces in vivo infection against avian coccidiosis. Br. J. Nutr. 2011, 106, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Bober, Z.; Stępień, A.; Aebisher, D.; Ożog, Ł.; Bartusik-Aebisher, D. medicinal benefits from the use of black pepper, curcuma and ginger. Eur. J. Clin. Exp. Med. 2018, 16, 133–145. [Google Scholar] [CrossRef]
- Karami, M.; Alimon, A.R.; Sazili, A.Q.; Goh, Y.M.; Ivan, M. Effects of dietary antioxidants on the quality, fatty acid profile, and lipid oxidation of longissimus muscle in kacang goat with aging time. Meat Sci. 2011, 88, 102–108. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solís-Cruz, B.; Patrin Pontin, K.; Latorre, J.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Merino-Guzman, R.; Méndez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; et al. Evaluation of the dietary supplementation of a formulation containing ascorbic acid and a solid dispersion of curcumin with boric acid against salmonella enteritidis and necrotic enteritis in broiler chickens. Animals 2019, 9, 184. [Google Scholar] [CrossRef]
- Leyva-Diaz, A.A.; Hernandez-Patlan, D.; Solis-Cruz, B.; Adhikari, B.; Kwon, Y.M.; Latorre, J.D.; Hernandez-Velasco, X.; Fuente-Martinez, B.; Hargis, B.M.; Lopez-Arellano, R.; et al. Evaluation of curcumin and copper acetate against salmonella typhimurium infection, intestinal permeability, and cecal microbiota composition in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 23. [Google Scholar] [CrossRef]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.M.; Pontin, K.P.; Latorre, J.D.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Arreguin, M.A.; Hargis, B.M.; et al. Evaluation of a Bacillus-based direct-fed microbial on aflatoxin b1 toxic effects, performance, immunologic status, and serum biochemical parameters in broiler chickens. Avian Dis. 2019, 63, 659–669. [Google Scholar] [CrossRef]
- Petrone-Garcia, V.M.; Lopez-Arellano, R.; Patiño, G.R.; Rodríguez, M.A.C.; Hernandez-Patlan, D.; Solis-Cruz, B.; Hernandez-Velasco, X.; Alba-Hurtado, F.; Vuong, C.N.; Castellanos-Huerta, I.; et al. Curcumin reduces enteric isoprostane 8-Iso-PGF2α and prostaglandin GF2α in Specific pathogen-free leghorn chickens challenged with Eimeria maxima. Sci. Rep. 2021, 11, 11609. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Hong, Y.H.; Jang, S.I.; Lillehoj, E.P.; Ionescu, C.; Mazuranok, L.; Bravo, D. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br. Poult. Sci. 2010, 51, 213–221. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Effects of Capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Anim. Sci. 2014, 92, 3426–3440. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Lee, J.J.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection. J. Anim. Sci. 2014, 92, 2050–2062. [Google Scholar] [CrossRef]
- Liu, Y.; Che, T.M.; Song, M.; Lee, J.J.; Almeida, J.a.S.; Bravo, D.; Van Alstine, W.G.; Pettigrew, J.E. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2013, 91, 5668–5679. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.-Y.; Souza dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019, 98, 422–429. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crops Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.V.; Murthy, P.S.; Manjunatha, J.R.; Bettadaiah, B.K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World 2016, 9, 245–250. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.B.; Yang, W.R.; Wang, Y.; Jiang, S.Z.; Zhang, G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011, 90, 1720–1727. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Nakatani, N. Cyclic diarylheptanoids from rhizomes of Zingiber officinale. Phytochemistry 1996, 43, 273–277. [Google Scholar] [CrossRef]
- Fuhrman, B.; Rosenblat, M.; Hayek, T.; Coleman, R.; Aviram, M. Ginger extract consumption reduces plasma cholesterol, inhibits ldl oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J. Nutr. 2000, 130, 1124–1131. [Google Scholar] [CrossRef]
- An, S.; Liu, G.; Guo, X.; An, Y.; Wang, R. Ginger Extract Enhances Antioxidant Ability and Immunity of Layers. Anim. Nutr. 2019, 5, 407–409. [Google Scholar] [CrossRef]
- Karthikeyan, J.; Rani, P. Enzymatic and non-enzymatic antioxidants in selected piper species. Indian J. Exp. Biol. 2003, 41, 135–140. [Google Scholar]
- Abou-Elkhair, R.; Ahmed, H.A.; Selim, S. Effects of black pepper (Piper Nigrum), turmeric powder (Curcuma Longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J. Anim Sci. 2014, 27, 847–854. [Google Scholar] [CrossRef]
- Khalaf, N.A.; Shakya, A.; Al-Othman, A.; Elagbar, Z.; Farah, H.S. Antioxidant activity of some common plants. Turk. J. Biol. 2008, 32, 51–55. [Google Scholar]
- Reen, R.K.; Roesch, S.F.; Kiefer, F.; Wiebel, F.J.; Singh, J. Piperine impairs cytochrome P4501A1 activity by direct interaction with the enzyme and not by down regulation of CYP1A1 gene expression in the rat hepatoma 5L cell line. Biochem. Biophys. Res. Commun. 1996, 218, 562–569. [Google Scholar] [CrossRef]
- Malini, T.; Arunakaran, J.; Aruldhas, M.M.; Govindarajulu, P. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem. Mol. Biol. Int. 1999, 47, 537–545. [Google Scholar] [CrossRef]
- Moorthy, M.; Ravi, S.; Ravikumar, M.; Viswanathan, K.; Edwin, S.C. Ginger, pepper and curry leaf powder as feed additives in broiler diet. Int. J. Poult. Sci. 2009, 8, 779–782. [Google Scholar] [CrossRef]
- Reynoso-Moreno, I.; Najar-Guerrero, I.; Escareño, N.; Flores-Soto, M.E.; Gertsch, J.; Viveros-Paredes, J.M. An endocannabinoid uptake inhibitor from black pepper exerts pronounced anti-inflammatory effects in mice. J. Agric. Food Chem. 2017, 65, 9435–9442. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.-G.; Lee, M.M.Y.; Lee, Y.-M.; Bae, J.-S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef]
- Ginzburg, S.; Golovine, K.V.; Makhov, P.B.; Uzzo, R.G.; Kutikov, A.; Kolenko, V.M. Piperlongumine inhibits NF-ΚB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate 2014, 74, 177–186. [Google Scholar] [CrossRef]
- Ghazalah, A.A.; El-Hakim, A.S.A.; Refaie, A.M. Response of broiler chicks to some dietary growth promoters throughout different growth period. Egypt. Poult. Sci. J. 2007, 27, 53–57. [Google Scholar]
- Tollba, A.A.H.; Azouz, H.M.M.; El-Samad, A.M.H. Antioxidants supplementation to diet of egyptian chicken under different environmental condition: 2-The Growth during cold winter stress. Egypt. Poult. Sci. J. 2007, 27, 727–748. [Google Scholar]
- Mansoub, N.H. Comparison of using different level of black pepper with probiotic on performance and serum composition of broiler chickens. J. Basic Appl. Sci. Res. 2011, 1, 2425–2428. [Google Scholar]
- Akbarian, A. influence of turmeric rhizome and black pepper on blood constituents and performance of broiler chickens. Afr. J. Biotechnol. 2012, 11, 8606–8611. [Google Scholar] [CrossRef]
- Al-Kassie, G.A.M.; Butris, G.Y.; Ajeena, S.J. The potency of feed supplemented mixture of hot red pepper and black pepper on the performance and some hematological blood traits on broiler diet. Int. J. Adv. Biol. Res. 2012, 2, 53–57. [Google Scholar]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef] [PubMed]
- Al-fataftah, A.; Abdelqader, A. Effect of Salix babylonica, Populus nigra and Eucalyptus camaldulensis extracts in drinking water on performance and heat tolerance of broiler chickens during heat stress. Am.-Eurasian J. Agric. Environ. Sci. 2013, 10, 1309–1313. [Google Scholar]
- Panaite, T.D.; Saracila, M.; Papuc, C.P.; Predescu, C.N.; Soica, C. Influence of dietary supplementation of salix alba bark on performance, oxidative stress parameters in liver and gut microflora of broilers. Animals 2020, 10, 958. [Google Scholar] [CrossRef]
- Saracila, M.; Tabuc, C.; Panaite, T.D.; Papuc, C.P.; Olteanu, M.; Criste, R.D. Effect of the dietary willow bark extract (Salix alba) on the caecal microbial population of broilers (14–28 Days) reared at 32 °C. Agric. Life Life Agric. Conf. Proc. 2018, 1, 155–161. [Google Scholar] [CrossRef]
- Yalçin, S.; Eser, H.; Onbaşilar, İ.; Yalçin, S. Effects of dried thyme (Thymus vulgaris L.) leaves on performance, some egg quality traits and immunity in laying hens. Ank. Üniversitesi Vet. Fakültesi Derg. 2020. [Google Scholar] [CrossRef]
- Ocaña, A.; Reglero, G. Effects of thyme extract oils (from Thymus Vulgaris, Thymus Zygis and Thymus hyemalis) on cytokine production and gene expression of OxLDL-stimulated THP-1-macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef]
- Amirghofran, Z.; Ahmadi, H.; Karimi, M.H.; Kalantar, F.; Gholijani, N.; Malek-Hosseini, Z. In vitro inhibitory effects of thymol and carvacrol on dendritic cell activation and function. Pharm. Biol. 2016, 54, 1125–1132. [Google Scholar]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-ΚB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef]
- Hassan, F.A.M.; Awad, A. Impact of thyme powder (Thymus vulgaris L.) supplementation on gene expression profiles of cytokines and economic efficiency of broiler diets. Environ. Sci. Pollut. Res. Int. 2017, 24, 15816–15826. [Google Scholar] [CrossRef]
- Lee, K.W.; Everts, H.; Kappert, H.J.; Frehner, M.; Losa, R.; Beynen, A.C. Effects of Dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003, 44, 450–457. [Google Scholar] [CrossRef]
- Shehata, A.A.; Attia, Y.; Khafaga, A.F.; Farooq, M.Z.; El-Seedi, H.R.; Eisenreich, W.; Tellez-Isaias, G. Restoring healthy gut microbiome in poultry using alternative feed additives with particular attention to phytogenic substances: Challenges and prospects. Ger. J. Vet. Res. 2022, 2, 32–42. [Google Scholar] [CrossRef]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms: Polyphenols extending meat shelf-life. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets: In vitro degradation and in Vivo passage kinetics of essential oils in piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential oils as feed additives—Future perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, J.; Yu, H.; Guo, Q.; Defelice, C.; Hernandez, M.; Yin, Y.; Wang, Q. Alginate-whey protein dry powder optimized for target delivery of essential oils to the intestine of chickens. Poult. Sci. 2014, 93, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Zhu, W.-Y. Bioactivation of flavonoid diglycosides by chicken cecal bacteria. FEMS Microbiol. Lett. 2009, 295, 30–41. [Google Scholar] [CrossRef]

| Mycotoxin | Downregulation of Intracellular Antioxidants | Upregulation of Pro-Inflammatory Cytokines |
|---|---|---|
| AFB1 | Nrf2, CAT, GPx; SOD | Cytokines, NO; NO2 |
| DON | CAT, GPx; SOD | AP-1; ERK-MAPK |
| OTA | Nrf2, CAT, GPx; SOD | Fenton reaction |
| ZEN | CAT, GPx; SOD | CoX-2, cytokines; iNOS |
| T-2 | Nrf2, CAT, GPx, GPx; SOD | Cytokines, iNOS; NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalçın, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Vet. Sci. 2023, 10, 55. https://doi.org/10.3390/vetsci10010055
Basiouni S, Tellez-Isaias G, Latorre JD, Graham BD, Petrone-Garcia VM, El-Seedi HR, Yalçın S, El-Wahab AA, Visscher C, May-Simera HL, et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Veterinary Sciences. 2023; 10(1):55. https://doi.org/10.3390/vetsci10010055
Chicago/Turabian StyleBasiouni, Shereen, Guillermo Tellez-Isaias, Juan D. Latorre, Brittany D. Graham, Victor M. Petrone-Garcia, Hesham R. El-Seedi, Sakine Yalçın, Amr Abd El-Wahab, Christian Visscher, Helen L. May-Simera, and et al. 2023. "Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects" Veterinary Sciences 10, no. 1: 55. https://doi.org/10.3390/vetsci10010055
APA StyleBasiouni, S., Tellez-Isaias, G., Latorre, J. D., Graham, B. D., Petrone-Garcia, V. M., El-Seedi, H. R., Yalçın, S., El-Wahab, A. A., Visscher, C., May-Simera, H. L., Huber, C., Eisenreich, W., & Shehata, A. A. (2023). Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Veterinary Sciences, 10(1), 55. https://doi.org/10.3390/vetsci10010055












