Improving Fermentation Rate during Use of Corn Grits in Beverage Alcohol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corn Grits and Germ Composition
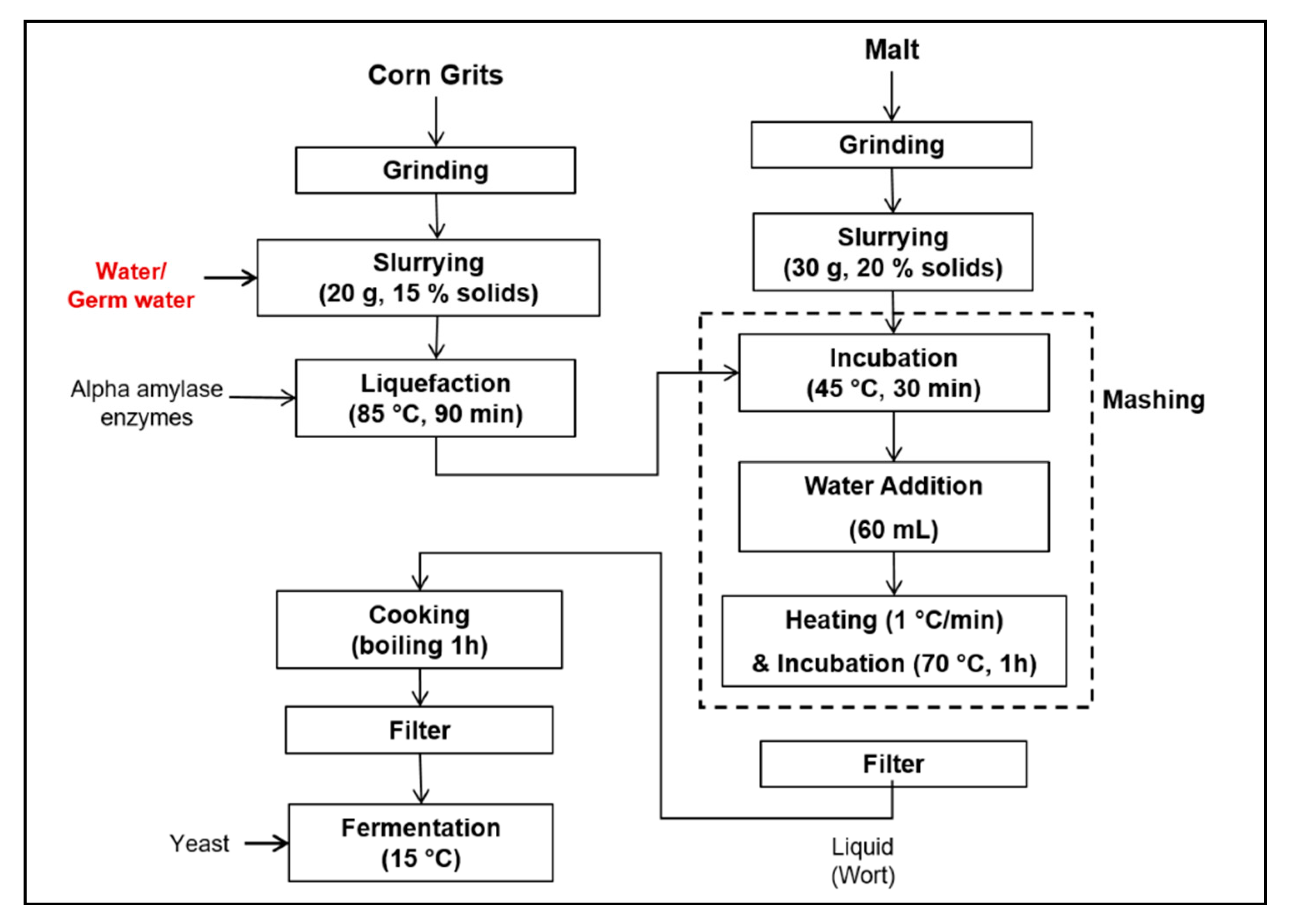
2.3. Brewing Process
2.4. Sample Analysis and Ethanol Production Rate
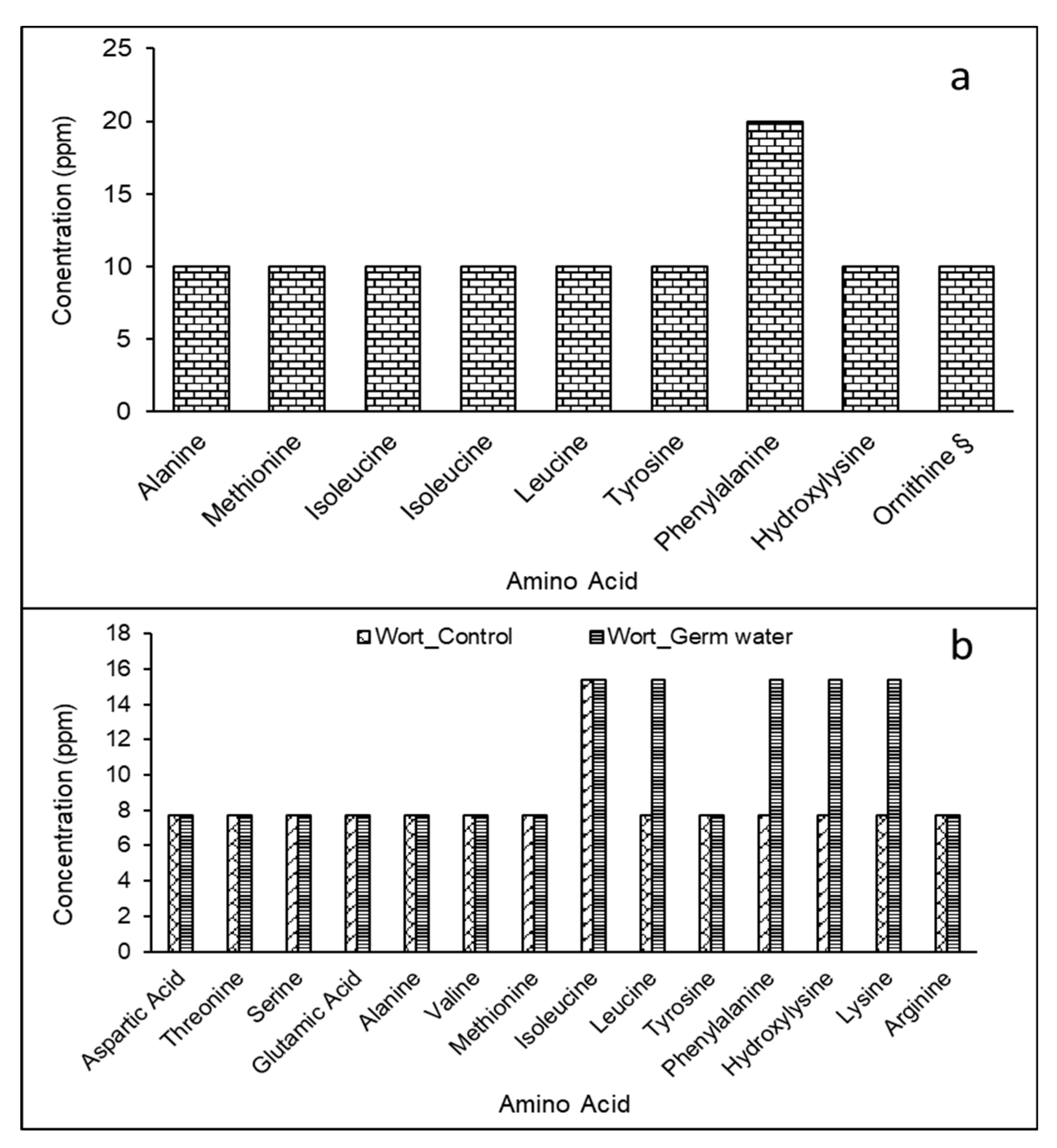
2.5. Total Free Amino Nitrogen and Amino Acid Profile
2.6. Zinc Determination
3. Results and Discussion
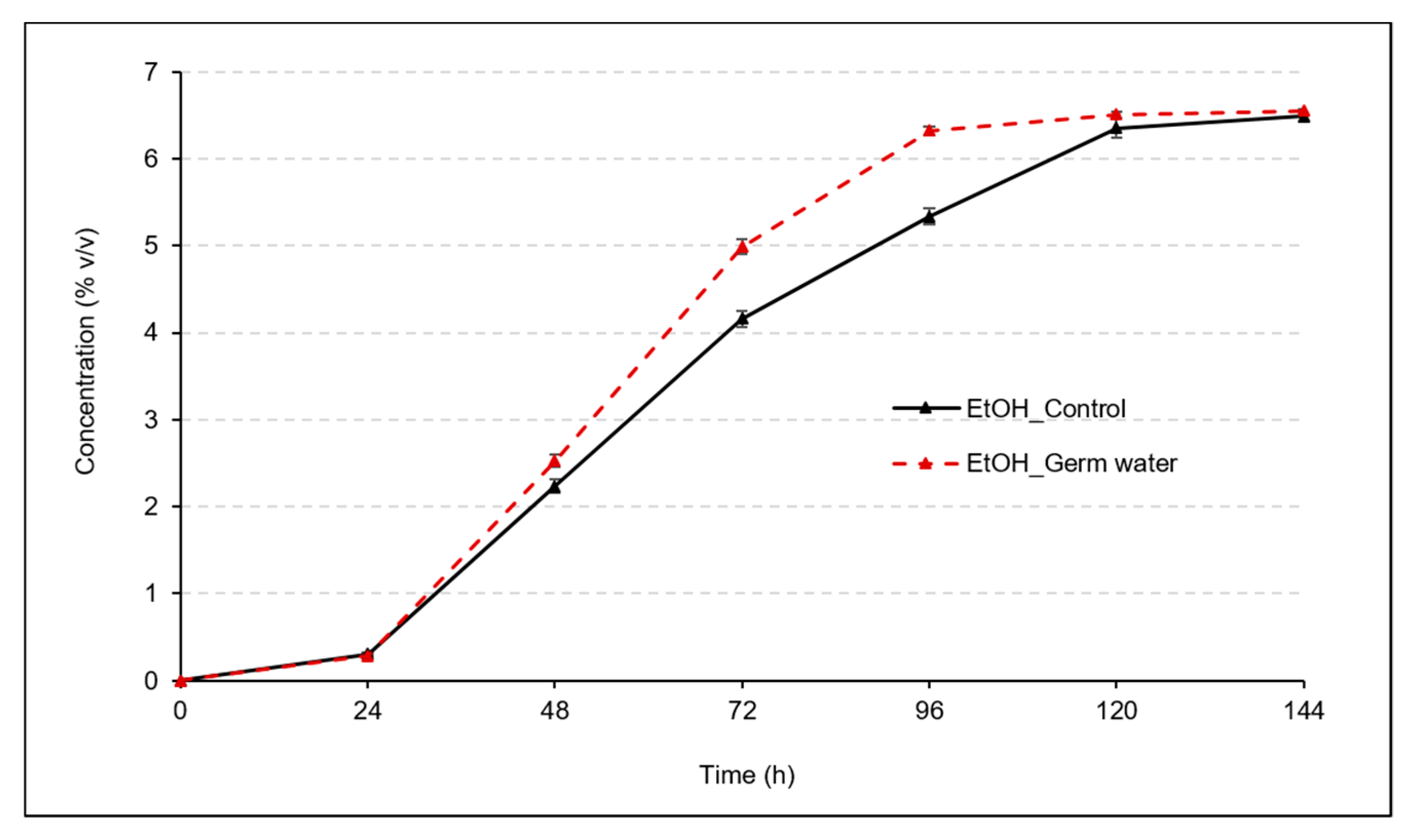
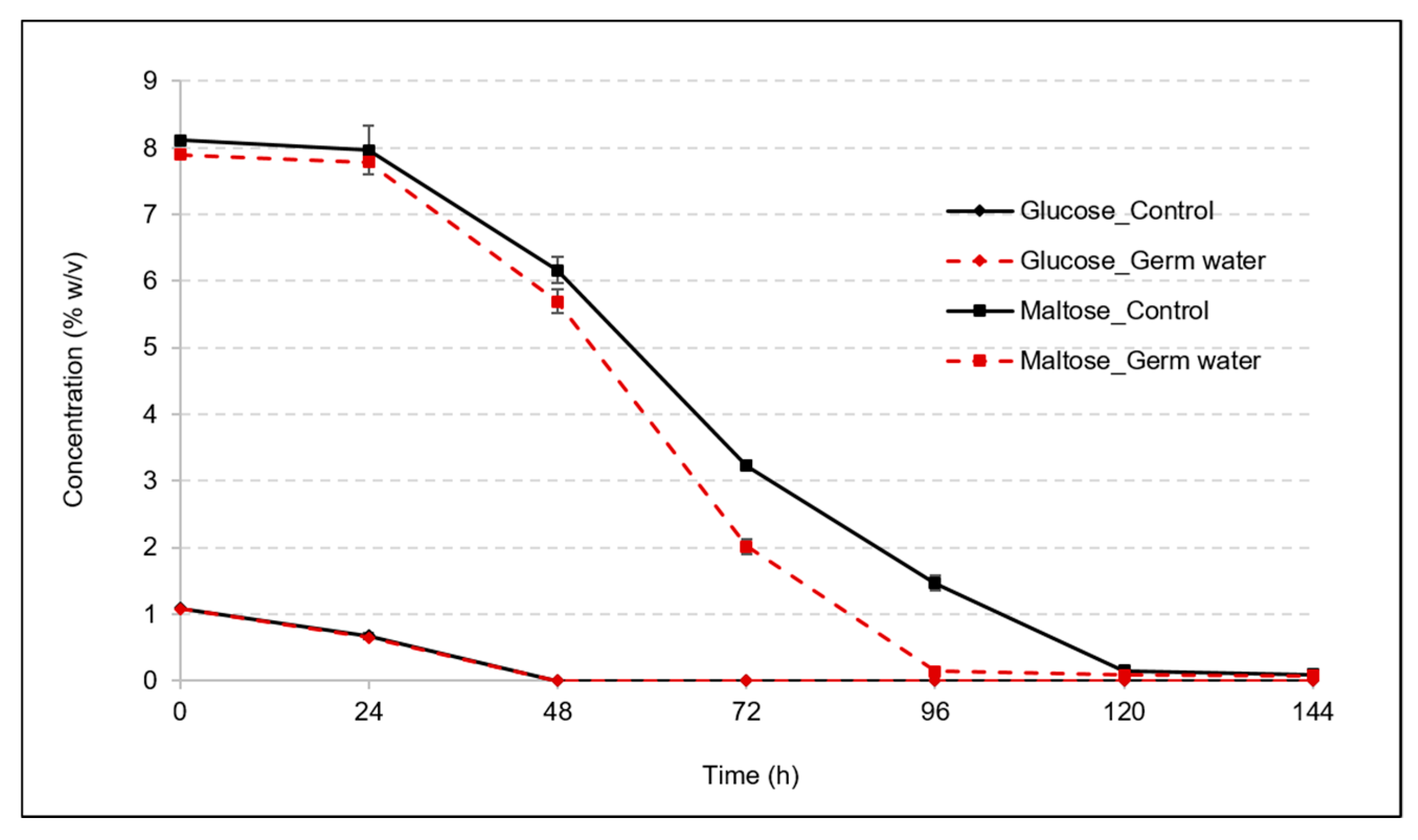
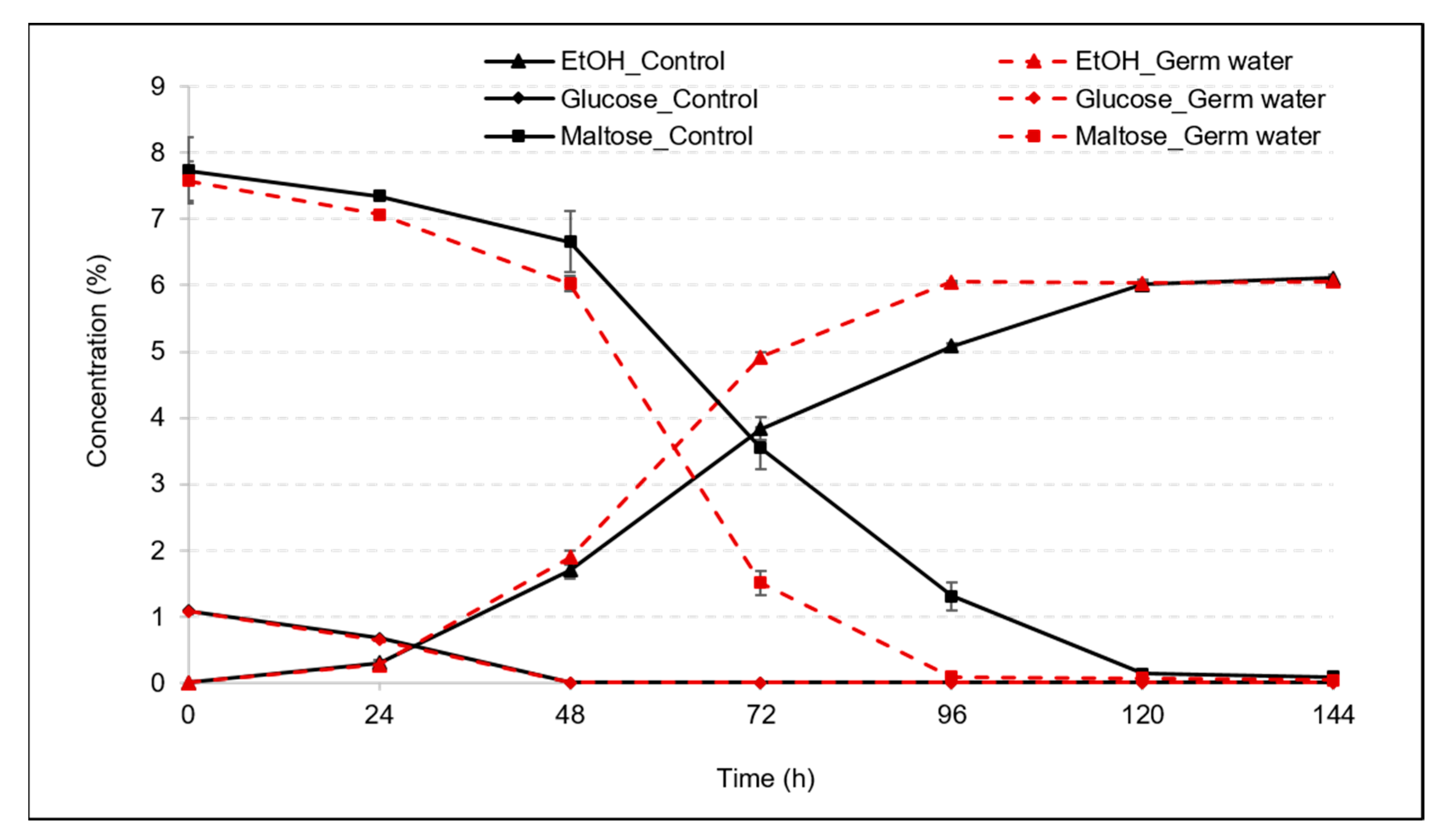
3.1. Effect of Germ Water Addition
3.2. Effect of Germ Water Addition from Flint Corn Germ
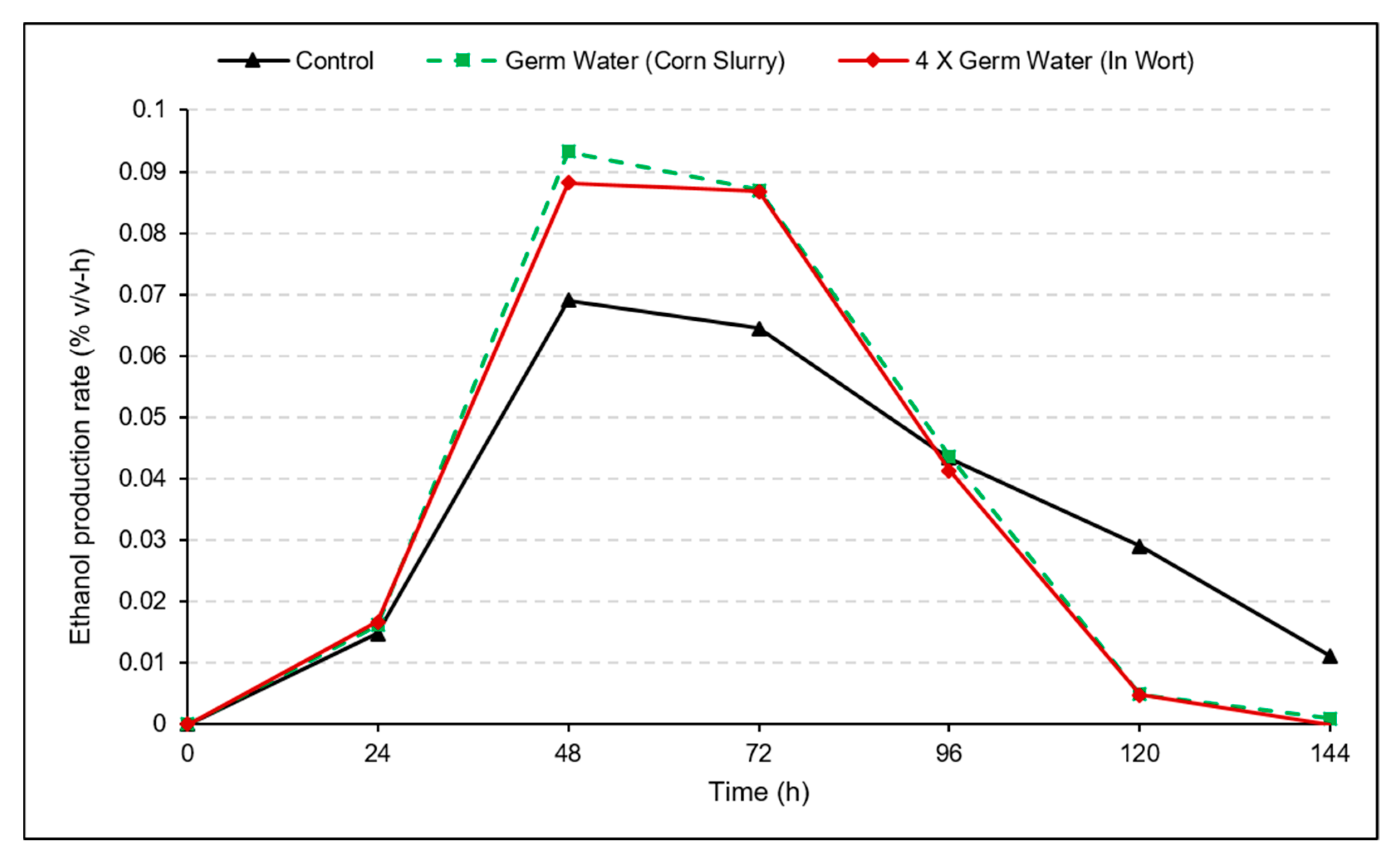
3.3. Use of Concentrated Germ Water in Wort
3.4. Change in Composition of Germ
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Wang, J.; Liu, C. Beers. In Current Developments in Biotechnology and Bioengineering; Elsevier: New York, NY, USA, 2017; pp. 305–351. Available online: https://www.sciencedirect.com/book/9780444636669/current-developments-in-biotechnology-and-bioengineering (accessed on 11 January 2019).
- Bravi, E.; Sensidoni, M.; Floridi, S.; Perretti, G. Fatty Acids Composition Differences between Beers Made With All-Malt and Brewer’s Corn Grits and Malt; Technical quarterly; Food and Agriculture Organization (FAO): Rome, Italy, 2009. [Google Scholar]
- Zhuang, S.; Shetty, R.; Hansen, M.; Fromberg, A.; Hansen, P.B.; Hobley, T.J. Brewing with 100% Unmalted Grains: Barley, Wheat, Oat and Rye. Eur. Food Res. Technol. 2017, 243, 447–454. [Google Scholar] [CrossRef]
- Poreda, A.; Czarnik, A.; Zdaniewicz, M.; Jakubowski, M.; Antkiewicz, P. Corn grist adjunct–application and influence on the brewing process and beer quality. J. Inst. Brew. 2014, 120, 77–81. [Google Scholar] [CrossRef]
- Bogdan, P.; Kordialik-Bogacka, E. Alternatives to malt in brewing. Trends Food Sci. Technol. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Cooper, C.; Evans, D.; Yousif, A.; Metz, N.; Koutoulis, A. Comparison of the impact on the performance of small-scale mashing with different proportions of unmalted barley, Ondea Pro®, malt and rice. J. Inst. Brew. 2016, 122, 218–227. [Google Scholar] [CrossRef]
- USDA-NAAS. Grain Crushings and CoProducts Production 2017 Summary; United States Department of Agriculture: Washington, DC, USA, 2018.
- Lekkas, C.; Stewart, G.; Hill, A.; Taidi, B.; Hodgson, J. The importance of free amino nitrogen in wort and beer. Tech. Q.-Master Brew. Assoc. Am. 2005, 42, 113. [Google Scholar]
- Zembold-Gula, A.; Blazewicz, J.; Wojewodzka, K. Protein Compounds in Brewing Worts Produced with Addition of Naked Barley Grain; Zywnosc Nauka Technologia Jakosc (Poland); Food and Agriculture Organization (FAO): Rome, Italy, 2008. [Google Scholar]
- Stewart, G.G.; Hill, A.E.; Russell, I. 125th anniversary review: Developments in brewing and distilling yeast strains. J. Inst. Brew. 2013, 119, 202–220. [Google Scholar] [CrossRef]
- Yano, M.; Tsuda, H.; Imai, T.; Ogawa, Y.; Ohkochi, M. The effect of barley adjuncts on free amino nitrogen contents in wort. J. Inst. Brew. 2008, 114, 230–238. [Google Scholar] [CrossRef]
- Murthy, G.S.; Singh, V.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M. Improvement in fermentation characteristics of degermed ground corn by lipid supplementation. J. Ind. Microbiol. Biotechnol. 2006, 33, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Ramchandran, D.; Wang, P.; Dien, B.; Liu, W.; Cotta, M.A.; Singh, V. Improvement of Dry-Fractionation Ethanol Fermentation by Partial Germ Supplementation. Cereal Chem. 2015, 92, 218–223. [Google Scholar] [CrossRef]
- Murthy, G.S.; Singh, V.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M. Evaluation and strategies to improve fermentation characteristics of modified dry-grind corn processes. Cereal Chem. 2006, 83, 455–459. [Google Scholar] [CrossRef]
- Juneja, A.; Kumar, D.; Singh, V. Germ soak water as nutrient source to improve fermentation of corn grits from modified corn dry grind process. Bioresour. Bioprocess. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.D.; Pruiett, L.E.; Wang, P.; Xu, L.; Belyea, R.L.; Tumbleson, M. Laboratory Measurement of Yield and Composition of Dry-Milled Corn Fractions Using a Shortened, Single-Stage Tempering Procedure. Cereal Chem. 2009, 86, 434–438. [Google Scholar] [CrossRef]
- Vidal, B.C., Jr.; Rausch, K.D.; Tumbleson, M.; Singh, V. Protease treatment to improve ethanol fermentation in modified dry grind corn processes. Cereal Chem. 2009, 86, 323–328. [Google Scholar] [CrossRef]
- AOAC. Official Methods of the Association of Official Analytical Chemists Washington, DC; Vol. Methods 920.39, 924.05, 973.18, 990.03; AOAC: Rockville, MD, USA, 2003. [Google Scholar]
- Vidal, B.C., Jr.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M.; Singh, V. Germ-derived FAN as nitrogen source for corn endosperm fermentation. Cereal Chem. 2011, 88, 328–332. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International: Arlington, VA.; Vol. Method 982.30 E(a, b, c) chp. 45.3.05; AOAC: Rockville, MD, USA, 2006. [Google Scholar]
- Lekkas, C.; Stewart, G.; Hill, A.; Taidi, B.; Hodgson, J. Elucidation of the role of nitrogenous wort components in yeast fermentation. J. Inst. Brew. 2007, 113, 3–8. [Google Scholar] [CrossRef]
- Rowell, J.L. Exploring Fermentation Rate in Beer and Hard Cider Brewing; Western Carolina University: Cullowhee, NC, USA, 2015. [Google Scholar]
- Walker, G.M. Metals in yeast fermentation processes. Adv. Appl. Microbiol. 2004, 54, 197–230. [Google Scholar] [PubMed]
- De Nicola, R.; Walker, G.M. Accumulation and cellular distribution of zinc by brewing yeast. Enzyme Microb. Technol. 2009, 44, 210–216. [Google Scholar] [CrossRef]
- Bromberg, S.K.; Bower, P.A.; Duncombe, G.; Fehring, J.; Gerber, L.; Lau, V.K.; Tata, M. Requirements for zinc, manganese, calcium, and magnesium in wort. J. Am. Soc. Brew. Chem. 1997, 55, 123–128. [Google Scholar] [CrossRef]
- Johnston, D.B.; McAloon, A.J.; Moreau, R.A.; Hicks, K.B.; Singh, V. Composition and economic comparison of germ fractions from modified corn processing technologies. J. Am. Oil Chem. Soc. 2005, 82, 603–608. [Google Scholar] [CrossRef]
| Soaking (Germ:Water by Weight) | Concentration (ppm) |
|---|---|
| 1:10 | 127 ± 8 |
| 1:5 | 250 ± 12 |
| 1:2.5 | 517 ± 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Hager, A.-S.; Sun, A.; Debyser, W.; Javier Guagliano, B.; Singh, V. Improving Fermentation Rate during Use of Corn Grits in Beverage Alcohol Production. Beverages 2019, 5, 5. https://doi.org/10.3390/beverages5010005
Kumar D, Hager A-S, Sun A, Debyser W, Javier Guagliano B, Singh V. Improving Fermentation Rate during Use of Corn Grits in Beverage Alcohol Production. Beverages. 2019; 5(1):5. https://doi.org/10.3390/beverages5010005
Chicago/Turabian StyleKumar, Deepak, Anna-Sophie Hager, Alberto Sun, Winok Debyser, Bruno Javier Guagliano, and Vijay Singh. 2019. "Improving Fermentation Rate during Use of Corn Grits in Beverage Alcohol Production" Beverages 5, no. 1: 5. https://doi.org/10.3390/beverages5010005
APA StyleKumar, D., Hager, A.-S., Sun, A., Debyser, W., Javier Guagliano, B., & Singh, V. (2019). Improving Fermentation Rate during Use of Corn Grits in Beverage Alcohol Production. Beverages, 5(1), 5. https://doi.org/10.3390/beverages5010005







