A Comparative Study on COVID-19 Dynamics: Mathematical Modeling, Predictions, and Resource Allocation Strategies in Romania, Italy, and Switzerland
Abstract
1. Introduction
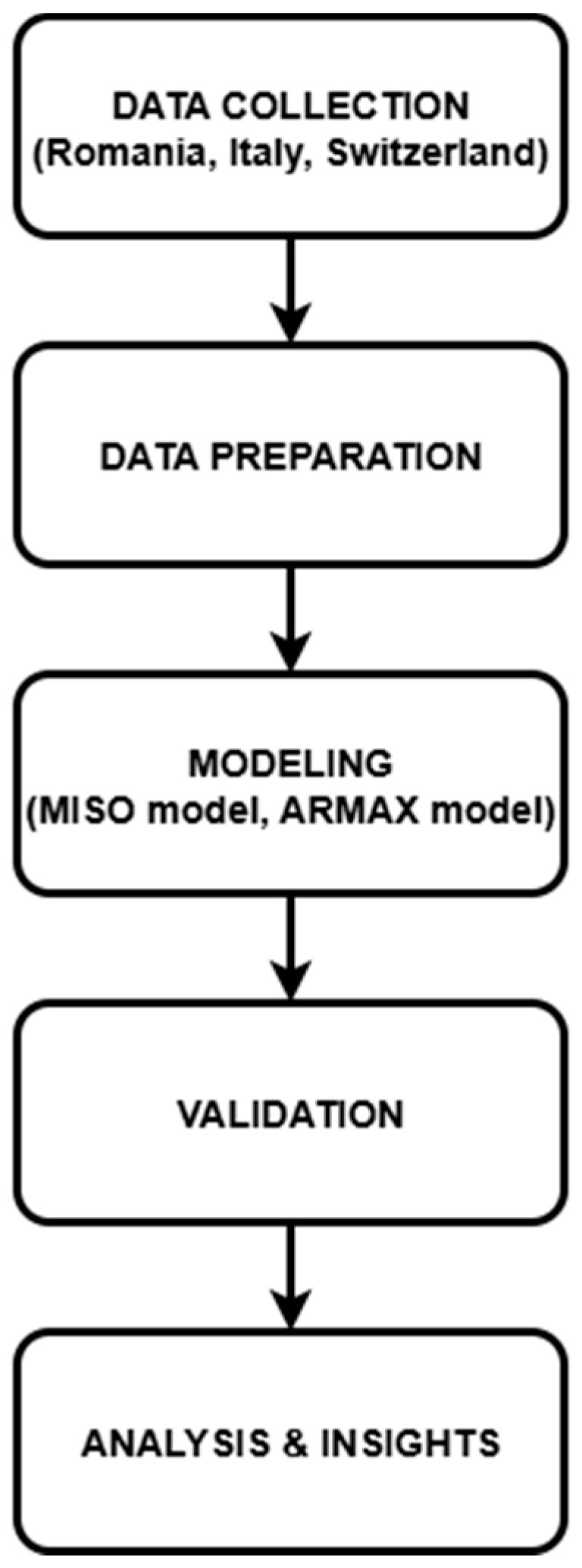
2. Data Processing and Mathematical Model Implementation
- Preparing the Data: Imputation methods were used in order to successfully identify and rectify any missing information. By way of illustration, the values of temperature and humidity that were absent were filled in by utilizing interpolated meteorological data. To prevent the findings from being skewed, duplicate records were eliminated.
- Standardization and Normalization of the Data: As a result of the fact that our data included variables that were measured in multiple units (for example, temperature in Celsius, testing numbers as absolute counts). Additionally, standardization was carried out in order to guarantee consistency, especially with regard to the quantifiable values of official measurements, which presented a large degree of variation across the three nations.
- Utilizing Categorical Data Encoding: Measures taken by the government were first documented in the form of written descriptions. For example, we gave numerical values to these measurements by utilizing a preset scoring system that was dependent on the severity of the situation (for example, lockdown = 100%, social distance requirements = 50%, and no limits = 0%).
- The Engineering of Features: For the purpose of improving predictive analysis, new characteristics were developed. As an example, the rate of rise in testing was computed in order to gain an understanding of the responsiveness of testing procedures over the course of several years.
- Trends in Temperature and Case Analysis [48]: There was a correlation between lower temperatures outdoors and an increase in the number of COVID-19 cases in Italy and Romania, which suggests that seasonal influences may be at play. Because of Switzerland’s varied climate, the country displayed a variety of patterns, which necessitated a more in-depth examination on a regional level.
- Function of Humidity [48]: There was a modest decrease in the number of cases with higher humidity levels, which lends credence to the findings of the previous study that implies humidity may have an effect on the spread of viruses.
- Evaluation of the Effectiveness: A general correlation was found between an increase in the number of tests and an increase in the number of cases that were recognized [49]. This highlights the significance of extensive testing in the process of early detection and control.
- Impact of the Measures taken by the Government: It was shown that countries with tighter measurements (higher quantifiable values) had slower growth rates of infection, especially when paired with high testing rates.
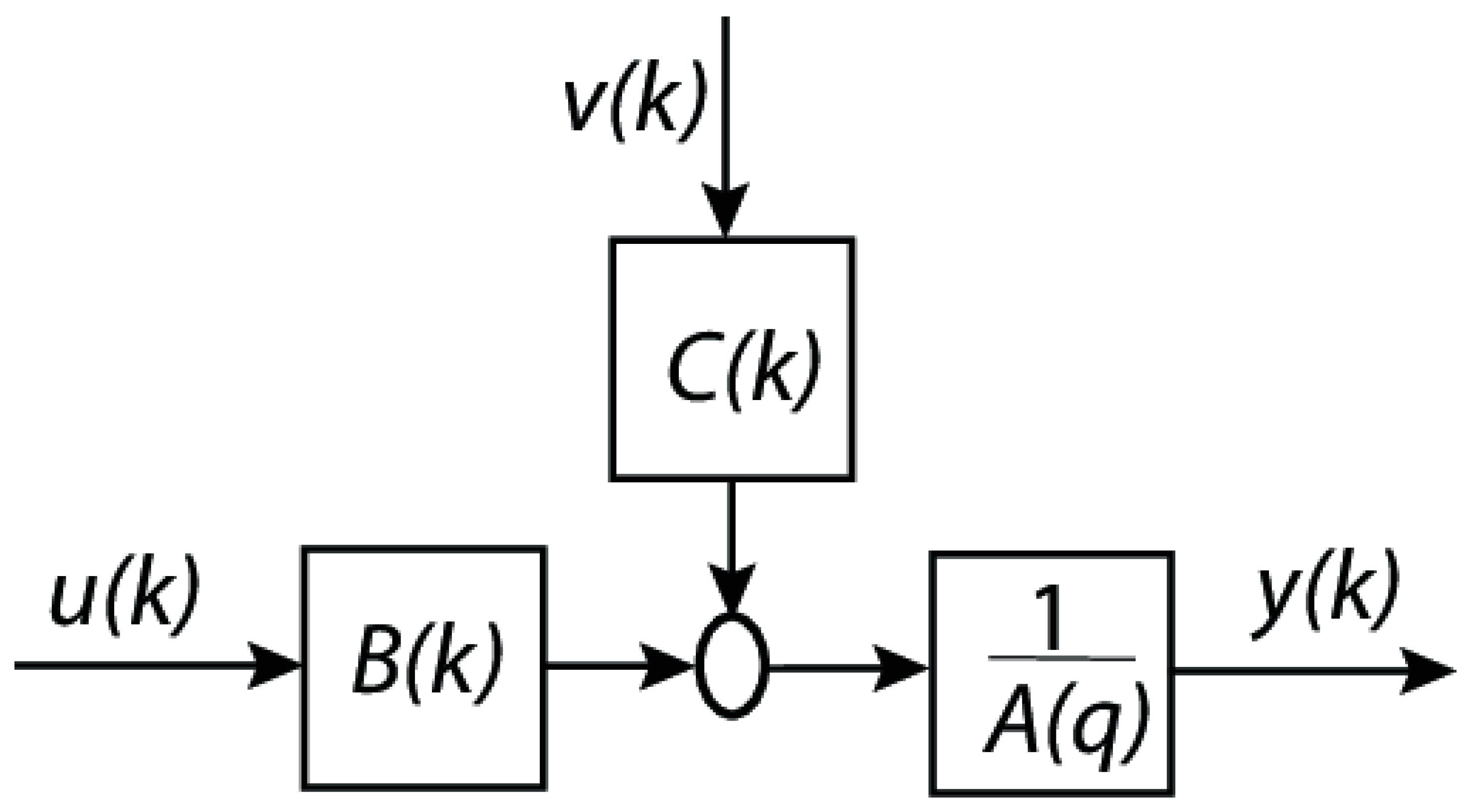
- Autoregressive part (AR) described in (3):
- Moving average part (MA) captures past predictions error (4):
- Exogenous part (X) includes the external factors which affect the outcome (5):
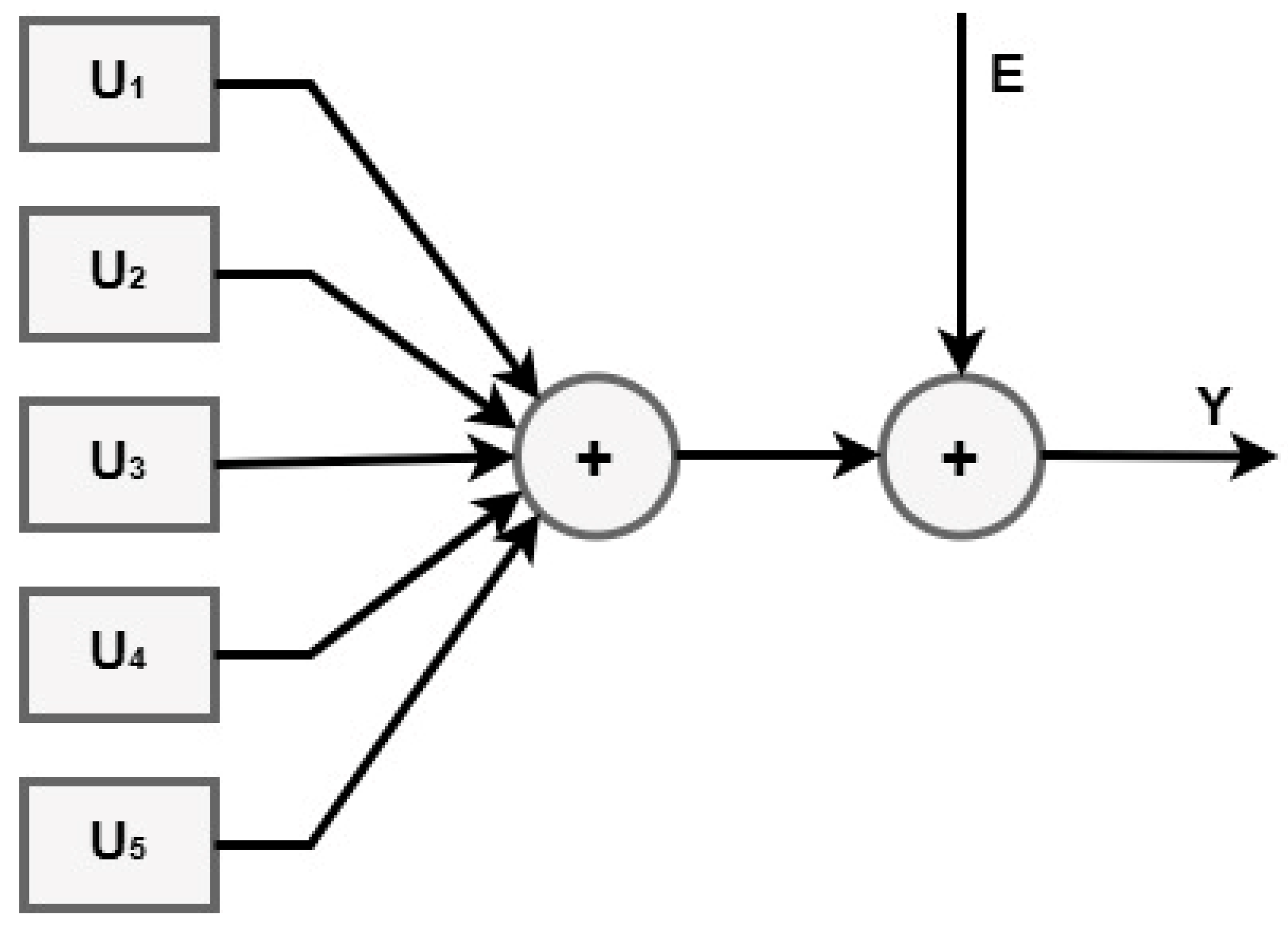
- u(k): exogenous input signal (for example, an external control input or an external effect).
- Exogenous input filter, denoted by B(k), which simulates the way in which u(k) affects the system.
- Noise or disturbance (random noise or unmodeled effects that impact the system) is denoted by the variable v(k).
- C(k) is the moving average (MA) component, which describes the way in which noise from the past affects the system.
- By adding the contributions of B(k)u(k) and C(k)v(k), the summation block is completed.
- is the autoregressive (AR) component (which represents the dynamics of the system).
- The output of the system (y(k)), which may be either the anticipated or observed value.
- A(q) is the autoregressive polynomial in the delay operator .
- B(q) is the exogenous input polynomial (how u(k) affects y(k)).
- C(q) is the moving average polynomial (how past noise affects y(k)).
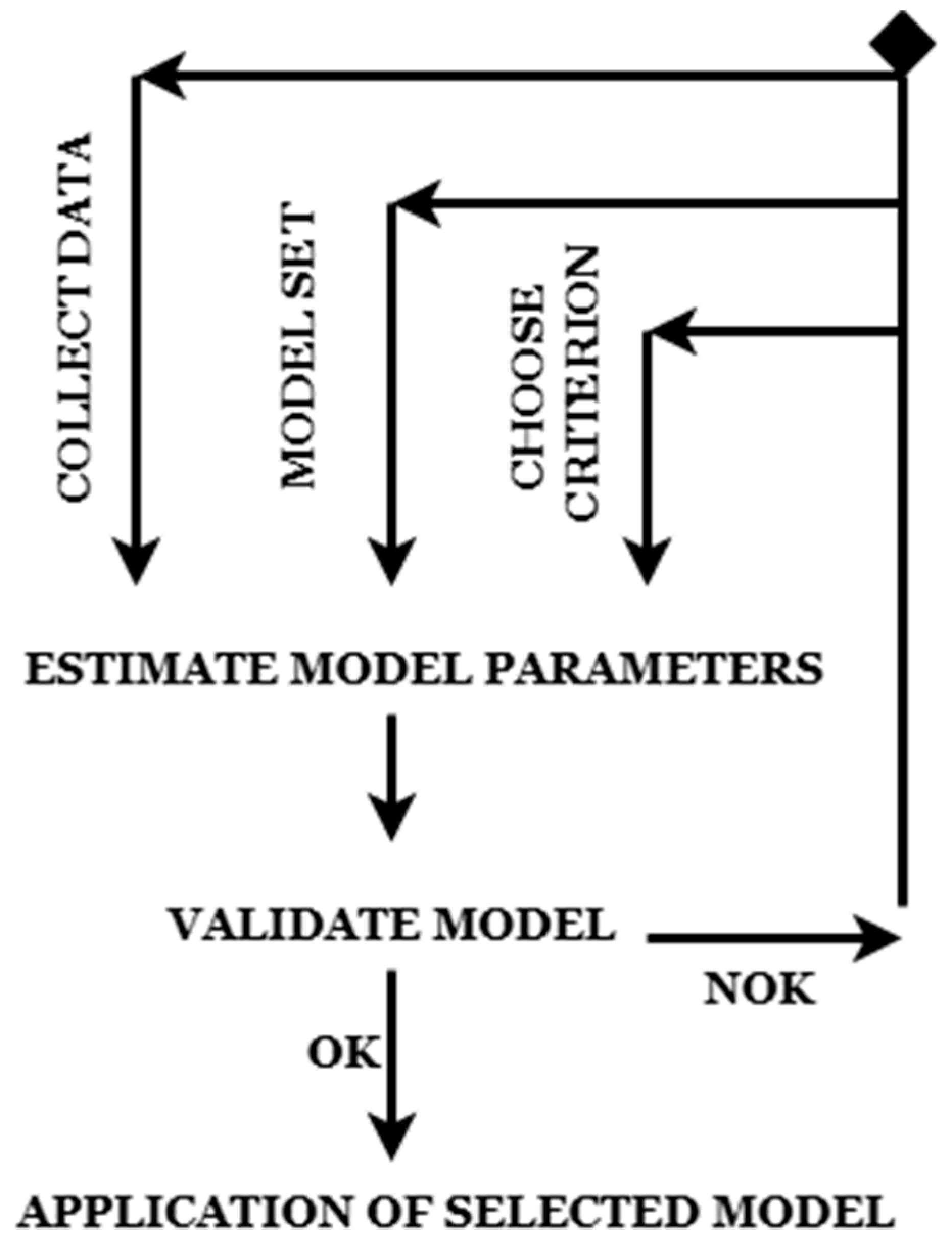
- Re-collecting data (if data quality is poor).
- Once validated, the final model is deployed for real-world predictions.
3. Results
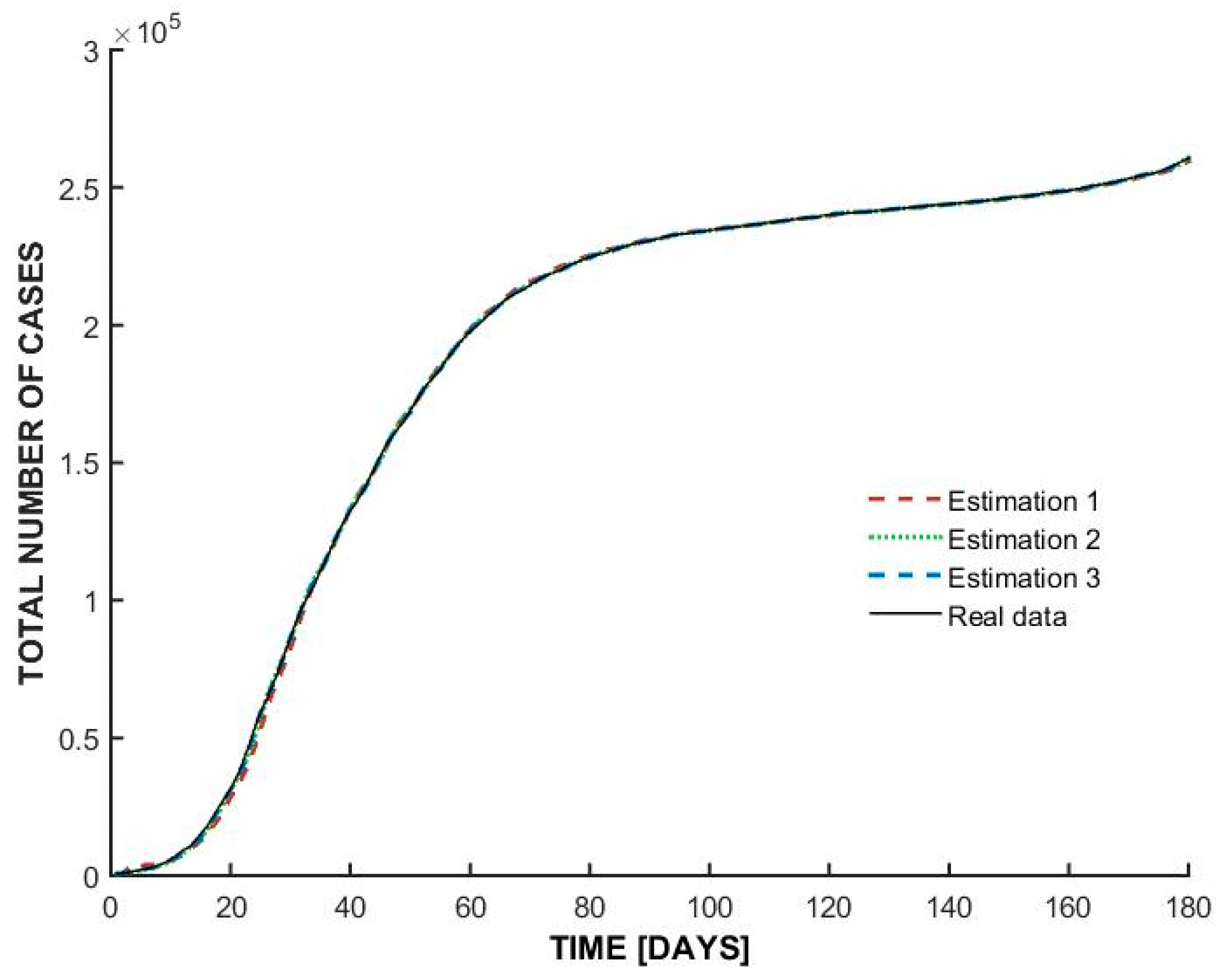
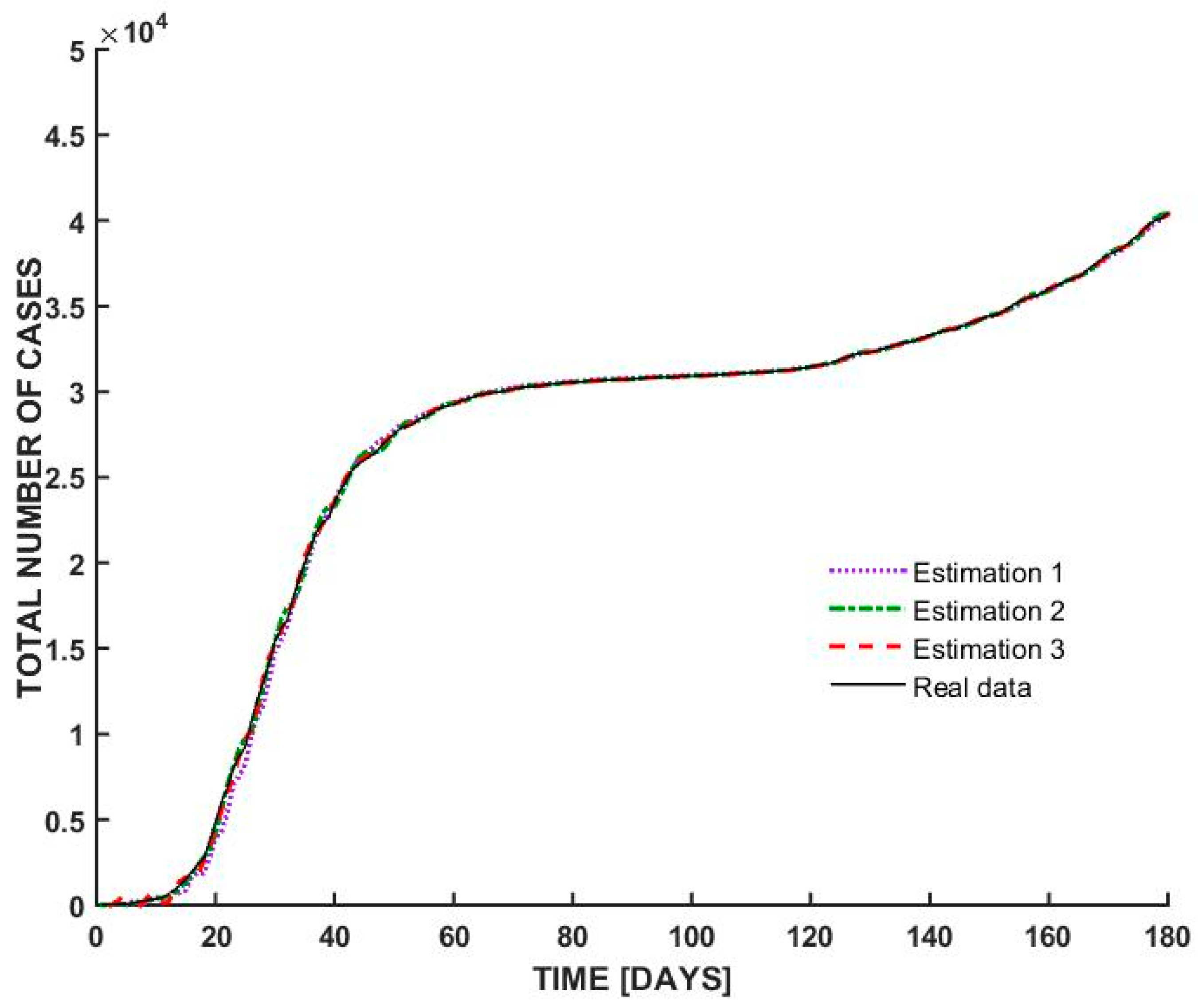
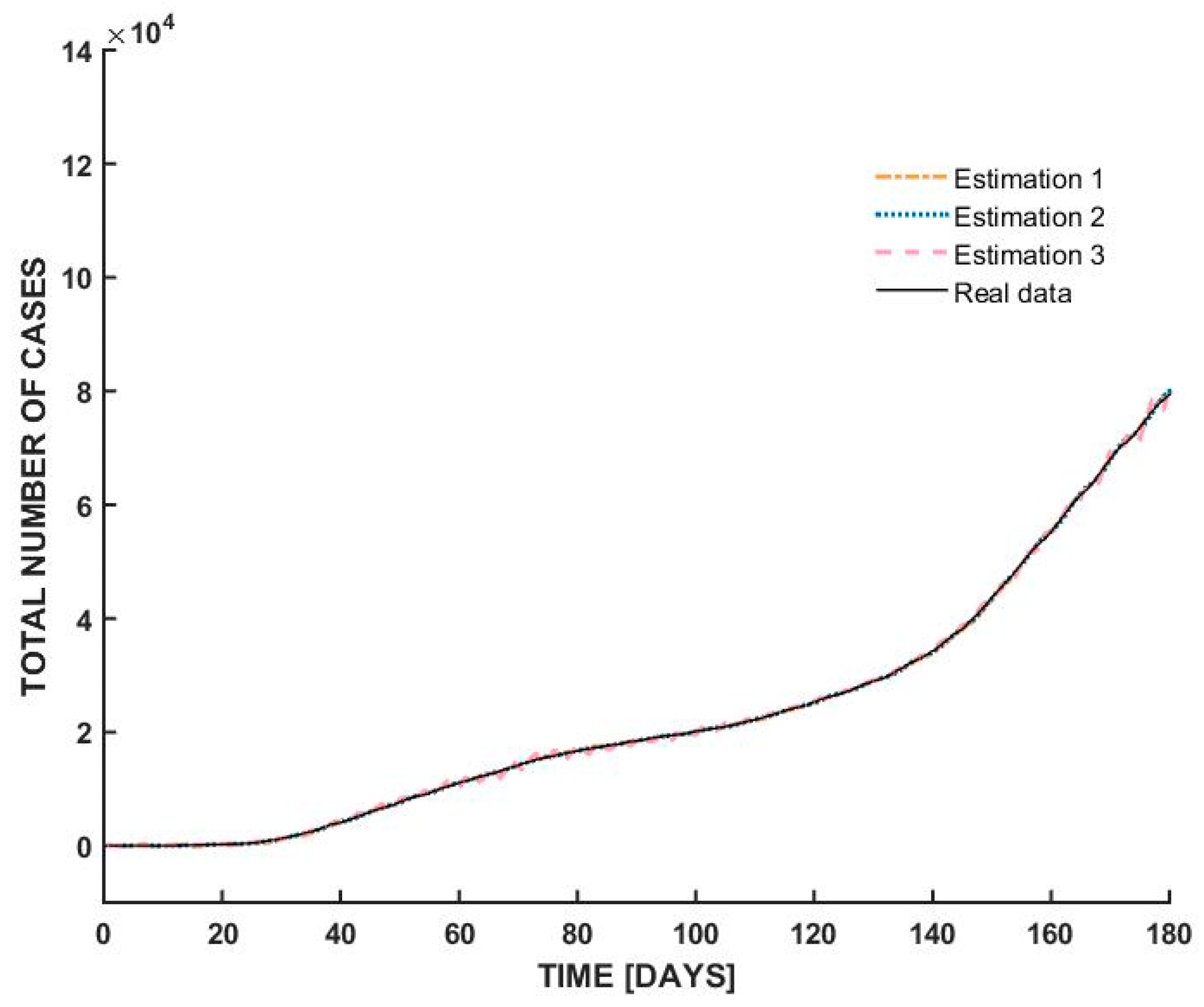
- At the beginning of the outbreak, the total number of cases increases sharply, indicating an exponential rise in infections. This suggests a highly contagious spread, typical of the early stages of a pandemic before interventions take effect.
- Around day 30, the curve begins to flatten, suggesting that the spread of the virus slowed down significantly. This could be attributed to government interventions such as lockdown measures, social distancing, and mask mandates. The plateau phase indicates that new infections were occurring at a lower rate, possibly due to public health measures successfully reducing transmission.
- After a prolonged period of stabilization, the curve starts rising again after day 100. This suggests a second wave or resurgence of cases, possibly due to relaxed restrictions, increased mobility, or changes in public behavior. Seasonal effects or new variants may have also played a role in driving up cases.
4. Conclusions
- Intervention by the government is absolutely necessary: in nations where the regulations were more stringent and were more effectively implemented, transfer rates were lower.
- There is no doubt that testing is an indispensable instrument: increased testing rates led to more precise case monitoring and improved overall management of outbreaks.
- There is a role played by environmental factors: there were observable and quantitative impacts of temperature and humidity on the propagation of the virus, which supported findings of seasonal trends.
- Policies that are driven by data are more effective: governments should make use of predictive models in order to make dynamic adjustments to their policies.
- Expanding the dataset to include new nations in order to validate it more thoroughly.
- Including other factors such as the population density, statistics on mobility, and vaccination rates in the analysis.
- Deep learning models are being used for the purpose of detecting more complicated patterns.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MISO | Multiple Inputs Single Output |
| MIMO | Multiple Inputs Multiple Outputs |
| SISO | Single Input Single Output |
| WHO | World Health Organization |
| FFNN | Feed-Forward Neural Network |
| PID | Proportional Integral Derivative |
| PHEIC | Public Health Emergency of International Concern |
References
- Dascalu, S. The Successes and Failures of the Initial COVID-19 Pandemic Response in Romania. Front. Public Health 2020, 8, 344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hâncean, M.-G.; Perc, M.; Lerner, J. Early spread of COVID-19 in Romania: Imported cases from Italy and human-to-human transmission networks. R. Soc. Open Sci. 2020, 7, 7200780. [Google Scholar] [CrossRef]
- Enciu, B.G.; Tănase, A.A.; Drăgănescu, A.C.; Aramă, V.; Pițigoi, D.; Crăciun, M.-D. The COVID-19 Pandemic in Romania: A Comparative Description with Its Border Countries. Healthcare 2022, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dascalu, S.; Flammer, P.G.; Ghafari, M.; Henson, S.C.; Nascimento, R.; Bonsall, M.B. Engaging Religious Institutions and Faith-Based Communities in Public Health Initiatives: A Case Study of the Romanian Orthodox Church During the COVID-19 Pandemic. Front. Public Health 2021, 9, 768091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cernicova-Buca, M.; Palea, A. An Appraisal of Communication Practices Demonstrated by Romanian District Public Health Authorities at the Outbreak of the COVID-19 Pandemic. Sustainability 2021, 13, 2500. [Google Scholar] [CrossRef]
- Chiruţă, C.; Bulgariu, E.; Avsec, J.; Ferčec, B.; Mencinger, M. Comparison of the Evolution of the COVID-19 Disease between Romania and Italy. Appl. Syst. Innov. 2020, 3, 44. [Google Scholar] [CrossRef]
- Salaris, L.; Iacob, A.; Anghel, V.; Contu, G. The Impact of the First Covid-19 Wave on Migrant Workers: The Case of Romanians in Italy. Cent. East. Eur. Migr. Rev. 2022, 11, 23–48. [Google Scholar] [CrossRef]
- Gatto, M.; Bertuzzo, E.; Mari, L.; Miccoli, S.; Carraro, L.; Casagrandi, R.; Rinaldo, A. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc. Natl. Acad. Sci. USA 2020, 117, 10484–10491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. Coronavirus (COVID-19) Cases. Published online at OurWorldinData.org. 2020. Available online: https://ourworldindata.org/covid-cases (accessed on 14 September 2025).
- Enciu, B.G.; Pițigoi, D.; Zaharia, A.; Popescu, R.; Niculcea, A.; Crăciun, M.-D.; Pistol, A. COVID-19 Vaccination in Romania and the Benefits of the National Electronic Registry of Vaccinations. Vaccines 2023, 11, 370. [Google Scholar] [CrossRef]
- Romania and Bulgaria Occupy the Last Two Places in the Ranking Regarding the Number of Vaccinated People in the European Union. Available online: https://www.digi24.ro/stiri/externe/cnn-cum-au-ajuns-romania-si-bulgaria-sa-aiba-cele-mai-mici-rate-de-vaccinare-din-ue-trecutul-comunist-este-una-din-cauze-1687669 (accessed on 7 February 2022).
- Mărcău, F.-C.; Purec, S.; Niculescu, G. Study on the Refusal of Vaccination against COVID-19 in Romania. Vaccines 2022, 10, 261. [Google Scholar] [CrossRef]
- Fedele, F.; Aria, M.; Esposito, V.; Micillo, M.; Cecere, G.; Spano, M.; De Marco, G. COVID-19 vaccine hesitancy: A survey in a population highly compliant to common vaccinations. Hum. Vaccines Immunother. 2021, 17, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Gentile, S.; Mambro, A.; Strollo, F. Parallel epidemics, or nearly so: Certainties and uncertainties about SARS-CoV-2 in Italy. Diabetes Res. Clin. Pract. 2020, 164, 108195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Signorelli, C.; Odone, A.; Gianfredi, V.; Balzarini, F.; Bucci, D.; Croci, R.; Gaetti, G.; Stirparo, G.; Guerra, R. Epidemiological assessment of the first COVID-19 epidemic wave in Lombardy. A systematic review. Acta Biomed. 2021, 92, e2021462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paciullo, F.; Giannandrea, D.; Gianfredi, V.; Borgognoni, F.; Verdecchia, P.; L’Angiocola, P.D.; Monti, M. Epidemiology of emergency calls for time-dependent acute illnesses during COVID-19 outbreak in Umbria region (Italy). Ann. Ig. 2020, 33, 198–200. [Google Scholar] [CrossRef]
- Cereda, D.; Tirani, M.; Rovida, F.; Demicheli, V.; Ajelli, M.; Poletti, P.; Trentini, F.; Guzzetta, G.; Marziano, V.; Barone, A. The early phase of the COVID-19 outbreak in Lombardy, Italy. arXiv 2020, arXiv:2003.09320. [Google Scholar] [CrossRef]
- Alteri, C.; Cento, V.; Piralla, A.; Costabile, V.; Tallarita, M.; Colagrossi, L.; Renica, S.; Giardina, F.; Novazzi, F.; Gaiarsa, S.; et al. Genomic epidemiology of SARS-CoV-2 reveals multiple lineages and early spread of SARS-CoV-2 infections in Lombardy, Italy. Nat. Commun. 2021, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Giardina, F.; Galli, C.; Pellegrinelli, L.; Paolucci, S.; Tallarita, M.; Pariani, E.; Piralla, A.; Baldanti, F. No evidence of SARS-CoV-2 circulation in the framework of influenza surveillance between October 2019 and February 2020 in Lombardy, Italy. Travel Med. Infect. Dis. 2021, 40, 102002. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. J. Am. Med. Assoc. 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. COVID-19 Lombardy ICU Network Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345. [Google Scholar] [CrossRef]
- Parotto, E.; Lamberti-Castronuovo, A.; Censi, V.; Valente, M.; Atzori, A.; Ragazzoni, L. Exploring Italian healthcare facilities response to COVID-19 pandemic: Lessons learned from the Italian Response to COVID-19 initiative. Front. Public Health 2023, 10, 1016649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giancotti, M. Responses of Italian Public Hospitals to COVID-19 Pandemic: Analysis of Supply and Demand of Hospital ICU Beds. Med. Sci. Forum 2021, 4, 16. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Stefanizzi, P.; Gesualdo, L.; Migliore, G.; Fucilli, F.I.M.; Cavone, D.; Delfino, M.C.; et al. COVID-19 hospital outbreaks: Protecting healthcare workers to protect frail patients. An Italian observational cohort study. Int. J. Infect. Dis. 2021, 102, 532–537. [Google Scholar] [CrossRef]
- Fagoni, N.; Perone, G.; Villa, G.F.; Celi, S.; Bera, P.; Sechi, G.M.; Mare, C.; Zoli, A.; Botteri, M. The Lombardy Emergency Medical System Faced with COVID-19: The Impact of Out-of-Hospital Outbreak. Prehospital Emerg. Care 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Murphy, C.; Lim, W.W.; Mills, C.; Wong, J.Y.; Chen, D.; Xie, Y.; Li, M.; Gould, S.; Xin, H.; Cheung, J.K.; et al. Effectiveness of social distancing measures and lockdowns for reducing transmission of COVID-19 in non-healthcare, community-based settings. Philos. Trans. A Math. Phys. Eng. Sci. 2023, 381, 20230132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merkaj, E.; Santolini, R. Italian national policies in response to the COVID-19 pandemic: The case of the Friuli-Venezia-Giulia and Umbria Regions. Health Policy 2022, 126, 287–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bosa, I.; Castelli, A.; Castelli, M.; Ciani, O.; Compagni, A.; Galizzi, M.M.; Garofano, M.; Ghislandi, S.; Giannoni, M.; Marini, G.; et al. Response to COVID-19: Was Italy (un)prepared? Health Econ. Policy Law 2022, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torri, E.; Sbrogiò, L.G.; Di Rosa, E.; Cinquetti, S.; Francia, F.; Ferro, A. Italian Public Health Response to the COVID-19 Pandemic: Case Report from the Field, Insights and Challenges for the Department of Prevention. Int. J. Environ. Res. Public Health 2020, 17, 3666. [Google Scholar] [CrossRef]
- Chabloz, Y.M.; Gouveia, A.; Berger, J.; Cohidon, C. COVID-19 pandemic in Switzerland: A brief overview of the role and response of primary care. La Presse Médicale Open 2024, 5, 100056. [Google Scholar] [CrossRef]
- Menon, L.K.; Richard, V.; de Mestral, C.; Baysson, H.; Wisniak, A.; Guessous, I.; Stringhini, S.; Specchio-COVID19 Study Group. Forgoing healthcare during the COVID-19 pandemic in Geneva, Switzerland—A cross-sectional population-based study. Prev. Med. 2022, 156, 106987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cariti, F.; Tuñas Corzon, A.; Fernandez-Cassi, X.; Ganesanandamoorthy, P.; Ort, C.; Julian, T.R.; Kohn, T. Wastewater Reveals the Spatiotemporal Spread of SARS-CoV-2 in the Canton of Ticino (Switzerland) during the Onset of the COVID-19 Pandemic. ACS ES T Water 2022, 2, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schumacher, L.; Dhif, Y.; Bonnabry, P.; Widmer, N. Managing the COVID-19 health crisis: A survey of Swiss hospital pharmacies. BMC Health Serv. Res. 2023, 23, 1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schumacher, L.; Tinguely Casserini, J.; Bonnabry, P.; Widmer, N. Management of the COVID-19 Health Crisis: A Survey of Swiss Health Authorities’ Responses. Disaster Med. Public Health Prep. 2023, 17, e406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hintermann, B.; Schoeman, B.; Molloy, J.; Schatzmann, T.; Tchervenkov, C.; Axhausen, K.W. The impact of COVID-19 on mobility choices in Switzerland. Transp. Res. Part A Policy Pract. 2023, 169, 103582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riou, J.; Panczak, R.; Althaus, C.L.; Junker, C.; Perisa, D.; Schneider, K.; Criscuolo, N.G.; Low, N.; Egger, M. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: A population-based analysis. Lancet Public Health 2021, 6, e683–e691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Büchel, K.; Legge, S.; Pochon, V.; Wegmüller, P. Swiss trade during the COVID-19 pandemic: An early appraisal. Swiss J. Econ. Stat. 2020, 156, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brülhart, M.; Lalive, R.; Lehmann, T.; Siegenthaler, M. COVID-19 financial support to small businesses in Switzerland: Evaluation and outlook. Swiss J. Econ. Stat. 2020, 156, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antohi, V.M.; Ionescu, R.V.; Zlati, M.L.; Mirica, C. Approaches related to the effects of Covid-19 pandemics on financing of the healthcare system in Romania. Front. Public Health 2022, 10, 940021. [Google Scholar] [CrossRef]
- Available online: https://www.cbc.ca/news/world/romania-hospital-icu-covid19-1.6216291 (accessed on 14 September 2025).
- Coronavirus (COVID-19) in Romania—Statistics & Facts|Statista. Available online: https://www.statista.com/topics/6240/coronavirus-covid-19-in-romania/ (accessed on 14 September 2025).
- Benvenuto, D.; Giovanetti, M.; Vassallo, L.; Angeletti, S.; Ciccozzi, M. Application of the ARIMA model on the COVID-2019 epidemic dataset. Infect. Dis. Model. 2020, 5, 194–199. [Google Scholar] [CrossRef]
- Ceylan, Z. Estimation of COVID-19 prevalence in Italy, Spain, and France. Sci. Total Environ. 2020, 729, 138817. [Google Scholar] [CrossRef]
- Pinter, G.; Felde, I.; Mosavi, A.; Ghamisi, P.; Gloaguen, R. COVID-19 pandemic prediction for Hungary; a hybrid machine learning approach. Mathematics 2020, 8, 890. [Google Scholar] [CrossRef]
- Rustam, F.; Reshi, A.A.; Mehmood, A.; Ullah, S.; On, B.W.; Aslam, W.; Choi, G.S. COVID-19 future forecasting using supervised machine learning models. IEEE Access 2020, 8, 101489–101499. [Google Scholar] [CrossRef]
- Ribeiro, M.H.D.M.; da Silva, R.G.; Mariani, V.C.; dos Santos Coelho, L. Short-term forecasting COVID-19 cumulative confirmed cases: Perspectives for Brazil. Chaos Solitons Fractals 2020, 135, 109853. [Google Scholar] [CrossRef] [PubMed]
- Hersbach, H.; Bell, B.; Berrisford, P.; Biavati, G.; Horányi, A.; Muñoz Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Rozum, I.; et al. ERA5 Hourly Data on Single Levels from 1940 to Present; Copernicus Climate Change Service (C3S) Climate Data Store (CDS). 2023. Available online: https://cds.climate.copernicus.eu/datasets/reanalysis-era5-single-levels?tab=overview (accessed on 14 September 2025).
- Official Data Collated by Our World in Data (2022)—With Major Processing by Our World in Data. “Total Tests per Million People” [Dataset]. Official Data Collated by Our World in Data, “COVID-19, testing”. Available online: https://archive.ourworldindata.org/20250915-063610/grapher/number-of-covid-19-tests-per-confirmed-case-bar-chart.html (accessed on 14 September 2025).
- World Health Organization (2025)—Processed by Our World in Data. “Cumulative Confirmed Deaths”. World Health Organization, “COVID-19 Dashboard WHO COVID-19 Dashboard—Daily Cases and Deaths”. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 14 September 2025).
- CARNOTCYCLE—the Classical Blog on Thermodynamics. How to Convert Relative Humidity to Absolute Humidity. 2020. Available online: https://carnotcycle.wordpress.com/2012/08/04/how-to-convert-relative-humidity-to-absolute-humidity/ (accessed on 14 September 2025).
- Arshad, M.Z.; Algarni, A. Exploring time complexity and machine learning scalability for COVID-19 Predictions: A case study. J. Comput. Appl. Math. 2025, 474, 116933. [Google Scholar] [CrossRef]
- Yin, C.-Y.; Meng, X.-Y.; Zuo, J.-M. Modeling the effects of vaccinating strategies and periodic outbreaks on dengue in singapore. J. Appl. Anal. Comput. 2025, 15, 1284–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, Y.; Lim, S.; Seo, G.; Kim, J.; Park, H.; Jung, J.; Song, K. COVID-19 prediction with doubly multi-task Gaussian Process. J. Biomed. Inform. 2025, 169, 104872. [Google Scholar] [CrossRef] [PubMed]
| Factor | Italy | Switzerland | Romania |
|---|---|---|---|
| Healthcare Capacity | Overwhelmed, especially in Lombardy | Well-prepared, efficient ICU distribution | Underfunded, ICU shortages |
| Lockdown Measures | Strict nationwide lockdown | Partial restrictions, fewer lockdowns | Strict lockdowns but difficult enforcement |
| Testing and Tracing | Improved over time | Early adoption of digital tools | Limited due to resource constraints |
| Economic Support | Financial aid but deep economic hit | Strong financial packages for businesses | Struggled with economic relief |
| Vaccine Rollout | Rapid but faced some hesitancy | Well-organized and efficient | Slow due to hesitancy and misinformation |
| Estimation | Coefficients |
|---|---|
| Estimation 1 | [2 2 2 1] |
| Estimation 2 | [4 4 4 1] |
| Estimation 3 | [3 3 3 1] |
| Estimation | Coefficients |
|---|---|
| Estimation 1 | [2 2 2 1] |
| Estimation 2 | [4 2 2 1] |
| Estimation 3 | [6 4 4 1] |
| Estimation | Coefficients |
|---|---|
| Estimation 1 | [2 2 2 1] |
| Estimation 2 | [8 1 4 1] |
| Estimation 3 | [6 1 2 1] |
| Model | Best Fit Obtained |
|---|---|
| Italy | 99.01% |
| Switzerland | 98.67% |
| Romania | 98.59% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stăncioi, C.-M.; Ștefan, I.A.; Briciu, V.; Mureșan, V.; Clitan, I.; Abrudean, M.; Ungureșan, M.-L.; Miron, R.; Stativă, E.; Cordoș, R.C.; et al. A Comparative Study on COVID-19 Dynamics: Mathematical Modeling, Predictions, and Resource Allocation Strategies in Romania, Italy, and Switzerland. Bioengineering 2025, 12, 991. https://doi.org/10.3390/bioengineering12090991
Stăncioi C-M, Ștefan IA, Briciu V, Mureșan V, Clitan I, Abrudean M, Ungureșan M-L, Miron R, Stativă E, Cordoș RC, et al. A Comparative Study on COVID-19 Dynamics: Mathematical Modeling, Predictions, and Resource Allocation Strategies in Romania, Italy, and Switzerland. Bioengineering. 2025; 12(9):991. https://doi.org/10.3390/bioengineering12090991
Chicago/Turabian StyleStăncioi, Cristina-Maria, Iulia Adina Ștefan, Violeta Briciu, Vlad Mureșan, Iulia Clitan, Mihail Abrudean, Mihaela-Ligia Ungureșan, Radu Miron, Ecaterina Stativă, Roxana Carmen Cordoș, and et al. 2025. "A Comparative Study on COVID-19 Dynamics: Mathematical Modeling, Predictions, and Resource Allocation Strategies in Romania, Italy, and Switzerland" Bioengineering 12, no. 9: 991. https://doi.org/10.3390/bioengineering12090991
APA StyleStăncioi, C.-M., Ștefan, I. A., Briciu, V., Mureșan, V., Clitan, I., Abrudean, M., Ungureșan, M.-L., Miron, R., Stativă, E., Cordoș, R. C., Topan, A., & Nanu, I. (2025). A Comparative Study on COVID-19 Dynamics: Mathematical Modeling, Predictions, and Resource Allocation Strategies in Romania, Italy, and Switzerland. Bioengineering, 12(9), 991. https://doi.org/10.3390/bioengineering12090991









