Abstract
Feeding a growing global population requires sustainable, innovative, and cost-effective solutions, especially in light of the environmental damage and nutrient imbalances caused by excessive chemical fertilizer use. Microalgae have gained prominence due to their phylogenetic diversity, physiological adaptability, eco-compatible characteristics, and potential to support regenerative agriculture and mitigate climate change. Functioning as biofertilizers, biostimulants, and bioremediators, microalgae accelerate nutrient cycling, improve soil aggregation through extracellular polymeric substances (EPSs), and stimulate rhizospheric microbial diversity. Empirical studies demonstrate their ability to increase crop yields by 5–25%, reduce chemical nitrogen inputs by up to 50%, and boost both organic carbon content and enzymatic activity in soils. Their application in saline and degraded lands further promotes resilience and ecological regeneration. Microalgal cultivation platforms offer scalable in situ carbon sequestration, converting atmospheric carbon dioxide (CO2) into biomass with potential downstream vaporization into biofuels, bioplastics, and biochar, aligning with circular economy principles. While the commercial viability of microalgae is challenged by high production costs, technical complexities, and regulatory gaps, recent breakthroughs in cultivation systems, biorefinery integration, and strain optimization highlight promising pathways forward. This review highlights the strategic importance of microalgae in enhancing climate resilience, promoting agricultural sustainability, restoring soil health, and driving global bioeconomic transformation.
1. Introduction
Soil is a complex ecosystem that hosts a vast array of microbes, which play a crucial role in decomposing organic matter and driving biogeochemical nutrient cycles [1]. Modern agriculture faces significant challenges due to the uncontrolled use of synthetic agrochemicals aimed at boosting crop yields [2]. Environmentally friendly bio-inoculants, such as symbiotic nitrogen (N) fixers, rhizobacteria, mycorrhizae, and microalgae, offer a sustainable alternative, delivering multiple benefits to plants and reducing the reliance on synthetic inputs [3,4,5]. Agricultural practices and conventional farming systems are under increasing pressure due to global issues such as climate change, rapid population growth, and environmental degradation. This highlights the pressing need for sustainable solutions that improve resource efficiency and mitigate ecological impacts [6]. Within this context, microalgae have gained attention for their potential contributions to carbon sequestration, climate change mitigation, soil health improvement, and sustainable farming systems [7,8,9].
Microalgal biomass enhances soil health by improving structure, increasing water retention capacity, stimulating microbial activity, and supplying essential nutrients [9]. These microorganisms possess a remarkable ability to absorb CO2 and nutrients from wastewater, thereby boosting soil fertility [10]. Their integration into agricultural systems presents a viable alternative to chemical fertilizers, offering economic benefits and supporting the overall nitrogen cycle [11,12]. Bioactive compounds produced by microalgae [13] act as biostimulants, promoting microbial activity, nutrient cycling, and plant development by releasing growth hormones, polysaccharides, and antimicrobial substances [9]. Thanks to their adaptability, microalgae are also ideal candidates for the sustainable production of biofuels and other high-value products such as bioplastics, pharmaceuticals, and nutraceuticals [14].
Application of microalgae as foliar sprays, seed primers, or soil amendments significantly enhances grain yields, root development, shoot dry matter, germination rates, carotenoid and chlorophyll accumulation, nitrate reduction, and overall plant height [15]. In addition, they produce allelopathic compounds that function as bioherbicides or biological control agents, positioning them as valuable tools for environmentally sustainable pest management [16].
Microalgae encompass both prokaryotic cyanobacteria (also known as blue-green algae) and eukaryotic green algae. These photosynthetic organisms play a vital role in converting atmospheric CO2 into organic biomass [9]. They are responsible for approximately 50% of Earth’s oxygen production by transforming sunlight into bioenergy and contributing to CO2 stabilization [17].
Additionally, microalgae play a vital role in mitigating climate change through carbon sequestration by absorbing atmospheric CO2 via photosynthesis [18]. In fact, for every kilogram of biomass produced, microalgae can sequester approximately 1.3 kg of CO2 [19]. Their carbon adsorption capacity is 10–50 times greater than that of terrestrial plants, without competing with food sources for humans or animals [20]. This efficiency is largely due to a specialized mechanism known as the carbon concentration mechanism (CCM). Through this process, the pyrenoid—an organelle located near the thylakoid membranes—elevates CO2 concentrations in its vicinity. This localized CO2 accumulation enhances the performance of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), a key enzyme involved in photosynthesis that facilitates CO2 fixation. Despite its fundamental role, Rubisco exhibits a low affinity for CO2 due to its evolutionary adaptation to ancient high-CO2 and low-O2 environments. The pyrenoid helps to compensate this inefficiency by continuously creating a favorable microenvironment for enhanced carbon assimilation [21,22].
Given their multifunctional composition, microalgae are considered as a promising feedstock for both CO2 sequestration and bioenergy production [20]. However, scaling up algal cultivation for large-scale agriculture and wastewater treatment remains challenging. Species selection is crucial, as algal performance varies with the types of pollutants and environmental conditions [23]. Some microalgal species also show potential in phytoremediation, effectively removing contaminants such as heavy metals from polluted soils and contributing to land rehabilitation for agricultural purposes [24].
In this context, the circular bioeconomy paradigm has emerged, emphasizing sustainable production, harnessing solar energy, and converting renewable resources into valuable bioproducts [25]. Microalgae-based biotechnology plays a pivotal role in advancing this paradigm. These organisms can transform solid, liquid, and gaseous waste residues into nutrient-rich biomass for producing high-value bioproducts. The sequential extraction of such bioproducts using microalgae-based biorefinery offers a viable strategy to improve the economic feasibility of the process [26]. Chlorella vulgaris is able to eliminate pollutants from undiluted textile wastewater to below legal discharge limits, with 0.234–0.290 d−1 growth rates, 78–112.39 mg DW L−1 d−1 productivities, and valuable pigment production for circular economy applications [27]. The use of 20 g L−1 organic fertilizer combined with 20 mM urea for Chlorella sp. cultivation can enhance biomass productivity to 1.04 g L−1 d−1 and lutein content to 6.03 mg g−1 while reducing production costs by ~96–97%, and ≥1 μM lutein greatly reduced reactive oxygen species (ROS) in mammal cells subjected to blue-light irradiation [28]. In spite of their elevated production costs, Chlorella sp. and Spirulina (Arthrospira) sp., representing more than 90% of the world’s microalgal biomass production, are gaining wider utilization outside nutraceuticals in wastewater treatment, biofuel production, innovative medical treatments, and green industrial technologies [29].
This review highlights the diverse applications of microalgae in waste remediation, biofuel development, and biofertilizer production within the framework of a circular bioeconomy. It also explores current challenges, limitations, and future perspectives [30], underscoring the potential benefits of microalgal technologies in enhancing carbon sequestration, improving soil health, and advancing sustainable agriculture. Furthermore, it addresses the key barriers and future opportunities in adopting microalgae for climate-smart soil management practices.
2. Contribution to Soil Health Improvement
Microalgae are an effective tool in regenerative and sustainable agriculture due to their remarkable biological and biochemical capabilities for enhancing soil health [31]; microalgae serve diverse ecological functions, including biological nitrogen fixation, carbon sequestration, pollutant remediation, soil stabilization, and augmentation of organic matter [4].
Microalgal biomass contributes to soil enrichment through decomposition processes that increase organic matter content [9], improve soil structure [32], enhance water-holding capacity [33], and stimulate microbial enzymatic activity [31]. A key mechanism underlying these effects is the secretion of extracellular polymeric substances (EPSs), which bind soil particles, improve aggregate stability [32], reduce evaporation losses [33], and increase porosity [32].
Microalgae contribute significantly to soil aggregation not only through EPS secretion but also via physical entanglement, mucilage formation, and flagellar interactions. These mechanisms promote better gas exchange, water retention, erosion resistance, and overall soil fertility, all crucial for crop productivity [32]. While microaggregates (<250 μm) form through mineral interactions, microbial and algal exudates bind them into macroaggregates (>250 μm), making aggregation a key indicator of soil health [34]. Cyanobacteria and soil algae support topsoil stabilization by forming biocrusts through EPS secretion, trichome entanglement, and mucilaginous sheath formation, thereby enhancing soil texture and integrity [32,35].
Microalgae also secrete plant growth regulators such as gibberellins, cytokinins, and auxins to enhance root development and promote exudation [8,36]. These processes stimulate microbial proliferation and further contribute to aggregate formation [37]. Specific strains, including Chlorococcum mexicana and C. sajao, are known to improve soil aggregate stability in temperate agricultural environments [38]. Acidophilic species such as Desmodesmus and Heterochlorella form algal crusts on soil surfaces, stabilize acidic soils, and raise pH levels [39]. Chamizo (2018) demonstrated that both the nitrogen-fixing Scytonema javanicum and the non-N-fixer Phormidium ambiguum enhance EPS production and contribute to biocrust formation, improving soil integrity and erosion resistance [40].
EPS produced by cyanobacteria also bind soil particles and metal ions (Ca, Fe, and Zn), forming stable organo-mineral complexes that enhance aggregation [9,41]. Chlamydomonas species adhere to soil particles via electrostatic forces and flagella to facilitate aggregation [42]. Overall, cyanobacteria and green algae influence soil physical properties, promote root growth, and contribute to carbon sequestration through biochemical secretion and physical wrapping mechanisms [43,44].
Specific microalgae strains, in degraded or saline soils, improve the soil quality by enhancing organic matter, lowering pH, enhancing catalase, sucrase, and urease activities, boosting chlorophyll, and improving microbial performance [45]. In tomato cultivation, microalgae-based biofertilizers can substantially increase soil nutrients such as phosphorus by 27.4%, dissolved organic carbon by 231.3%, organic nitrogen by 403.4%, ammonium-N by 125.2%, nitrate-N by 215.6%, and magnesium by 73.4% [31,45,46,47,48,49,50,51].
Biofertilizers derived from microalgae are recognized as sustainable, affordable, and eco-friendly alternatives to synthetic inputs. Their versatility spans multiple ecosystems, from enhancing nitrogen fixation in tropical lowlands to mitigating erosion in temperate zones [4,7]. Widely used strains in paddy rice cultivation include Nostoc, Anabaena, Tolypothrix, and Aulosira, while symbiotic combinations such as Azolla-Anabaena and Rhizobium species are known to improve both soil fertility and crop performance [52,53]. In oil palm farming, microalgal biofertilizers have led to notable growth improvements [54]. Similarly, experiments with maize demonstrated that Nostoc piscinale increased humus content (a proxy for soil organic matter) by 17–20% compared to control conditions, indicating significant enhancement of soil fertility [55].
From this finding, it is clear that EPS-producing green algae, nitrogen-fixing cyanobacteria, and plant growth regulators are combined to form a multi-strain microalgal biofertilizer, releasing species to improve crop yields, salvage degraded soils, and store carbon in a variety of agricultural environments.
3. Contribution to Sustainable Agriculture
The application of microalgae such as Chlorella vulgaris and Spirulina platensis has shown notable improvements in soil quality and crop yields, with up to a 20.9% increase reported in rice cultivation [56]. In maize, microalgal treatments have resulted in enhanced germination rates, early-stage growth, and improved yield parameters [57]. For example, inoculation with Monoraphidium sp. in tomato (Solanum lycopersicum) resulted in a 32% increase in shoot biomass and a 12% rise in chlorophyll-a content, thereby improving photosynthetic efficiency [58]. Similarly, Nostoc piscinale positively influenced vegetative growth and grain yield in maize (Zea mays) [59]. In the case of Anabaena species, mass yield, nitrogen fixation, disease resistance, nutrient uptake, and soil fertility in wheat and other crops are significantly increased [60].
A wheat field experiment showed that wastewater-grown microalgae plus compost increased microbial biomass carbon by 31.8–67.0%, cut nitrogen fertilizer needs by 25%, and improved grain nitrogen content (3.56%), plant dry weight (7.4–33.1%), spike weight (≤10%), and thousand-grain weight (5.6–8.4%) [61]. In Brazil, co-inoculation of Chlorella vulgaris with Rhizobium tropici and Azospirillum brasilense improved bean yields by 219.7 kg ha−1 in clay soil and 656.0 kg ha−1 in sandy soil, yielding 25.6% higher profits compared to nitrogen-fertilized controls [62] (Figure 1 and Table 1).
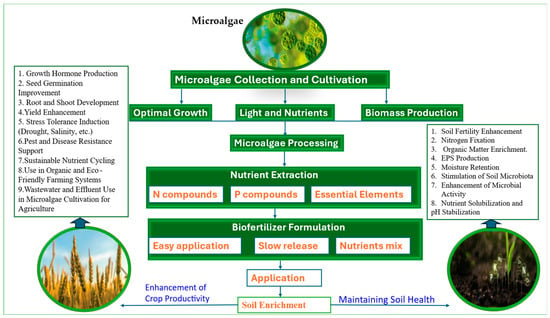
Figure 1.
A schematic representation contribution of microalgae in soil improvement and sustainable agriculture.
Cyanobacteria such as Nostoc and Anabaena have demonstrated the ability to reduce synthetic nitrogen fertilizer requirements by 25–50%, enhance microbial activity and crop yields by 5–25%, fix biologically 25–40 kg ha−1 of nitrogen, and minimize nitrogen leaching to around 7%, compared to 50% under conventional systems [63]. Their secretion of EPS significantly improves soil structure by 85–160% across loam, silty clay, and sandy soils, enhancing aeration, water infiltration, and moisture retention [64]. Moreover, co-inoculation with Bacillus sp. and other species increases microbial diversity and suppresses soil pathogens in continuous tomato cultivation, reinforcing the system’s resilience against biological stress [65,66].
Microalgae also act as potent bio-stimulants. Extracts from Scenedesmus obliquus have been reported to increase seed germination by 40%, auxin-like activity by 60%, and cytokinin-like activity by 187.5% in crops like mung bean and watercress [67]. Mixed algal treatments further improve shoot and root length, biomass accumulation, flower count, and overall yield, with increases of 46–57% relative to controls [68,69].
Microalgae can improve crop yields by 15.7–29.6%, reduce chemical fertilizer use, and restore degraded agroecosystems via nutrient cycling, nitrogen fixation, soil improvement, microorganism activation, and stress alleviation [70]. These findings confirm that microalgae are a robust, versatile, and environmentally sustainable resource for enhancing soil health, stress tolerance, and agricultural productivity. Figure 1 and Table 1 summarize how microalgae contribute to soil health improvement and sustainable agriculture.

Table 1.
Contribution of microalgae in soil improvement and sustainable agriculture.
Table 1.
Contribution of microalgae in soil improvement and sustainable agriculture.
| Species | Function/Method | Effect/Benefits | References |
|---|---|---|---|
| Arthrospira platensis, Chlorella vulgaris, Nostoc muscorum, Anabaena azollae, Scenedesmus spp., Dunaliella salina | Soil enrichment, EPS secretion, biomass decomposition | Enhance soil health, increase organic matter, improve soil structure, water-holding capacity, and microbial enzymatic activity | [4,9,31,32,33,37] |
| Nostoc muscorum, Tolypothrix tenuis, Anabaena spp. | EPS secretion (soil particle binding, mucilage, flagella) | Improve aggregation, porosity, reduce evaporation, enhance gas exchange, water retention, erosion resistance, and soil fertility | [32,33,34,44] |
| Nostoc calcicole, Cyanobacteria, Scytonema spp., Anabaena spp. | Biocrust formation (EPS, trichomes, mucilage) | Stabilize topsoil, improve soil texture, and integrity | [32,35,71] |
| Nostoc commune, Tolypothrix distorta, Trichocoleus desertorum, Leptolyngbya frigida, Chlorella vulgaris, Nannochloropsis salina, Arthrospira platensis, Spirulina platensis | Secretion of plant growth regulators (gibberellins, cytokinins, auxins) and seed germination | Enhance root development, stimulate microbial activity, and increase aggregate formation | [8,36,37,72] |
| Chlorococcum mexicana, C. sajao | EPS and soil aggregation | Improve soil aggregate stability in temperate agriculture | [38] |
| Desmodesmus, Heterochlorella | Acidophilic biocrust formation | Stabilize acidic soils, raise pH levels | [39] |
| Scytonema javanicum (N-fixer), Phormidium ambiguum (non-N-fixer) | EPS production, biocrust formation | Enhance soil integrity, erosion resistance | [40] |
| Chlorella spp., Scenedesmus spp., Nannochloropsis spp., Dunaliella salina | Binding soil particles and metals (Ca, Fe, Zn) | Form organo-mineral complexes, enhance aggregation | [9,41] |
| Chlamydomonas spp. | Electrostatic/flagellar adhesion | Facilitate soil particle aggregation | [42] |
| Tetradesmus obliquus and Chlorella sorokiniana, Chlorella pyrenoidosa, Azotobacter beijerinckii, Leptolyngbya spp., Dunaliella salina | Soil improvement in degraded/saline soils | Enhance organic matter, lower pH, improve enzyme activity, and microbial performance | [45,73,74,75,76] |
| Tribonema spp., Thermonaerobaculia, Subgroup_10, Sordariomycetes, Microascaceae, Pseudomonas, Togniniaceae, and Phaeoacremonium, Chlorella ellipsoidea, Arthrospira maxima | Soil nutrient enrichment in tomato cultivation | ↑ Phosphorus (27.4%), organic N (403.4%), ammonium-N (125.2%), nitrate-N (215.6%), Mg (73.4%) | [31,45,46,47,48,49,50,51] |
| Nostoc, Anabaena, Tolypothrix, Aulosira | Nitrogen fixation in paddy rice | Improve soil fertility, reduce synthetic inputs | [4,7,52,53] |
| Azolla–Anabaena, Rhizobium spp. (symbiotic) | Biofertilizer combinations | Enhance fertility, crop performance | [52,53] |
| Nostoc piscinale | Biofertilizer in maize | ↑ Humus 17–20%, improve soil fertility and crop yield | [55,59] |
| Chlorella vulgaris, Spirulina platensis | Biofertilizer application | Improve soil quality, ↑ rice yield by 20.9% | [56] |
| Monoraphidium spp. | Tomato inoculation | ↑ Shoot biomass 32%, ↑ Chlorophyll-a 12% | [58] |
| Anabaena spp. | Nitrogen fixation, growth regulator secretion | ↑ Yield, nitrogen fixation, disease resistance, soil fertility (wheat and others) | [60] |
| Microalgae (Chlorella spp.) + compost (wheat field) | Wastewater-grown algal application | ↑ Microbial biomass C (31.8–67%), ↓ fertilizer use 25%, ↑ grain N (3.56%), ↑ yield components | [61,77] |
| Chlorella vulgaris + Rhizobium tropici + Azospirillum brasilense | Co-inoculation (bean cultivation) | ↑ Yield (219.7–656 kg ha−1), ↑ profit by 25.6% | [62] |
| Nostoc spp., Anabaena | EPS secretion, N fixation | ↓ Fertilizer use by 25–50%, ↑ microbial activity 5–25%, fix 25–40 kg N ha−1, reduce leaching | [63,64] |
| Burkholderia vietnamiensis + Trichoderma harzianum | Co-inoculation in tomato | ↑ Microbial diversity, suppress pathogens, enhance resilience | [65,66] |
| Scenedesmus obliquus | Bio-stimulant activity | ↑ Germination 40%, auxin-like activity 60%, cytokinin-like activity 187.5% | [67] |
| Trichormus variabilis, Auxenochlorella pyrenoidosa, Spirulina platensis Anabaena spp., Tribonema spp., Chlorella vulgaris | Biofertilizer and soil restoration | ↑ Crop yields 15.7–29.6%, ↓ fertilizer use, restore degraded soils | [70] |
Note: (↑) Increase and (↓) Decrease.
4. Contribution to Carbon Sequestration and Climate Change Mitigation
Microalgae play a pivotal role in the global carbon cycle and carbon sequestration, contributing both to natural ecosystems and engineered systems. Through biological carbon fixation, they significantly influence atmospheric CO2 concentrations [78]. Marine microalgae, especially phytoplankton such as diatoms, are responsible for approximately 50 gigatons (Gt) of CO2 fixation annually, representing half of Earth’s total biological carbon assimilation [79]. Remarkably, diatoms alone account for 20% of global CO2 sequestration, a capacity equivalent to that of all the world’s rainforests combined [80].
Their ability to capture atmospheric CO2 is further amplified by their tolerance to other greenhouse gases, including hydrocarbons (HC), sulfur dioxide (SO2), methane, and nitrogen oxides (NOx). It is estimated that microalgae can absorb up to 77% of total greenhouse gas emissions [20], operating with a carbon capture efficiency 10–50 times higher than terrestrial plants [81].
Photosynthesis is their primary mechanism for capturing carbon. Benefiting from a high surface area-to-volume ratio and accelerated growth rates, microalgae can fix CO2 up to 50 times faster than land flora [82,83]. Certain species, such as Chlorella vulgaris, have demonstrated sequestration rates of 1.6–2 tons of CO2 per ton of biomass produced [84]. In photobioreactor and open raceway pond systems, biomass yields can reach up to 82 tons per hectare annually [85]. Emerging technologies, including spray absorption towers, have enhanced CO2 fixation performance, achieving improvements of 50%, compared to the 11.17% efficiency of conventional bubbling systems [86].
Research in China’s karst wetlands indicates that microalgae can fix over 4200 tons of carbon annually, with 28.7% of bicarbonate converted into organic carbon via photosynthesis [87], underscoring their effectiveness as natural carbon sinks in aquatic and wetland environments. Global estimates of microalgal biomass production indicate 93,756 tons in 2010, 87,000 tons in 2018, and 56,465 tons in 2019 [88], which correlates with CO2 sequestration totals of approximately 187,500 tons, 174,000 tons, and 112,900 tons, respectively [20,89].
Engineered systems designed for industrial-scale CO2 capture include photobioreactors, bioenergy facilities, and biochar platforms [90], which can be integrated with factories and power plants to extract CO2 directly from flue gas emissions [91]. The harvested biomass supports the production of biofuels [19], bioplastics [92], fertilizers [90], and biochar [90], a soil amendment that not only enhances long-term carbon storage but also reinforces the circular carbon economy [91].
Under optimal conditions in photobioreactors, Chlorella species can achieve CO2 uptake rates of 160–175 mg of biomass per liter per day [93]. Some systems have reached capture efficiencies of 93.7% [94], with comparable results obtained in continuously stirred tank reactors, which report up to 178 mg L−1 d−1 and 96% CO2 removal efficiency [95].
In agricultural contexts, microalgae provide synergistic benefits, enhancing crop productivity while contributing to carbon sequestration and mitigating greenhouse gas emissions. One study in hawthorn orchards found that microalgal biofertilizers increased fruit yields by 29.6%, improved soil organic carbon content, and maintained stable greenhouse gas emission levels [70]. Figure 2 and Table 2 summarize how microalgae contribute to carbon sequestration and climate change mitigation.
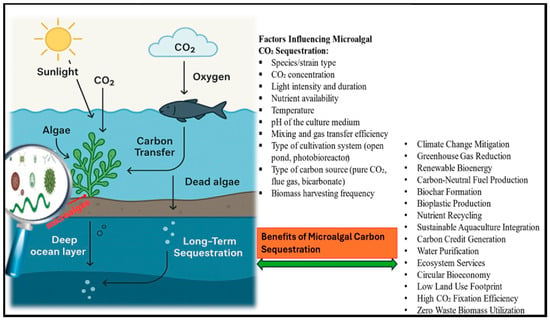
Figure 2.
A schematic representation of the contribution of microalgae to carbon sequestration.

Table 2.
Contribution of microalgae to carbon sequestration and climate change mitigation.
5. Contribution to Environmental Benefits
As previously noted, atmospheric CO2 can be effectively sequestered by microalgae, which convert it into biomass through photosynthesis [9]. This biological process is crucial for reducing greenhouse gas emissions and mitigating climate change. Due to their adaptability to aquatic environments and rapid growth rates, microalgae fix atmospheric CO2 more efficiently than terrestrial plants [18]. They are particularly effective at absorbing excess CO2 from industrial emissions while simultaneously producing oxygen [98]. For example, in photobioreactors, microalgae utilize CO2 from flue gases and other waste streams to generate valuable biomass [86].
Algal biomass serves as a sustainable alternative to fossil fuels and synthetic fertilizers, helping to reduce emissions and dependency on resources [99,100]. Biofuels derived from microalgae enable closed-loop carbon recycling by reusing absorbed CO2 and generating oxygen, offering a renewable energy source with significantly lower carbon emissions compared to conventional fossil fuels [83].
Additionally, microalgae can absorb airborne pollutants such as nitrogen oxides (NOx) and sulfur oxides (SOx), contributing to reductions in smog and acid rain. These pollutants are metabolized in biofiltration systems, making microalgae effective tools for air purification in urban and industrial settings [101].
Microalgae cultivation has shown promise in areas affected by soil degradation or desertification, improving agricultural productivity and restoring soil fertility [102]. Species adapted to saline environments have been successfully grown in brackish water and degraded soils to enhance organic matter content and improve soil structure [103].
Thanks to their resilience, microalgae thrive in diverse environmental conditions, including arid, nutrient-poor, and saline soils [103]. By assimilating excess nutrients such as phosphorus and nitrogen, which contribute to eutrophication, microalgae efficiently purify wastewater [104]. They also enhance water quality through the biosorption and bioaccumulation of heavy metals, such as cadmium, lead, and mercury [24,105]. These features make microalgae especially suitable for agricultural development in regions facing water scarcity or high salinity [106].
Furthermore, microalgae can be processed into biodegradable and renewable products such as building materials, textiles, and bioplastics, advancing circular economy principles and reducing long-term pollution [107].
Microalgae-powered systems can capture industrial CO2, purify air and wastewater, restore degraded soils, and produce renewable fuels, fertilizers, and biodegradable materials, creating a single, self-sustaining solution for climate, energy, and resource challenges.
Figure 3 and Table 3 summarize how microalgae sequester atmospheric CO2, purify wastewater, improve soil health in degraded environments, and produce renewable bioproducts supporting sustainable agriculture and circular bioeconomy strategies.
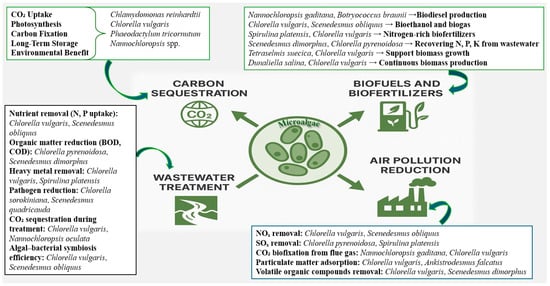
Figure 3.
Roles of microalgae in climate mitigation and ecosystem restoration. Microalgae sequester atmospheric CO2, purify wastewater, improve soil health in degraded environments, and produce renewable bioproducts supporting sustainable agriculture and circular bioeconomy strategies.

Table 3.
Contribution of microalgae to environmental benefits.
6. Contribution to Circular Economy and Waste Valorization
Microalgae such as Chlorella and Scenedesmus are highly efficient in wastewater treatment, capable of removing nutrients like nitrate, phosphate, and ammonium [108], as well as heavy metals (Cu, Zn, Pb, Cr, Cd), dyes, and organic pollutants, with removal rates ranging from 90% to 98% [109]. By assimilating these compounds into their biomass, they purify water while generating a valuable feedstock [26]. Additionally, microalgae utilize CO2 from flue gases to accelerate growth, transforming industrial emissions into useful bioresources [110]. For example, Micractinium pusillum has demonstrated CO2 fixation rates of ~137 mg L−1day−1, lipid yields of nearly 32%, and biomass production of 1.3 g L−1, even when cultivated directly in flue gas streams [111].
This capacity supports co-treatment strategies, turning pollutants and effluents into productive outputs [112]. Integrated biorefineries offer a sustainable solution, producing biofuels such as biodiesel, biogas, and bioethanol, alongside high-value co-products including omega-3 oils, proteins, phycobiliproteins, biofertilizers, and bioplastics, aligning with a zero-waste valorization model [26]. Certain strains like Schizochytrium sp. and Nannochloropsis spp. exhibit lipid content exceeding 45% dry weight. When cultivated on food waste hydrolysates, they can reach 49% lipid accumulation and 32% FAME yield [112].
The cultivation of Nostoc sp., Arthrospira platensis, and Porphyridium purpureum in industrial wastewater has demonstrated removal rates of 98% COD, 94% nitrogen, and 100% phosphate, with phycocyanin yields up to 103 mg g−1 dry weight [113].
Residual biomass can be converted into biochar, which improves soil fertility, enhances water retention, and contributes to long-term carbon sequestration—key components of circular and net-zero strategies [114]. In the EU Horizon project WWTBP-by-Microalgae, Arthrospira platensis treated brewery effluents by removing 90% of nutrients and CO2, producing phycocyanin pigments valued at EUR 221 kg−1, biogas (93 mL CH4 g−1 VS), and biochar [115].
Pilot-scale systems demonstrate nitrogen and phosphorus removal above 90% in both municipal and industrial wastewater treatments [116]. In warmer regions, hybrid pond systems for biogas and biofertilizer production offer more sustainable alternatives to conventional wastewater methods [105]. Cascading biorefinery models maximize resource recovery beginning with lipid extraction, followed by protein, pigment, and carbohydrate recovery [112,117].
Economic viability remains a challenge but is improving. In Almeria, Spain, a tubular photobioreactor using Scenedesmus almeriensis reported a cost of ~EUR 69 kg−1 dry biomass, projected to fall to EUR 12–13 kg−1 at 200 t y−1 scale [118]. In Italy, a 1-hectare Green Wall Panel system with Tetraselmis suecica reported costs of EUR 12.4 kg−1, reducing to EUR 5.1 kg−1 at 100 ha scale and potentially EUR 3.2 kg−1 under optimal climate conditions [119]. Scale-up involves logistical complexities in harvesting, drying, and extraction, requiring optimization to lower energy demand. Additionally, regulatory gaps and limited market infrastructure hinder widespread adoption [120].
Nonetheless, industry outlooks remain positive. According to Fortune Business Insights (June 2025), the global microalgae market is expected to grow at a CAGR of 7.29%, rising from USD 782.6 million in 2024 to USD 1.38 billion by 2032 [121]. Table 4 summarizes how microalgae integrate CO2 mitigation, wastewater purification, high-value product generation, and validation of the circular economy, alongside current limitations in cost, scaling, and regulation.

Table 4.
Contribution of microalgae to circular economy and waste valorization.
7. Challenges, Limitations, Solutions, and Future Directions
The widespread application of microalgae faces considerable technical, economic, and environmental hurdles that hinder its commercial deployment across various sectors, including biofuels, food, pharmaceuticals, and wastewater treatment [124,125]. While low-cost open raceway ponds are easy to implement, they are prone to contamination, have low productivity, and offer limited environmental control, resulting in inconsistent yields [23,126,127]. In contrast, photobioreactors (PBRs) ensure operational control and high biomass productivity, yet their elevated capital and energy costs restrict scalability [128,129,130]. Additional challenges in closed systems include insufficient light penetration in dense cultures and photoinhibition, which reduces efficiency [91,131,132].
Downstream processing remains complex and energy-intensive, as extracting pigments, lipids, and proteins often requires cell wall disruption that can affect microalgal viability [133,134]. Microalgae also exhibit high sensitivity to variations in temperature, light, salinity, and pH, confining large-scale outdoor cultivation to specific geographic zones [135,136]. From an economic standpoint, the cost per liter of algal biofuels and derivatives still exceeds that of other biomass sources and fossil alternatives—even when factoring in co-products [137,138].
Despite these constraints, microalgae show strong promise as biofertilizers and soil amendments in regenerative agriculture [4,7,139]. Species such as Spirulina and Chlorella are rich in nitrogen, phosphorus, potassium, amino acids, and micronutrients. These compounds improve soil structure, promote microbial communities, and boost nutrient availability [4,140,141]. Their application reduces reliance on synthetic fertilizers, preventing contamination of soil and water systems [141,142,143]. Integrating algal biomass into biochar further enhances carbon retention, presenting a natural method for long-term soil carbon storage [19,144].
Large-scale microalgae cultivation in PBRs or open ponds can be coupled with industrial CO2 capture systems, thereby aiding in emission reductions from fossil fuel combustion and manufacturing processes [145,146]. In sustainable farming, microalgae also contribute to stress resilience and pest control through the production of natural antimicrobial and antifungal metabolites [8,147]. Their ability to grow on non-arable land or wastewater positions them as resource-efficient inputs that do not compete with food crops [148]. This circular approach reinforces their potential in climate-smart agriculture, placing microalgae at the core of resilient agroecosystems [149,150].
The advancement of genetic engineering and synthetic biology offers pathways to develop stress-tolerant strains with enhanced metabolite yields and growth rates [151,152,153]. Parallel improvements in cultivation and harvesting techniques, focusing on energy savings and cost reduction, are vital for scaling operations. Additionally, integrated algal biorefineries should be developed to produce biofertilizers, biofuels, feedstocks, bioplastics, and nutraceuticals, ensuring economic feasibility [154,155,156,157].
Supportive policies will be instrumental in accelerating adoption: Financial incentives, increased research funding, and regulatory frameworks are needed to safeguard ecological integrity [158]. Public engagement and agricultural education will also be key in fostering acceptance at the community level. Through coordinated scientific and societal efforts, microalgae can become a cornerstone in climate-resilient agriculture, enabling carbon neutrality, soil restoration, and a global transition to sustainable bioeconomy systems (Table 5).

Table 5.
A comprehensive table compiles all algal species mentioned throughout the paper, alongside their documented uses and purposes.
From the discussion, it is clear that Spirulina spp. and Chlorella spp. are nutrient-rich for biofertilizers, stress-tolerant strains like engineered Chlorella vulgaris and Dunaliella salina suit harsh conditions, and high-biomass species Nannochloropsis. Scenedesmus enables biofuels and CO2 capture, Haematococcus and Spirulina provide bioactive compounds, versatile Chlorella and Nannochloropsis support multi-product biorefineries for a circular bioeconomy [4,26].
8. Conclusions
Microalgae are increasingly recognized as a versatile and sustainable biological resource, offering transformative potential across agriculture, environmental remediation, energy production, and industrial biotechnology. Their exceptional ability to sequester atmospheric CO2, eliminate pollutants, purify wastewater, and rehabilitate degraded soils positions them as a strategic asset in confronting the interlinked global crises of climate change, soil degradation, and food insecurity. In agriculture systems, microalgae act as biofertilizers and biostimulants, contributing to enhanced crop productivity, improved soil health, and more efficient nutrient cycling. These benefits are achieved while simultaneously reducing dependence on synthetic agrochemicals, thereby promoting more ecologically sound farming practices. Moreover, their capacity to thrive on non-arable land and utilize waste and carbon streams underscores their suitability for integration into circular bioeconomy frameworks. The emergence of algal biorefineries presents a scalable and economically viable approach to the production of high-value commodities, including biofuels, bioplastics, pharmaceuticals, and functional foods. These systems support zero-waste valorization strategies and offer promising avenues for diversifying revenue streams within sustainable industries. However, despite these advantages, widespread commercialization remains constrained by several challenges—namely, energy-intensive downstream processing, cultivation limitations, elevated production costs, and insufficient regulatory support. Encouragingly, recent innovations in genetic engineering, hybrid cultivation platforms, and cascading extraction technologies are paving the way to overcome these barriers. Realizing the full potential of microalgae will require targeted investments in research and development, infrastructure, and the establishment of coherent policy frameworks. Equally important are public awareness, interdisciplinary collaboration, and educational initiatives to foster acceptance and long-term integration. With coordinated global efforts, microalgae stand poised not only to revolutionize agricultural paradigms but also to play a pivotal role in advancing carbon neutrality, restoring ecological balance, and building a resilient, bio-based economy for future generations.
Author Contributions
Conceptualization, A.S., G.M. and M.D.; methodology, A.S., G.M. and M.D.; software, M.M.H., V.I. and F.P.; validation, A.S., R.P.R. and M.D.; formal analysis, M.M.H. and V.I.; investigation, M.M.H. and V.I.; resources, M.M.H., V.I. and F.P.; data curation, A.S., G.M. and M.D.; writing—original draft preparation, M.M.H. and V.I.; writing—review and editing, M.M.H., B.K.S., A.S. and M.D.; visualization, A.S., M.D., G.M., B.K.S. and M.M.H.; supervision, M.D., G.M. and A.S.; project administration, M.M.H. and M.D.; funding acquisition, M.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Program on Agricultural and Rural Transformation for Nutrition, Entrepreneurship and Resilience in Bangladesh (PARTNER)-APCU, BARC, Bangladesh Agricultural Research Council, New Airport Road, Farmgate, Dhaka-1215. Memo No: PARTNER/APCU-BARC 02/HRD/2024/673.
Acknowledgments
The authors would like to thank the support of the Agricultural and Rural Transformation for Nutrition, Entrepreneurship and Resilience in Bangladesh (PARTNER)-APCU, BARC, Bangladesh, for the PhD scholarship granted to Md Muzammal Hoque, AlgaeBiomed for the PhD scholarship granted to Valeria Iannelli, and Bioinnova srls for the PhD scholarship granted to Francesca Padula. We also thank to Bangladesh Institute of Nuclear Agriculture (BINA), Mymensingh, Bangladesh and DAFE and DiSBA, UNIBAS, Potenza, Italy.
Conflicts of Interest
Author Valeria Iannelli was employed by the company AlgaeBioMed srl. Authors Francesca Padula and Rosa Paola Radice were employed by the company Bioinnova srls. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| EPS | Extracellular polymeric substances |
| CO2 | Carbon dioxide |
| CCM | Carbon concentration mechanism |
| Gt | Gigatons |
| HC | Hydrocarbons |
| SO2 | Sulfur dioxide |
| PHB | Polyhydroxybutyrate |
| PLA | Polylactic acid |
| PBR | Photobioreactor |
| ROS | Reactive oxygen species |
References
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for Feeding the World More Sustainably with Organic Agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as Promising Complement of Chemical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Peng, C.; Li, D. Replacing Nitrogen Fertilizer with Nitrogen-Fixing Cyanobacteria Reduced Nitrogen Leaching in Red Soil Paddy Fields. Agric. Ecosyst. Environ. 2021, 312, 107320. [Google Scholar] [CrossRef]
- Viana, C.M.; Freire, D.; Abrantes, P.; Rocha, J.; Pereira, P. Agricultural Land Systems Importance for Supporting Food Security and Sustainable Development Goals: A Systematic Review. Sci. Total Environ. 2022, 806, 150718. [Google Scholar] [CrossRef]
- Reisoglu, Ş.; Aydin, S. Microalgae as a Promising Candidate for Mitigating Climate Change and Biodiversity Loss. In Microalgae—Current and Potential Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next Generation Plant Growth Additives: Functions, Applications, Challenges and Circular Bioeconomy Based Solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Gurau, S.; Imran, M.; Ray, R.L. Algae: A Cutting-Edge Solution for Enhancing Soil Health and Accelerating Carbon Sequestration—A Review. Environ. Technol. Innov. 2025, 37, 103980. [Google Scholar] [CrossRef]
- Mutum, L.; Janda, T.; Ördög, V.; Molnár, Z. Biologia Futura: Potential of Different Forms of Microalgae for Soil Improvement. Biol. Futur. 2022, 73, 1–8. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-Fertilizers: Between Current Situation and Future Prospective: The Role of Algae as a Bio-Fertilizer in Serving of Ecosystem. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Grina, F.; Lorentz, J.F.; Silva, I.; Castaño-Sánchez, O.; Marks, E.A.N. Green Agriculture: A Review of the Application of Micro- and Macroalgae and Their Impact on Crop Production on Soil Quality. J. Soil Sci. Plant Nutr. 2022, 22, 4627–4641. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Alam, M.A.; Xu, J.L.; Wang, Z. Microalgae Biotechnology for Food, Health and High Value Products; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–483. [Google Scholar] [CrossRef]
- Youssef, S.M.; El-Serafy, R.S.; Ghanem, K.Z.; Elhakem, A.; Abdel Aal, A.A. Foliar Spray or Soil Drench: Microalgae Application Impacts on Soil Microbiology, Morpho-Physiological and Biochemical Responses, Oil and Fatty Acid Profiles of Chia Plants under Alkaline Stress. Biology 2022, 11, 1844. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of Microalgae as Biopesticides to Contribute to Sustainable Agriculture and Environmental Development. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 366–375. [Google Scholar] [CrossRef]
- Sekerc, Y.; Ozarslan, R. Oxygen-Plankton Model under the Effect of Global Warming with Nonsingular Fractional Order. Chaos Solitons Fractals 2020, 132, 109532. [Google Scholar] [CrossRef]
- Khambalkar, P.A.; Yadav, S.S.; Sadawart, M.J.; Shivansh. Innovative Use of Algae for Carbon Sequestration and Renewable Energy Generation. Environ. Rep. 2023, 5, 10–14. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal Biomass Valorization for Biofuel Production and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Ashour, M.; Mansour, A.T.; Alkhamis, Y.A.; Elshobary, M. Usage of Chlorella and Diverse Microalgae for CO2 Capture-towards a Bioenergy Revolution. Front. Bioeng. Biotechnol. 2024, 12, 1387519. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Adapting from Low to High: An Update to CO2-Concentrating Mechanisms of Cyanobacteria and Microalgae. Plants 2023, 12, 1569. [Google Scholar] [CrossRef]
- Barrett, J.; Girr, P.; Mackinder, L.C.M. Pyrenoids: CO2-Fixing Phase Separated Liquid Organelles. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118949. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Moreira, J.B.; Santos, T.D.; Duarte, J.H.; Bezerra, P.Q.M.; de Morais, M.G.; Costa, J.A.V. Role of Microalgae in Circular Bioeconomy: From Waste Treatment to Biofuel Production. Clean Technol. Environ. Policy 2023, 25, 427–437. [Google Scholar] [CrossRef]
- Ezhumalai, G.; Arun, M.; Manavalan, A.; Rajkumar, R.; Heese, K. A Holistic Approach to Circular Bioeconomy Through the Sustainable Utilization of Microalgal Biomass for Biofuel and Other Value-Added Products. Microb. Ecol. 2024, 87, 61. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.A.; Salgado, E.M.; Gonçalves, A.L.; Esteves, A.F.; Pires, J.C.M. Microalgae-Based Remediation of Real Textile Wastewater: Assessing Pollutant Removal and Biomass Valorisation. Bioengineering 2024, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.M.; Yang, Y.C.; Zhang, W.X.; Wu, J.X.; Chen, Y.T.; Lin, C.H.; Lin, M.W.; Lin, C.S. A Low-Cost Fertilizer Medium Supplemented with Urea for the Lutein Production of Chlorella Sp. and the Ability of the Lutein to Protect Cells against Blue Light Irradiation. Bioengineering 2023, 10, 594. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef]
- Sarma, S.; Sharma, S.; Rudakiya, D.; Upadhyay, J.; Rathod, V.; Patel, A.; Narra, M. Valorization of Microalgae Biomass into Bioproducts Promoting Circular Bioeconomy: A Holistic Approach of Bioremediation and Biorefinery. 3 Biotech 2021, 11, 378. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Feng, Y.; Zhou, C.; Li, X.; Yan, X.; Ruan, R.; Cheng, P. Microalgae-Based Biofertilizers Improve Fertility and Microbial Community Structures in the Soil of Potted Tomato. Front. Plant Sci. 2024, 15, 1461945. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Li, C.; Liang, Y.; Miao, Q.; Ji, X.; Duan, P.; Quan, D. The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes. Agronomy 2024, 14, 2102. [Google Scholar] [CrossRef]
- Mikha, M.M.; Jin, V.L.; Johnson, J.M.F.; Lehman, R.M.; Karlen, D.L.; Jabro, J.D. Land Management Effects on Wet Aggregate Stability and Carbon Content. Soil Sci. Soc. Am. J. 2021, 85, 2149–2168. [Google Scholar] [CrossRef]
- Crouzet, O.; Consentino, L.; Pétraud, J.P.; Marrauld, C.; Aguer, J.P.; Bureau, S.; Le Bourvellec, C.; Touloumet, L.; Bérard, A. Soil Photosynthetic Microbial Communities Mediate Aggregate Stability: Influence of Cropping Systems and Herbicide Use in an Agricultural Soil. Front. Microbiol. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of Microalgae and Cyanobacteria to Improve Soil Health and Agricultural Productivity: A Critical View. Environ. Sci. Adv. 2023, 2, 586–611. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal Metabolites: An Inevitable Substitute for Antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Shanthakumar, S.; Abinandan, S.; Venkateswarlu, K.; Subashchandrabose, S.R.; Megharaj, M. Algalization of Acid Soils with Acid-Tolerant Strains: Improvement in PH, Carbon Content, Exopolysaccharides, Indole Acetic Acid and Dehydrogenase Activity. Land Degrad. Dev. 2021, 32, 3157–3166. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Adessi, A.; Cruz de Carvalho, R.; De Philippis, R.; Branquinho, C.; Marques da Silva, J. Microbial Extracellular Polymeric Substances Improve Water Retention in Dryland Biological Soil Crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Kreis, C.T.; Grangier, A.; Bäumchen, O. In Vivo Adhesion Force Measurements of Chlamydomonas on Model Substrates. Soft Matter 2019, 15, 3027–3035. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. [Google Scholar] [CrossRef]
- De Silva, A.G.S.D.; Hashim, Z.K.; Solomon, W.; Zhao, J.-B.; Kovács, G.; Kulmány, I.M.; Molnár, Z. Unveiling the Role of Edaphic Microalgae in Soil Carbon Sequestration: Potential for Agricultural Inoculants in Climate Change Mitigation. Agriculture 2024, 14, 2065. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial Fertilizers Improve Soil Quality and Crop Yield in Coastal Saline Soils by Regulating Soil Bacterial and Fungal Community Structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Abinandan, S.; Praveen, K.; Venkateswarlu, K.; Megharaj, M. Seed Priming with Microalgae Enhances Plant Productivity and Rhizosphere Health. Soil Ecol. Lett. 2025, 7, 240271. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Laipan, M.; Wei, T.; Guo, J. Soil Health Improvement by Inoculation of Indigenous Microalgae in Saline Soil. Environ. Geochem. Health 2024, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Mutum, L.; Janda, T.; Molnar, Z. Microalgae–Bacteria Interaction: A Catalyst to Improve Maize (Zea mays L.) Growth and Soil Fertility. Cereal Res. Commun. 2025, 53, 1037–1049. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Li, Y.; Chen, L.; Liu, Z.; Wang, X.; Yan, P.; Qin, S. Responses of Soil Microbial Communities to a Short-Term Application of Seaweed Fertilizer Revealed by Deep Amplicon Sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Abideen, Z.; Waqif, H.; Munir, N.; El-Keblawy, A.; Hasnain, M.; Radicetti, E.; Mancinelli, R.; Nielsen, B.L.; Haider, G. Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy 2022, 12, 1788. [Google Scholar] [CrossRef]
- Nawaz, T.; Joshi, N.; Nelson, D.; Saud, S.; Abdelsalam, N.R.; Abdelhamid, M.M.A.; Jaremko, M.; Rahman, T.U.; Fahad, S. Harnessing the Potential of Nitrogen-Fixing Cyanobacteria: A Rich Bio-Resource for Sustainable Soil Fertility and Enhanced Crop Productivity. Environ. Technol. Innov. 2024, 36, 103886. [Google Scholar] [CrossRef]
- Samantaray, A.; Chattaraj, S.; Mitra, D.; Ganguly, A.; Kumar, R.; Gaur, A.; Mohapatra, P.K.D.; Santos-Villalobos, S.d.l.; Rani, A.; Thatoi, H. Advances in Microbial Based Bio-Inoculum for Amelioration of Soil Health and Sustainable Crop Production. Curr. Res. Microb. Sci. 2024, 7, 100251. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Sani, I.; Kurnia, Y.W.; Sinthya, H.C.; Anthony, R.; Situmorang, E.C.; Utomo, C.; Liwang, T. Exploring The Potency of Microalgae-Based Biofertilizer and Its Impact on Oil Palm Seedlings Growth. Agrivita 2022, 44, 139–151. [Google Scholar] [CrossRef]
- Solomon, W.; Mutum, L.; Rakszegi, M.; Janda, T.; Molnár, Z. Harnessing the Synergy of the Cyanobacteria-Plant Growth Promoting Bacteria for Improved Maize (Zea mays) Growth and Soil Health. Sustainability 2023, 15, 16660. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-Fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Jimenez, R.; Markou, G.; Tayibi, S.; Barakat, A.; Chapsal, C.; Monlau, F. Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Appl. Sci. 2020, 10, 3890. [Google Scholar] [CrossRef]
- Ördög, V.; Stirk, W.A.; Takács, G.; Pőthe, P.; Illés, Á.; Bojtor, C.; Széles, A.; Tóth, B.; van Staden, J.; Nagy, J. Plant Biostimulating Effects of the Cyanobacterium Nostoc Piscinale on Maize (Zea mays L.) in Field Experiments. South African J. Bot. 2021, 140, 153–160. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biofertilizing Effect of Soil Cyanobacterium Anabaena Cylindrica–Based Formulations on Wheat Growth, Physiology, and Soil Fertility. Agriculture 2025, 15, 189. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the Efficacy of Wastewater-Grown Microalgal Biomass as a Biofertilizer for Wheat. Environ. Sci. Pollut. Res. 2016, 23, 6608–6620. [Google Scholar] [CrossRef]
- De Oliveira, K.S.; Volsi, B.; Telles, T.S.; Mendes, A.D.R.; Yunes, J.S.; Andrade, D.S. Co-Inoculation with Rhizobium, Azospirillum, and Microalgae Increases Common Bean Yield and Profitability. Agron. J. 2025, 117, e21719. [Google Scholar] [CrossRef]
- Solomon, W.; Mutum, L.; Janda, T.; Molnár, Z. Potential Benefit of Microalgae and Their Interaction with Bacteria to Sustainable Crop Production. Plant Growth Regul. 2023, 101, 53–65. [Google Scholar] [CrossRef]
- Song, X.; Bo, Y.; Feng, Y.; Tan, Y.; Zhou, C.; Yan, X.; Ruan, R.; Xu, Q.; Cheng, P. Potential Applications for Multifunctional Microalgae in Soil Improvement. Front. Environ. Sci. 2022, 10, 1035332. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Li, J.; Hu, J.; Wei, Y.; Wang, Y.; Yang, H.; Zhou, Y.; Wu, Y.; Zhang, S. Co-Inoculating Burkholderia Vietnamiensis B418 and Trichoderma Harzianum T11W Reduced Meloidogyne Incognita Infestation of Tomato Plants. Microorganisms 2025, 13, 1337. [Google Scholar] [CrossRef]
- Tang, T.; Sun, X.; Liu, Q.; Dong, Y.; Zha, M. Treatment with Organic Manure Inoculated with a Biocontrol Agent Induces Soil Bacterial Communities to Inhibit Tomato Fusarium Wilt Disease. Front. Microbiol. 2023, 13, 1006878. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Gabriel Acién, F.; Gouveia, L. Biostimulant Potential of Scenedesmus Obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef]
- Mignoni, D.S.B.; Nonato, J.D.S.; Alves, J.S.; Michelon, W.; de Oliveira Nunes, E.; Pandolfi, J.R.; Luchessi, A.D. Agriculture Application, Comparison, and Functional Association between Macrophytes and Microalgae: A Review. Discov. Agric. 2025, 3, 82. [Google Scholar] [CrossRef]
- Sanodiya, L.K.; Kevat, P.; Tiwari, M. Seaweed Extract: Usable for Plants Growth and Yield. Vigyan Varta 2022, 3, 80–84. [Google Scholar]
- Ma, F.; Li, Y.; Han, X.; Li, K.; Zhao, M.; Guo, L.; Li, S.; Wang, K.; Qin, K.; Duan, J.; et al. Microalgae-Based Biofertilizer Improves Fruit Yield and Controls Greenhouse Gas Emissions in a Hawthorn Orchard. PLoS ONE 2024, 19, e0307774. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Hashem, A.; Abd_Allah, E.F.; Santoyo, G.; Kumar, A.; Gupta, R.K. Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms 2023, 11, 2507. [Google Scholar] [CrossRef]
- Alameda-Martín, A.; Chamizo, S.; Rodríguez-Caballero, E.; Muñoz-Rojas, M.; Cantón, Y. The Potential of Biocrust-Forming Cyanobacteria to Enhance Seedling Growth of Native Semi-Arid Plants Through Seed Biopriming. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Malla, M.A.; Ansari, F.A.; Featherston, J.; Hassan, H.; Owaes, M.; Osman, A.; Heintz-Buschart, A.; Eisenhauer, N.; Ismail, A.; Bux, F.; et al. Microalgae Inoculation Increases Bacterial Diversity and Gene Abundances Related to Nutrient Removal While Decreasing Antibiotic Resistant Genes in Municipal Wastewater. Algal Res. 2025, 91, 104217. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Duan, H.; Dong, H.; Li, J.; Zhang, S.; Zhang, J.; Ding, S.; Xu, T.; Guo, B. Improved Effects of Combined Application of Nitrogen-Fixing Bacteria Azotobacter Beijerinckii and Microalgae Chlorella Pyrenoidosa on Wheat Growth and Saline-Alkali Soil Quality. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Mao, Q.; Xie, Z.; Pinzon-Nuñez, D.A.; Issaka, S.; Liu, T.; Zhang, L.; Irshad, S. Leptolyngbya Sp. XZMQ and Bacillus XZM Co-Inoculation Reduced Sunflower Arsenic Toxicity by Regulating Rhizosphere Microbial Structure and Enzyme Activity. Environ. Pollut. 2024, 341, 123001. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Li, S.M.; Wang, X.J.; Yu, X.; Wang, Y.C.; Qin, S.; Cui, H.L. Symbiotic Microalgae and Microbes: A New Frontier in Saline Agriculture. Front. Microbiol. 2025, 16, 1540274. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Hong, Y.; Liu, X.; Zhao, G.; Zhang, H.; Zhai, Q. Microalgae Cultivation in Domestic Wastewater for Wastewater Treatment and High Value-Added Production: Species Selection and Comparison. Biochem. Eng. J. 2022, 185, 108493. [Google Scholar] [CrossRef]
- Li, G.; Xiao, W.; Yang, T.; Lyu, T. Optimization and Process Effect for Microalgae Carbon Dioxide Fixation Technology Applications Based on Carbon Capture: A Comprehensive Review. C-J. Carbon Res. 2023, 9, 35. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Yin, Z.; Wang, X.; Wang, S.; Ye, Z. Quantifying Photosynthetic Performance of Phytoplankton Based on Photosynthesis–Irradiance Response Models. Environ. Sci. Eur. 2020, 32, 24. [Google Scholar] [CrossRef]
- Shimakawa, G.; Demulder, M.; Flori, S.; Kawamoto, A.; Tsuji, Y.; Nawaly, H.; Tanaka, A.; Tohda, R.; Ota, T.; Matsui, H.; et al. Diatom Pyrenoids Are Encased in a Protein Shell That Enables Efficient CO2 Fixation. Cell 2024, 187, 5919–5934.e19. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.; Hou, W.; Wang, S.; Kong, F. Effects of Substrate Type on Enhancing Pollutant Removal Performance and Reducing Greenhouse Gas Emission in Vertical Subsurface Flow Constructed Wetland. J. Environ. Manage. 2021, 280, 111674. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; He, B. Biodiesel from Microalgae. In Handbook of Microalgae-Based Processes and Products; Academic Press: Cambridge, MA, USA, 2020; pp. 329–371. [Google Scholar] [CrossRef]
- Li, G.; Yao, J. A Review of Algae-Based Carbon Capture, Utilization, and Storage (Algae-Based CCUS). Gases 2024, 4, 468–503. [Google Scholar] [CrossRef]
- Adamczyk, M.; Lasek, J.; Skawińska, A. CO2 Biofixation and Growth Kinetics of Chlorella Vulgaris and Nannochloropsis Gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Acién-Fernández, F.G.; Gómez-Serrano, C.; González-López, C.V. Annual Production of Microalgae in Wastewater Using Pilot-Scale Thin-Layer Cascade Photobioreactors. J. Appl. Phycol. 2021, 33, 3861–3871. [Google Scholar] [CrossRef]
- Politaeva, N.; Ilin, I.; Velmozhina, K.; Shinkevich, P. Carbon Dioxide Utilization Using Chlorella Microalgae. Environments 2023, 10, 109. [Google Scholar] [CrossRef]
- Yan, Z.; Shen, T.; Li, W.; Cheng, W.; Wang, X.; Zhu, M.; Yu, Q.; Xiao, Y.; Yu, L. Contribution of Microalgae to Carbon Sequestration in a Natural Karst Wetland Aquatic Ecosystem: An in-Situ Mesocosm Study. Sci. Total Environ. 2021, 768, 144387. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Mohammadi, A.; Mashhadi, H.; Mahmoudnia, F. Evaluation of Effective Environmental Parameters on Lipid, Protein and Beta-Carotene Production in Spirulina Platensis Microalga. Results Eng. 2023, 18, 101102. [Google Scholar] [CrossRef]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, F.; Wang, H.; Ho, S.H. The Magic of Algae-Based Biochar: Advantages, Preparation, and Applications. Bioengineered 2023, 14, 2252157. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Elkamel, A.; Babar, Z. Bin Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods. BioTech 2023, 12, 10. [Google Scholar] [CrossRef]
- Chaudry, S.; Hurtado-McCormick, V.; Cheng, K.Y.; Willis, A.; Speight, R.; Kaksonen, A.H. Microalgae to Bioplastics—Routes and Challenges. Clean. Eng. Technol. 2025, 25, 100922. [Google Scholar] [CrossRef]
- Kim, K.H.; Parrow, M.W.; Kheirkhah Sangdeh, P. Microalgae-Integrated Building Enclosures: A Nature-Based Solution for Carbon Sequestration. Front. Built Environ. 2025, 11, 1574582. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in Microalgae Application for CO2 Sequestration. Clean. Chem. Eng. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Tsai, D.D.W.; Chen, P.H.; Ramaraj, R. The Potential of Carbon Dioxide Capture and Sequestration with Algae. Ecol. Eng. 2017, 98, 17–23. [Google Scholar] [CrossRef]
- Raeesossadati, M.J.; Ahmadzadeh, H.; McHenry, M.P.; Moheimani, N.R. CO2 Environmental Bioremediation by Microalgae. In Biomass and Biofuels from Microalgae. Biofuel and Biorefinery Technologies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 117–136. [Google Scholar] [CrossRef]
- Yahya, L.; Harun, R.; Abdullah, L.C. Screening of Native Microalgae Species for Carbon Fixation at the Vicinity of Malaysian Coal-Fired Power Plant. Sci. Rep. 2020, 10, 22355. [Google Scholar] [CrossRef]
- Abraham, J.; Prigiobbe, V.; Abimbola, T.; Christodoulatos, C. Integrating Biological and Chemical CO2 Sequestration Using Green Microalgae for Bioproducts Generation. Front. Clim. 2023, 4, 949411. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Montero, N.; Ferrer, I.; Acién, F.G.; Gómez, C.; Garfí, M. Life Cycle Assessment of High Rate Algal Ponds for Wastewater Treatment and Resource Recovery. Sci. Total Environ. 2018, 622–623, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.N.D.; Mukhtar, H.; Le, L.T.; Tran, D.P.H.; Ngo, M.T.T.; Pham, M.D.T.; Nguyen, T.B.; Vo, T.K.Q.; Bui, X.T. Roles of Microalgae-Based Biofertilizer in Sustainability of Green Agriculture and Food-Water-Energy Security Nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef]
- Bagh, S.S.K.K.P. Application of Algae in Air Pollution Control Technique. Bachelor Thesis, National Institue of Technology, Rourkela, India, 2018; pp. 1–30. [Google Scholar]
- Díaz-Pérez, F.J.; Díaz, R.; Valdés, G.; Valdebenito-Rolack, E.; Hansen, F. Effects of Microalgae and Compost on the Yield of Cauliflower Grown in Low Nutrient Soil. Chil. J. Agric. Res. 2023, 83, 181–194. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Yu, Z.; Ma, M.; Liu, M.; Pei, H. Phycoremediation Potential of Salt-Tolerant Microalgal Species: Motion, Metabolic Characteristics, and Their Application for Saline–Alkali Soil Improvement in Eco-Farms. Microorganisms 2024, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Sarma, U.; Hoque, M.E.; Thekkangil, A.; Venkatarayappa, N.; Rajagopal, S. Microalgae in Removing Heavy Metals from Wastewater—An Advanced Green Technology for Urban Wastewater Treatment. J. Hazard. Mater. Adv. 2024, 15, 100444. [Google Scholar] [CrossRef]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Lim, H.R.; Manickam, S.; Ang, W.L.; Show, P.L. Towards a Sustainable Circular Economy: Algae-Based Bioplastics and the Role of Internet-of-Things and Machine Learning. ChemBioEng Rev. 2024, 11, 39–59. [Google Scholar] [CrossRef]
- Popa, M.D.; Simionov, I.A.; Petrea, S.M.; Georgescu, P.L.; Ifrim, G.A.; Iticescu, C. Efficiency of Microalgae Employment in Nutrient Removal (Nitrogen and Phosphorous) from Municipal Wastewater. Water 2025, 17, 260. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Jacob, A.; Dey, P.; Somu, P.; Haldar, D. Biomass as Eco-Friendly Adsorbents for the Removal of Emerging Pollutants from Wastewater: A Review. Discov. Appl. Sci. 2025, 7, 771. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Mataya, D.; Cobb, K.; Chen, P.; Ruan, R. Enhanced Sustainable Integration of CO2 Utilization and Wastewater Treatment Using Microalgae in Circular Economy Concept. Bioresour. Technol. 2022, 366, 128188. [Google Scholar] [CrossRef]
- Singh Chauhan, D.; Sahoo, L.; Mohanty, K. Maximize Microalgal Carbon Dioxide Utilization and Lipid Productivity by Using Toxic Flue Gas Compounds as Nutrient Source. Bioresour. Technol. 2022, 348, 126784. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chong, J.W.R.; Khoo, K.S.; Tang, D.Y.Y.; Show, P.L. Upcycling Food Waste for Microalgae Cultivation toward Lipid Production in a Closed-Loop and System-Integrated Circular Bioeconomy. Biotechnol. Biofuels Bioprod. 2025, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural Pigments from Microalgae Grown in Industrial Wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef]
- Wu, G.; Tham, P.E.; Chew, K.W.; Munawaroh, H.S.H.; Tan, I.S.; Wan-Mohtar, W.A.A.Q.I.; Sriariyanun, M.; Show, P.L. Net Zero Emission in Circular Bioeconomy from Microalgae Biochar Production: A Renewed Possibility. Bioresour. Technol. 2023, 388, 129748. [Google Scholar] [CrossRef]
- CORDIS Webpage. A Circular Economy Platform for Treatment of Wastewater by Blue Green Microalgae. Available online: https://cordis.europa.eu/project/id/101064763 (accessed on 1 October 2022).
- Velásquez-Orta, S.B.; Yáñez-Noguez, I.; Ramírez, I.M.; Ledesma, M.T.O. Pilot-Scale Microalgae Cultivation and Wastewater Treatment Using High-Rate Ponds: A Meta-Analysis. Environ. Sci. Pollut. Res. 2024, 31, 46994–47021. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.G.; Mehmood, M.A. A Novel Wastewater-Derived Cascading Algal Biorefinery Route for Complete Valorization of the Biomass to Biodiesel and Value-Added Bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production Cost of a Real Microalgae Production Plant and Strategies to Reduce It. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Tredici, M.R.; Rodolfi, L.; Biondi, N.; Bassi, N.; Sampietro, G. Techno-Economic Analysis of Microalgal Biomass Production in a 1-Ha Green Wall Panel (GWP®) Plant. Algal Res. 2016, 19, 253–263. [Google Scholar] [CrossRef]
- Rumin, J.; de Oliveira Junior, R.G.; Bérard, J.B.; Picot, L. Improving Microalgae Research and Marketing in the European Atlantic Area: Analysis of Major Gaps and Barriers Limiting Sector Development. Mar. Drugs 2021, 19, 319. [Google Scholar] [CrossRef]
- Fortune Business Insights. Microalgae Market Size, Share & Industry Analysis, By Species (Spirulina, Chlorella, Nannochloropsis, Haematococcus, Isochrysis, Chlamydomonas, and Others), By Application (Food and Feed), and Regional Forecast, 2024–2032. 2023, pp. 1–163. Available online: https://www.fortunebusinessinsights.com/microalgae-market-110314 (accessed on 4 August 2025).
- Hoque, M.M.; Saha, B.K.; Scopa, A.; Drosos, M. Biochar in Agriculture: A Review on Sources, Production, and Composites Related to Soil Fertility, Crop Productivity, and Environmental Sustainability. C 2025, 11, 50. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Salleh, M.A.M. Biochar Production from Microalgae Cultivation through Pyrolysis as a Sustainable Carbon Sequestration and Biorefinery Approach. Clean Technol. Environ. Policy 2018, 20, 2047–2055. [Google Scholar] [CrossRef]
- Webster, L.J.; Villa-Gomez, D.; Brown, R.; Clarke, W.; Schenk, P.M. A Synthetic Biology Approach for the Treatment of Pollutants with Microalgae. Front. Bioeng. Biotechnol. 2024, 12, 1379301. [Google Scholar] [CrossRef]
- Piyatilleke, S.; Thevarajah, B.; Nimarshana, P.H.V.; Ariyadasa, T.U. Microalgal Biofuels: Challenges and Prospective in the Framework of Circular Bioeconomy. Energy Nexus 2025, 17, 100338. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Dalgleish, P. Sustainability Research Project: Research of Suitable Locations, Design and Operation of Microalgae Production Plants for Biofuel’s. Nat. Resour. 2017, 8, 671–708. [Google Scholar] [CrossRef][Green Version]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent Advancements in Photo-Bioreactors for Microalgae Cultivation: A Brief Overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Banerjee, S.; Ramaswamy, S. Comparison of Productivity and Economic Analysis of Microalgae Cultivation in Open Raceways and Flat Panel Photobioreactor. Bioresour. Technol. Rep. 2019, 8, 100328. [Google Scholar] [CrossRef]
- Yu, B.S.; Pyo, S.; Lee, J.; Han, K. Microalgae: A Multifaceted Catalyst for Sustainable Solutions in Renewable Energy, Food Security, and Environmental Management. Microb. Cell Fact. 2024, 23, 308. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dias, R.R.; Zepka, L.Q.; Jacob-Lopes, E. Tackling Old Challenges in Microalgal Biotechnology: The Role of Photobioreactors to Advance the Technology Readiness Level. Processes 2025, 13, 51. [Google Scholar] [CrossRef]
- Cañedo, J.C.G.; Lizárraga, G.L.L. Considerations for Photobioreactor Design and Operation for Mass Cultivation of Microalgae. In Algae: Organisms for Imminent Biotechnology; Intech Open: London, UK, 2016. [Google Scholar] [CrossRef]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Nunes, A.L.F.; Lima, V.S.; Júnior, J.R.M.; Resende, M.E.T.; da Silva, C.A.S.; Martins, M.A.; Coimbra, J.S.D.R. Cell Disruption of Microalgae: Advances and Perspectives. Cienc. Rural 2024, 54, e20220330. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, H. Microalgae Cultivation. Adv. Bioenergy 2021, 6, 37–115. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Moreno-Garrido, I. Influence of Salinity and Temperature on the Growth, Productivity, Photosynthetic Activity and Intracellular ROS of Two Marine Microalgae and Cyanobacteria. Mar. Environ. Res. 2023, 186, 105932. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.M.; Martins, A.A.; Freitas, M.A.V.; Caetano, N.S. Economic Analysis of Microalgae Biodiesel Production in a Small-Scale Facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front. Energy Res. 2021, 9, 749968. [Google Scholar] [CrossRef]
- Nichols, K.; Olson, M.; Ayers, A.D.; Nichols, K. Microalgae As a Beneficial Soil Amendment; MyLand Company, LLC: Phoenix, AZ, USA, 2020; pp. 1–22. [Google Scholar]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Díez-Montero, R. Can Microalgae Grown in Wastewater Reduce the Use of Inorganic Fertilizers? J. Environ. Manag. 2022, 323, 116224. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Fan, Y.; Zhang, L.; Wang, H. Using Microalgae to Reduce the Use of Conventional Fertilizers in Hydroponics and Soil-Based Cultivation. Sci. Total Environ. 2024, 912, 169424. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Riddech, N.; Theerakulpisut, P.; Ma, Y.N.; Sarin, P. Bioorganic Fertilizers from Agricultural Waste Enhance Rice Growth under Saline Soil Conditions. Sci. Rep. 2025, 15, 8979. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Skifa, I.; Chauchat, N.; Cocquet, P.-H.; Guer, Y. Le Microalgae Cultivation in Raceway Ponds: Advances, Challenges, and Hydrodynamic Considerations. EFB Bioeconomy J. 2025, 5, 100073. [Google Scholar] [CrossRef]
- Frongia, F.; Arru, L.; Cramarossa, M.R.; Forti, L. Microalgae Potential in the Capture of CO2 Emission. Acta Innov. 2021, 41, 19–27. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Yadav, A.; Sahoo, A.; Kumari, P.; Singh, L.A.; Swapnil, P.; Meena, M.; Kumar, S. Microalgal-Based Sustainable Bio-Fungicides: A Promising Solution to Enhance Crop Yield. Discov. Sustain. 2025, 6, 39. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource Recovery through Bioremediation of Wastewaters and Waste Carbon by Microalgae: A Circular Bioeconomy Approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the Potential of Microalgae Cultivated on Wastewater Combined with Salinity Stress to Improve Biodiesel Production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Claassens, N.J.; D’Adamo, S.; van der Oost, J.; Barbosa, M.J. Synthetic Biology Approaches To Enhance Microalgal Productivity. Trends Biotechnol. 2021, 39, 1019–1036. [Google Scholar] [CrossRef]
- Ahmad Kamal, A.H.; Mohd Hamidi, N.F.; Zakaria, M.F.; Ahmad, A.; Harun, M.R.; Chandra Segaran, T.; Jusoh, M. Genetically Engineered Microalgae for Enhanced Bioactive Compounds. Discov. Appl. Sci. 2024, 6, 482. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, B. Algal Biorefinery: An Integrated Approach for Sustainable Biodiesel Production. Biomass and Bioenergy 2019, 131, 105398. [Google Scholar] [CrossRef]
- Barik, S.K.; Kumar, P.; Pal, U.J.; Aikat, K. Integrated Biorefinery Approach for Sustainable Biofuel Production from Algal Biomass. Clean Technol. Environ. Policy 2024. [Google Scholar] [CrossRef]
- Grzesik, M.; Pszczółkowski, W.; Pszczółkowska, A.; Romanowska-Duda, Z. Microalgae as Efficient Feedstock for Biorefinery. Acta Innov. 2015, 14, 17–25. [Google Scholar]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Macroalgae Farming for Sustainable Future: Navigating Opportunities and Driving Innovation. Heliyon 2024, 10, e28208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).