Plant-Derived Bone Substitute Presents Effective Osteointegration in Several Clinical Settings: A Pilot Study from a Single Center
Abstract
1. Introduction
2. Materials and Methods
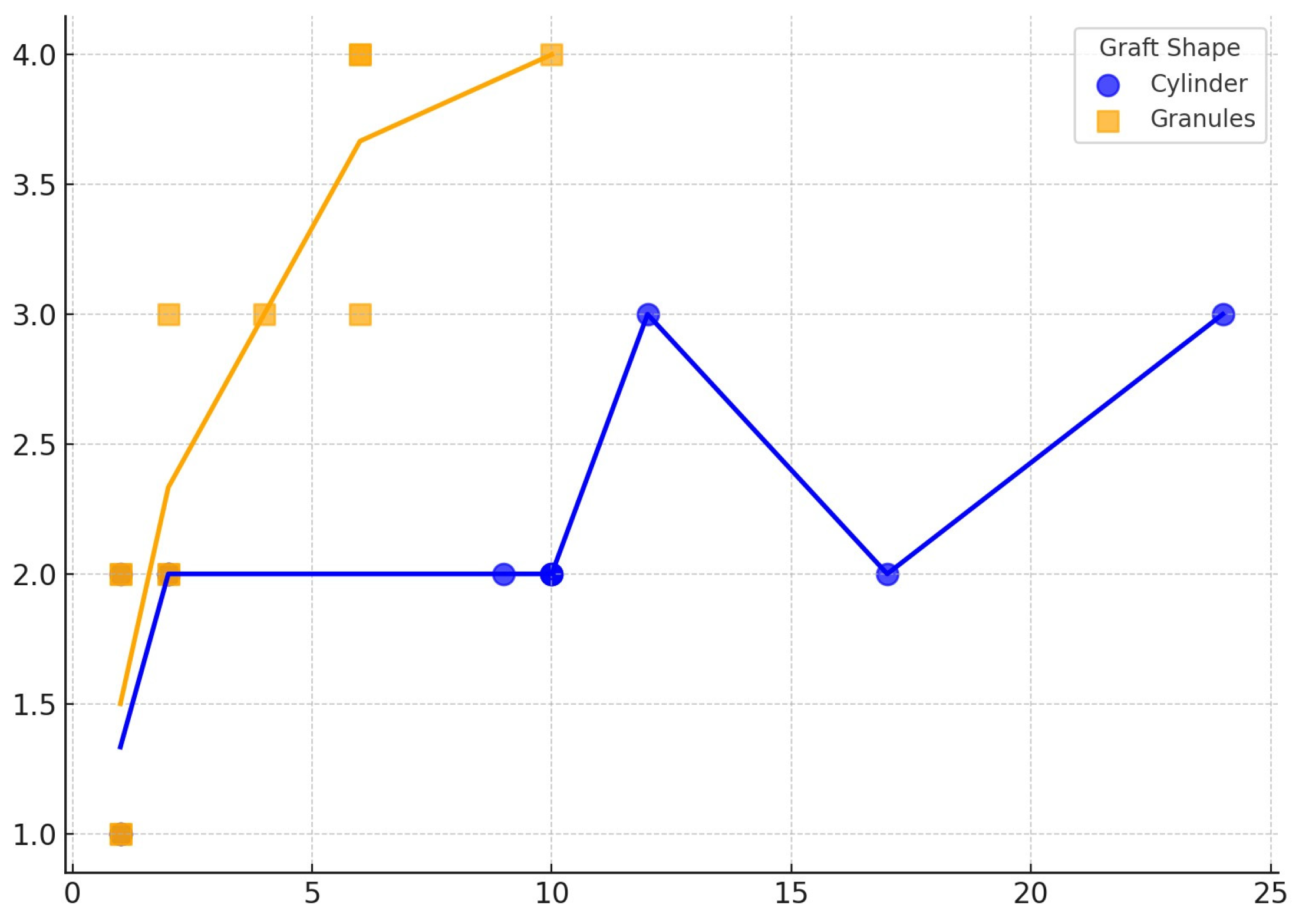
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| TCP | Tricalcium Phosphate |
| HA | Hydroxyapatite |
| rhPDGF-BB | Recombinant human platelet-derived growth factor |
| MSC | Mesenchymal stem cells |
| IMT | Induced membrane technique |
| TKA | Total knee arthroplasty |
| THA | Total hip arthroplasty |
| ORIF | Open reduction and internal fixation |
| TAA | Total ankle arthroplasty |
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- De Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone Grafts and Bone Graft Substitutes in Orthopaedic Trauma Surgery: A Critical Analysis. J. Bone Jt. Surg. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Galois, L.; Mainard, D.; Delagoutte, J. Beta-Tricalcium Phosphate Ceramic as a Bone Substitute in Orthopaedic Surgery. Int. Orthop. 2002, 26, 109–115. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Alt, V.; Walter, N.; Rupp, M.; Begué, T.; Plecko, M. Bone Defect Filling with a Novel Rattan-Wood Based Not-Sintered Hydroxyapatite and Beta-Tricalcium Phosphate Material (b.BoneTM) after Tricortical Bone Graft Harvesting—A Consecutive Clinical Case Series of 9 Patients. Trauma Case Rep. 2023, 44, 100805. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the Osteoinductive Potential of a Bio-Inspired Scaffold Mimicking the Osteogenic Niche for Bone Augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tosounidis, T.H.; Pape, H.-C. The Use of a New Grafting Material (b.BoneTM) for the Management of Severely Depressed Tibial Plateau Fractures: Preliminary Report of Three Cases. Trauma Case Rep. 2023, 47, 100893. [Google Scholar] [CrossRef]
- McNamara, M.G.; Heckman, J.D.; Corley, F.G. Severe Open Fractures of the Lower Extremity: A Retrospective Evaluation of the Mangled Extremity Severity Score (MESS). J. Orthop. Trauma 1994, 8, 81–87. [Google Scholar] [CrossRef]
- Metsemakers, W.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-Related Infection: A Consensus on Definition from an International Expert Group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://Greenbone.It/Publication/Tecnica-Operatoria-It (accessed on 10 July 2025).
- Aktas, M.; Radke, T.F.; Strauer, B.E.; Wernet, P.; Kogler, G. Separation of Adult Bone Marrow Mononuclear Cells Using the Automated Closed Separation System Sepax. Cytotherapy 2008, 10, 203–211. [Google Scholar] [CrossRef]
- Mfarrej, B.; Vicari, O.; Ouffai, S.; Malenfant, C.; Granata, A.; Thevenet, S.; Chabannon, C.; Lemarié, C.; Calmels, B. Sepax-2 Cell Processing Device: A Study Assessing Reproducibility of Concentrating Thawed Hematopoietic Progenitor Cells. J. Transl. Med. 2022, 20, 503. [Google Scholar] [CrossRef]
- Ozalay, M.; Sahin, O.; Akpinar, S.; Ozkoc, G.; Cinar, M.; Cesur, N. Remodeling Potentials of Biphasic Calcium Phosphate Granules in Open Wedge High Tibial Osteotomy. Arch. Orthop. Trauma Surg. 2009, 129, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Checklists. Available online: https://www.strobe-statement.org/checklists/ (accessed on 3 March 2025).
- Kim, T.K. Understanding One-Way ANOVA Using Conceptual Figures. Korean J. Anesthesiol. 2017, 70, 22. [Google Scholar] [CrossRef]
- Toro, G.; Cecere, A.B.; Braile, A.; Cicco, A.D.; Liguori, S.; Tarantino, U.; Iolascon, G. New Insights in Lower Limb Reconstruction Strategies. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231189008. [Google Scholar] [CrossRef]
- Loveland, J.D. A Retrospective Review of Recombinant Human Platelet-Derived Growth Factor with Beta-Tricalcium Phosphate Bone Graft Substitute Use in Hindfoot and/or Ankle Arthrodesis. J. Orthop. 2023, 44, 93–98. [Google Scholar] [CrossRef]
- DiGiovanni, C.W.; Lin, S.S.; Baumhauer, J.F.; Daniels, T.; Younger, A.; Glazebrook, M.; Anderson, J.; Anderson, R.; Evangelista, P.; Lynch, S.E.; et al. Recombinant Human Platelet-Derived Growth Factor-BB and Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP): An Alternative to Autogenous Bone Graft. JBJS 2013, 95, 1184. [Google Scholar] [CrossRef] [PubMed]
- Toro, G.; Lepore, F.; Calabrò, G.; Toro, G.; Rossini, M.; Vasso, M.; Schiavone Panni, A. Humeral Shaft Non-Union in the Elderly: Results with Cortical Graft plus Stem Cells. Injury 2019, 50 (Suppl. S2), S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Lane, J.; Watson, J.T.; Giannoudis, P. Bone Graft Substitution and Augmentation. J. Orthop. Trauma 2015, 29 (Suppl. S12), S34–S38. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The “diamond Concept” for Long Bone Non-Union Management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Conza, G.; Braile, A.; Iodice, G.; Di Cristofaro, N.; Trotta, M.C.; D’Amico, G.; D’Arienzo, A.; Toro, G. Current Perspectives on Regenerative Medicine in Early Osteoarthritis. Minerva Orthop. 2024, 75, 307–315. [Google Scholar] [CrossRef]
- Fayaz, H.C.; Giannoudis, P.V.; Vrahas, M.S.; Smith, R.M.; Moran, C.; Pape, H.C.; Krettek, C.; Jupiter, J.B. The Role of Stem Cells in Fracture Healing and Nonunion. Int. Orthop. 2011, 35, 1587–1597. [Google Scholar] [CrossRef]
- Pountos, I.; Giannoudis, P.V. Biology of Mesenchymal Stem Cells. Injury 2005, 36, S8–S12. [Google Scholar] [CrossRef]
- Zheng, M.; Weng, M.; Zhang, X.; Li, R.; Tong, Q.; Chen, Z. Beta-Tricalcium Phosphate Promotes Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells through Macrophages. Biomed. Mater. 2021, 16, 025005. [Google Scholar] [CrossRef] [PubMed]
- Knapp, G.; Pawelke, J.; Heiss, C.; Elmas, S.; Vinayahalingam, V.; ElKhassawna, T. Traumatic Fracture Treatment: Calcium Phosphate Bone Substitute Case–Control Study in Humerus, Radius, Tibia Fractures—Assessing Efficacy and Recovery Outcomes. Biomedicines 2023, 11, 2862. [Google Scholar] [CrossRef]
- Sasaki, G.; Watanabe, Y.; Yasui, Y.; Nishizawa, M.; Saka, N.; Kawano, H.; Miyamoto, W. Clinical and Radiological Assessment of the Induced Membrane Technique Using Beta-Tricalcium Phosphate in Reconstructive Surgery for Lower Extremity Long Bone Defects. Bone Jt. J. 2021, 103-B, 456–461. [Google Scholar] [CrossRef]
- Ji, C.; Qiu, M.; Ruan, H.; Li, C.; Cheng, L.; Wang, J.; Li, C.; Qi, J.; Cui, W.; Deng, L. Transcriptome Analysis Revealed the Symbiosis Niche of 3D Scaffolds to Accelerate Bone Defect Healing. Adv. Sci. 2022, 9, 2105194. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Srioudom, J.R.; Sun, W.; Xing, M.; Yan, S.; Yu, L.; Yang, J. The Use of Hydrogel Microspheres as Cell and Drug Delivery Carriers for Bone, Cartilage, and Soft Tissue Regeneration. Biomater. Transl. 2024, 5, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Altunbek, M.; Bartolo, P.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B. 3D Printed Scaffold Design for Bone Defects with Improved Mechanical and Biological Properties. J. Mech. Behav. Biomed. Mater. 2022, 134, 105418. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Salamanna, F.; Filardo, G.; Di Matteo, B.; Shabshin, N.; Shani, J.; Fini, M.; Perdisa, F.; Parrilli, A.; Sprio, S.; et al. Bone Regeneration in Load-Bearing Segmental Defects, Guided by Biomorphic, Hierarchically Structured Apatitic Scaffold. Front. Bioeng. Biotechnol. 2021, 9, 734486. [Google Scholar] [CrossRef]
- Ma, W.; Lu, H.; Xiao, Y.; Wu, C. Advancing Organoid Development with 3D Bioprinting. Organoid Res. 2025, 1, 025040004. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Z.; Su, J. Organoid Research Breakthroughs in 2024: A Review. Organoid Res. 2025, 1, 025040005. [Google Scholar] [CrossRef]
- Li, Z.; Lu, M.; Zou, S.; Liu, R.; Lei, H.; Wang, Y.; Zhang, Y.; Zhou, Y.; Zhou, C.; Luo, Y.; et al. Four-Dimensional-Printed Personalized Shape Memory NiTi Implant for Minimally Invasive Delivery in Cavitary Bone Defect Reconstruction. Biomater. Transl. 2025. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Ruffini, A.; Celotti, G.; Lesci, I.G.; Roveri, N. From Wood to Bone: Multi-Step Process to Convert Wood Hierarchical Structures into Biomimetic Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Mater. Chem. 2009, 19, 4973. [Google Scholar] [CrossRef]
- Filardo, G.; Roffi, A.; Fey, T.; Fini, M.; Giavaresi, G.; Marcacci, M.; Martínez-Fernández, J.; Martini, L.; Ramírez-Rico, J.; Salamanna, F.; et al. Vegetable Hierarchical Structures as Template for Bone Regeneration: New Bio-ceramization Process for the Development of a Bone Scaffold Applied to an Experimental Sheep Model. J. Biomed. Mater. Res. 2020, 108, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Pitsilos, C.; Giannoudis, P.V. Distal Femoral Bone Defect Treatment Using an Engineered Hydroxyapatite Cylinder Scaffold Made from Rattan Wood. BMJ Case Rep. 2025, 18, e264131. [Google Scholar] [CrossRef] [PubMed]
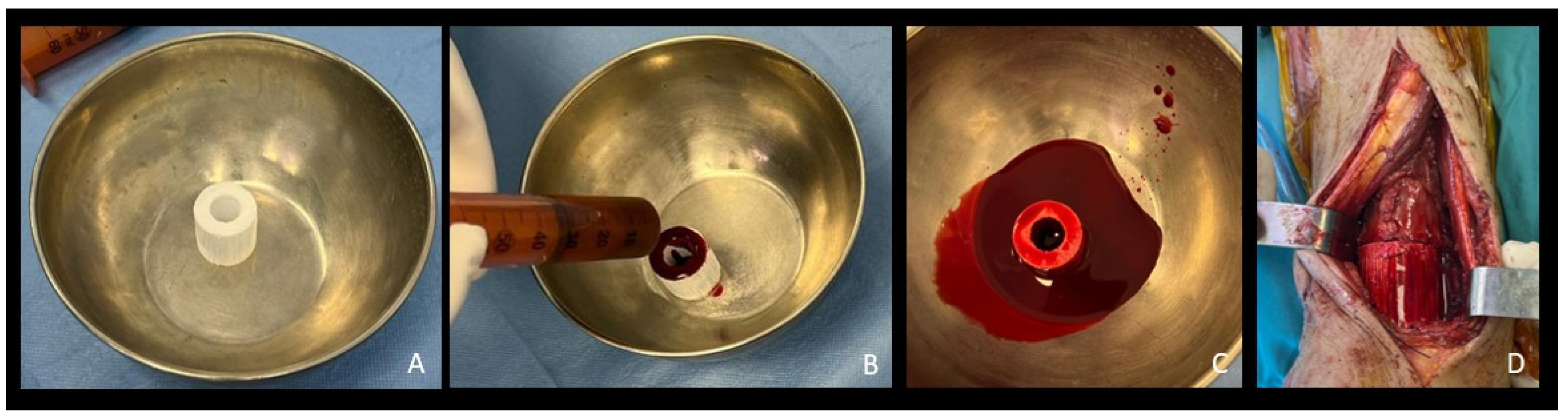

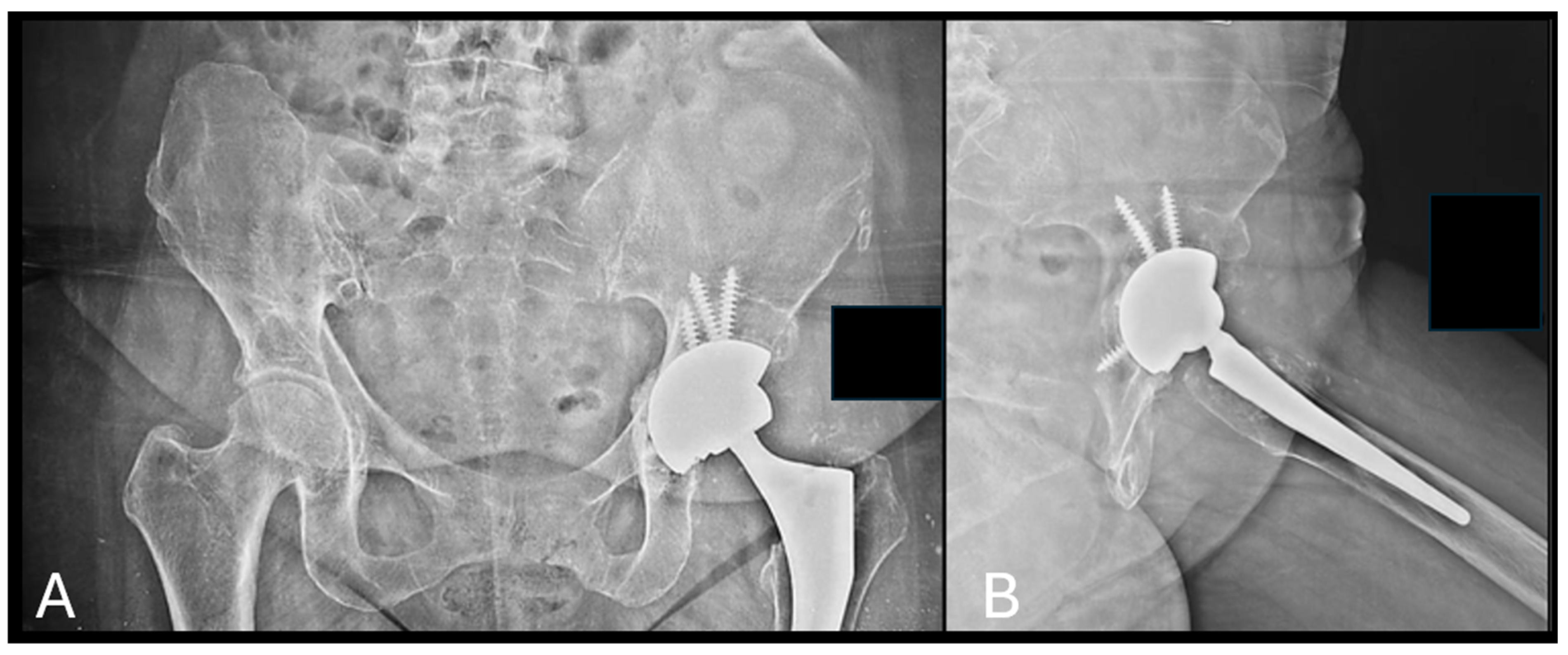

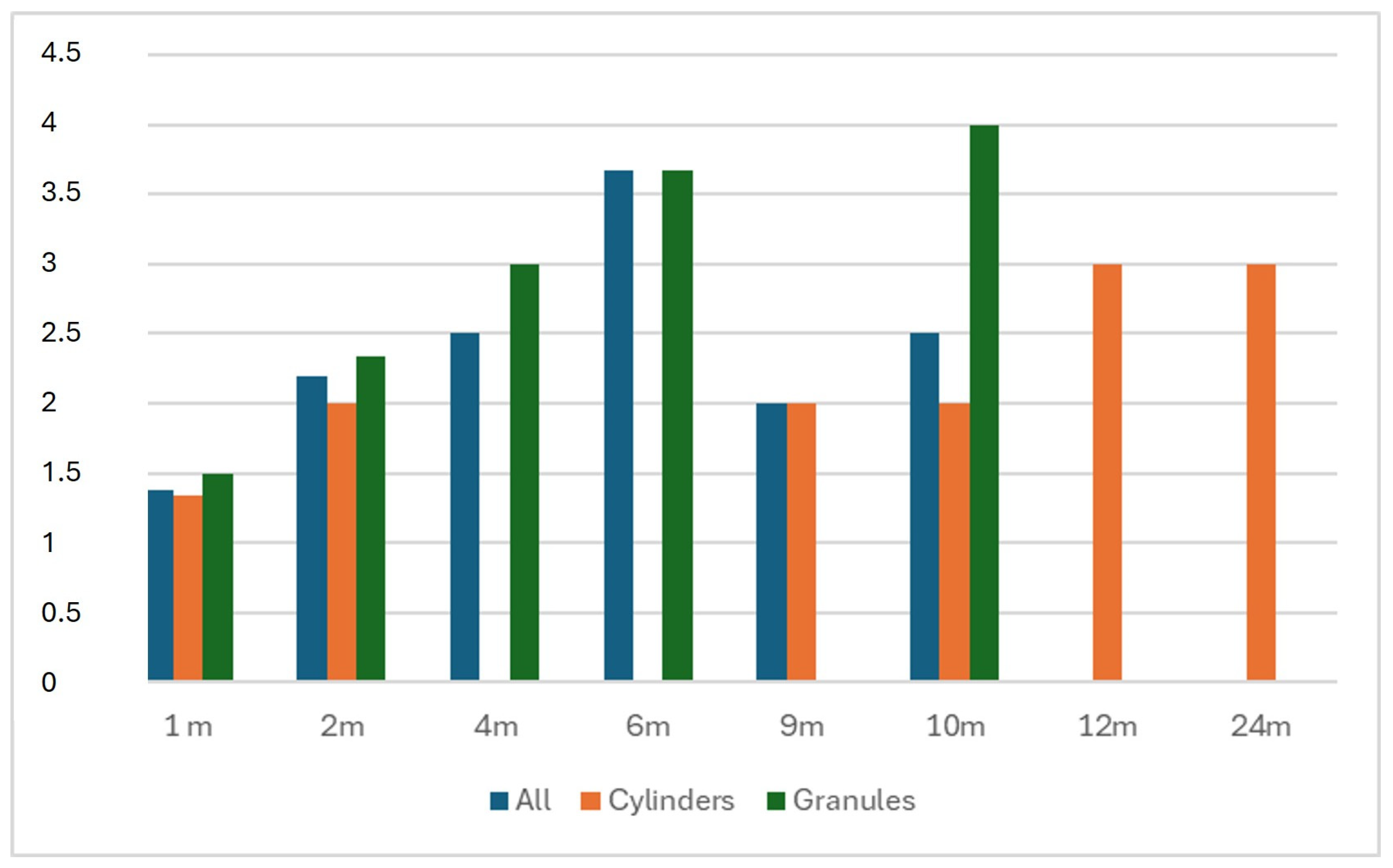

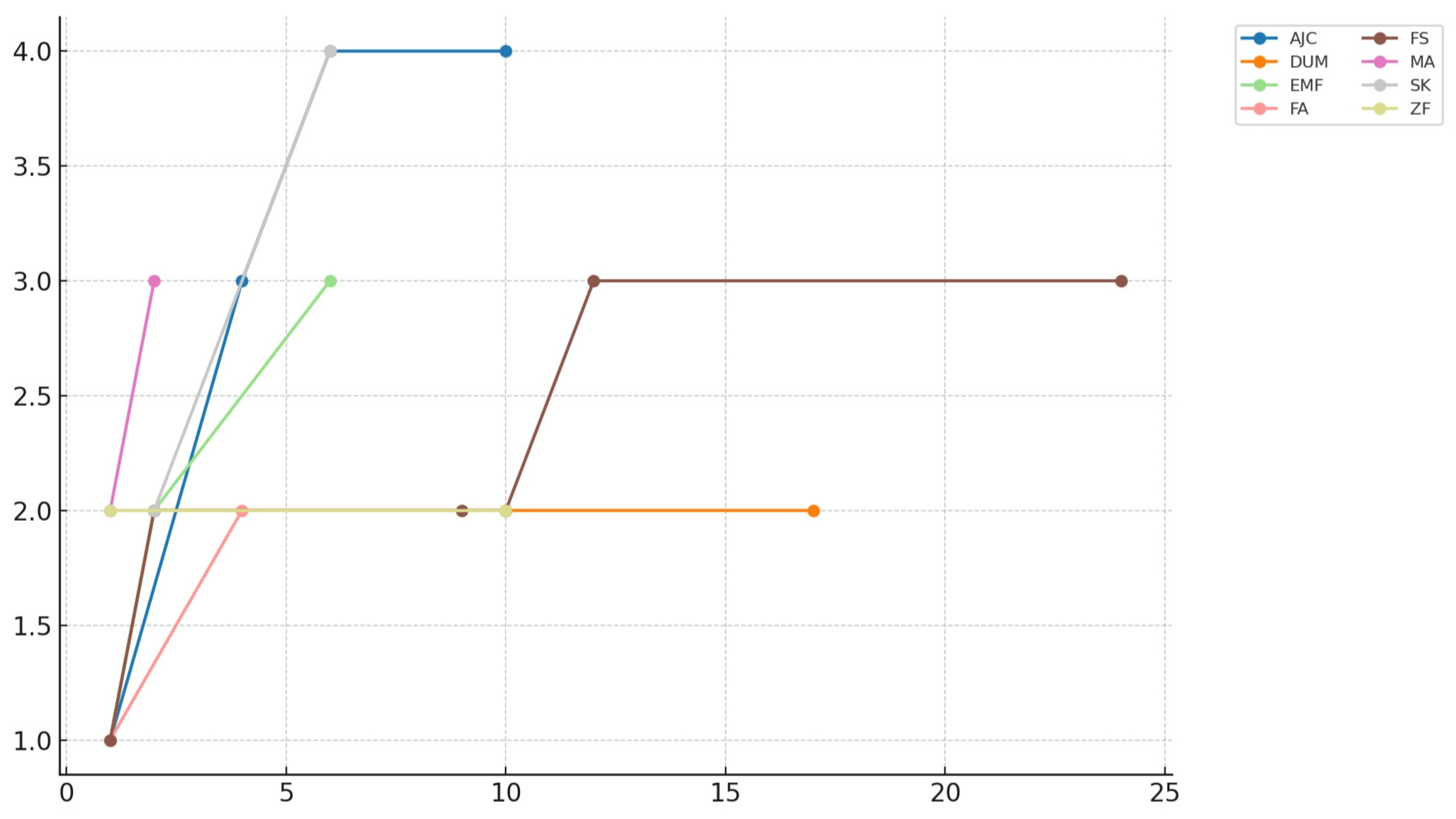
|
|
|
|
| Phases of BCP Remodeling | Radiological Definition |
|---|---|
| 0—Direct postoperative | No sign |
| 1—Vascular phase | Rounded osteotomy sites, clear distinction between BCP and bone |
| 2—Osteoblastic phase | Whitened osteotomy sites, distinction between BCP and bone still visible |
| 3—Consolidation phase | Distinction between BCP and bone not visible, cloudy bone formation |
| 4A—Full reformation | No sign of osteotomy, BCP visible |
| 4B—Full reformation | Disappearance of BCP |
| Patient | Sex | Age | Comorbidities | Diagnosis | Surgical Treatment | Graft Shape (Dimension in cm) | Follow-Up (Months) | Van Hemert Grading |
|---|---|---|---|---|---|---|---|---|
| M.A. | F | 66 | Rheumatoid arthritis, type II diabetes, obesity | Aseptic loosening of TKA + periprosthetic fracture | Revision TKA + ORIF | Granules (n.a.) | 4 | 3 |
| A.J.C. | F | 52 | n.a. | Pelvic instability | Pubic fusion | Granules (n.a.) | 10 | 4 |
| E.M.F. | M | 63 | Smoker, chronic liver disease, previous drug abuse | Septic arthritis | Two-stage THA | Granules (n.a.) | 5 | 3 |
| D.U.M. | M | 84 | Former smoker, hypertension | Distal tibia osteomyelitis | Resection and ankle fusion | Cylinder (5) | 17 | 2 |
| S.K. | F | 56 | n.a. | Aseptic loosening of THA | Acetabular revision | Granules (n.a.) | 6 | 4 |
| F.S. | M | 45 | Smoker, previous drug abuse | Distal tibia osteomyelitis | Resection and ankle fusion | Cylinder (3) | 23 | 3 |
| Z.F. | M | 19 | Neurofibromatosis type 1 | Skewfoot | Medial column lengthening | Cylinder (1.7) | 10 | 2 |
| F.A. | M | 45 | Previous drug abuse, rheumatoid arthritis, HIV infection, chronic liver disease | Ankle ankylosis | TAA | Prism (2.4) | 4 | 2 |
| Timepoint | ANOVA F (df) | ANOVA p-Value | Welch’s t-Test t | Welch’s t-Test p-Value |
|---|---|---|---|---|
| 1 m | 4.53 (3.17) | 0.0165 | 2.65 | 0.0331 |
| 2 m | 15.75 (3.12) | 0.0004 | 1.58 | 0.1747 |
| 10 m | 45.08 (2.10) | 4.4 × 10−5 | ∞ | <0.0001 |
| Shape | ANOVA F (df) | ANOVA p-Value | Kruskal–Wallis H | Kruskal–Wallis p-Value |
|---|---|---|---|---|
| Granules | 21.54 (4.8) | >0.000001 | 19.01 | 0.00078 |
| Cylinder | ∞ (3, 5) | >0.0001 | 19.00 | 0.00416 |
| Graft Shape | Spearman ρ | p-Value | 95% CI |
|---|---|---|---|
| Cylinder | 0.765 | 0.0038 | nan–nan |
| Granules | 0.905 | 0.0001 | 0.772–0.972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conza, G.; Braile, A.; Vittoria, A.D.; Di Cristofaro, N.; Itro, A.; Martin, G.; Toro, G.; Indelli, P.F.; Salini, V.; Toro, G. Plant-Derived Bone Substitute Presents Effective Osteointegration in Several Clinical Settings: A Pilot Study from a Single Center. Bioengineering 2025, 12, 861. https://doi.org/10.3390/bioengineering12080861
Conza G, Braile A, Vittoria AD, Di Cristofaro N, Itro A, Martin G, Toro G, Indelli PF, Salini V, Toro G. Plant-Derived Bone Substitute Presents Effective Osteointegration in Several Clinical Settings: A Pilot Study from a Single Center. Bioengineering. 2025; 12(8):861. https://doi.org/10.3390/bioengineering12080861
Chicago/Turabian StyleConza, Gianluca, Adriano Braile, Antonio Davide Vittoria, Nicola Di Cristofaro, Annalisa Itro, Gabriele Martin, Gabriella Toro, Pier Francesco Indelli, Vincenzo Salini, and Giuseppe Toro. 2025. "Plant-Derived Bone Substitute Presents Effective Osteointegration in Several Clinical Settings: A Pilot Study from a Single Center" Bioengineering 12, no. 8: 861. https://doi.org/10.3390/bioengineering12080861
APA StyleConza, G., Braile, A., Vittoria, A. D., Di Cristofaro, N., Itro, A., Martin, G., Toro, G., Indelli, P. F., Salini, V., & Toro, G. (2025). Plant-Derived Bone Substitute Presents Effective Osteointegration in Several Clinical Settings: A Pilot Study from a Single Center. Bioengineering, 12(8), 861. https://doi.org/10.3390/bioengineering12080861








