Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Assumptions
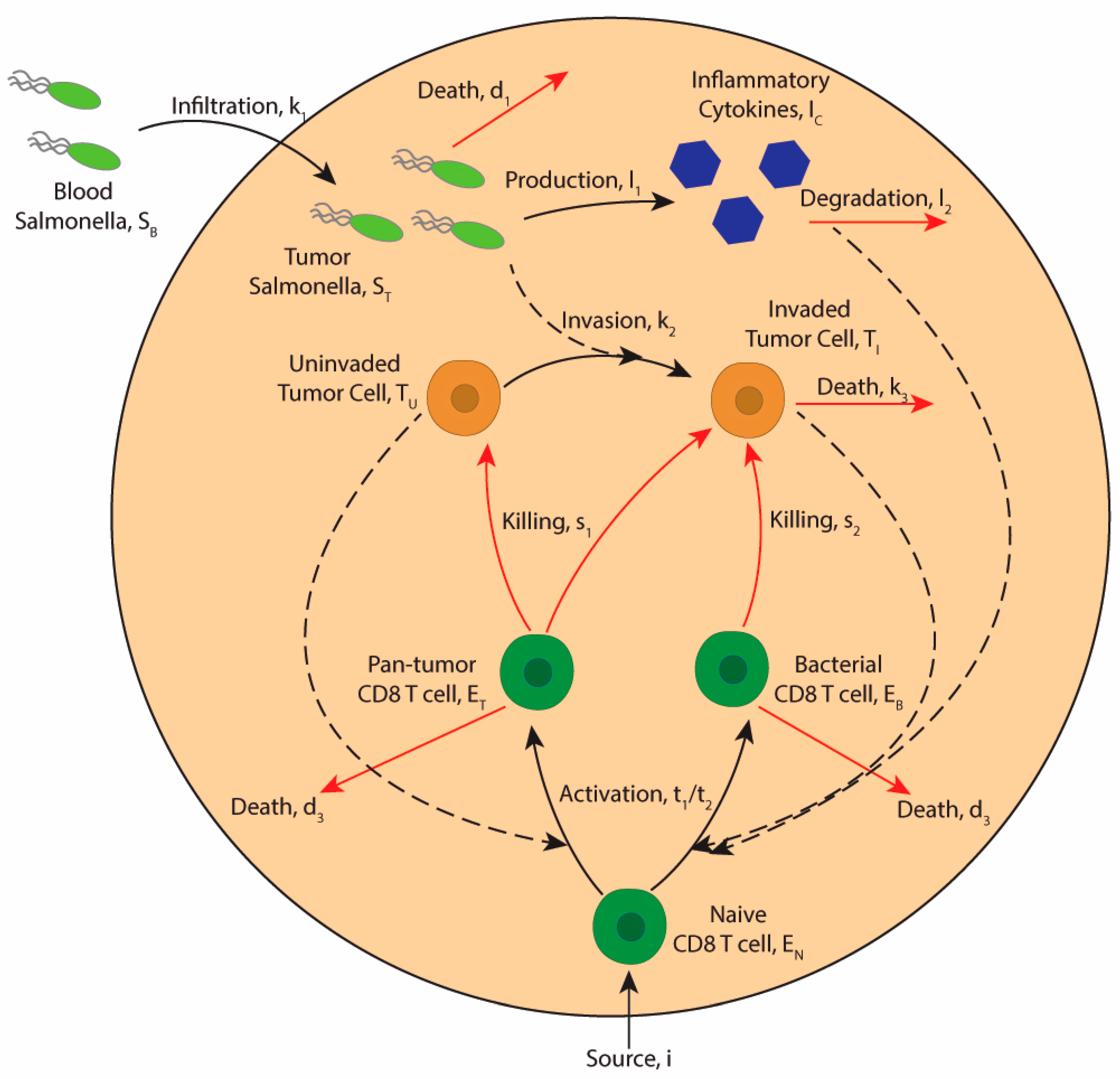
2.2. Model Description
2.2.1. Bacterial Dynamics
2.2.2. Tumor Cell Dynamics
2.2.3. Immune Cell Dynamics
2.2.4. Cytokine Dynamics
2.3. Parameter Estimations
| Parameter | Description | Units | Starting Value | Sources |
|---|---|---|---|---|
| k1 | Bacterial tumor invasion rate | day−1 | 0.5 | [6] |
| k2 | Bacterial cell invasion rate | cell−1 day−1 | 2.97 × 10−6 | [4] |
| d1 | Bacterial half-life | day−1 | 0.001 | [55] |
| a | Tumor growth rate | day−1 | 0.43 | [32,56,57] |
| b | Tumor carrying capacity | cells−1 | 1.02 × 10−9 | [32,56] |
| s1 | Tumor epitope specific T cell killing rate of cancer cells | day−1 | 2.87 | [32,40,49,63] |
| h1 | Half-max concentration for T cell to cancer cell killing | cells | 5 × 103 | [33] |
| k3 | Bacteria-based tumor cell death | day−1 | 3.20 | [47] |
| s2 | Bacterial antigen-specific T cell rate of killing invaded cells | day−1 | 2.13 | [32,40,49,63] |
| t1 | Rate of bacterial antigen memory T cell activation | day−1 | 1.50 | [58,59,60,61] |
| h2 | Half-max conc for bacterial antigen memory T cell activation | cells | 2.50 × 103 | [61,62] |
| d2 | Tumor killing of immune cells | cell−1 day−1 | 3.03 × 10−10 | [57,61,66] |
| d3 | Immune cell half-life | day−1 | 0.11 | [33,64] |
| t2 | Rate of pan-tumor naïve T cell activation | day−1 | 1.23 | [58,59,60,61] |
| h3 | Half-max conc for pan-tumor naïve T cell activation | cells | 2.50 × 103 | [61] |
| i | Source of naïve T cells | cells day−1 | 90 | [65] |
| l1 | Production of cytokines in response to bacteria | ng mL−1 cell−1 day−1 | 2.88–5.76 | [67,68,69] |
| l2 | Degradation of cytokines | day−1 | 0.51 | [35,74] |
| g1 | Half-max cytokine conc for T cell activation | ng mL−1 | 120 | [32,35,70,71,72] |
2.4. Sensitivity Analysis
3. Results
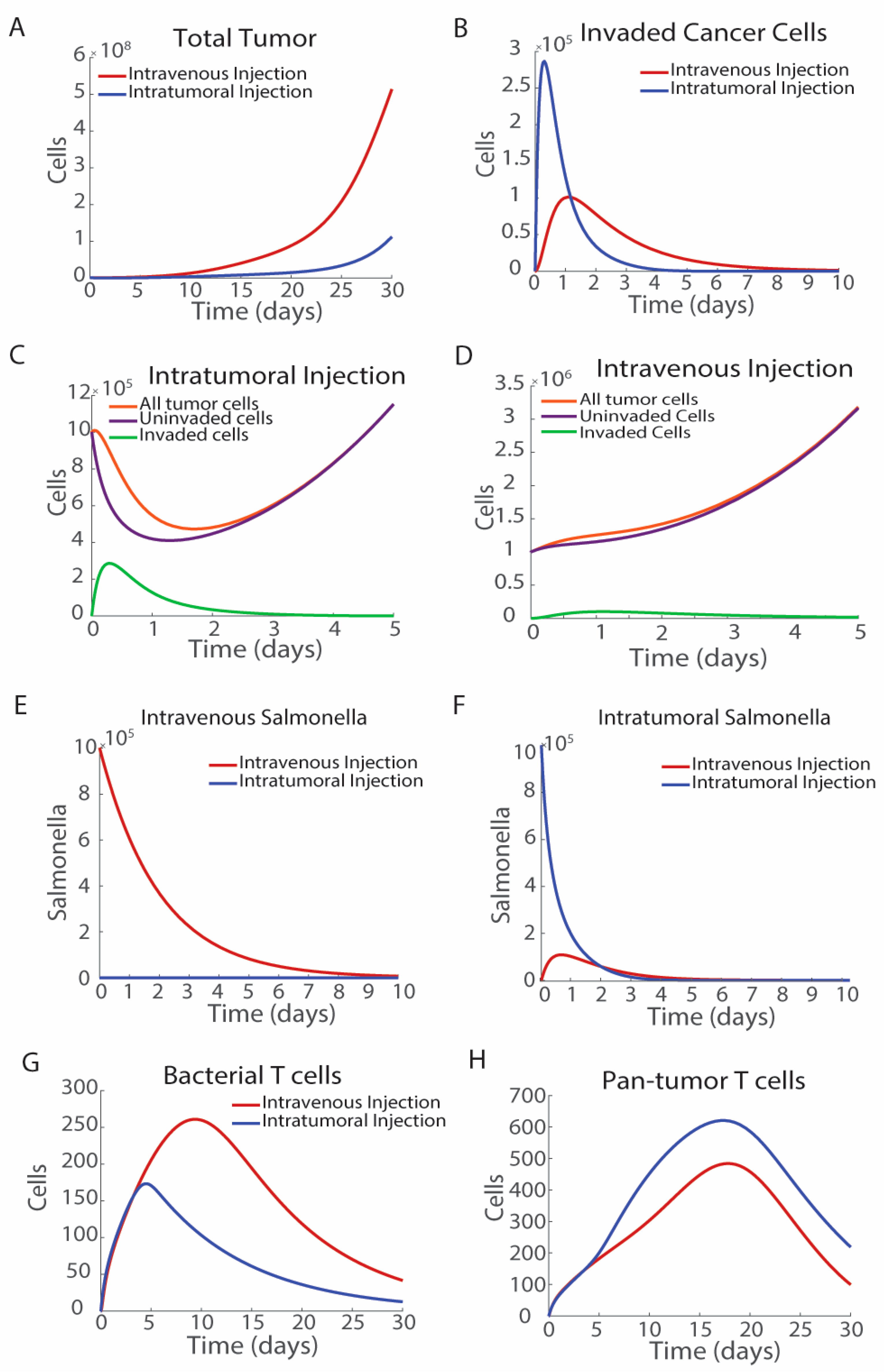
3.1. The Route of Bacterial Administration Impacts Tumor Growth
3.2. Characterization of Hot and Cold Tumor Models
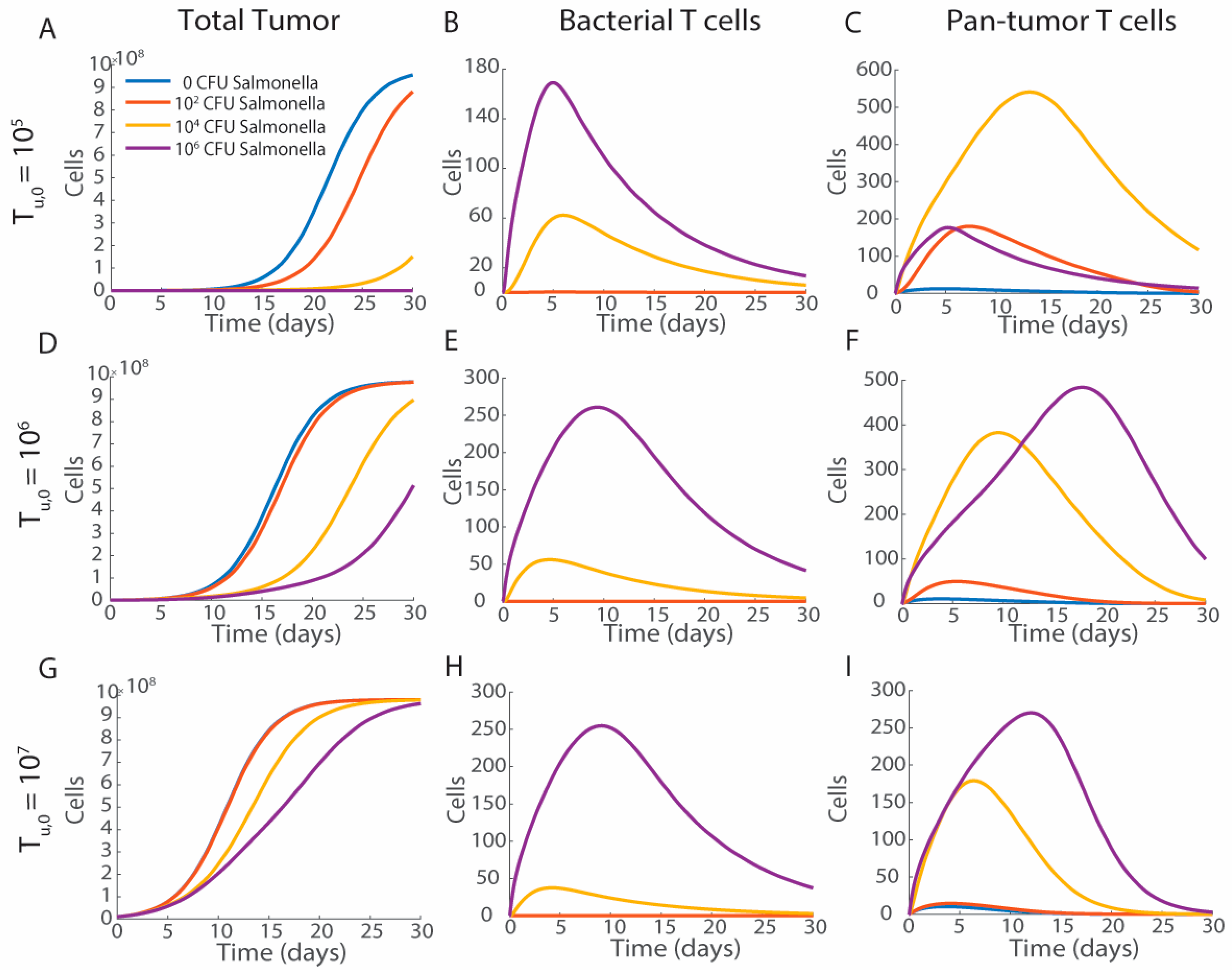
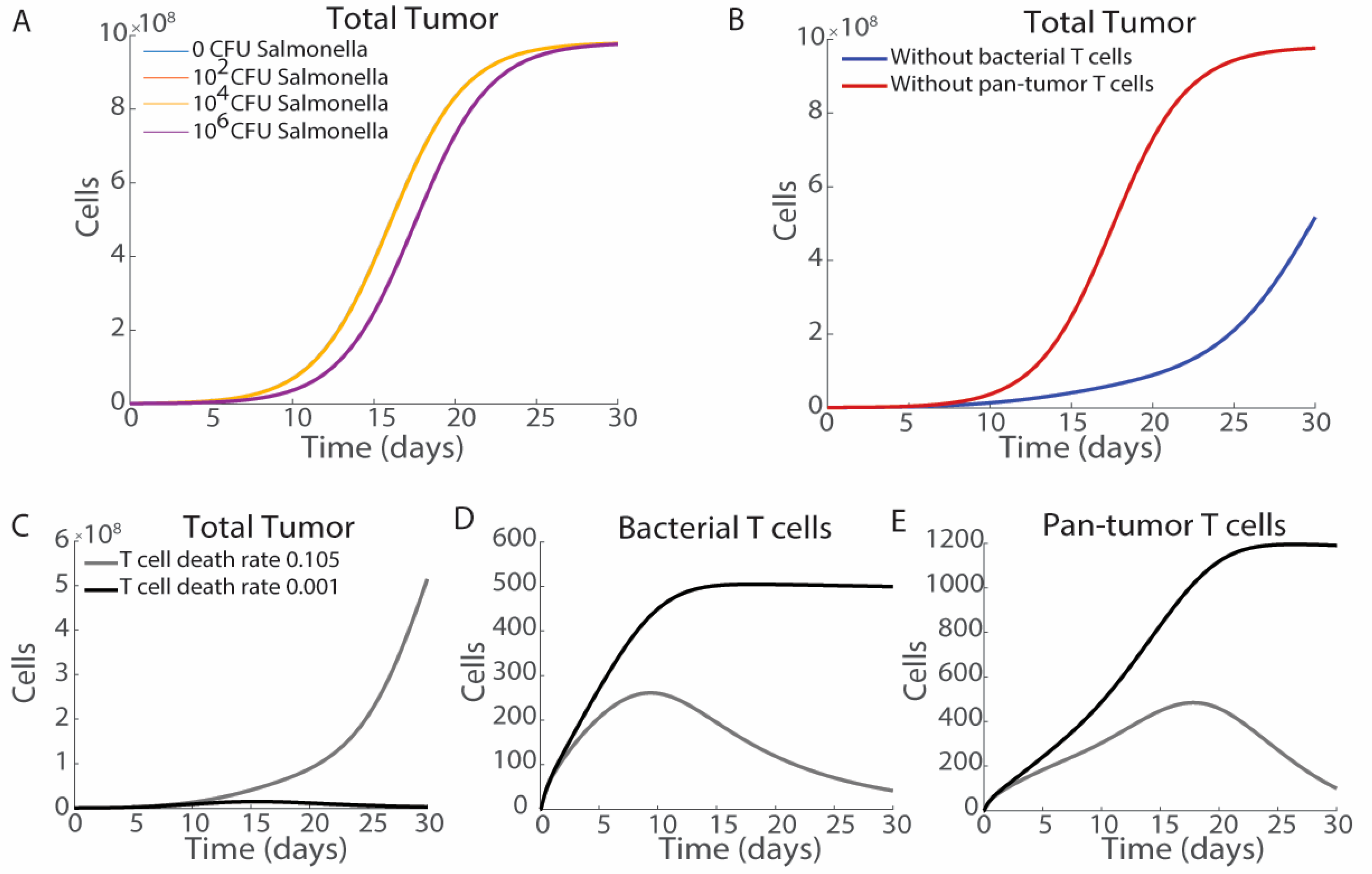
3.3. Tumor Volume and Bacterial Injection Density
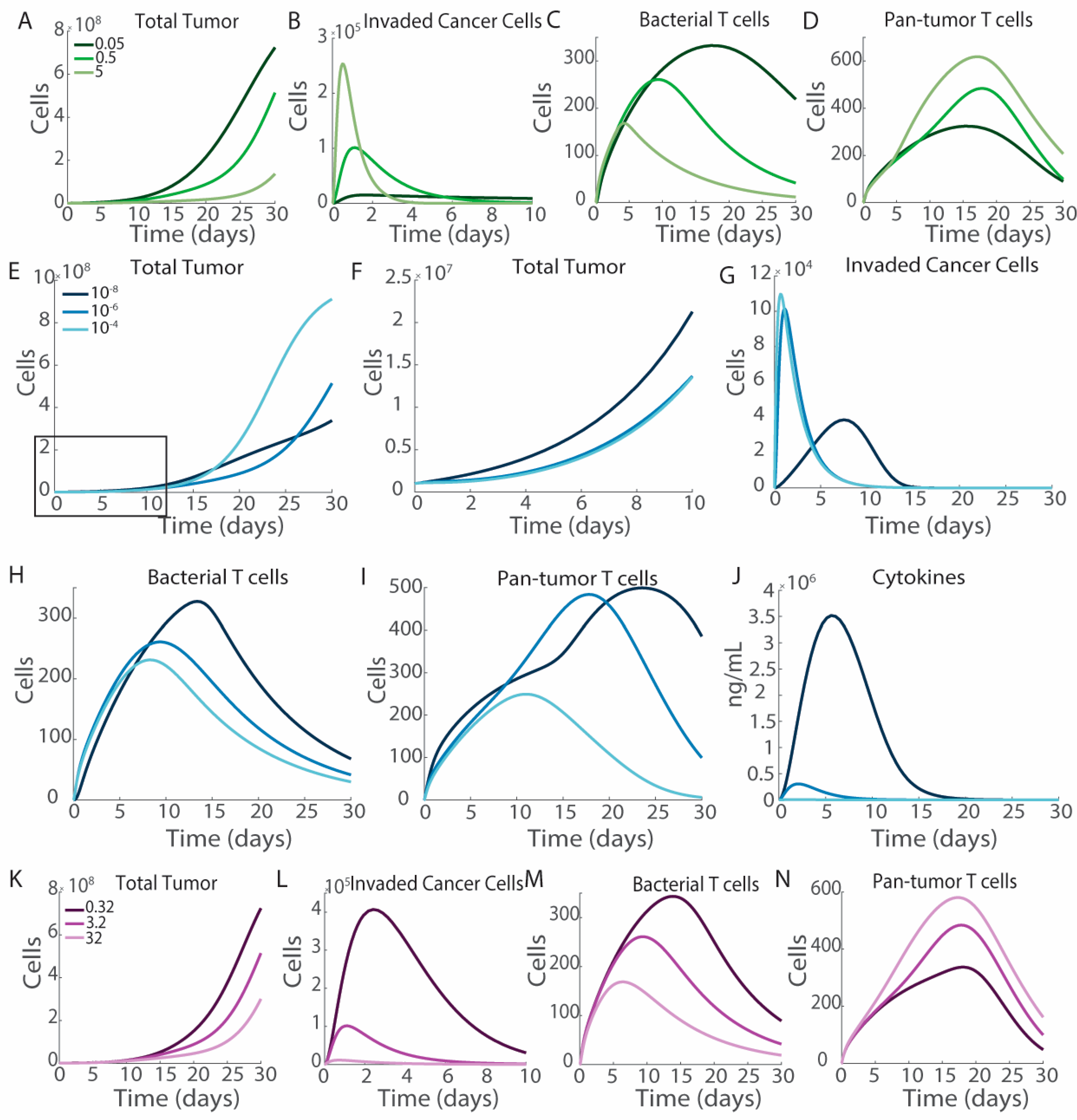
3.4. Engineering Salmonella to Improve Therapeutic Efficacy
3.5. Cytokine Production
3.6. Impact of T Cell Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howell, L.M.; Forbes, N.S. Bacteria-Based Immune Therapies for Cancer Treatment. Semin. Cancer Biol. 2021, 86, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.Q.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-Cancer Interactions: Bacteria-Based Cancer Therapy. Exp. Mol. Med. 2019, 51, 152. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Van Dessel, N.; Hall, C.L.; Wetherby, V.E.; Whitney, S.A.; Kolewe, E.L.; Bloom, S.M.K.; Sharma, A.; Hardy, J.A.; Bollen, M.; et al. Intracellular Delivery of Protein Drugs with an Autonomously Lysing Bacterial System Reduces Tumor Growth and Metastases. Nat. Commun. 2021, 12, 6116. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Van Dessel, N.; O’Connor, O.M.; Forbes, N.S. The Motility Regulator flhDC Drives Intracellular Accumulation and Tumor Colonization of Salmonella. J. Immunother. Cancer 2019, 7, 44. [Google Scholar] [CrossRef]
- Raman, V.; Howell, L.M.; Bloom, S.M.K.; Hall, C.L.; Wetherby, V.E.; Minter, L.M.; Kulkarni, A.A.; Forbes, N.S. Intracellular Salmonella Delivery of an Exogenous Immunization Antigen Refocuses CD8 T Cells Against Cancer Cells, Eliminates Pancreatic Tumors and Forms Antitumor Immunity. Front. Immunol. 2023, 14, 1228532. [Google Scholar] [CrossRef]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I Study of the Intravenous Administration of Attenuated Salmonella typhimurium to Patients with Metastatic Melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef]
- Forbes, N.S.; Coffin, R.S.; Deng, L.; Evgin, L.; Fiering, S.; Giacalone, M.; Gravekamp, C.; Gulley, J.L.; Gunn, H.; Hoffman, R.M.; et al. White Paper on Microbial Anti-Cancer Therapy and Prevention. J. Immunother. Cancer 2018, 6, 78. [Google Scholar] [CrossRef]
- Shams, H.; Poblete, F.; Rüssmann, H.; Galán, J.E.; Donis, R.O. Induction of Specific CD8+ Memory T cells and Long Lasting Protection Following Immunization with Salmonella typhimurium Expressing a Lymphocytic Choriomeningitis MHC Class I-Restricted Epitope. Vaccine 2001, 20, 577–585. [Google Scholar] [CrossRef]
- Fensterle, J.; Bergmann, B.; Yone, C.L.R.P.; Hotz, C.; Meyer, S.R.; Spreng, S.; Goebel, W.; Rapp, U.R.; Gentschev, I. Cancer Immunotherapy Based on Recombinant Salmonella enterica Serovar Typhimurium aroA Strains Secreting Prostate-Specific Antigen and Cholera Toxin Subunit B. Cancer Gene Ther. 2008, 15, 85–93. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sato, E.; Briones, G.; Chen, L.-M.; Matsuo, M.; Nagata, Y.; Ritter, G.; Jäger, E.; Nomura, H.; Kondo, S.; et al. In Vivo Antigen Delivery by a Salmonella typhimurium Type III Secretion System for Therapeutic Cancer Vaccines. J. Clin. Investig. 2006, 116, 1946–1954. [Google Scholar] [CrossRef]
- Flickinger, J.C.; Singh, J.; Yarman, Y.; Carlson, R.D.; Barton, J.R.; Waldman, S.A.; Snook, A.E. T-Cell Responses to Immunodominant Listeria Epitopes Limit Vaccine-Directed Responses to the Colorectal Cancer Antigen, Guanylyl Cyclase C. Front. Immunol. 2022, 13, 855759. [Google Scholar] [CrossRef]
- Meng, J.Z.; Dong, Y.J.; Huang, H.; Li, S.; Zhong, Y.; Liu, S.L.; Wang, Y.D. Oral Vaccination with Attenuated Salmonella enterica Strains Encoding T-Cell Epitopes from Tumor Antigen NY-ESO-1 Induces Specific Cytotoxic T-Lymphocyte Responses. Clin. Vaccine Immunol. 2010, 17, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Yrlid, U.; Wick, M.J. Salmonella-Induced Apoptosis of Infected Macrophages Results in Presentation of a Bacteria-Encoded Antigen after Uptake by Bystander Dendritic Cells. J. Exp. Med. 2000, 191, 613–623. [Google Scholar] [CrossRef]
- Avogadri, F.; Martinoli, C.; Petrovska, L.; Chiodoni, C.; Transidico, P.; Bronte, V.; Longhi, R.; Colombo, M.P.; Dougan, G.; Rescigno, M. Cancer immunotherapy Based on Killing of Salmonella-Infected Tumor Cells. Cancer Res. 2005, 65, 3920–3927. [Google Scholar] [CrossRef]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and Genetic Stability of the Novel Antitumor Agent VNP20009, a Genetically Modified Strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef]
- Arrach, N.; Zhao, M.; Porwollik, S.; Hoffman, R.M.; McClelland, M. Salmonella Promoters Preferentially Activated inside Tumors. Cancer Res. 2008, 68, 4827–4832. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Li, X.M.; Jiang, P.; Baranov, E.; Li, S.; Xu, M.; Penman, S.; Hoffman, R.M. Tumor-Targeting Bacterial Therapy with Amino Acid Auxotrophs of GFP-Expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2005, 102, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Fattinger, S.A.; Sellin, M.E.; Hardt, W.-D. Salmonella Effector Driven Invasion of the Gut Epithelium: Breaking in and Setting the House on Fire. Curr. Opin. Microbiol. 2021, 64, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.M.; Mesa-Pereira, B.; Medina, C.; Flores, A.; Santero, E. Engineering Salmonella as Intracellular Factory for Effective Killing of Tumour Cells. Sci. Rep. 2016, 6, 30591. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Zhang, P.; Piao, R.; Wang, Y. Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. Front. Cell. Infect. Microbiol. 2019, 9, 270. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The Innate Immune Response to Bacterial Flagellin Is Mediated by Toll-like Receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.M.; Manole, S.; Reitter, A.R.; Forbes, N.S. Controlled Production of Lipopolysaccharides Increases Immune Activation in Salmonella Treatments of Cancer. Microb. Biotechnol. 2024, 17, e14461. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Mohamed, Y.A.; Nader, N.; Issac, J.; Elkord, E.; Chouaib, S.; Fernandez-Cabezudo, M.J.; al-Ramadi, B.K. Salmonella-Mediated Tumor Regression Involves Targeting of Tumor Myeloid Suppressor Cells Causing a Shift to M1-like Phenotype and Reduction in Suppressive Capacity. Cancer Immunol. Immunother. 2014, 63, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Mittal, D.; Saccheri, F.; Sarrafiore, M.; Ciocca, M.; Larghi, P.; Orecchia, R.; Rescigno, M. Intra-tumoral Salmonella typhimurium Induces a Systemic Anti-tumor Immune Response That Is Directed by Low-dose Radiation to Treat Distal Disease. Eur. J. Immunol. 2008, 38, 1937–1947. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. IL-18-Producing Salmonella Inhibit Tumor Growth. Cancer Gene Ther. 2008, 15, 787–794. [Google Scholar] [CrossRef]
- Sorenson, B.S.; Banton, K.L.; Frykman, N.L.; Leonard, A.S.; Saltzman, D.A. Attenuated Salmonella typhimurium with IL-2 Gene Reduces Pulmonary Metastases in Murine Osteosarcoma. Clin. Orthop. Relat. Res. 2008, 466, 1285–1291. [Google Scholar] [CrossRef]
- Kim, J.-E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, P.Y.; Shiau, A.L. T Cell Augments the Antitumor Activity of Tumor-Targeting Salmonella. Appl. Microbiol. Biotechnol. 2011, 90, 1381–1388. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Hodge, J.W.; Sharp, H.J.; Gameiro, S.R. Abscopal Regression of Antigen Disparate Tumors by Antigen Cascade after Systemic Tumor Vaccination in Combination with Local Tumor Radiation. Cancer Biother. Radiopharm. 2012, 27, 12–22. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Walter, U.; Bourgeois, C.; McLean, A.; Rocha, B. Response of Naïve and Memory CD8+ T Cells to Antigen Stimulation in Vivo. Nat. Immunol. 2000, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- De Pillis, L.G.; Radunskaya, A.E.; Wiseman, C.L. A Validated Mathematical Model of Cell-Mediated Immune Response to Tumor Growth. Cancer Res. 2005, 65, 7950–7958. [Google Scholar] [CrossRef] [PubMed]
- Dickman, L.R.; Milliken, E.; Kuang, Y. Tumor Control, Elimination, and Escape Through a Compartmental Model of Dendritic Cell Therapy for Melanoma. SIAM J. Appl. Math. 2020, 80, 906–928. [Google Scholar] [CrossRef]
- De Boer, R.J.; Hogeweg, P.; Dullens, H.F.; De Weger, R.A.; Den Otter, W. Macrophage T Lymphocyte Interactions in the Anti-Tumor Immune Response: A Mathematical Model. J. Immunol. 1985, 134, 2748–2758. [Google Scholar] [CrossRef]
- Harkos, C.; Stylianopoulos, T.; Jain, R.K. Mathematical Modeling of Intratumoral Immunotherapy Yields Strategies to Improve the Treatment Outcomes. PLoS Comput. Biol. 2023, 19, e1011740. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium Specifically Chemotax and Proliferate in Heterogeneous Tumor Tissue in Vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef]
- Suh, S.; Leaman, E.J.; Zhan, Y.; Behkam, B. Mathematical Modeling of Bacteria-Enabled Drug Delivery System Penetration into Multicellular Tumor Spheroids. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 6162–6165. [Google Scholar]
- Bunimovich-Mendrazitsky, S.; Claude Gluckman, J.; Chaskalovic, J. A Mathematical Model of Combined Bacillus Calmette-Guerin (BCG) and Interleukin (IL)-2 Immunotherapy of Superficial Bladder Cancer. J. Theor. Biol. 2011, 277, 27–40. [Google Scholar] [CrossRef]
- Lazebnik, T.; Yanetz, S.; Bunimovich-Mendrazitsky, S. PDE Modeling of Bladder Cancer Treatment Using BCG Immunotherapy. In Functional Differential Equations and Applications; Domoshnitsky, A., Rasin, A., Padhi, S., Eds.; Springer Proceedings in Mathematics & Statistics; Springer Nature: Singapore, 2021; Volume 379, pp. 119–129. ISBN 9789811662966. [Google Scholar]
- Eftimie, R.; Dushoff, J.; Bridle, B.W.; Bramson, J.L.; Earn, D.J.D. Multi-Stability and Multi-Instability Phenomena in a Mathematical Model of Tumor-Immune-Virus Interactions. Bull. Math. Biol. 2011, 73, 2932–2961. [Google Scholar] [CrossRef]
- Mahasa, K.J.; Eladdadi, A.; de Pillis, L.; Ouifki, R. Oncolytic Potency and Reduced Virus Tumor-Specificity in Oncolytic Virotherapy. A Mathematical Modelling Approach. PLoS ONE 2017, 12, e0184347. [Google Scholar] [CrossRef]
- Forbes, N.S.; Munn, L.L.; Fukumura, D.; Jain, R.K. Sparse Initial Entrapment of Systemically Injected Salmonella typhimurium Leads to Heterogeneous Accumulation Within Tumors. Cancer Res. 2003, 63, 5188–5193. [Google Scholar]
- Deisboeck, T.S.; Wang, Z. Cancer Dissemination: A Consequence of Limited Carrying Capacity? Med. Hypotheses 2007, 69, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Novel Tools to Analyze the Function of Salmonella Effectors Show That SvpB Ectopic Expression Induces Cell Cycle Arrest in Tumor Cells. PLoS ONE 2013, 8, e78458. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-Targeted Salmonella as a Novel Anticancer Vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar] [PubMed]
- Guiney, D.G. The Role of Host Cell Death in Salmonella Infections. Curr. Top. Microbiol. Immunol. 2005, 289, 131–150. [Google Scholar] [CrossRef]
- Toley, B.J.; Forbes, N.S. Motility Is Critical for Effective Distribution and Accumulation of Bacteria in Tumor Tissue. Integr. Biol. 2012, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Viola, A.; Lanzaecchia, A. T Cell Activation Determined by T Cell Receptor Number and Tunable Thresholds. Science 1996, 273, 104–106. [Google Scholar] [CrossRef]
- Thompson, E.D.; Enriquez, H.L.; Fu, Y.X.; Engelhard, V.H. Tumor Masses Support Naive T Cell Infiltration, Activation, and Differentiation into Effectors. J. Exp. Med. 2010, 207, 1791–1804. [Google Scholar] [CrossRef]
- Leschner, S.; Westphal, K.; Dietrich, N.; Viegas, N.; Jablonska, J.; Lyszkiewicz, M.; Lienenklaus, S.; Falk, W.; Gekara, N.; Loessner, H.; et al. Tumor Invasion of Salmonella enterica Serovar Typhimurium Is Accompanied by Strong Hemorrhage Promoted by TNF-α. PLoS ONE 2009, 4, e6692. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Attenuated Salmonella Engineered to Produce Human Cytokine LIGHT Inhibit Tumor Growth. Proc. Natl. Acad. Sci. USA 2007, 104, 12879–12883. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of Specific Cytokine Expression Patterns in Patients with Breast Cancer. Sci. Rep. 2019, 9, 2924. [Google Scholar] [CrossRef]
- Frankenberg, T.; Kirschnek, S.; Häcker, H.; Häcker, G. Phagocytosis-Induced Apoptosis of Macrophages Is Linked to Uptake, Killing and Degradation of Bacteria. Eur. J. Immunol. 2008, 38, 204–215. [Google Scholar] [CrossRef]
- Diefenbach, A.; Jensen, E.R.; Jamieson, A.M.; Raulet, D.H. Rae1 and H60 Ligands of the NKG2D Receptor Stimulate Tumour Immunity. Nature 2001, 413, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Okuneye, K.; Bergman, D.; Bloodworth, J.C.; Pearson, A.T.; Sweis, R.F.; Jackson, T.L. A Validated Mathematical Model of FGFR3-Mediated Tumor Growth Reveals Pathways to Harness the Benefits of Combination Targeted Therapy and Immunotherapy in Bladder Cancer. Comput. Syst. Oncol. 2021, 1, e1019. [Google Scholar] [CrossRef]
- Zimmermann, C.; Prévost-Blondel, A.; Blaser, C.; Pircher, H. Kinetics of the Response of Naive and Memory CD8 T Cells to Antigen: Similarities and Differences. Eur. J. Immunol. 1999, 29, 284–290. [Google Scholar] [CrossRef]
- Lee, P.P.; Yee, C.; Savage, P.A.; Fong, L.; Brockstedt, D.; Weber, J.S.; Johnson, D.; Swetter, S.; Thompson, J.; Greenberg, P.D.; et al. Characterization of Circulating T Cells Specific for Tumor-Associated Antigens in Melanoma Patients. Nat. Med. 1999, 5, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Colonna, M.; Ayyoub, M.; Cella, M.; Pittet, M.J.; Batard, P.; Valmori, D.; Guillaume, P.; Liénard, D.; Cerottini, J.-C.; et al. The Activatory Receptor 2B4 Is Expressed In Vivo by Human CD8+ Effector Aβ T Cells1. J. Immunol. 2001, 167, 6165–6170. [Google Scholar] [CrossRef]
- de Pillis, L.; Fister, K.R.; Gu, W.; Collins, C.; Daub, M.; Gross, D.; Moore, J.; Preskill, B. Mathematical Model Creation for Cancer Chemo-Immunotherapy. Comput. Math. Methods Med. 2009, 10, 165–184. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Makalkin, I.A.; Taylor, M.A.; Perelson, A.S. Nonlinear Dynamics of Immunogenic Tumors: Parameter Estimation and Global Bifurcation Analysis. Bull. Math. Biol. 1994, 56, 295–321. [Google Scholar] [CrossRef]
- Vasconcelos, Z.; Müller, S.; Guipouy, D.; Yu, W.; Christophe, C.; Gadat, S.; Valitutti, S.; Dupré, L. Individual Human Cytotoxic T Lymphocytes Exhibit Intraclonal Heterogeneity during Sustained Killing. Cell Rep. 2015, 11, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, B.; Krebs, P.; Junt, T.; Metters, H.; Ford, N.J.; Anderson, R.M.; Bocharov, G. Determining Control Parameters for Dendritic Cell-Cytotoxic T Lymphocyte Interaction. Eur. J. Immunol. 2004, 34, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Mold, J.E.; Réu, P.; Olin, A.; Bernard, S.; Michaëlsson, J.; Rane, S.; Yates, A.; Khosravi, A.; Salehpour, M.; Possnert, G.; et al. Cell Generation Dynamics Underlying Naive T-Cell Homeostasis in Adult Humans. PLoS Biol. 2019, 17, e3000383. [Google Scholar] [CrossRef] [PubMed]
- Radunskaya, A.; de Pillis, L.; Gallegos, A. A Model of Dendritic Cell Therapy for Melanoma. Front. Oncol. 2013, 3, 41772. [Google Scholar] [CrossRef]
- Fernandez-Cabezudo, M.J.; Mechkarska, M.; Azimullah, S.; al-Ramadi, B.K. Modulation of Macrophage Proinflammatory Functions by Cytokine-Expressing Salmonella Vectors. Clin. Immunol. 2009, 130, 51–60. [Google Scholar] [CrossRef]
- Savage, T.M.; Vincent, R.L.; Rae, S.S.; Huang, L.H.; Ahn, A.; Pu, K.; Li, F.; de Los Santos-Alexis, K.; Coker, C.; Danino, T.; et al. Chemokines Expressed by Engineered Bacteria Recruit and Orchestrate Antitumor Immunity. Sci. Adv. 2023, 9, eadc9436. [Google Scholar] [CrossRef]
- VanEngelenburg, S.B.; Palmer, A.E. Quantification of Real-Time Salmonella Effector Type-III Secretion Kinetics Reveals Differential Secretion Rates for SopE2 and SptP. Chem. Biol. 2008, 15, 619–628. [Google Scholar] [CrossRef]
- Glassman, C.R.; Su, L.; Majri-Morrison, S.S.; Winkelmann, H.; Mo, F.; Li, P.; Pérez-Cruz, M.; Ho, P.P.; Koliesnik, I.; Nagy, N.; et al. Calibration of Cell-Intrinsic Interleukin-2 Response Thresholds Guides Design of a Regulatory T Cell Biased Agonist. eLife 2021, 10, e65777. [Google Scholar] [CrossRef]
- Levin, A.M.; Bates, D.L.; Ring, A.M.; Krieg, C.; Lin, J.T.; Su, L.; Moraga, I.; Raeber, M.E.; Bowman, G.R.; Novick, P.; et al. Exploiting a Natural Conformational Switch to Engineer an Interleukin-2 ‘Superkine’. Nature 2012, 484, 529–533. [Google Scholar] [CrossRef]
- Cheever, M.A.; Thompson, J.A.; Kern, D.E.; Greenberg, P.D. Interleukin 2 (IL 2) Administered in Vivo: Influence of IL 2 Route and Timing on T Cell Growth. J. Immunol. 1985, 134, 3895–3900. [Google Scholar] [CrossRef]
- Lems, C.M.; Burger, G.A.; Beltman, J.B. Tumor-Mediated Immunosuppression and Cytokine Spreading Affects the Relation Between EMT and PD-L1 Status. Front. Immunol. 2023, 14, 1219669. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, T. Elimination Half-Lives of Interleukin-6 and Cytokine-Induced Neutrophil Chemoattractant-1 Synthesized in Response to Inflammatory Stimulation in Rats. Lab. Anim. Res. 2018, 34, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A Methodology for Performing Global Uncertainty and Sensitivity Analysis in Systems Biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Bai, Y.; Wang, Z. Elevated T Cell Activation Score Is Associated with Improved Survival of Breast Cancer. Breast Cancer Res. Treat. 2017, 164, 689–696. [Google Scholar] [CrossRef]
- Gilley, R.P.; Dube, P.H. Checkpoint Blockade Inhibitors Enhances the Effectiveness of a Listeria Monocytogenes-Based Melanoma Vaccine. Oncotarget 2020, 11, 740–754. [Google Scholar] [CrossRef]
- Binder, D.C.; Engels, B.; Arina, A.; Yu, P.; Slauch, J.M.; Fu, Y.-X.; Karrison, T.; Burnette, B.; Idel, C.; Zhao, M.; et al. Antigen-Specific Bacterial Vaccine Combined with Anti-PD-L1 Rescues Dysfunctional Endogenous T Cells to Reject Long-Established Cancer. Cancer Immunol. Res. 2013, 1, 123–133. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, L.M.; Forbes, N.S. Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation. Bioengineering 2025, 12, 751. https://doi.org/10.3390/bioengineering12070751
Howell LM, Forbes NS. Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation. Bioengineering. 2025; 12(7):751. https://doi.org/10.3390/bioengineering12070751
Chicago/Turabian StyleHowell, Lars M., and Neil S. Forbes. 2025. "Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation" Bioengineering 12, no. 7: 751. https://doi.org/10.3390/bioengineering12070751
APA StyleHowell, L. M., & Forbes, N. S. (2025). Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation. Bioengineering, 12(7), 751. https://doi.org/10.3390/bioengineering12070751






