Enhanced Bioactivity of Cu-Doped Bioactive Glass Coatings on Human Freeze-Dried Cortical Bone: An In Vitro Study
Abstract
1. Introduction
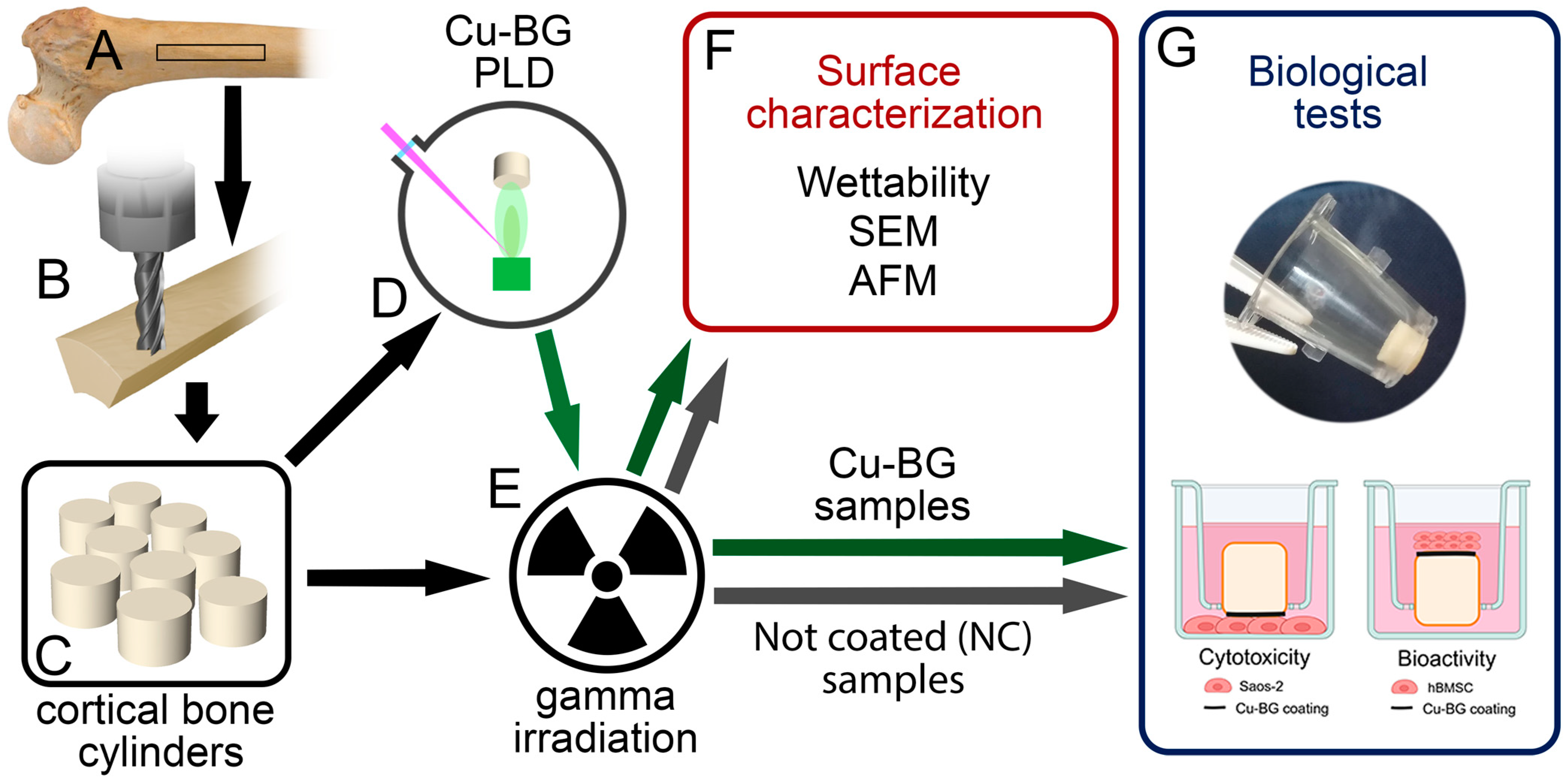
2. Materials and Methods
2.1. Preparation of Cortical Bone Specimens
2.2. Cu-BG Deposition
2.3. Sample Sterilization
2.4. Surface Characterization
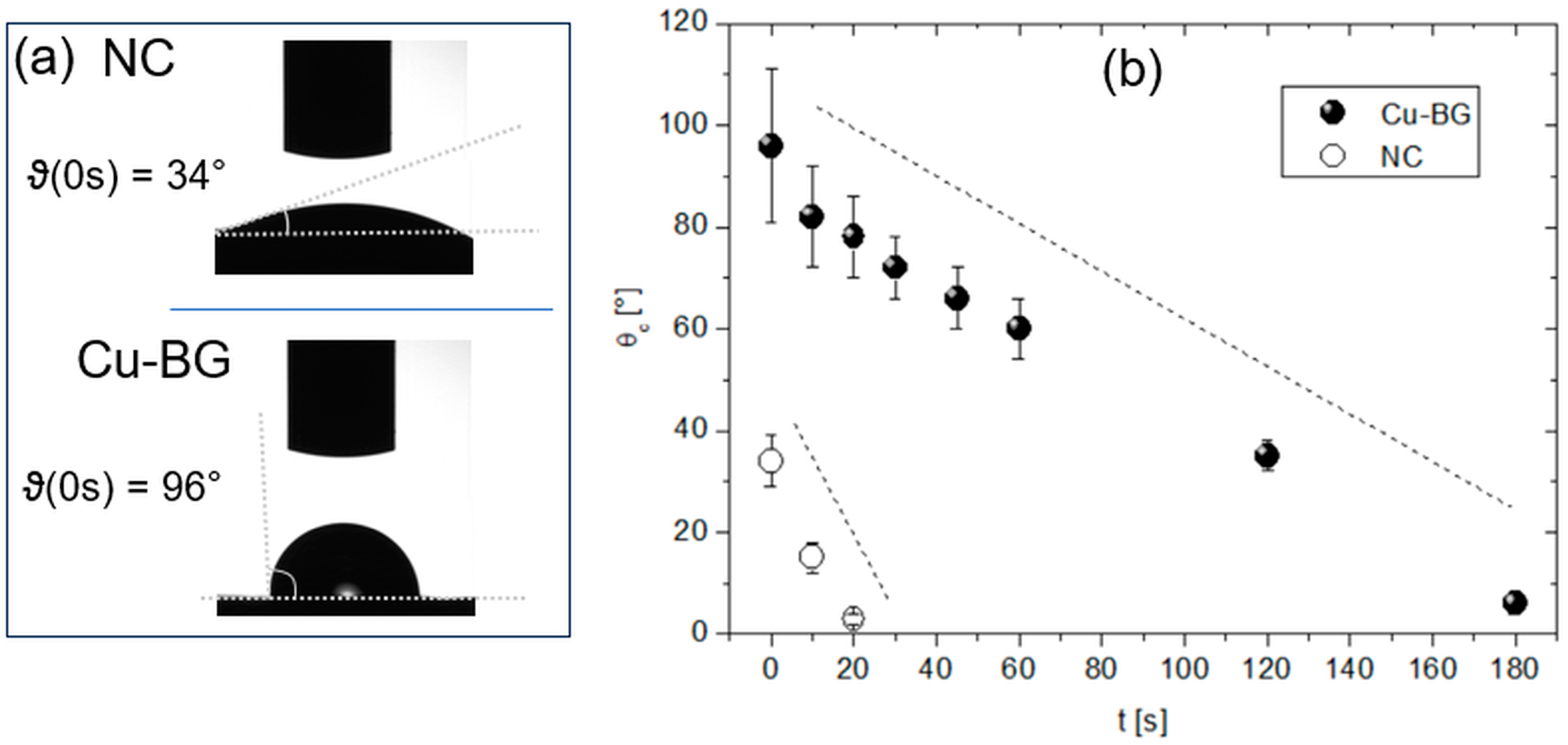
2.4.1. Wettability
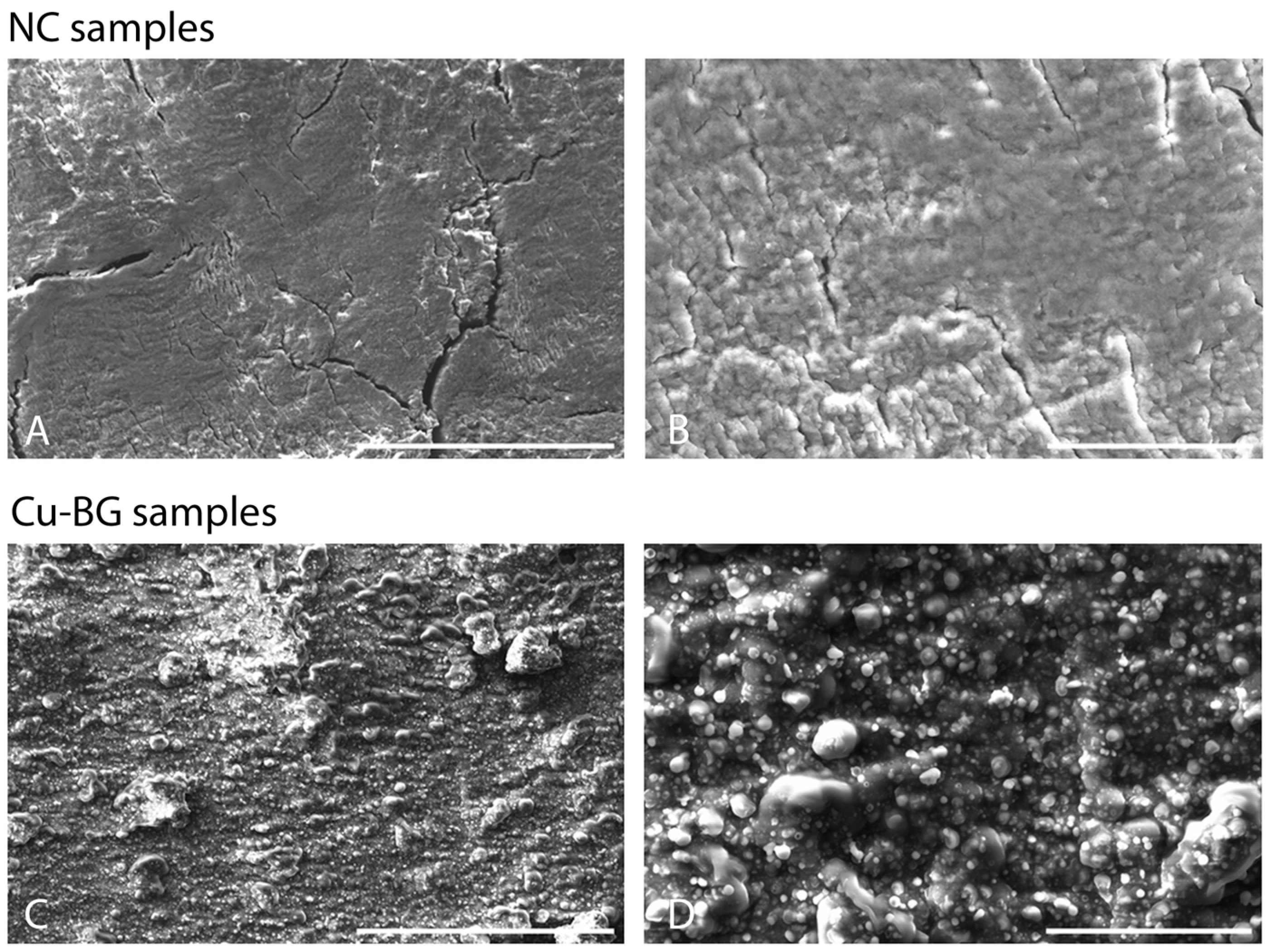
2.4.2. Scanning Electron Microscopy
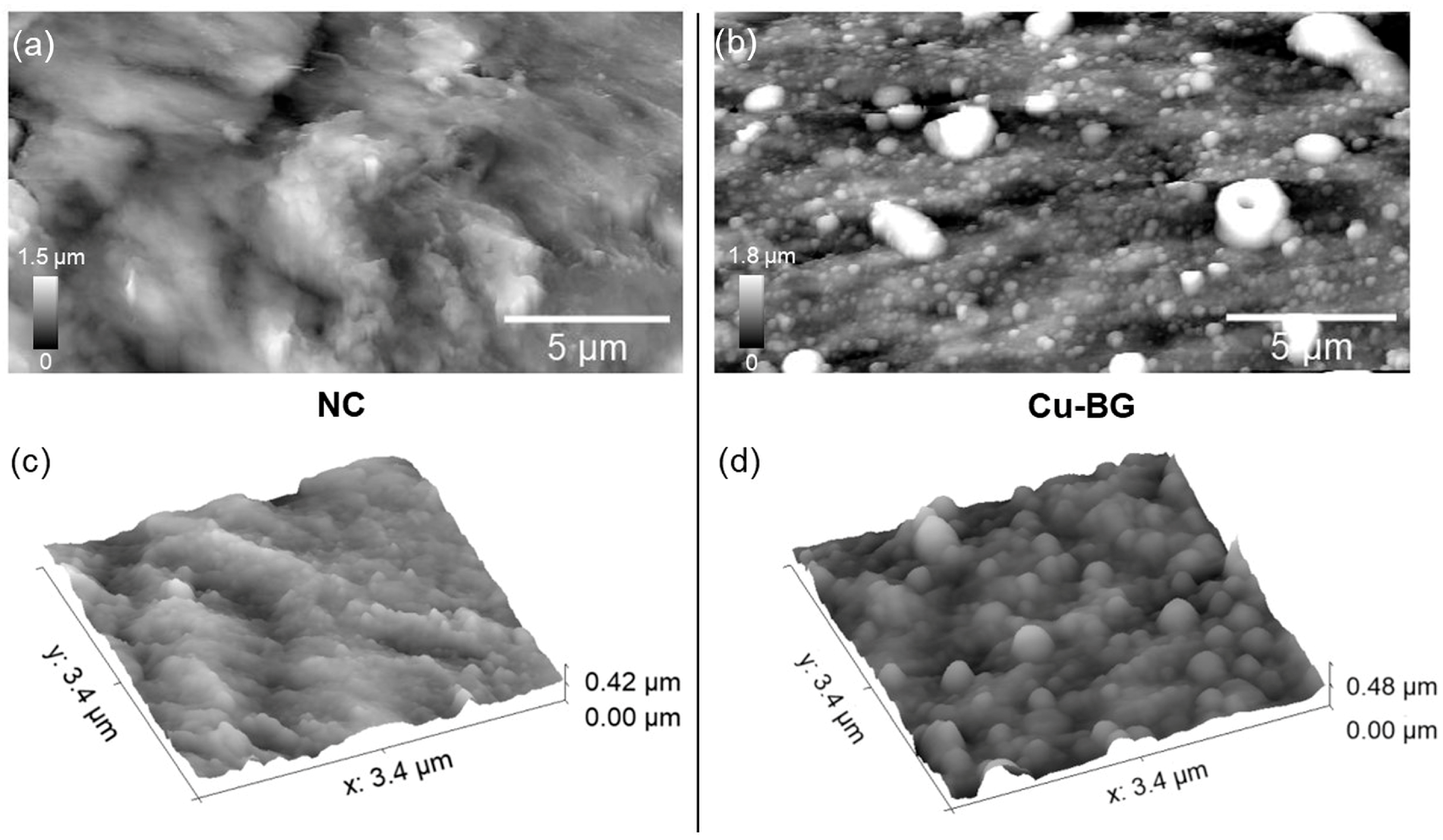
2.4.3. Atomic Force Microscopy
2.5. Biological Tests
2.5.1. Cytotoxicity Evaluation
Cell Proliferation and LDH Release
Neutral Red Staining
2.5.2. Bioactivity Evaluation
hBMSC Viability
hBMSC Osteogenic Differentiation
2.6. Statistical Analysis
3. Results
3.1. Surface Analyses
3.1.1. Wettability
3.1.2. Scanning Electron Microscopy
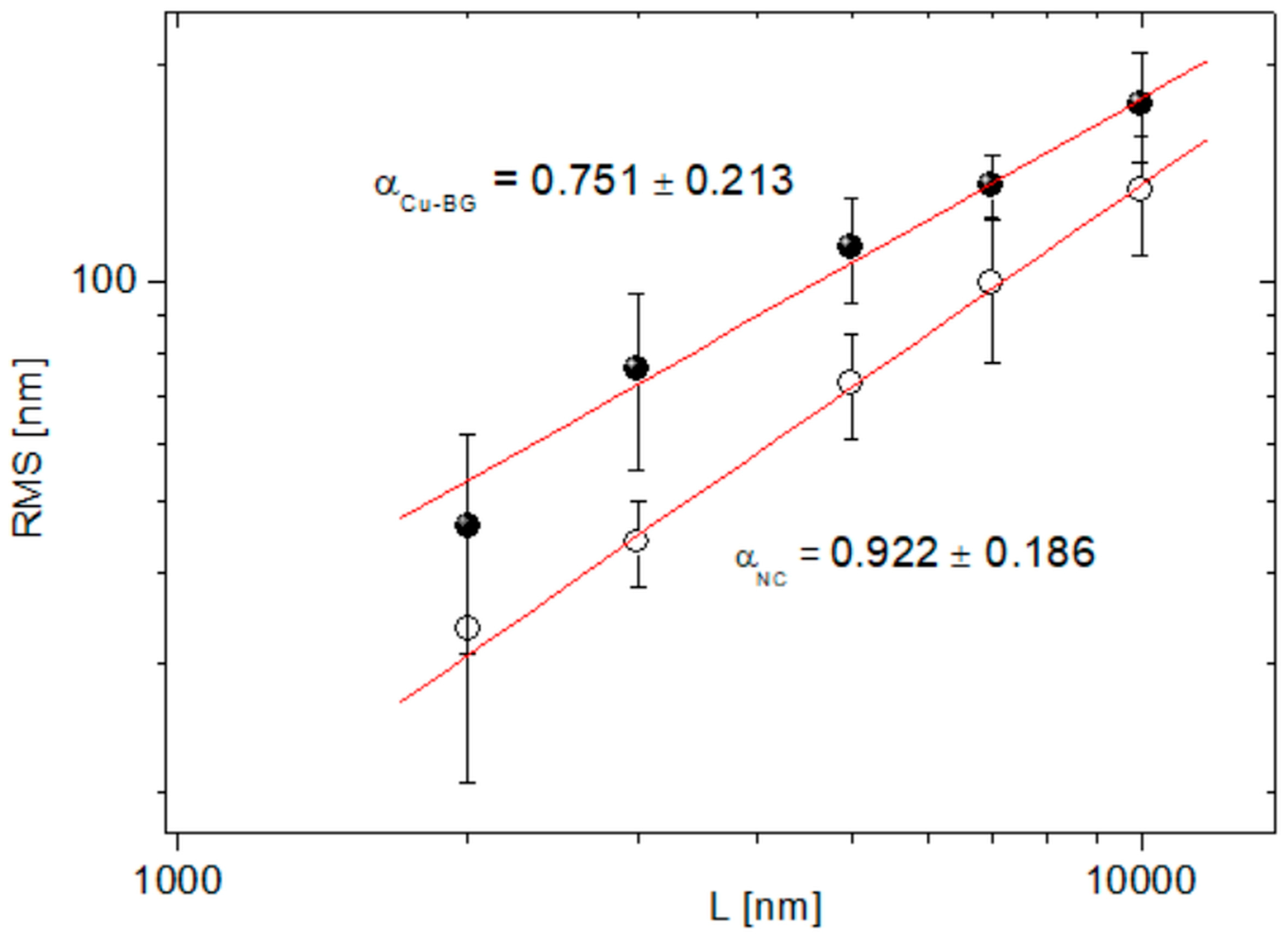
3.1.3. Atomic Force Microscopy
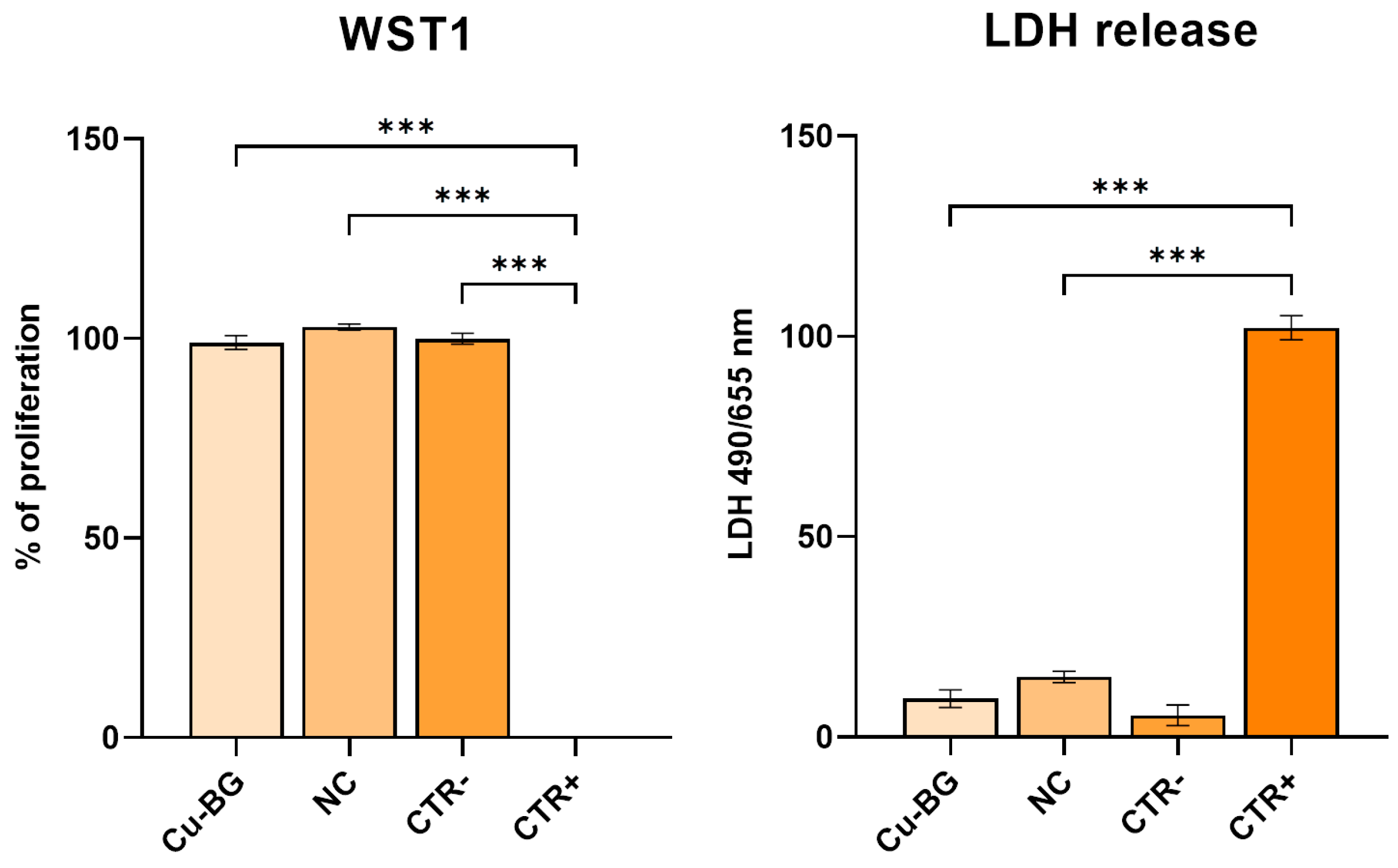
3.2. Cytotoxicity Evaluation
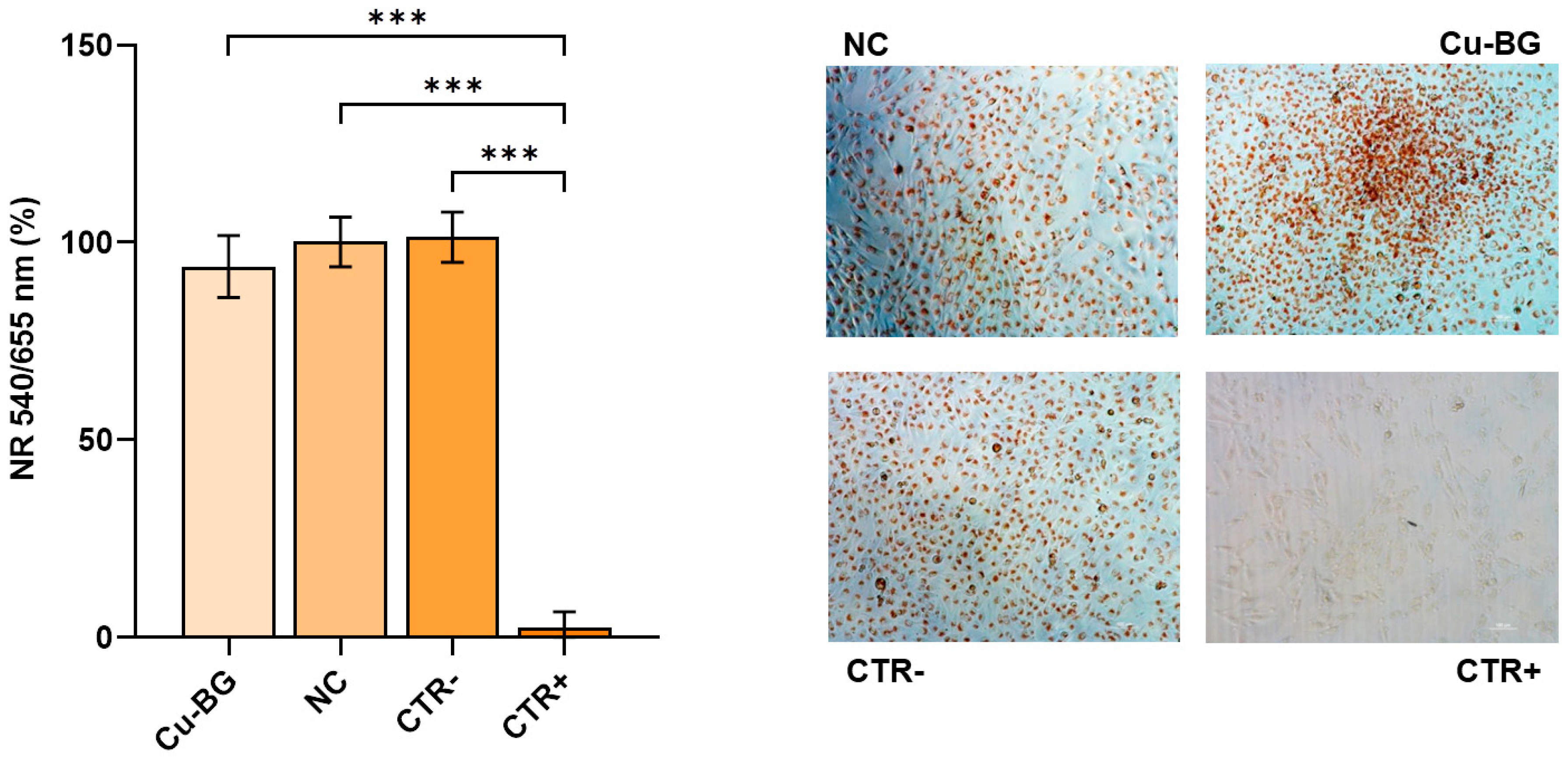
3.3. Bioactivity Evaluation
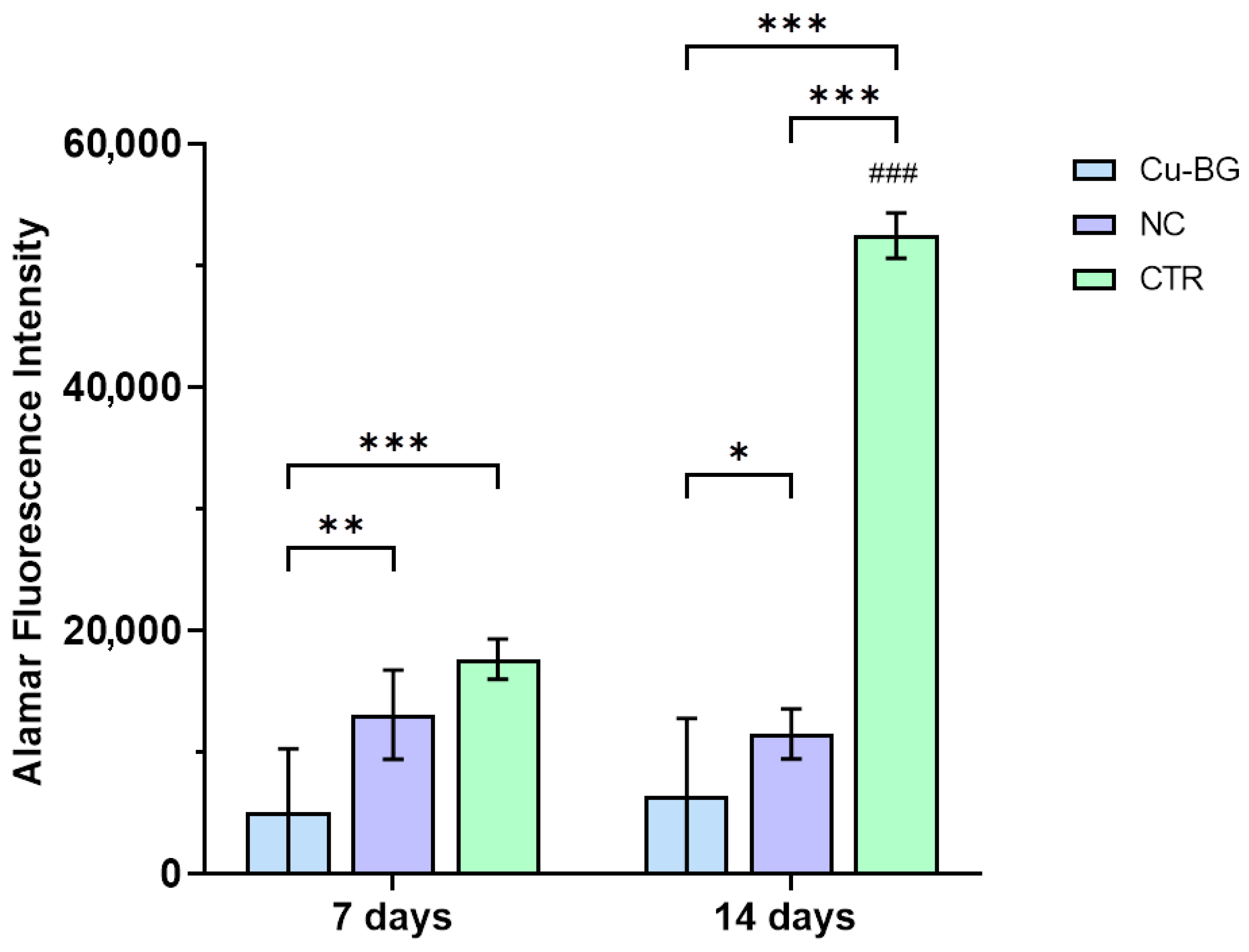
3.3.1. hBMSC Viability
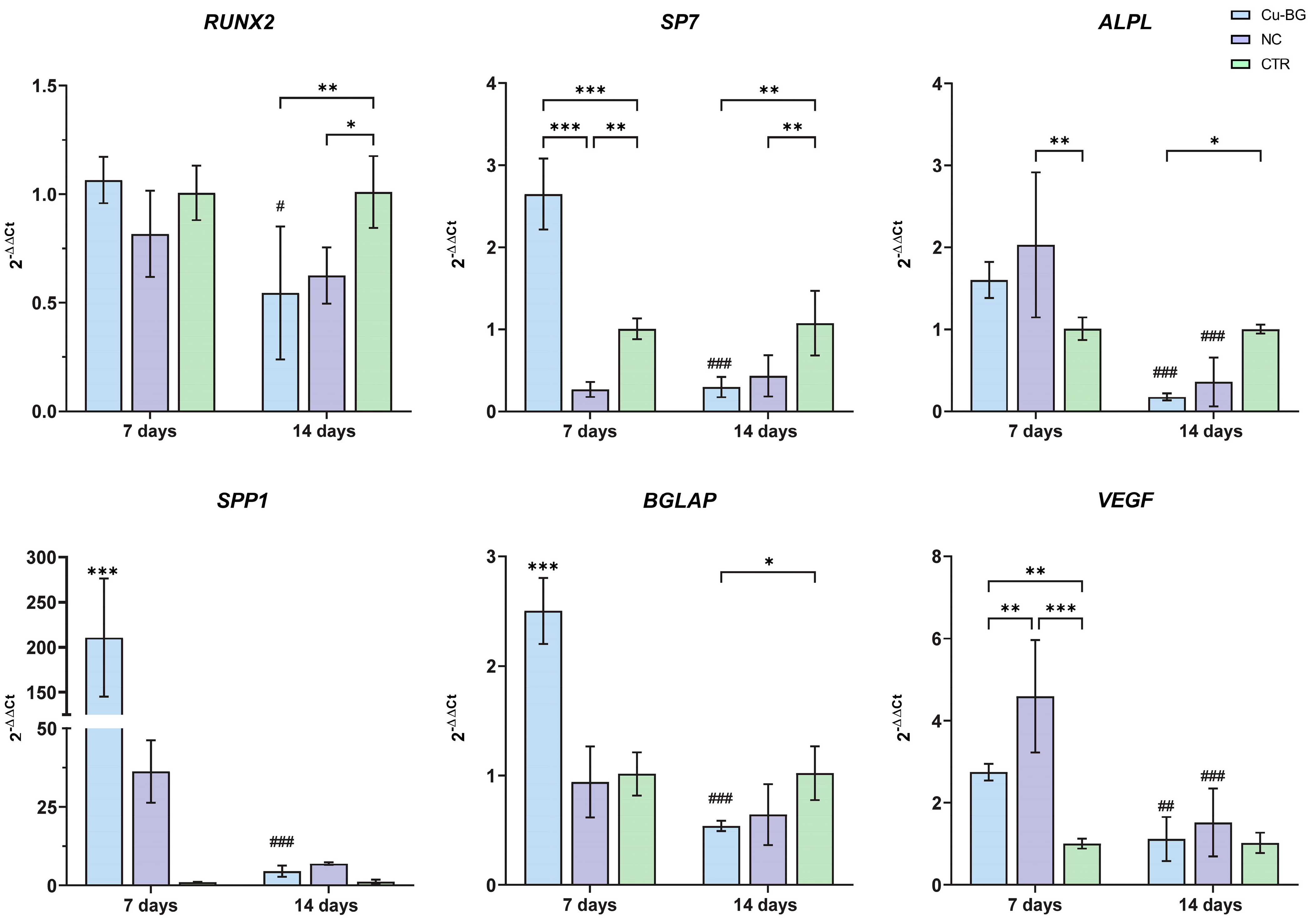
3.3.2. hBMSC Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archunan, M.W.; Petronis, S.; Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Mastnak, T.; Maver, U.; Finšgar, M. Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 2786. [Google Scholar] [CrossRef] [PubMed]
- Samsell, B.; Softic, D.; Qin, X.; McLean, J.; Sohoni, P.; Gonzales, K.; Moore, M.A. Preservation of Allograft Bone Using a Glycerol Solution: A Compilation of Original Preclinical Research. Biomater. Res. 2019, 23, 5. [Google Scholar] [CrossRef]
- Bucciarelli, A.; Pedranz, A.; Gambari, L.; Petretta, M.; Vivarelli, L.; Dallari, D.; Grigolo, B.; Maniglio, D.; Grassi, F. Modeling the Osteogenic Potential of Decellularized Human Bone Particles by Tuning Their Size Distribution Through a Sonic Microfragmentation Approach. Adv. Mater. Technol. 2023, 8, 2300635. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Funamoto, S.; Kimura, T.; Nam, K.; Fujisato, T.; Kishida, A. The Effect of Decellularized Bone/Bone Marrow Produced by High-Hydrostatic Pressurization on the Osteogenic Differentiation of Mesenchymal Stem Cells. Biomaterials 2011, 32, 7060–7067. [Google Scholar] [CrossRef]
- de Roeck, N.J.; Drabu, K.J. Impaction Bone Grafting Using Freeze-Dried Allograft in Revision Hip Arthroplasty. J. Arthroplast. 2001, 16, 201–206. [Google Scholar] [CrossRef]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Mansor, A.; Ariffin, A.F.; Yusof, N.; Mohd, S.; Ramalingam, S.; Md Saad, A.P.; Baharin, R.; Min, N.W. Effects of Processing and Gamma Radiation on Mechanical Properties and Organic Composition of Frozen, Freeze-Dried and Demineralised Human Cortical Bone Allograft. Cell Tissue Bank. 2023, 24, 25–35. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. A Review of Techniques for the Application of Bioactive Coatings on Metal-Based Implants to Achieve Controlled Release of Active Ingredients. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive Glass Coatings on Metallic Implants for Biomedical Applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Wu, C.; Zreiqat, H. Orthopedic Coating Materials: Considerations and Applications. Expert Rev. Med. Devices 2009, 6, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-Based Composites for Bone Tissue Engineering and Functional Applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Taye, M.B. Biomedical Applications of Ion-Doped Bioactive Glass: A Review. Appl. Nanosci. 2022, 12, 3797–3812. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Kanchanadumkerng, P.; Ruenraroengsak, P. Multifunctional Zn and Ag Co-Doped Bioactive Glass Nanoparticles for Bone Therapeutic and Regeneration. Sci. Rep. 2023, 13, 6775. [Google Scholar] [CrossRef]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef]
- Liang, J.; Lu, X.; Zheng, X.; Li, Y.R.; Geng, X.; Sun, K.; Cai, H.; Jia, Q.; Jiang, H.B.; Liu, K. Modification of Titanium Orthopedic Implants with Bioactive Glass: A Systematic Review of in Vivo and in Vitro Studies. Front. Bioeng. Biotechnol. 2023, 11, 1269223. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Brouki Milan, P.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium- and Cobalt-Substituted Bioactive Glasses Seeded with Human Umbilical Cord Perivascular Cells to Promote Bone Regeneration via Enhanced Osteogenic and Angiogenic Activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef]
- Hammami, I.; Graça, M.P.F.; Gavinho, S.R.; Jakka, S.K.; Borges, J.P.; Silva, J.C.; Costa, L.C. Exploring the Impact of Copper Oxide Substitution on Structure, Morphology, Bioactivity, and Electrical Properties of 45S5 Bioglass®. Biomimetics 2024, 9, 213. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-Based Biomaterials for Bone and Cartilage Tissue Engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Xu, S.; Wang, K.; Yu, S. Effects of Cu2+ and pH on Osteoclastic Bone Resorption in Vitro. Prog. Nat. Sci. 2003, 13, 266–270. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Ramamurthi, A. Copper Nanoparticle Cues for Biomimetic Cellular Assembly of Crosslinked Elastin Fibers. Acta Biomater. 2009, 5, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D.; Rayton, J.K.; Balthrop, J.E.; Di Silvestro, R.A.; Garcia-de-Quevedo, M. Copper and the Synthesis of Elastin and Collagen. In Ciba Foundation Symposium 79—Biological Roles of Copper; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1980; pp. 163–182. ISBN 978-0-470-72062-2. [Google Scholar]
- Noori, A.; Hoseinpour, M.; Kolivand, S.; Lotfibakhshaiesh, N.; Azami, M.; Ai, J.; Ebrahimi-Barough, S. Synergy Effects of Copper and L-Arginine on Osteogenic, Angiogenic, and Antibacterial Activities. Tissue Cell 2022, 77, 101849. [Google Scholar] [CrossRef]
- Rau, J.V.; Curcio, M.; Raucci, M.G.; Barbaro, K.; Fasolino, I.; Teghil, R.; Ambrosio, L.; De Bonis, A.; Boccaccini, A.R. Cu-Releasing Bioactive Glass Coatings and Their in Vitro Properties. ACS Appl. Mater. Interfaces 2019, 11, 5812–5820. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S. Sputter Depth Profiling: Past, Present, and Future. Surf. Interface Anal. 2014, 46, 654–662. [Google Scholar] [CrossRef]
- Stan, G.E.; Montazerian, M.; Shearer, A.; Stuart, B.W.; Baino, F.; Mauro, J.C.; Ferreira, J.M.F. Critical Advances in the Field of Magnetron Sputtered Bioactive Glass Thin-Films: An Analytical Review. Appl. Surf. Sci. 2024, 646, 158760. [Google Scholar] [CrossRef]
- Wen, J.; Lu, T.; Wang, X.; Xu, L.; Wu, Q.; Pan, H.; Wang, D.; Liu, X.; Jiang, X. In Vitro and in Vivo Evaluation of Silicate-Coated Polyetheretherketone Fabricated by Electron Beam Evaporation. ACS Appl. Mater. Interfaces 2016, 8, 13197–13206. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A Practical Guide to Pulsed Laser Deposition. Chem. Soc. Rev. 2023, 52, 2294–2321. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef]
- Torchio, P.; Gatto, A.; Alvisi, M.; Albrand, G.; Kaiser, N.; Amra, C. High-Reflectivity HfO2/SiO2 Ultraviolet Mirrors. Appl. Opt. AO 2002, 41, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11, 811. [Google Scholar] [CrossRef]
- Curcio, M.; De Stefanis, A.; De Bonis, A.; Teghil, R.; Rau, J.V. Pulsed Laser Deposited Bioactive RKKP-Mn Glass-Ceramic Coatings on Titanium. Surf. Coat. Technol. 2019, 357, 122–128. [Google Scholar] [CrossRef]
- Schitea, R.-I.; Nitu, A.; Ciobota, A.-A.; Munteanu, A.-L.; David, I.-M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef]
- Grassi, F.R.; Grassi, R.; Vivarelli, L.; Dallari, D.; Govoni, M.; Nardi, G.M.; Kalemaj, Z.; Ballini, A. Design Techniques to Optimize the Scaffold Performance: Freeze-Dried Bone Custom-Made Allografts for Maxillary Alveolar Horizontal Ridge Augmentation. Materials 2020, 13, 1393. [Google Scholar] [CrossRef]
- Vivarelli, L.; Govoni, M.; Attala, D.; Zoccali, C.; Biagini, R.; Dallari, D. Custom Massive Allograft in a Case of Pelvic Bone Tumour: Simulation of Processing with Computerised Numerical Control vs. Robotic Machining. J. Clin. Med. 2022, 11, 2781. [Google Scholar] [CrossRef]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J.; et al. In Vitro Reactivity of Cu Doped 45S5 Bioglass® Derived Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2013, 1, 5659–5674. [Google Scholar] [CrossRef]
- Rau, J.V.; De Bonis, A.; Curcio, M.; Schuhladen, K.; Barbaro, K.; De Bellis, G.; Teghil, R.; Boccaccini, A.R. Borate and Silicate Bioactive Glass Coatings Prepared by Nanosecond Pulsed Laser Deposition. Coatings 2020, 10, 1105. [Google Scholar] [CrossRef]
- D’Alessio, L.; Galasso, A.; Santagata, A.; Teghil, R.; Villani, A.R.; Villani, P.; Zaccagnino, M. Plume Dynamics in TiC Laser Ablation. Appl. Surf. Sci. 2003, 208–209, 113–118. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, D.A.F.; Forwood, M.R. Sterilization of Allograft Bone: Effects of Gamma Irradiation on Allograft Biology and Biomechanics. Cell Tissue Bank. 2007, 8, 93–105. [Google Scholar] [CrossRef]
- ISO 11137-2:2013; Sterilization of Health Care Products—Radiation. Part 2: Establishing the Sterilization Dose. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/62442.html (accessed on 24 February 2025).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 24 February 2025).
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; De Bonis, A.; Fosca, M.; Santagata, A.; Teghil, R.; Rau, J.V. Pulsed Laser-Deposited Composite Carbon–Glass–Ceramic Films with Improved Hardness. J. Mater. Sci. 2017, 52, 9140–9150. [Google Scholar] [CrossRef]
- De Bonis, A.; Uskoković, V.; Barbaro, K.; Fadeeva, I.; Curcio, M.; Imperatori, L.; Teghil, R.; Rau, J.V. Pulsed Laser Deposition Temperature Effects on Strontium-Substituted Hydroxyapatite Thin Films for Biomedical Implants. Cell Biol. Toxicol. 2020, 36, 537–551. [Google Scholar] [CrossRef]
- Puckett, S.; Pareta, R.; Webster, T.J. Nano Rough Micron Patterned Titanium for Directing Osteoblast Morphology and Adhesion. Int. J. Nanomed. 2008, 3, 229–241. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D Printing Method for Bone Tissue Engineering Scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Lovecchio, J.; Cortesi, M.; Zani, M.; Govoni, M.; Dallari, D.; Giordano, E. Fiber Thickness and Porosity Control in a Biopolymer Scaffold 3D Printed through a Converted Commercial FDM Device. Materials 2022, 15, 2394. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Borsari, V.; Fini, M.; Giavaresi, G.; Tschon, M.; Chiesa, R.; Chiusoli, L.; Salito, A.; Rimondini, L.; Giardino, R. Comparative in Vivo Evaluation of Porous and Dense Duplex Titanium and Hydroxyapatite Coating with High Roughnesses in Different Implantation Environments. J. Biomed. Mater. Res. Part A 2009, 89A, 550–560. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Russo, A.; Bianchi, M. A Comparative Study of the Growth Dynamics of Zirconia Thin Films Deposited by Ionized Jet Deposition onto Different Substrates. Surf. Coat. Technol. 2018, 337, 306–312. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Pagani, S.; Perut, F.; Ciapetti, G.; Baldini, N.; Mongiorgi, R.; Prati, C. Innovative Silicate-Based Cements for Endodontics: A Study of Osteoblast-like Cell Response. J. Biomed. Mater. Res. Part A 2008, 87A, 477–486. [Google Scholar] [CrossRef]
- Polo, L.; Díaz de Greñu, B.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial Activity of Commercial Calcium Phosphate Based Materials Functionalized with Vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Torricelli, P.; Veronesi, F.; Salamanna, F.; Cepollaro, S.; Fini, M. An Advanced Tri-Culture Model to Evaluate the Dynamic Interplay among Osteoblasts, Osteoclasts, and Endothelial Cells. J. Cell. Physiol. 2018, 233, 291–301. [Google Scholar] [CrossRef]
- Salamanna, F.; Sartori, M.; Brodano, G.B.; Griffoni, C.; Martini, L.; Boriani, S.; Fini, M. Mesenchymal Stem Cells for the Treatment of Spinal Arthrodesis: From Preclinical Research to Clinical Scenario. Stem Cells Int. 2017, 2017, 3537094. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Graziani, G.; Sassoni, E.; Pagani, S.; Boi, M.; Maltarello, M.C.; Baldini, N.; Fini, M. Nanostructure and Biomimetics Orchestrate Mesenchymal Stromal Cell Differentiation: An in Vitro Bioactivity Study on New Coatings for Orthopedic Applications. Mater. Sci. Eng. C 2021, 123, 112031. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the Proliferation and Differentiation of Human Mesenchymal Stem Cells by Copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, Function, and Phosphorylation in Osteoblast Differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-Derived Glass–Ceramic Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-Containing Mesoporous Bioactive Glass Scaffolds with Multifunctional Properties of Angiogenesis Capacity, Osteostimulation and Antibacterial Activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The Stimulation of Angiogenesis and Collagen Deposition by Copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The Bone Building Blues: Self-Hardening Copper-Doped Calcium Phosphate Cement and Its in Vitro Assessment against Mammalian Cells and Bacteria. Mater. Sci. Eng. C 2017, 79, 270–279. [Google Scholar] [CrossRef]
- Graziani, V.; Fosca, M.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Baranchikov, A.E.; Ortenzi, M.; Caminiti, R.; Komlev, V.S.; Rau, J.V. Zinc-Releasing Calcium Phosphate Cements for Bone Substitute Materials. Ceram. Int. 2016, 42, 17310–17316. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.V. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395. [Google Scholar] [CrossRef]
- Ghezzi, D.; Graziani, G.; Cappelletti, M.; Fadeeva, I.V.; Montesissa, M.; Sassoni, E.; Borciani, G.; Barbaro, K.; Boi, M.; Baldini, N.; et al. New Strontium-Based Coatings Show Activity against Pathogenic Bacteria in Spine Infection. Front. Bioeng. Biotechnol. 2024, 12, 1347811. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-Induced Vascular Endothelial Growth Factor Expression and Wound Healing. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Lazoryak, B.I.; Davidova, G.A.; Murzakhanov, F.F.; Gabbasov, B.F.; Petrakova, N.V.; Fosca, M.; Barinov, S.M.; Vadalà, G.; Uskoković, V.; et al. Antibacterial and Cell-Friendly Copper-Substituted Tricalcium Phosphate Ceramics for Biomedical Implant Applications. Mater. Sci. Eng. C 2021, 129, 112410. [Google Scholar] [CrossRef]
- Russo, T.; De Santis, R.; Gloria, A.; Barbaro, K.; Altigeri, A.; Fadeeva, I.V.; Rau, J.V. Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles. Polymers 2020, 12, 37. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative Investigation on the Adhesion of Hydroxyapatite Coating on Ti–6Al–4V Implant: A Review Paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
| Gene | Primer | Amplicon Length | Annealing Temperature |
|---|---|---|---|
| GAPDH | QuantiTect Primer Assay (Qiagen) Hs_GAPDH_1_SG | 95 bp | 55 °C |
| RUNX2 | Forward: CTTCACAAATCCTCCCCAAGT Reverse: AGGCGGTCAGAGAACAAAC | 212 bp | 60 °C |
| ALPL | QuantiTect Primer Assay (Qiagen) Hs_ALPL_1_SG | 110 bp | 55 °C |
| SP7 | QuantiTect Primer Assay (Qiagen) Hs_SP7_1_SG | 120 bp | 55 °C |
| BGLAP | QuantiTect Primer Assay (Qiagen) Hs_BGLAP_1_SG | 90 bp | 55 °C |
| VEGF | QuantiTect Primer Assay (Qiagen) Hs_VEGFA_6_SG | 99 bp | 55 °C |
| SPP1 | QuantiTect Primer Assay (Qiagen) Hs_SPP1_1_SG | 115 bp | 55 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogini, S.; Tschon, M.; Vivarelli, L.; Gambardella, A.; De Bonis, A.; Giavaresi, G.; Fini, M.; Dallari, D.; Rau, J.V.; Govoni, M. Enhanced Bioactivity of Cu-Doped Bioactive Glass Coatings on Human Freeze-Dried Cortical Bone: An In Vitro Study. Bioengineering 2025, 12, 354. https://doi.org/10.3390/bioengineering12040354
Brogini S, Tschon M, Vivarelli L, Gambardella A, De Bonis A, Giavaresi G, Fini M, Dallari D, Rau JV, Govoni M. Enhanced Bioactivity of Cu-Doped Bioactive Glass Coatings on Human Freeze-Dried Cortical Bone: An In Vitro Study. Bioengineering. 2025; 12(4):354. https://doi.org/10.3390/bioengineering12040354
Chicago/Turabian StyleBrogini, Silvia, Matilde Tschon, Leonardo Vivarelli, Alessandro Gambardella, Angela De Bonis, Gianluca Giavaresi, Milena Fini, Dante Dallari, Julietta V. Rau, and Marco Govoni. 2025. "Enhanced Bioactivity of Cu-Doped Bioactive Glass Coatings on Human Freeze-Dried Cortical Bone: An In Vitro Study" Bioengineering 12, no. 4: 354. https://doi.org/10.3390/bioengineering12040354
APA StyleBrogini, S., Tschon, M., Vivarelli, L., Gambardella, A., De Bonis, A., Giavaresi, G., Fini, M., Dallari, D., Rau, J. V., & Govoni, M. (2025). Enhanced Bioactivity of Cu-Doped Bioactive Glass Coatings on Human Freeze-Dried Cortical Bone: An In Vitro Study. Bioengineering, 12(4), 354. https://doi.org/10.3390/bioengineering12040354
















