Immunomodulation and Mechanical Characterization of Manuka Honey-Incorporated Near-Field Electrospun Bioresorbable Vascular Grafts
Abstract
1. Introduction
2. Materials and Methods
2.1. Vascular Template Fabrication
2.2. Mechanical Characterization
2.3. Longitudinal Uniaxial Elongation
2.4. Circumferential Uniaxial Elongation
2.5. Suture Retention
2.6. Burst Pressure
2.7. Template Sterilization
2.8. Manuka Honey Elution
2.9. NET Release
2.10. Statistical Methods
3. Results
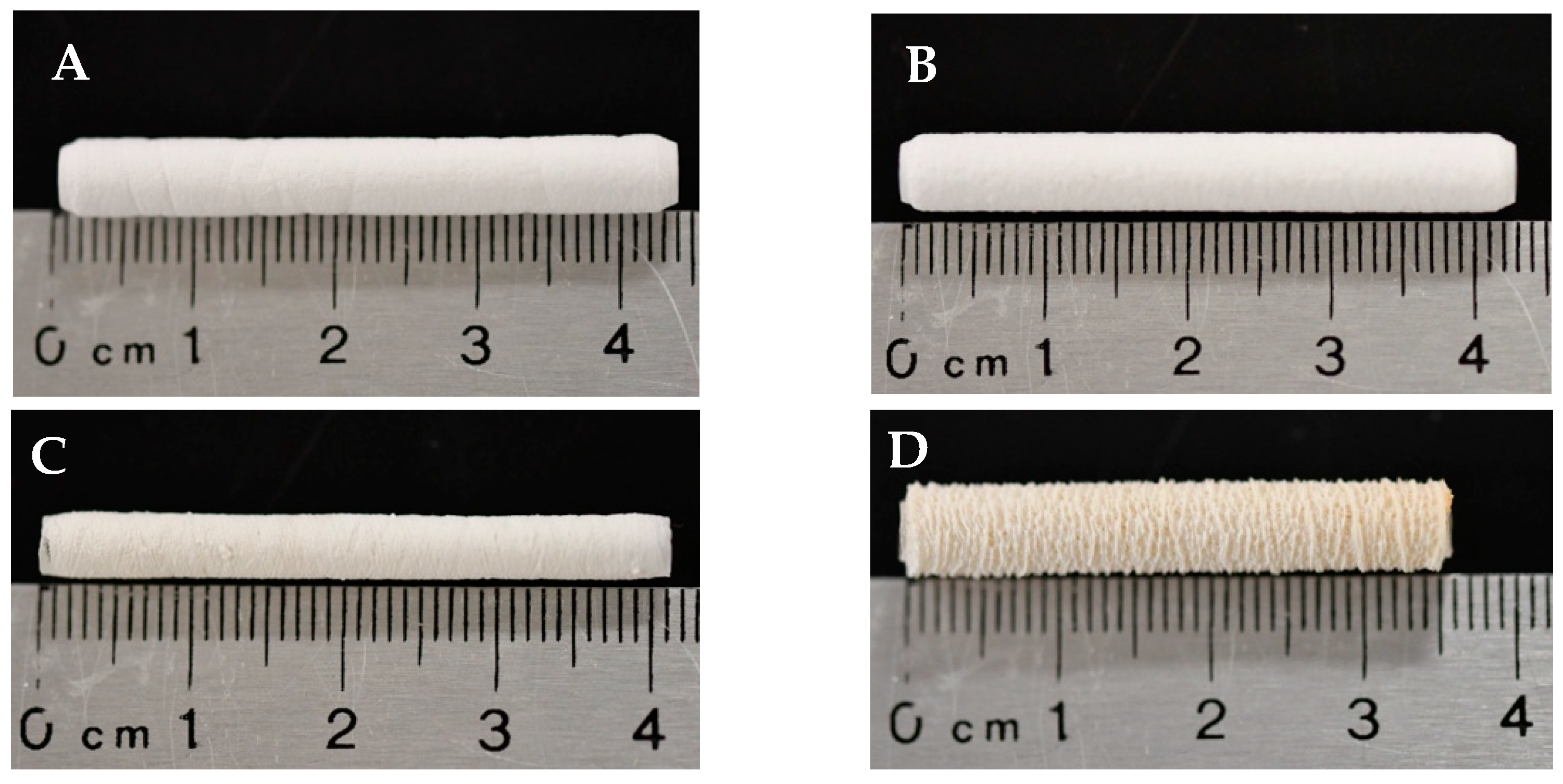
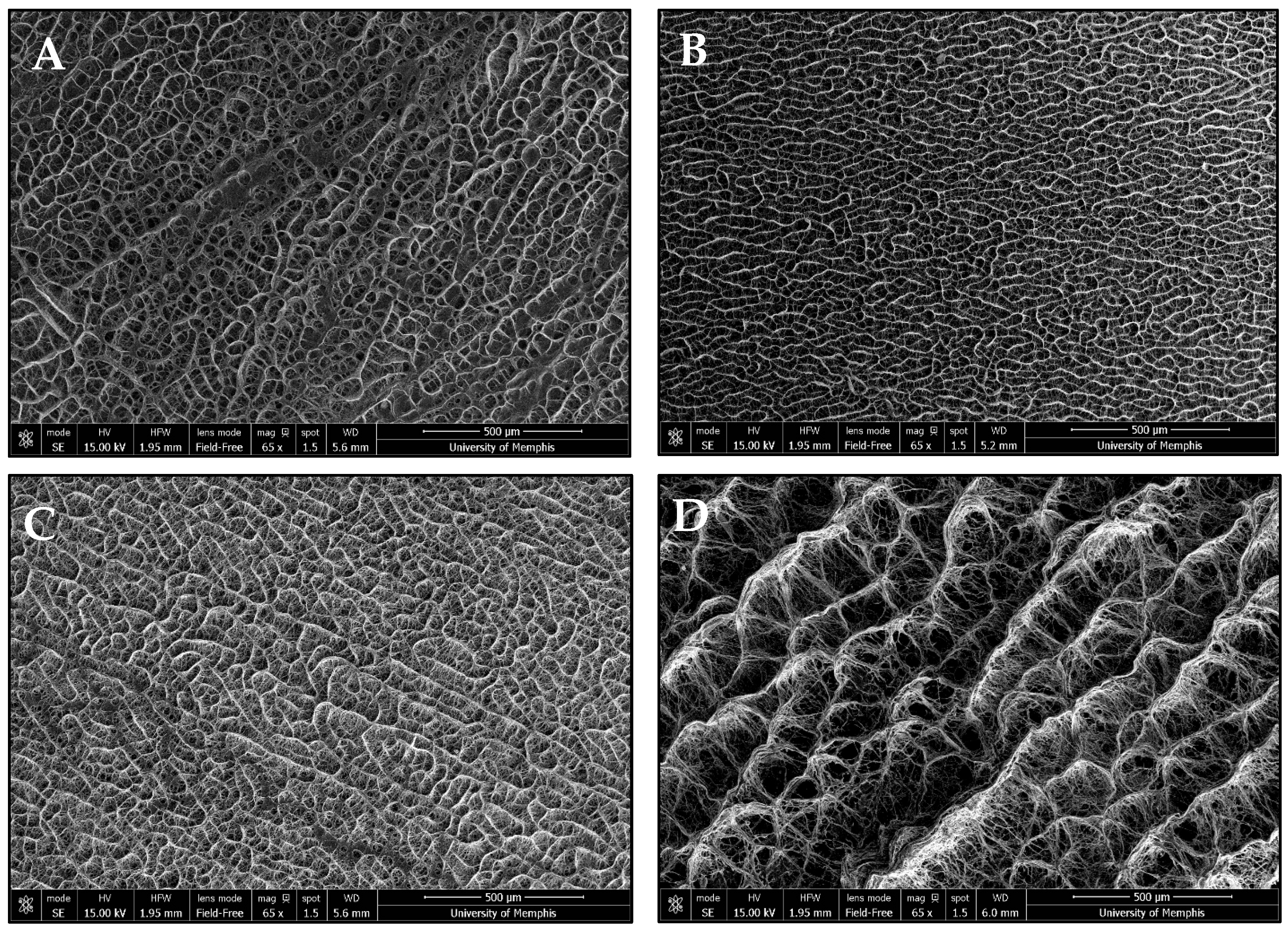
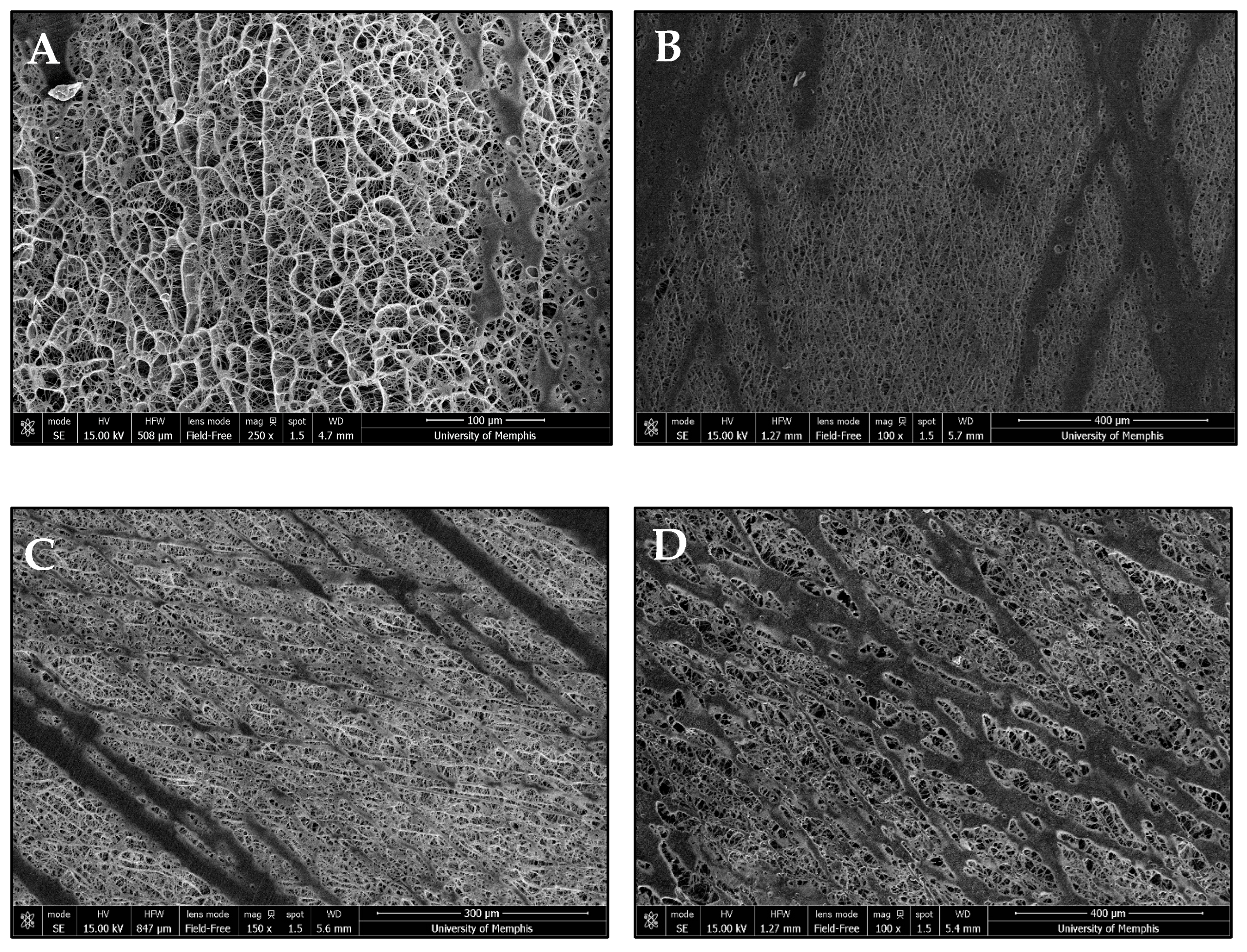
3.1. Template Morphology
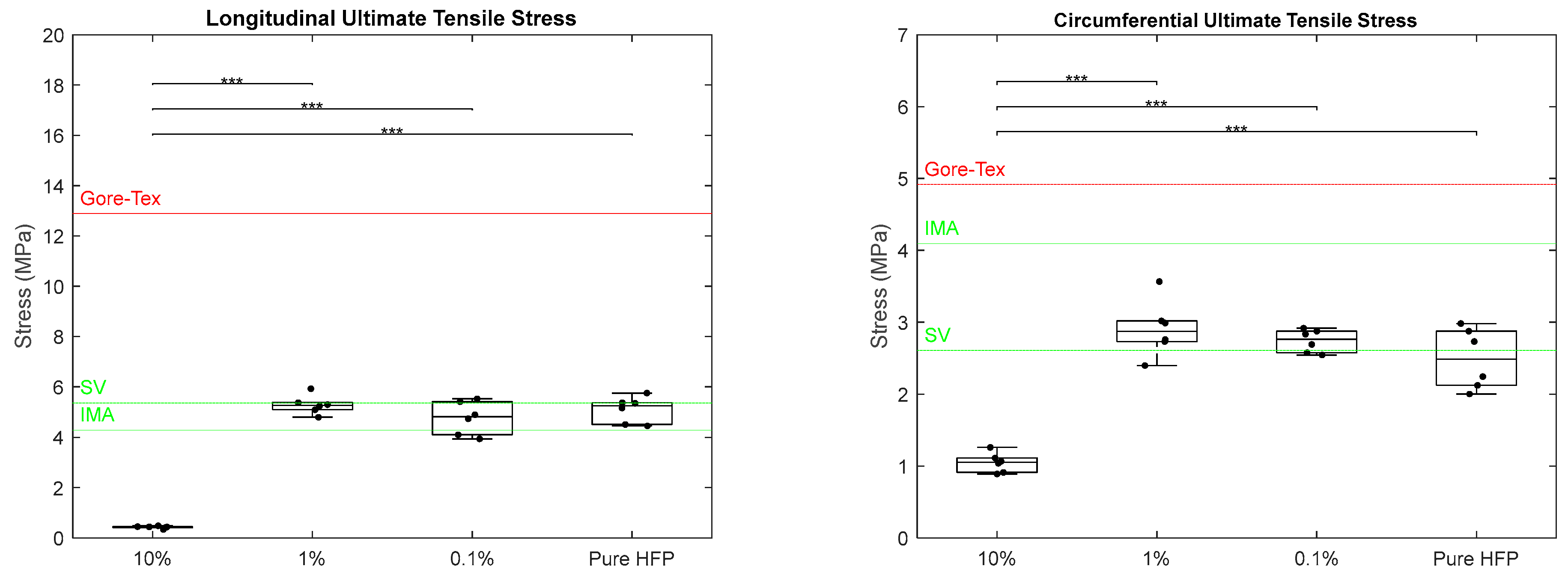
3.2. Mechanical Characterization
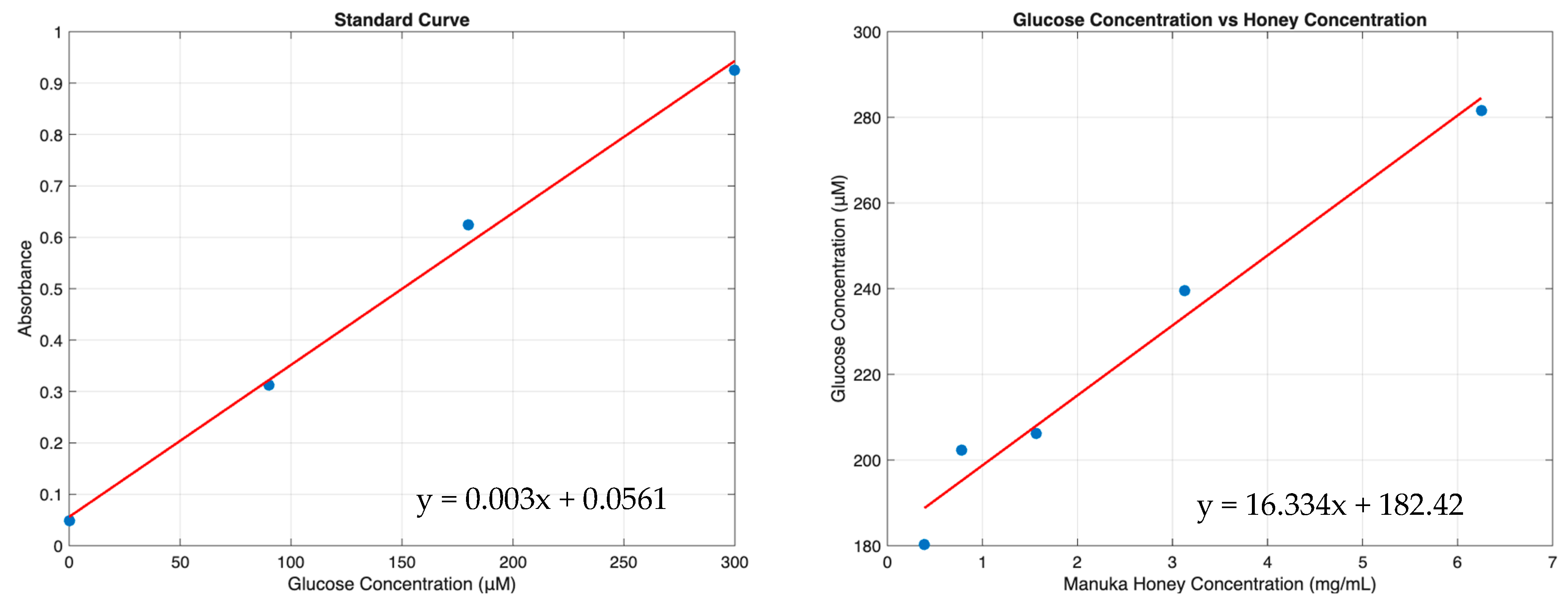
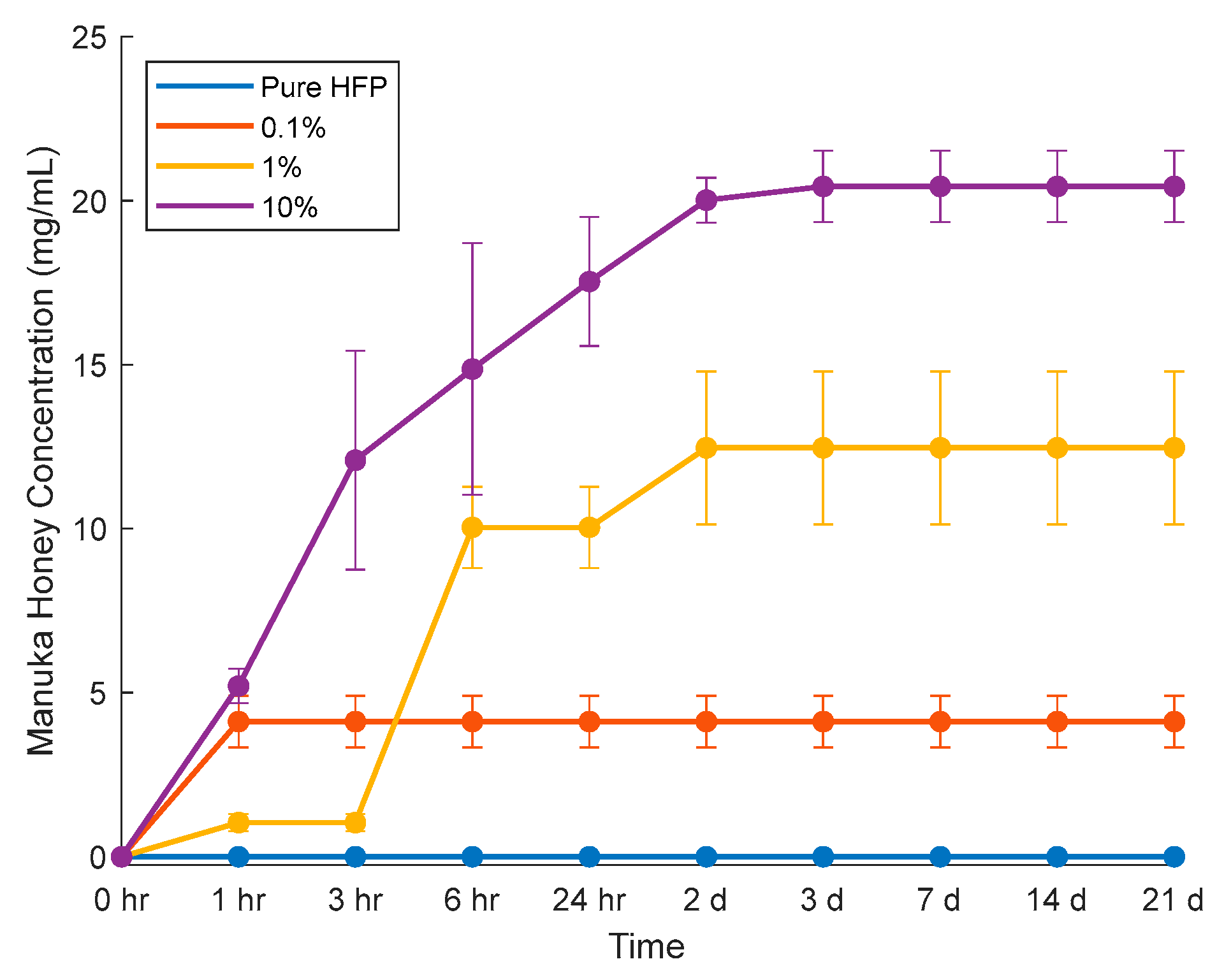
3.3. Manuka Honey Elution
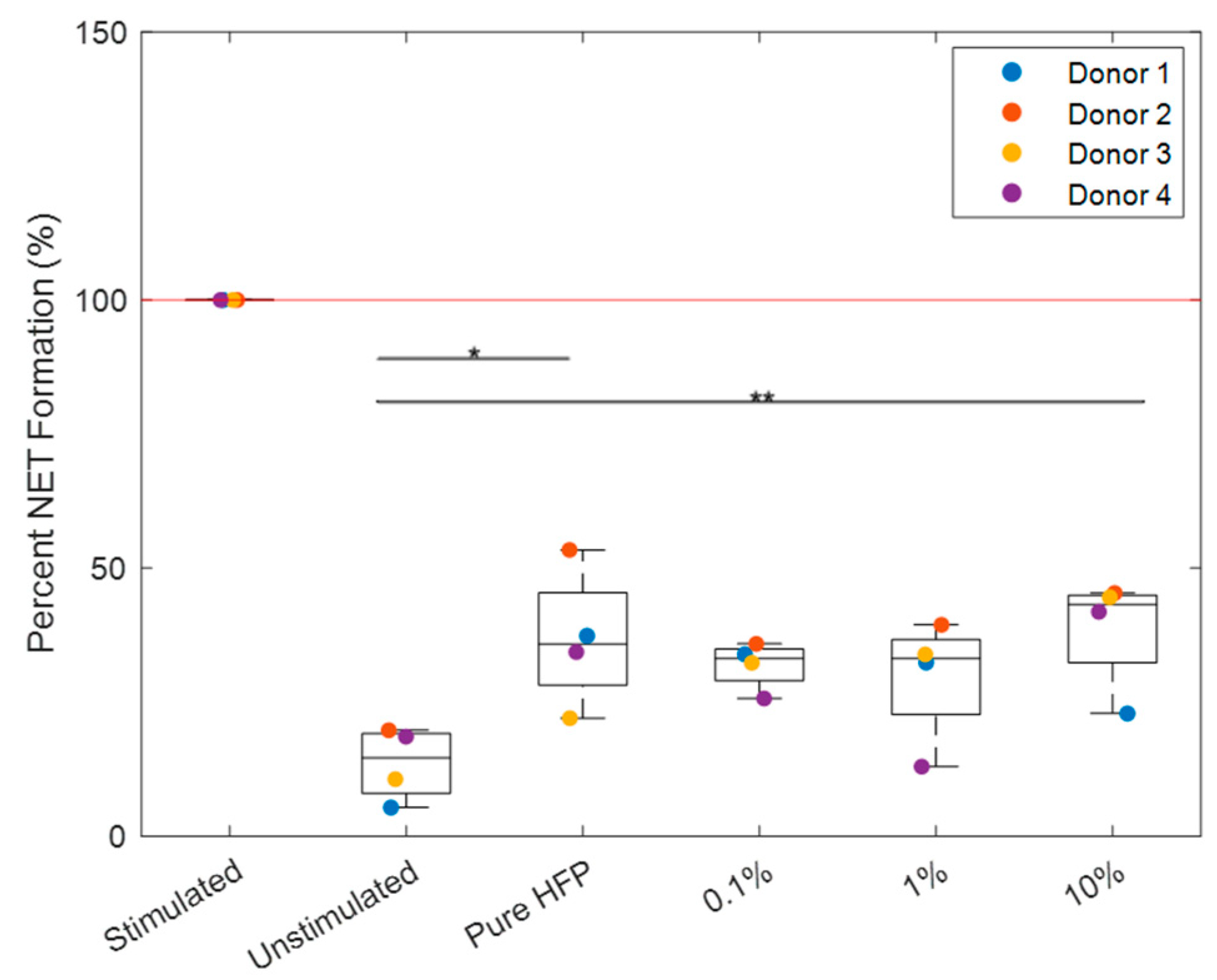
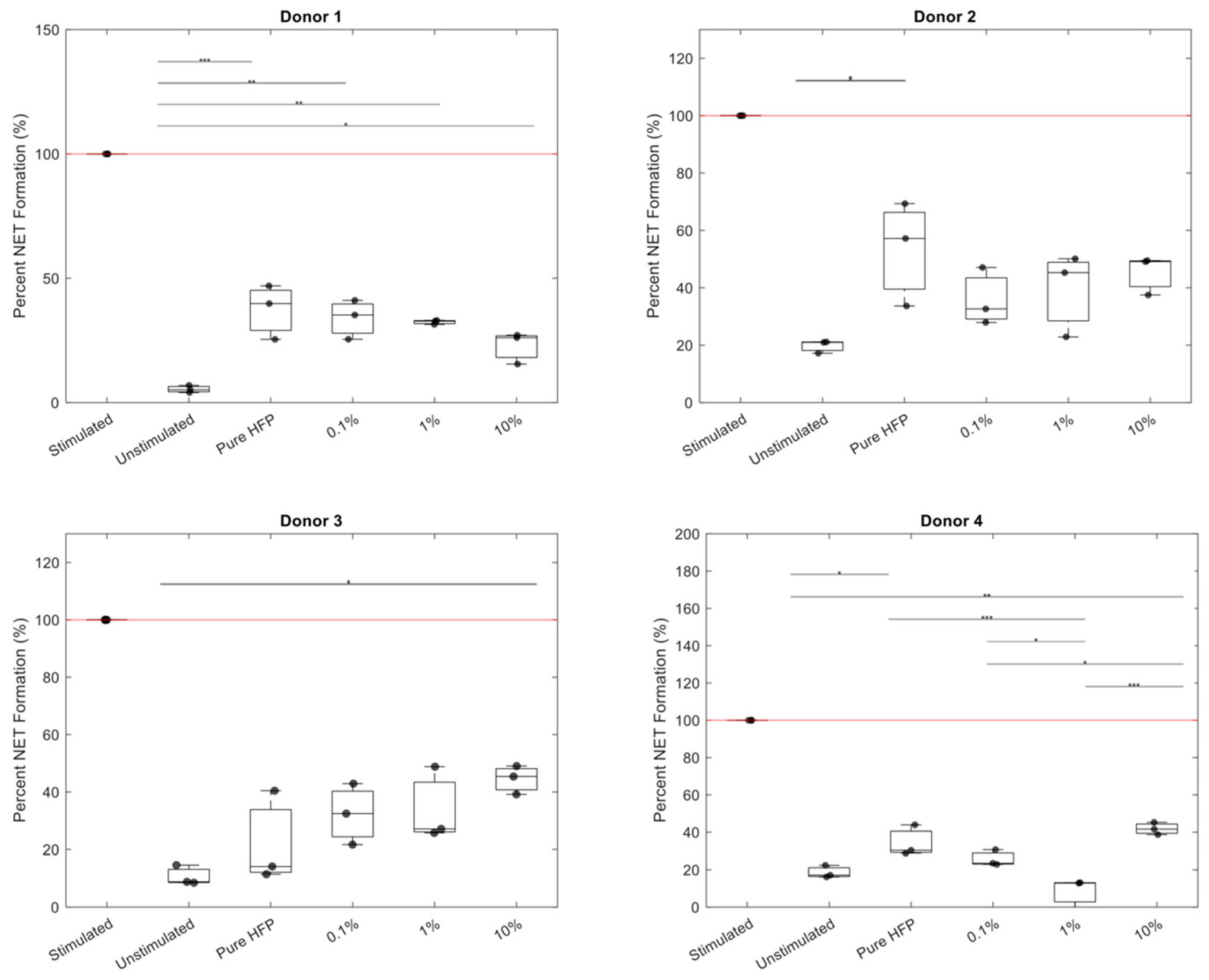
3.4. NET Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- What Is Atherosclerosis? Available online: https://www.nhlbi.nih.gov/health/atherosclerosis (accessed on 15 November 2023).
- Elliott, B.M.; Robison, J.G.; Brothers, T.E.; Cross, M.A. Limitations of peroneal artery bypass grafting for limb salvage. J. Vasc. Surg. 1993, 18, 881–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altshuler, P.; Nahirniak, P.; Welle, N.J. Saphenous Vein Grafts; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Saito, J.; Kaneko, M.; Ishikawa, Y.; Yokoyama, U. Challenges and Possibilities of Cell-Based Tissue-Engineered Vascular Grafts. Cyborg Bionic Syst. 2021, 2021, 1532103. [Google Scholar] [CrossRef]
- Bos, G.W.; Poot, A.A.; Beugeling, T.; van Aken, W.G.; Feijen, J. Small-Diameter Vascular Graft Prostheses: Current Status. J. Metab. Dis. 1998, 106, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Wu, L.-P.; Zhao, M. Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering. Polymers 2023, 15, 2015. [Google Scholar] [CrossRef]
- Johnson, W.C.; Lee, K.K. Comparative evaluation of externally supported Dacron and polytetrafluoroethylene prosthetic bypasses for femorofemoral and axillofemoral artrerial reconstructions. J. Vasc. Surg. 1999, 30, 1077–1083. [Google Scholar] [CrossRef]
- Ravari, H.; Kazemzade, G.H.; Modaghegh, M.H.S.; Khashayar, P. Patency rate and complications of polytetrafluoroethylen grafts compared with polyurethane grafts for hemodialysis. Upsala J. Med. Sci. 2010, 115, 245–248. [Google Scholar] [CrossRef]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and Modifications in the Development of Small-Diameter Vascular Grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Bush, H.L., Jr. Mechanisms of graft failure. J. Vasc. Surg. 1989, 9, 392–394. [Google Scholar] [CrossRef][Green Version]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M. The Mechanical Behavior of Vascular Grafts: A Review. J. Biomater. Appl. 2001, 15, 171–289. [Google Scholar] [CrossRef]
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jørgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680. [Google Scholar] [CrossRef]
- Cheng, H.; Clymer, J.W.; Chen, B.P.-H.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Lau, S.; Gossen, M.; Lendlein, A. Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane. Int. J. Mol. Sci. 2021, 22, 13120. [Google Scholar] [CrossRef] [PubMed]
- Kabootian, M.T.; Row, S.; Smith, R.J., Jr.; Koenigsknecht, C.; Adreadis, S.T.; Swartz, D.D. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 2016, 76, 344–358. [Google Scholar] [CrossRef]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Berger, K.; Sauvage, L.R.; Rao, A.M.; Wood, S.J. Healing of Arterial Prostheses in Man: Its Incompleteness. Ann. Surg. 1972, 175, 118–127. [Google Scholar] [CrossRef]
- Kim, S.-E.; Jeong, S.I.; Shim, K.-M.; Jang, K.; Park, J.S.; Lim, Y.M.; Kang, S.S. In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts With VEGF in Aged Rats. Polymers 2022, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Pennel, T.; Bezuidenhout, D.; Koehne, J.; Davies, N.H.; Zilla, P. Transmural Ingrowth is essential for confluent vascular graft healing. Acta Biomater. 2018, 65, 237–247. [Google Scholar] [CrossRef]
- Pennel, T.; Zilla, P.; Bezuidenhout, D. Differentiating transmural from transanastomotic prosthetic graft endothelialization throuh an isolation loop-graft model. J. Vasc. Surg. 2013, 58, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Row, S.; Peng, H.; Schlaich, E.M.; Koenigsknecht, C.; Adreadis, S.T.; Swartz, D.D. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: The role of cells in the vasclar wall. Biomaterials 2015, 50, 115–126. [Google Scholar] [CrossRef]
- Ratner, B. Vascular grafts: Technology Success/Technology Failure. BME Front. 2023, 4, 0003. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Baldrich, W.J.; Moore, W.S.; Ziomek, S.; Chvapil, M. Development of a “leak-proof,” knitted Dacron vascular prosthesis. J. Vasc. Surg. 1986, 3, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Hamilton, G. Graft materials past and future. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; The University of Adelaide Press: Adelaide, Australia, 2011; pp. 511–536. [Google Scholar]
- Sheehan, S.J.; Rajah, S.M.; Kester, R.C. Effect of preclotting on the porosity and thrombogenicity of knitted Dacron® grafts. Biomaterials 1989, 10, 75–79. [Google Scholar] [CrossRef]
- Laurens, N.; Koolwijk, P.; De Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef]
- Glynn, M.F.X.; Williams, W.G. A Technique for Preclotting Vascular Grafts. Ann. Thorac. Surg. 1980, 29, 182–183. [Google Scholar] [CrossRef]
- Jonas, R.A.; Schoen, F.J.; Levy, R.J.; Castaneda, A.R. Biological Sealants and Knitted Dacron: Porosity and Histological Comparisons of Vascular Graft Materials with and without Collagen and Fibrin Glue Pretreatments. Ann. Thorac. Surg. 1986, 41, 657–663. [Google Scholar] [CrossRef]
- Lucereau, B.; Koffhi, F.; Lejay, A.; Georg, Y.; Durand, B.; Thaveau, F.; Heim, F.; Chakfe, N. Compliance of Textile Vascular Prostheses Is a Fleeting Reality. European J. Vasc. Endovasc. Surg. 2020, 60, 773–779. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 32, 151–160. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Cho, Y.; Baek, J.W.; Sagong, M.; Ahn, S.; Nam, J.S.; Kim, I.-D. Electrospinning and Nanofiber Technology: Fundamentals, Innovations, and Applications. Adv. Mater. 2025, 37, 2500162. [Google Scholar] [CrossRef]
- Oliviero, O.; Ventre, M.; Netti, P.A. Functional porous hydrogels to study angiogenesis under the effect of controlled released of vascular endothelial growth factor. Acta Biomater. 2012, 8, 3294–3301. [Google Scholar] [CrossRef]
- Walthers, C.M.; Nazemi, A.K.; Patel, S.L.; Wu, B.M.; Dunn, J.C.Y. The effect of scaffold macroporousity on angiogenesis and cell survival in tissue-engineered smooth muscle. Biomaterials 2014, 35, 5129–5137. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Yuan, Y.; Lu, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015, 5, srep09409. [Google Scholar] [CrossRef]
- King, W.E.; Bowlin, G.L. Mechanical characterization and neutrophil NETs response of a novel hybrid geometry polydioxanone near-field electrospun scaffold. Biomed. Mater. 2021, 16, 065002. [Google Scholar] [CrossRef]
- Kameoka, J.; Orth, R.; Yang, Y.; Czaplewski, D.; Mathers, R.; Coates, G.W.; Craighead, H.G. A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology 2003, 14, 1124–1129. [Google Scholar] [CrossRef]
- Sun, D.; Chang, C.; Li, S.; Lin, L. Near-Field Electrospinning. Nano Lett. 2006, 6, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.M.; Khodabandeh, A.; Hadjizadeh, A. Near-Field Electrospinning: Crucial Parameters, Challenges, and Applications. ACS Appl. Bio Mater. 2022, 5, 394–412. [Google Scholar] [CrossRef]
- Snyder, A.E.; Sandridge, J.K.; Nordmoe, A.E.; Main, E.N.; Bowlin, G.L. Fabrication and mechanical characterization of near field electrospun bioresorbable vascular grafts with fibrous architecture mimicking the arterial extracellular matrix. J. Bioact. Compat. Polym. 2024, 39, 08839115241262038. [Google Scholar] [CrossRef] [PubMed]
- Niklason, L.E.; Lawson, J.H. Bioengineered human blood vessels. Science 2020, 370, eaaw8682. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Christoffersson, G.; Henriksnäs, J.; Johansson, L.; Rolny, C.; Ahlström, H.; Caballero-Corbalan, J.; Segersvärd, R.; Permert, J.; Korsgren, O.; Carlsson, P.-O.; et al. Clinical and Experimental Pancreatic Islet Transplantation to Striated Muscle: Establishment of a Vascular System Similar to That in Native Islets. Diabetes 2010, 59, 2569–2578. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosman, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef]
- Sousa, A.B.; Barbosa, J.N. The Role of Neutrophils in Biomaterial-Based Tissue Repair--Shifting Paradigms. J. Funct. Biomater. 2023, 14, 327. [Google Scholar] [CrossRef]
- Fetz, A.E.; Bowlin, G.L. Neutrophil Extracellular Traps: Inflammation and Biomaterial Preconditioning for Tissue Engineering. Tissue Eng. 2022, 28, 437–450. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and cardiovascular disease. Arter. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Main, E.N.; Bowlin, G.L. Potential for Manuka honey-inspired therapeutics to improve the host–biomaterial response. MedComm—Biomater. Appl. 2022, 1, e18. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Smith, R.A.; Radic, M.Z.; van der Merwe, M.; Bowlin, G.L. Manuka Honey Reduces NETosis on an Electrospun Template Within a Therapeutic Window. Polymers 2020, 12, 1430. [Google Scholar] [CrossRef]
- Main, E.N.; Huang, J.C.; Bowlin, G.L. Methyl Syringate: A Primary Driving Factor in Manuka Honeys Ability to Ameliorate Neutrophil Intracellular ROS Activity and NETosis. Front. Biosci. Landmark 2024, 29, 255. [Google Scholar] [CrossRef]
- King III, W.E.; Gillespie, Y.; Gilbert, K.; Bowlin, G.L. Characterization of Polydioxanone in Near-Field Electrospinning. Polymers 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Huling, J.; Gotz, A.; Grabow, N.; Illner, S. GIFT: An Image J macro for automated fiber diameter quantification. PLoS ONE 2022, 17, e0275528. [Google Scholar] [CrossRef]
- King, W.E.; Bowlin, G.L. Near-field electrospinning of polydioxanone small diameter vascular graft scaffolds. J. Mech. Behav. Biomed. Mater. 2022, 130, 105207. [Google Scholar] [CrossRef]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrida, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical Properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2008, 30, 1542–1550. [Google Scholar] [CrossRef]
- Johnson, J. Development of Novel, Bioresorbable, Small-Diameter Electrospun Vascular Grafts. J. Tissue Sci. Eng. 2015, 6, 2. [Google Scholar] [CrossRef]
- Camasão, D.B.; Mantaovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest ofr physiological-relevant performances. A critical reveiw. Mater. Today Bio 2021, 10, 100106. [Google Scholar] [CrossRef]
- Hamedani, B.A.; Navidbakhsh, M.; Tafti, H.A. Comparison between mechanical properties of human saphenous vein and umbililcal vein. Biomed. Eng. OnLine 2012, 11, 59. [Google Scholar] [CrossRef]
- ANSI/AAMI VP20:1994; Cardiovascular implants-Vascular Graft Prostheses. American National Standard: Washington, DC, USA, 1994.
- Fetz, A.E.; Wallace, S.E.; Bowlin, G.L. Electrospun Polydioxanone Loaded With Chloroquine Modulates Template-Induced NET Release and Inflammatory Responses From Human Neutrophils. Front. Bioeng. Biotechnol. 2021, 9, 652055. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fibers Fabr. 2015, 10, 155892501501000406. [Google Scholar] [CrossRef]
- Fetz, A.E.; Neeli, I.; Rodriguez, I.A.; Radic, M.Z.; Bowlin, G.L. Electrospun Template Architecture and Composition Regulate Neutrophil NETosis In Vitro and In Vivo. Tissue Eng. 2017, 23, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.; Neeli, I. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Fetz, A.E.; King, W.E.; Minden-Birkenmaier, B.A.; Bowlin, G.L. Methods for Quantifying Neutrophil Extracellular Traps on Biomaterials. In Biomedical Engineering Technologies; Methods in Molecular Biology; Rasooly, A., Baker, H., Ossandon, M.R., Eds.; Springer Protocols: Berlin/Heidelberg, Germany, 2022; Volume 2394, pp. 727–742. [Google Scholar]
- Roudier, G.; Hourques, M.; Da Silva, N.; Gluais, M.; Binyet, E.; Olive, J.M.; L’Heureux, N. Effects of weaving parameters on the properties of completely biological tissue-engineered vascular grafts. Biofabrication 2023, 16, 015015. [Google Scholar] [CrossRef]
- Li, Z.; Chen, E.; Parsons, J.; Turng, L.-S. Design, synthesis, and characterization of polymer-hydrogel composite vascular grafts using double-expanded polytetrafluoroethylene to achieve enhanced mechanical and biological properties. Polym. Eng. Sci. 2025, 65, 2893–2908. [Google Scholar] [CrossRef]
- Wang, S.; Qui, Y.; Zhu, F. An updated review of functional ingredients of Manuka honey and their value-added innovations. Food Chem. 2024, 440, 138060. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, K.; Zhang, L.; Zhang, S.; Liu, Q.; Wang, Y.; Wei, T.; Gao, G.; Hu, X. Impacts of Solvents on the Stability of the Biomass-Derived Sugars and Furans. Energy Fuels 2020, 34, 3250–3261. [Google Scholar] [CrossRef]
- Dantignana, V.; Milan, M.; Cussó, O.; Company, A.; Bietti, M.; Costas, M. Chemoselective Aliphatic C–H Bond Oxidation Enabled by Polarity Reversal. ACS Cent. Sci. 2017, 3, 1350–1358. [Google Scholar] [CrossRef]
- Kim, M.-H.; Liu, W.; Borjesson, D.L.; Curry, F.-R.E.; Miller, L.S.; Cheung, A.L.; Liu, F.-T.; Isseroff, R.R.; Simon, S.I. Dynamics of Neutrophil Infiltration during Cutaneous Wound Healing and Infection Using Fluorescence Imaging. J. Investig. Dermatol. 2009, 128, 1812–1820. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Tigner, A.; Ibrahim, S.A.; Murray, I.V. Histology, White Blood Cell. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Seo, K.; Ki, S.H.; Shin, S.M. Methylglyoxal Induces Mitochondrial Dysfunction and Cell Death in Liver. Toxicol. Res. 2014, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Berends, E.; van Oostengrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Ivanov, V.A.; Mikhalchik, E.V.; Gorduko, I.V.; Grigorieva, D.V.; Basyreva, L.Y.; Shmeleva, E.V.; Gusev, S.A.; Kostevich, V.A.; Gorbunov, N.P.; et al. Methylglyoxal-Modified Human Serum Albumin Binds to Leukocyte Myeloperoxidase and Inhibits its Enzymatic Activity. Antioxidants 2022, 11, 2263. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Kuster, B. 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture. Starch-Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef]
- Weingart, E.; Teevs, L.; Krieg, R.; Prüße, U. Hexafluoroisopropanol as a Low-Boiling Extraction Solvent for 5-Hydroxymethylfurfural Production. Energy Technol. 2018, 6, 432–440. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
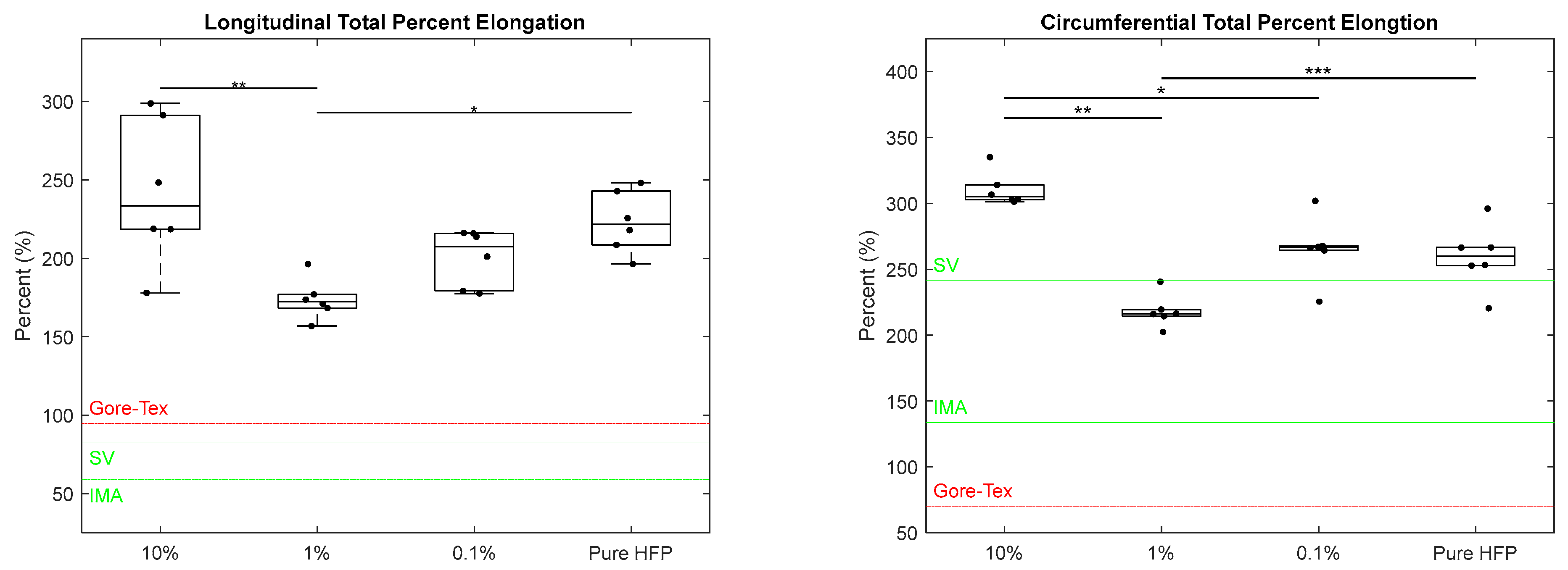
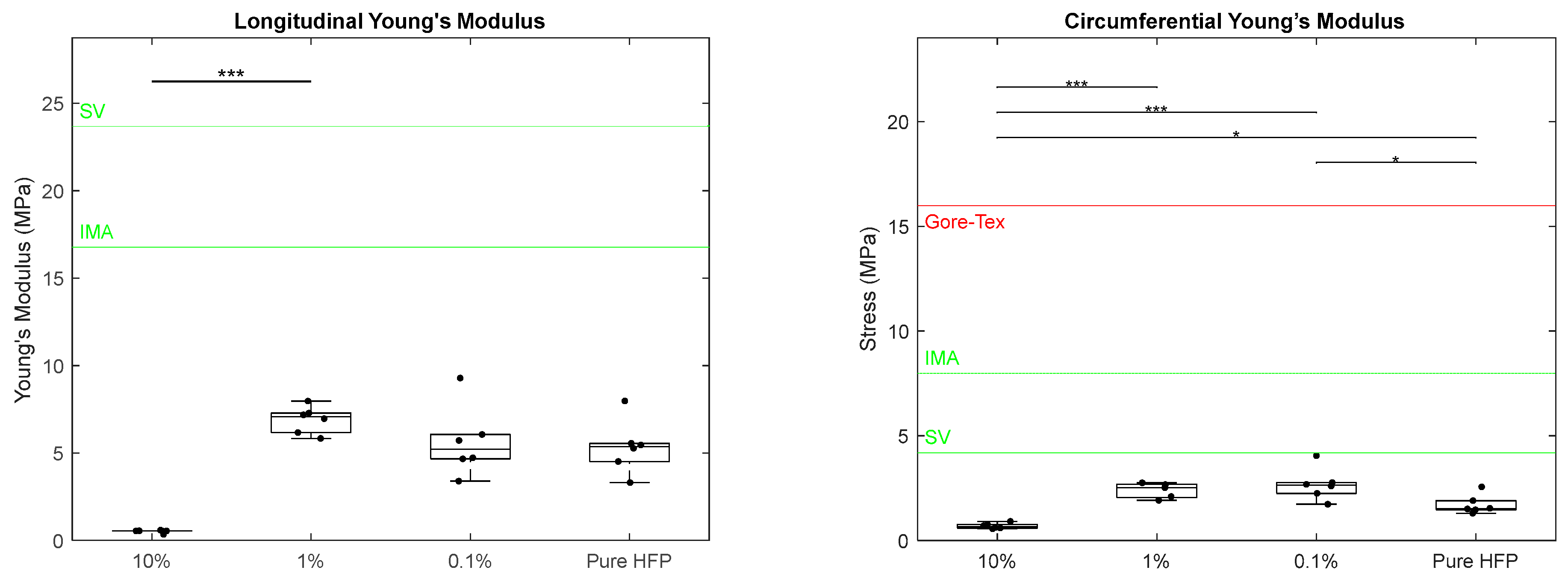
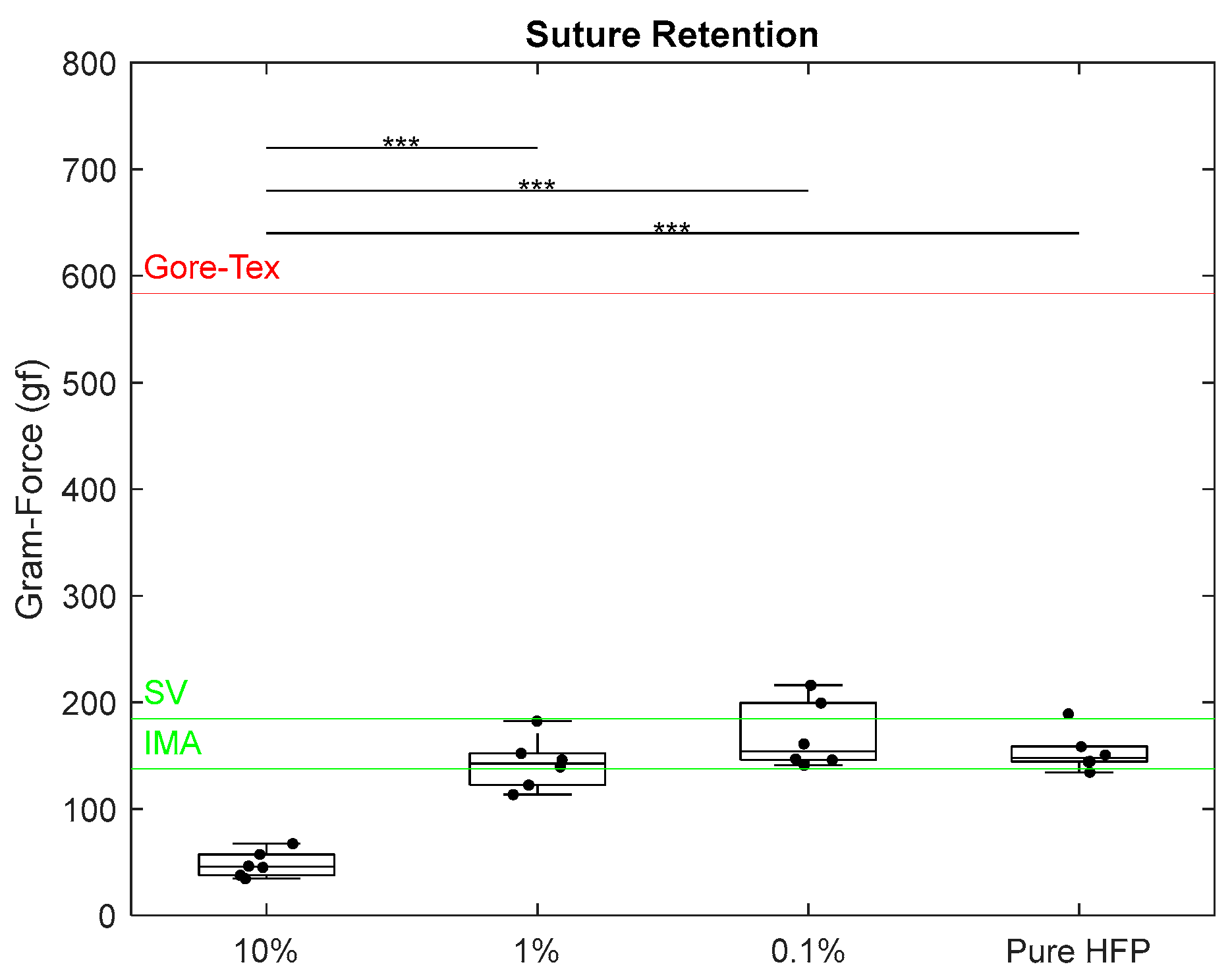
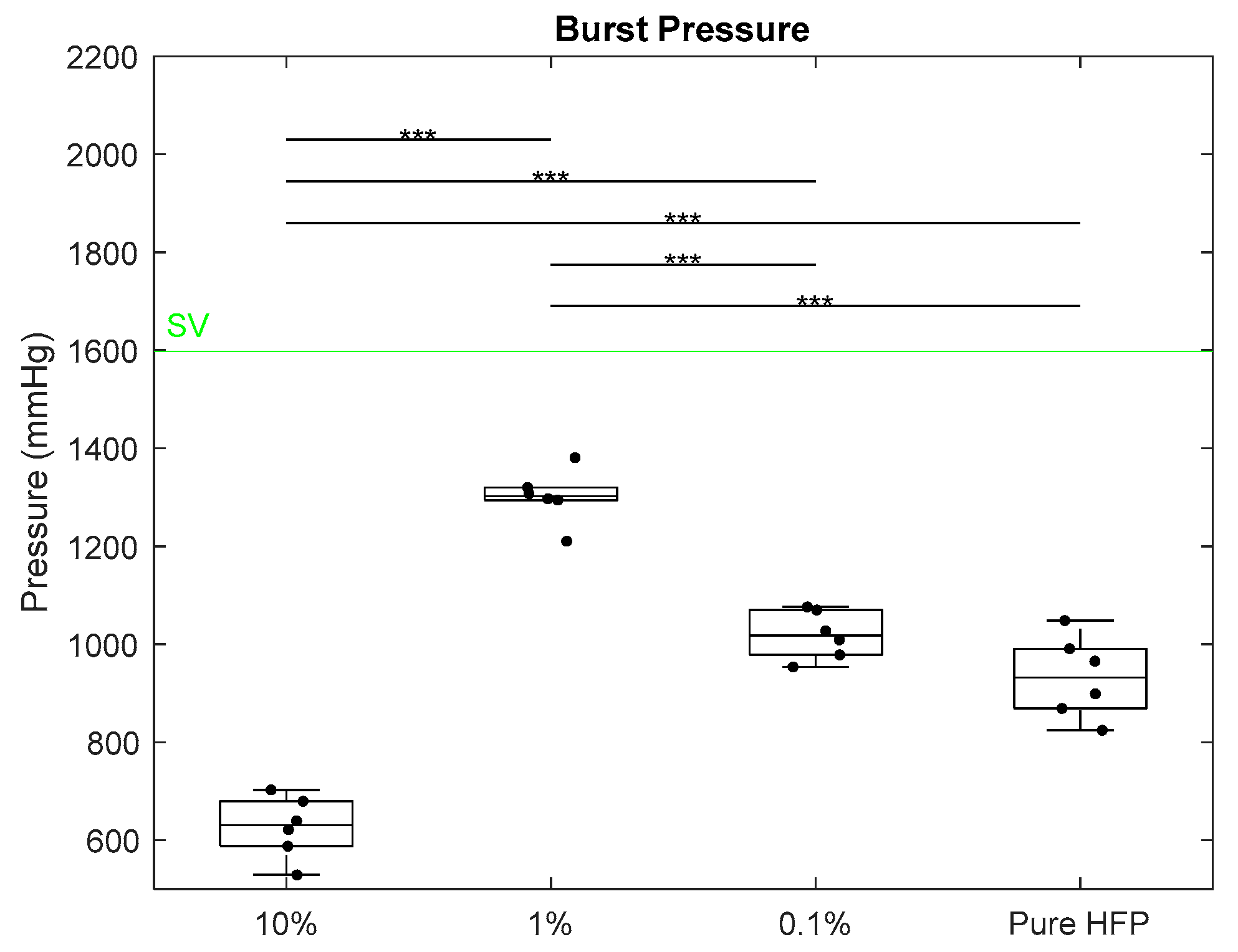
| Fiber Diameter (μm) | Pore Size (μm) | Wall Thickness (μm) | |
|---|---|---|---|
| Pure HFP | 3.44 ± 0.25 | 86.6 ± 21.2 | 207 ± 20 |
| 0.1% Manuka Honey | 3.39 ± 0.52 | 74.5 ± 23.8 | 214 ± 8.6 |
| 1% Manuka Honey | 3.38 ± 0.42 | 96.2 ± 19.7 | 236 ± 33 |
| 10% Manuka Honey | 3.40 ± 0.33 | 128 ± 38.8 | 197 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, A.E.; Main, E.N.; Bowlin, G.L. Immunomodulation and Mechanical Characterization of Manuka Honey-Incorporated Near-Field Electrospun Bioresorbable Vascular Grafts. Bioengineering 2025, 12, 1270. https://doi.org/10.3390/bioengineering12111270
Snyder AE, Main EN, Bowlin GL. Immunomodulation and Mechanical Characterization of Manuka Honey-Incorporated Near-Field Electrospun Bioresorbable Vascular Grafts. Bioengineering. 2025; 12(11):1270. https://doi.org/10.3390/bioengineering12111270
Chicago/Turabian StyleSnyder, Alexandra E., Evan N. Main, and Gary L. Bowlin. 2025. "Immunomodulation and Mechanical Characterization of Manuka Honey-Incorporated Near-Field Electrospun Bioresorbable Vascular Grafts" Bioengineering 12, no. 11: 1270. https://doi.org/10.3390/bioengineering12111270
APA StyleSnyder, A. E., Main, E. N., & Bowlin, G. L. (2025). Immunomodulation and Mechanical Characterization of Manuka Honey-Incorporated Near-Field Electrospun Bioresorbable Vascular Grafts. Bioengineering, 12(11), 1270. https://doi.org/10.3390/bioengineering12111270







