Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review
Abstract
1. Introduction
2. Pathology of DED
2.1. Inflammation in Dry Eye
2.2. Immune Response of Dry Eye
2.3. Other Pathologies of Dry Eye
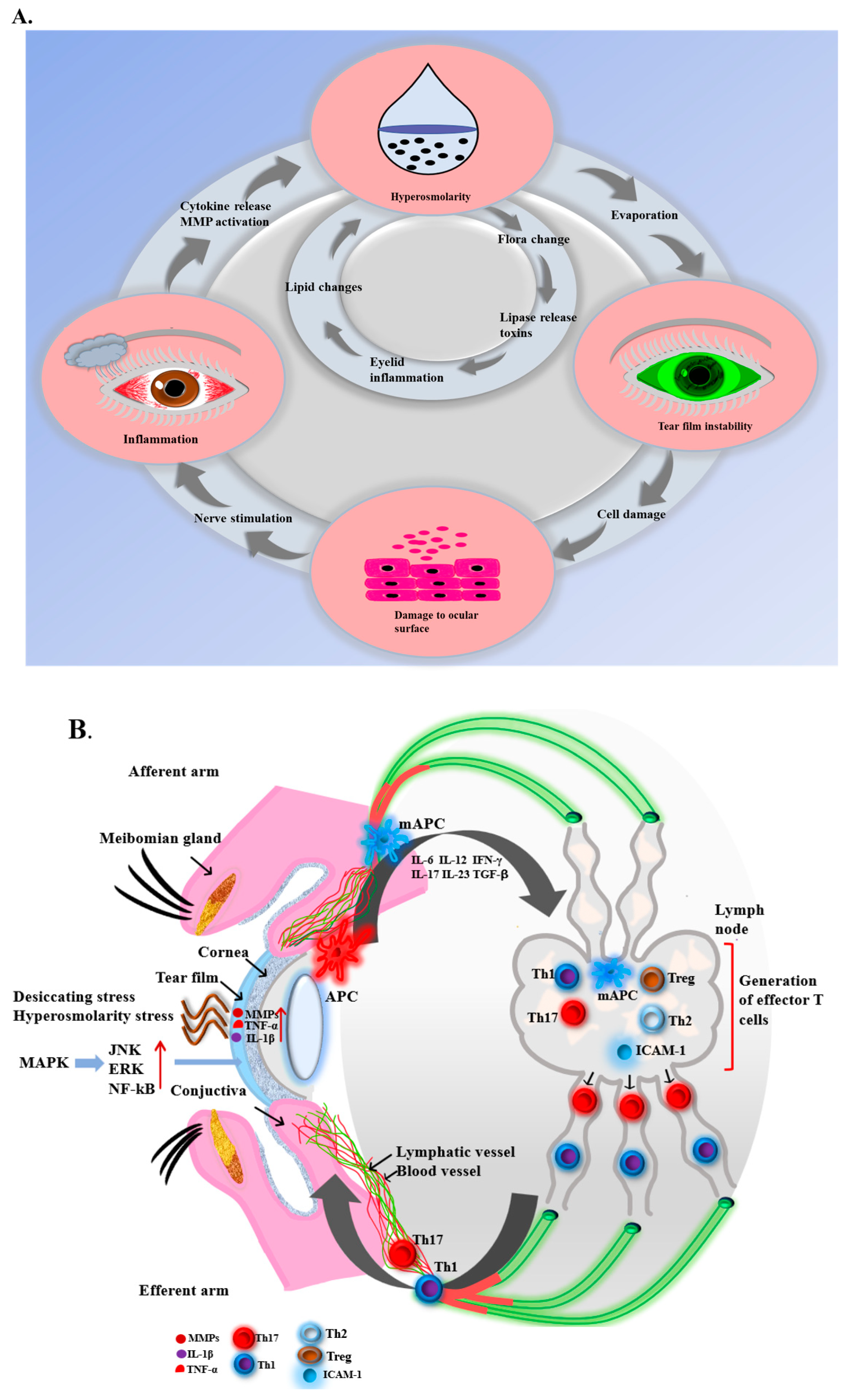
3. Treatments and Weaknesses of Existing Treatments for DED
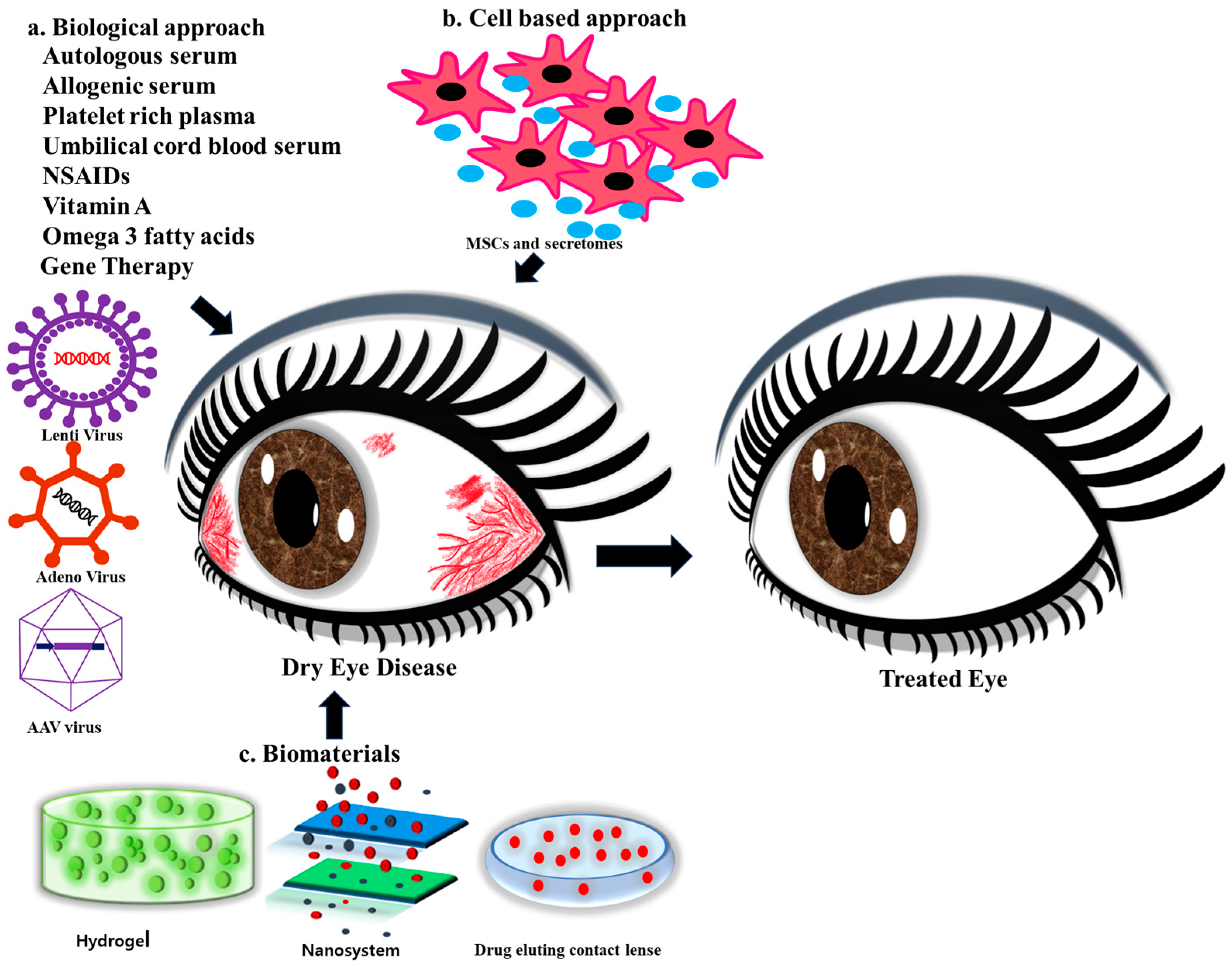
4. Biological Approaches in DED
4.1. Autologous Serum (AS)
4.2. Allogenic Serum (ALS)
4.3. Platelet-Rich Plasma (PRP)
4.4. Umbilical Cord Blood Serum (UCS)
4.5. Gene Therapy
4.6. Artificial Solutions with/without Anti-Inflammatory Drugs
4.7. Nonsteroidal Anti-Inflammatory Drugs and Antibiotics (NSAIDs)
4.8. Punctal Bags
4.9. Corticosteroids
4.10. Vitamin A
4.11. Omega 3 Fatty Acids
5. Cell-Based Regenerative Approaches
6. Biomaterials for DED Treatment
6.1. Scaffolds
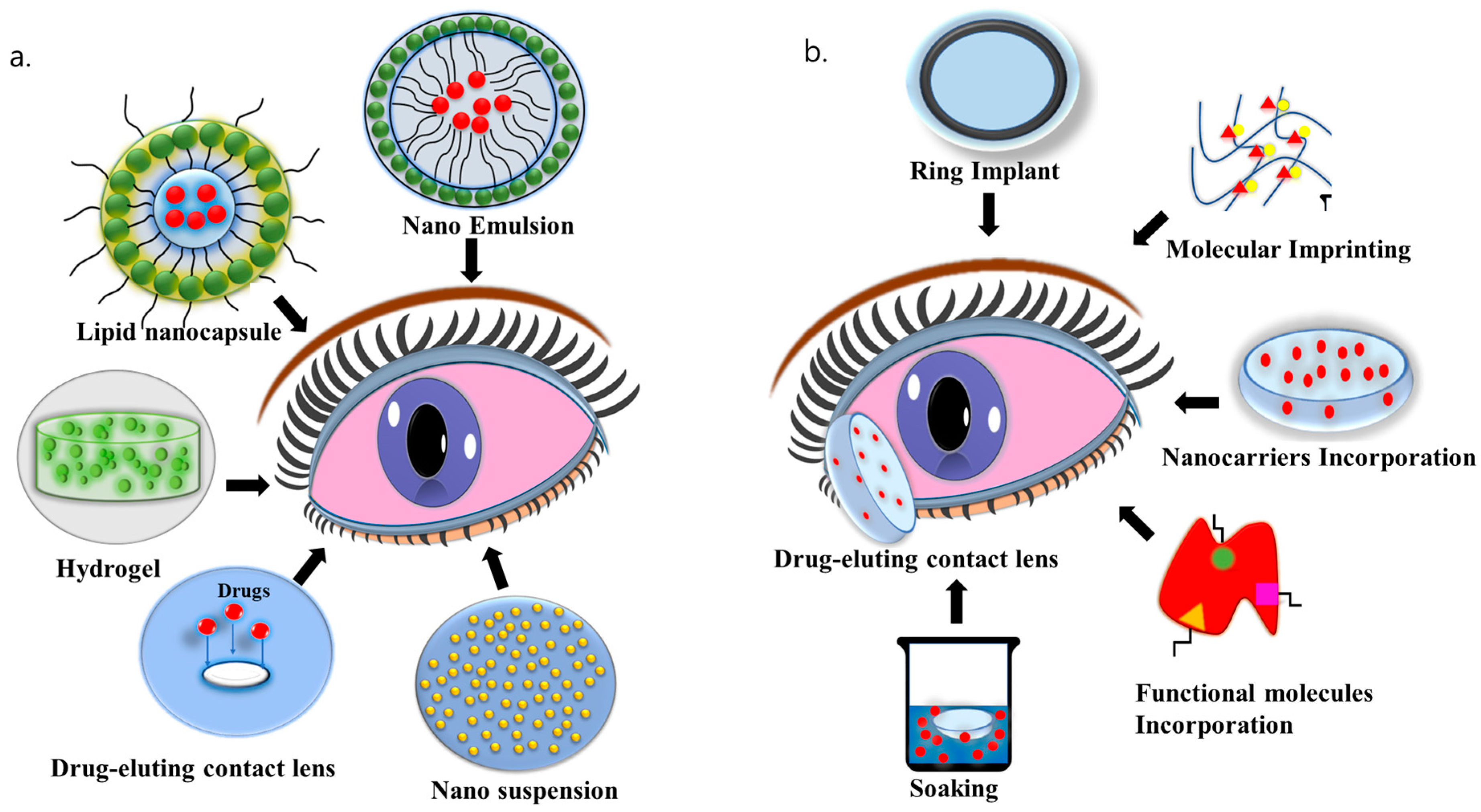
6.2. Nanosystems
6.3. Hydrogels
6.4. Drug-Eluting Contact Lenses
6.5. Contact Lens-Based Drug Delivery for DED
6.6. Biosensors Integrated Contact Lenses
| Contact Lens Biomaterials | Pros | Cons | References |
|---|---|---|---|
| Poly (vinyl alcohol) (PVA) |
|
| [159] |
| Silicon hydrogel |
|
| [160] |
| HEMA hydrogel |
|
| [161,162] |
| Polymethyl methacrylate (PMMA) |
|
| [161,163] |
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DED | Dry eye disease |
| DES | Dry eye syndrome |
| AS | Autologous serum |
| TGF-β | Transforming growth factor-beta |
| PDGF | Platelet-derived growth factor |
| EGF | Epidermal growth factor |
| NGF | Nerve growth factor |
| IGF-1 | Insulin-like growth factor 1 |
| VEGF | Vascular endothelial growth factor |
| PRP | Platelet-rich plasma |
| UCS | Umbilical cord blood serum |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| CGRP | Calcitonin gene-related peptide |
| ALS | Allogeneic serum |
| HGF | Hepatocyte growth factor |
| PF-4 | Platelet factor-4 |
| PRGF | Plasma rich in growth factors |
| PRPGF | Plasma rich in platelet and growth factors |
| PC | Platelet concentrate |
| LR-PRP | Leukocyte-rich platelet-rich plasma |
| LP-PRP | Leukocyte-poor platelet-rich plasma |
| MMP-9 | Matrix metallopeptidase 9 |
| MSCs | Mesenchymal stem cells or multipotent stromal cell |
| IFN-γ | Interferon-gamma |
| CL | Contact lenses |
| EGCG | Epi-gallocatechin gallate |
| HA | Hyaluronic acid |
| HEMA | Hydroxyethyl methacrylate |
| TSG-6 | Tumor necrosis factor-stimulated gene-6 |
| BAC | Benzalkonium chloride |
| FGF | Fibroblast growth factor |
| CCL3 | Chemokine (C-C motif) ligand 3 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
References
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A PICASSO board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kim, D.H.; Park, C.-K.; Kim, Y.H. Experimental Models, Induction Protocols, and Measured Parameters in Dry Eye Disease: Focusing on Practical Implications for Experimental Research. Int. J. Mol. Sci. 2021, 22, 12102. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.; Tseng, C.-L.; Chang, C.-Y.; Jakfar, S.; Chen, H.Y.; Lin, F.-H. Mucoadhesive Bletilla striata polysaccharide-based artificial tears to relieve symptoms and inflammation in rabbit with dry eyes syndrome. Polymers 2020, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Al-Sheikh, O.; Elisseeff, J.H.; Grant, M.P. Biomaterials and tissue engineering strategies for conjunctival reconstruction and dry eye treatment. Middle East Afr. J. Ophthalmol. 2015, 22, 428. [Google Scholar] [PubMed]
- Kersey, J.; Broadway, D. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: A systematic review and meta-analysis. Ocul. Surf. 2015, 13, 213–225. [Google Scholar] [CrossRef]
- Marshall, L.L.; Roach, J.M. Treatment of dry eye disease. Consult. Pharm.® 2016, 31, 96–106. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.; Karla, P.K.; Boddu, S.H. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact lenses: Promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V. Tfos dews ii pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, S.; Sakimoto, T.; Shoji, J.; Sawa, M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn. J. Ophthalmol. 2011, 55, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Han, S.J.; Ji, Y.W.; Choi, Y.J.; Jun, I.; Alotaibi, M.H.; Ko, B.Y.; Kim, E.K.; Kim, T.-I.; Nam, S.M. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci. Rep. 2019, 9, 7648. [Google Scholar] [CrossRef] [PubMed]
- Boehm, N.; Riechardt, A.I.; Wiegand, M.; Pfeiffer, N.; Grus, F.H. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Fujimoto, T.; Itaya-Hironaka, A.; Miyaoka, T.; Yoshimoto, K.; Sakuramoto-Tsuchida, S.; Yamauchi, A.; Takeda, M.; Tsujinaka, H.; Tanaka, Y. Significance of interleukin-6/STAT pathway for the gene expression of REG Iα, a new autoantigen in Sjögren’s syndrome patients, in salivary duct epithelial cells. Clin. Rev. Allergy Immunol. 2017, 52, 351–363. [Google Scholar] [CrossRef]
- Tan, X.; Sun, S.; Liu, Y.; Zhu, T.; Wang, K.; Ren, T.; Wu, Z.; Xu, H.; Zhu, L. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye 2014, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.K.; Mah, F.S. Inflammation in dry eye disease: How do we break the cycle? Ophthalmology 2017, 124, S14–S19. [Google Scholar] [CrossRef]
- Li, D.-Q.; Luo, L.; Chen, Z.; Kim, H.-S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef]
- Kodati, S.; Chauhan, S.K.; Chen, Y.; Dohlman, T.H.; Karimian, P.; Saban, D.; Dana, R. CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5871–5877. [Google Scholar] [CrossRef]
- Chen, Y.; Chauhan, S.K.; Soo Lee, H.; Saban, D.R.; Dana, R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014, 7, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 2008, 38, 2636–2649. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.; Zhang, S.; Shojaei, A. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J. Ocul. Pharmacol. Ther. 2017, 33, 5–12. [Google Scholar] [CrossRef]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.-Q.; Pflugfelder, S.C. Dry eye–induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Albertsmeyer, A.-C.; Kakkassery, V.; Spurr-Michaud, S.; Beeks, O.; Gipson, I.K. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp. Eye Res. 2010, 90, 444–451. [Google Scholar] [CrossRef]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Ling, J.; Chan, B.C.-L.; Tsang, M.S.-M.; Gao, X.; Leung, P.C.; Lam, C.W.-K.; Hu, J.-M.; Wong, C.K. Current Advances in mechanisms and treatment of Dry eye Disease: Toward Anti-inflammatory and immunomodulatory therapy and traditional Chinese medicine. Front. Med. 2022, 8, 815075. [Google Scholar] [CrossRef]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The immunological basis of dry eye disease and current topical treatment options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Ärzteblatt Int. 2015, 112, 71. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Kim, H.-J.; Mohanto, N.; Jee, J.-P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising therapeutic drug delivery systems for glaucoma: A comprehensive review. Ther. Adv. Ophthalmol. 2020, 12, 2515841420905740. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: A review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Maclennan, S.; Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A. Autologous serum and serum components. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES121–DES129. [Google Scholar] [CrossRef]
- Lekhanont, K.; Jongkhajornpong, P.; Choubtum, L.; Chuckpaiwong, V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. BioMed Res. Int. 2013, 2013, 521315. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef]
- Tsubota, K.; Goto, E.; Fujita, H.; Ono, M.; Inoue, H.; Saito, I.; Shimmura, S. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br. J. Ophthalmol. 1999, 83, 390–395. [Google Scholar] [CrossRef]
- Ogawa, Y.; Okamoto, S.; Mori, T.; Yamada, M.; Mashima, Y.; Watanabe, R.; Kuwana, M.; Tsubota, K.; Ikeda, Y.; Oguchi, Y. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003, 31, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Dogru, M.; Goto, E.; Ohashi, Y.; Kojima, T.; Ishida, R.; Tsubota, K. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology 2004, 111, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Lin, S.-L.; Chang, F.-J. Effects of autologous serum eye drops for treatment of keratoconjunctivitis sicca in dogs. Taiwan Vet. J. 2018, 44, 111–117. [Google Scholar] [CrossRef]
- Semeraro, F.; Forbice, E.; Braga, O.; Bova, A.; Di Salvatore, A.; Azzolini, C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. BioMed Res. Int. 2014, 2014, 826970. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Daniels, J.T.; Dart, J.K.; Cree, I.A.; Khaw, P.T. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 948–956. [Google Scholar] [CrossRef]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous serum eye drops in dry eye disease: Preferred practice pattern guidelines. Indian J. Ophthalmol. 2023, 71, 1357. [Google Scholar] [CrossRef] [PubMed]
- Stenwall, P.A.; Bergström, M.; Seiron, P.; Sellberg, F.; Olsson, T.; Knutson, F.; Berglund, D. Improving the anti-inflammatory effect of serum eye drops using allogeneic serum permissive for regulatory T cell induction. Acta Ophthalmol. 2015, 93, 654–657. [Google Scholar] [CrossRef]
- van der Meer, P.F.; Seghatchian, J.; Marks, D.C. Quality standards, safety and efficacy of blood-derived serum eye drops: A review. Transfus. Apher. Sci. 2016, 54, 164–167. [Google Scholar] [CrossRef]
- Kunz, M.; Ibrahim, S.M. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediat. Inflamm. 2009, 2009, 979258. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- Alio, J.L.; Rodriguez, A.E.; WróbelDudzinska, D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr. Opin. Ophthalmol. 2015, 26, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R.; Roides, B.; Hwang, J.; Mafilios, M.; Story, B.; Bhattacharyya, S. Normal platelet function in platelet concentrates requires non-platelet cells: A comparative in vitro evaluation of leucocyte-rich (type 1a) and leucocyte-poor (type 3b) platelet concentrates. BMJ Open Sport Exerc. Med. 2016, 2, e000071. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saita, Y.; Nishio, H.; Ikeda, H.; Takazawa, Y.; Nagao, M.; Takaku, T.; Komatsu, N.; Kaneko, K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J. Orthop. Sci. 2016, 21, 683–689. [Google Scholar] [CrossRef]
- Riestra, A.; Alonso-Herreros, J.; Merayo-Lloves, J. Platelet rich plasma in ocular surface. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2016, 91, 475–490. [Google Scholar] [CrossRef]
- Riestra, A.; Vazquez, N.; Chacón, M.; Berisa, S.; Sanchez-Avila, R.M.; Orive, G.; Anitua, E.; Meana, A.; Merayo-Lloves, J. Autologous method for ex vivo expansion of human limbal epithelial progenitor cells based on plasma rich in growth factors technology. Ocul. Surf. 2017, 15, 248–256. [Google Scholar] [CrossRef]
- López-Plandolit, S.; Morales, M.-C.; Freire, V.; Etxebarría, J.; Durán, J.A. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea 2010, 29, 843–848. [Google Scholar] [CrossRef]
- Merayo-Lloves, J.; Sanchez-Avila, R.M.; Riestra, A.C.; Anitua, E.; Begoña, L.; Orive, G.; Fernandez-Vega, L. Safety and efficacy of autologous plasma rich in growth factors eye drops for the treatment of evaporative dry eye. Ophthalmic Res. 2016, 56, 68–73. [Google Scholar] [CrossRef]
- Alio, J.L.; Rodriguez, A.E.; Ferreira-Oliveira, R.; Wróbel-Dudzińska, D.; Abdelghany, A.A. Treatment of dry eye disease with autologous platelet-rich plasma: A prospective, interventional, non-randomized study. Ophthalmol. Ther. 2017, 6, 285–293. [Google Scholar] [CrossRef]
- Yoon, K.C. Use of umbilical cord serum in ophthalmology. Chonnam Med. J. 2014, 50, 82–85. [Google Scholar] [CrossRef]
- Sharma, N.; Goel, M.; Velpandian, T.; Titiyal, J.S.; Tandon, R.; Vajpayee, R.B. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1087–1092. [Google Scholar] [CrossRef]
- Yoon, K.-C.; Heo, H.; Im, S.-K.; You, I.-C.; Kim, Y.-H.; Park, Y.-G. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am. J. Ophthalmol. 2007, 144, 86–92.e82. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Panikker, P.; D’Souza, S.; Shetty, R.; Mohan, R.R.; Ghosh, A. Corneal Regeneration Using Gene Therapy Approaches. Cells 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Trousdale, M.D.; Zhu, Z.; Stevenson, D.; Schechter, J.E.; Ritter, T.; Mircheff, A.K. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J. Autoimmune Dis. 2005, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.B.; Samant, D.M.; Selvam, S.; Wei, R.H.; Wang, Y.; Stevenson, D.; Schechter, J.E.; Apparailly, F.; Mircheff, A.K.; Trousdale, M.D. Adeno-associated virus–mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5137–5144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, Z.; Yin, H.; Cabrera-Pérez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Ghoraba, H.H.; Akhavanrezayat, A.; Karaca, I.; Yavari, N.; Lajevardi, S.; Hwang, J.; Regenold, J.; Matsumiya, W.; Pham, B.; Zaidi, M. Ocular gene therapy: A literature review with special focus on immune and inflammatory responses. Clin. Ophthalmol. 2022, 16, 1753–1771. [Google Scholar] [CrossRef]
- You, I.C.; Li, Y.; Jin, R.; Ahn, M.; Choi, W.; Yoon, K.C. Comparison of 0.1%, 0.18%, and 0.3% hyaluronic acid eye drops in the treatment of experimental dry eye. J. Ocul. Pharmacol. Ther. 2018, 34, 557–564. [Google Scholar] [CrossRef]
- Li, Z.; Choi, J.-H.; Oh, H.-J.; Park, S.-H.; Lee, J.-B.; Yoon, K.C. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr. Eye Res. 2014, 39, 871–878. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; de Paiva, C.S. Managing Sjögren’s Syndrome and non-Sjögren Syndrome dry eye with anti-inflammatory therapy. Clin. Ophthalmol. 2014, 8, 1447–1458. [Google Scholar] [PubMed]
- Perry, H.D.; Doshi-Carnevale, S.; Donnenfeld, E.D.; Solomon, R.; Biser, S.A.; Bloom, A.H. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea 2006, 25, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Gilbard, J.P. Ophtalmic Solution with Tetracycline for Topical Treatment of Dry Eye Disease. Google Patents W02000007601A1, 17 February 2000. [Google Scholar]
- Nguyen, A.; Kolluru, A.; Beglarian, T. Dry eye disease: A review of anti-inflammatory therapies. Taiwan J. Ophthalmol. 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Balaram, M.; Schaumberg, D.A.; Dana, M.R. Efficacy and tolerability outcomes after punctal occlusion with silicone plugs in dry eye syndrome. Am. J. Ophthalmol. 2001, 131, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N. Pharmacological management of dry eye in the elderly patient. Drugs Aging 2008, 25, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.E. Therapy of lacrimal keratoconjunctivitis. In Dry Eye and Ocular Surface Disorders; CRC Press: Boca Raton, FL, USA, 2004; pp. 321–336. [Google Scholar]
- Pinto-Fraga, J.; López-Miguel, A.; González-García, M.J.; Fernández, I.; López-de-la-Rosa, A.; Enríquez-de-Salamanca, A.; Stern, M.E.; Calonge, M. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: A randomized controlled clinical trial. Ophthalmology 2016, 123, 141–153. [Google Scholar] [CrossRef]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Al Mahmood, A.M.; Al-Swailem, S.A. Essential fatty acids in the treatment of dry eye syndrome: A myth or reality? Saudi J. Ophthalmol. 2014, 28, 195–197. [Google Scholar] [CrossRef]
- Miljanović, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. Relation between dietary n−3 and n−6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef]
- Rashid, S.; Jin, Y.; Ecoiffier, T.; Barabino, S.; Schaumberg, D.A.; Dana, M.R. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch. Ophthalmol. 2008, 126, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sen, S.; Datta, H. Comparative role of 20% cord blood serum and 20% autologous serum in dry eye associated with Hansen’s disease: A tear proteomic study. Br. J. Ophthalmol. 2015, 99, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Noda-Tsuruya, T.; Asano-Kato, N.; Toda, I.; Tsubota, K. Autologous Serum Eye Drops for Dry Eye after LASIK; Slack Incorporated: Thorofare, NJ, USA, 2006; Volume 22, pp. 61–66. [Google Scholar]
- Avila, M.Y.; Igua, A.M.; Mora, A.M. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. Br. J. Ophthalmol. 2019, 103, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Nadelmann, J.B.; Bunya, V.Y.; Ying, G.-S.; Hua, P.; Massaro-Giordano, M. Effect of Autologous Platelet-Rich Plasma Drops in the Treatment of Ocular Surface Disease. Clin. Ophthalmol. 2022, 16, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Postorino, E.I.; Gargiulo, L.; Aragona, P. Use of allogeneic platelet-rich plasma for the treatment of autoimmune ocular surface disorders: Case series. Front. Ophthalmol. 2023, 3, 1215848. [Google Scholar] [CrossRef]
- Vermeulen, C.; van der Burg, L.L.; van Geloven, N.; Eggink, C.A.; Cheng, Y.Y.; Nuijts, R.M.; Wisse, R.P.; van Luijk, C.M.; Nieuwendaal, C.; Remeijer, L. Allogeneic Serum Eye Drops: A Randomized Clinical Trial to Evaluate the Clinical Effectiveness of Two Drop Sizes. Ophthalmol. Ther. 2023, 12, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Na, K.-S.; Kim, M.S. Allogeneic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J. Ocul. Pharmacol. Ther. 2012, 28, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Buzzi, M.; Fresina, M.; Velati, C.; Versura, P. Efficacy of 2-month treatment with cord blood serum eye drops in ocular surface disease: An in vivo confocal microscopy study. Cornea 2017, 36, 915–921. [Google Scholar] [CrossRef]
- Campos, E.; Versura, P.; Buzzi, M.; Fontana, L.; Giannaccare, G.; Pellegrini, M.; Lanconelli, N.; Brancaleoni, A.; Moscardelli, F.; Sebastiani, S. Blood derived treatment from two allogeneic sources for severe dry eye associated to keratopathy: A multicentre randomised cross over clinical trial. Br. J. Ophthalmol. 2020, 104, 1142–1147. [Google Scholar] [CrossRef]
- Lan, Y.; Kodati, S.; Lee, H.S.; Omoto, M.; Jin, Y.; Chauhan, S.K. Kinetics and function of mesenchymal stem cells in corneal injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3638–3644. [Google Scholar] [CrossRef]
- Yao, L.; Bai, H. Mesenchymal stem cells and corneal reconstruction. Mol. Vis. 2013, 19, 2237. [Google Scholar] [PubMed]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood J. Am. Soc. Hematol. 2012, 120, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Beyazyıldız, E.; Pınarlı, F.A.; Beyazyıldız, Ö.; Hekimoğlu, E.R.; Acar, U.; Demir, M.N.; Albayrak, A.; Kaymaz, F.; Sobacı, G.; Delibaşı, T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014, 2014, 250230. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Ott, L.; Roth, M.; Witt, J.; Geerling, G.; Mertsch, S.; Schrader, S. MSC transplantation improves lacrimal gland regeneration after surgically induced dry eye disease in mice. Sci. Rep. 2019, 9, 18299. [Google Scholar] [CrossRef] [PubMed]
- Møller-Hansen, M.; Larsen, A.-C.; Toft, P.B.; Lynggaard, C.D.; Schwartz, C.; Bruunsgaard, H.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J.; Heegaard, S. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul. Surf. 2021, 19, 43–52. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W. miR-204–containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin. Med. J. 2018, 131, 704–712. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Long, H.; Gong, X.; Hu, S.; Gong, C. Exosomes derived from mouse adipose-derived mesenchymal stem cells alleviate benzalkonium chloride-induced mouse dry eye model via inhibiting NLRP3 inflammasome. Ophthalmic Res. 2022, 65, 40–51. [Google Scholar] [CrossRef]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef]
- Bhujel, B.; Oh, S.-H.; Kim, C.-M.; Yoon, Y.-J.; Kim, Y.-J.; Chung, H.-S.; Ye, E.-A.; Lee, H.; Kim, J.-Y. Mesenchymal Stem Cells and Exosomes: A Novel Therapeutic Approach for Corneal Diseases. Int. J. Mol. Sci. 2023, 24, 10917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, S.; Gao, Y. Mesenchymal Stromal Cell–Based Therapy for Dry Eye: Current Status and Future Perspectives. Cell Transplant. 2022, 31, 09636897221133818. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Ko, A.Y.; Ko, J.H.; Lee, H.J.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015, 23, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liang, Q.; He, Y.; Wang, C.; Jiang, J.; Chen, T.; Zhang, D.; Hu, K. Mesenchymal stromal cells-derived extracellular vesicles regulate dendritic cell functions in dry eye disease. Cells 2022, 12, 33. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Guo, D.; Yi, H.; Yang, L.; Liu, X.; Sun, D.; Nian, H.; Wei, R. Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype. Exp. Eye Res. 2020, 191, 107905. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; He, C.; Lai, P.; Luo, C.; Guo, R.; Wu, S.; Geng, S.; Xiangpeng, A.; Liu, X.; Du, X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 2012, 20, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Møller-Hansen, M. Mesenchymal Stem Cell Therapy in Aqueous Deficient Dry Eye Disease; Wiley Online Library: New York, NY, USA, 2023. [Google Scholar]
- Zhang, X.; Williams, D. Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Williams, D. Essential Biomaterials Science; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Byun, W.Y.; Frank, A.; Massaro-Giordano, M.; Lee, V.; Bunya, V.Y.; Huh, D. Human blinking ‘eye-on-a-chip’. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3872. [Google Scholar]
- Heidari, M.; Noorizadeh, F.; Wu, K.; Inomata, T.; Mashaghi, A. Dry eye disease: Emerging approaches to disease analysis and therapy. J. Clin. Med. 2019, 8, 1439. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L. Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Lynch, C.; Kondiah, P.P.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in biodegradable nano-sized polymer-based ocular drug delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.; Singh, V.; Basu, S.; Singh, S. Biomaterials for dry eye disease treatment: Current overview and future perspectives. Exp. Eye Res. 2023, 226, 109339. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Luo, L.-J.; Harroun, S.G.; Wei, S.-C.; Unnikrishnan, B.; Chang, H.-T.; Huang, Y.-F.; Lai, J.-Y.; Huang, C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale 2019, 11, 5580–5594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, R.; Ran, M.; Deng, Y.; Ge, Y.; Zhu, Y.; Tao, X.; Shang, L.; Gou, J.; He, H. A novel eyes topical drug delivery system: CsA-LNC for the treatment of DED. Pharm. Res. 2020, 37, 146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D. Formulation considerations for the management of dry eye disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef]
- Barber, L.D.; Pflugfelder, S.C.; Tauber, J.; Foulks, G.N. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology 2005, 112, 1790–1794. [Google Scholar] [CrossRef]
- Goldberg, D.F.; Malhotra, R.P.; Schechter, B.A.; Justice, A.; Weiss, S.L.; Sheppard, J.D. A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology 2019, 126, 1230–1237. [Google Scholar] [CrossRef]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-based natural polymers as smart carriers for the controlled delivery of Timolol Maleate through the cornea for glaucoma. Int. J. Biol. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.; Kim, C.E.; Choi, S.Y.; Lee, H.; Yang, J. Therapeutic efficacy of nanocomplex of poly (ethylene glycol) and catechin for dry eye disease in a mouse model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zheng, M.; Zhang, A.Y.; Han, Z. A cerium oxide loaded glycol chitosan nano-system for the treatment of dry eye disease. J. Control. Release 2019, 315, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Zorzi, G.; Hileeto, D.; Lopez-Garcia, A.; Calonge, M.; Seijo, B.; Sánchez, A.; Diebold, Y. A nanomedicine to treat ocular surface inflammation: Performance on an experimental dry eye murine model. Gene Ther. 2013, 20, 467–477. [Google Scholar] [CrossRef]
- Yu, Y.; Chow, D.W.Y.; Lau, C.M.L.; Zhou, G.; Back, W.; Xu, J.; Carim, S.; Chau, Y. A bioinspired synthetic soft hydrogel for the treatment of dry eye. Bioeng. Transl. Med. 2021, 6, e10227. [Google Scholar] [CrossRef]
- Tan, H.; DeFail, A.J.; Rubin, J.P.; Chu, C.R.; Marra, K.G. Novel multiarm PEG-based hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 979–987. [Google Scholar] [CrossRef]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater. 2018, 7, 1800225. [Google Scholar] [CrossRef]
- Ailincai, D.; Dorobanțu, A.M.; Dima, B.; Irimiciuc, Ș.A.; Lupașcu, C.; Agop, M.; Olguta, O. Poly(vinyl alcohol boric acid)-diclofenac sodium salt drug delivery systems: Experimental and theoretical studies. J. Immunol. Res. 2020, 2020, 3124304. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Williams, D.L.; Mann, B.K. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement: A masked controlled study. PLoS ONE 2014, 9, e99766. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Kambhampati, S.P.; Hsu, C.-C.; Kannan, R.M.; Yiu, S.C. Subconjunctival dendrimer-drug therapy for the treatment of dry eye in a rabbit model of induced autoimmune dacryoadenitis. Ocul. Surf. 2018, 16, 415–423. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, C.; Li, R.; Wu, Y.; Liu, W.; Wang, F.; Zhang, X.; Wang, X. Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen. Int. J. Nanomed. 2019, 14, 5125–5133. [Google Scholar] [CrossRef]
- Williams, D.L.; Mann, B.K. A crosslinked HA-based hydrogel ameliorates dry eye symptoms in dogs. Int. J. Biomater. 2013, 2013, 460437. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Long-acting mucoadhesive thermogels for improving topical treatments of dry eye disease. Mater. Sci. Eng. C 2020, 115, 111095. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, L.; Shi, H.; Xu, C.; Liu, M.; Li, Q.; Zheng, L.; Chi, H.; Wang, M.; Liu, Z. Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Bioact. Mater. 2022, 9, 77–91. [Google Scholar] [CrossRef]
- Jones, L.; Hui, A.; Phan, C.-M.; Read, M.L.; Azar, D.; Buch, J.; Ciolino, J.B.; Naroo, S.A.; Pall, B.; Romond, K. BCLA CLEAR–Contact lens technologies of the future. Contact Lens Anterior Eye 2021, 44, 398–430. [Google Scholar] [CrossRef]
- Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic ophthalmic lenses: A review. Pharmaceutics 2020, 13, 36. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Heidari Keshel, S. Preparation of Nanoparticle-Containing Ring-Implanted Poly(Vinyl Alcohol) Contact Lens for Sustained Release of Hyaluronic Acid. Macromol. Biosci. 2021, 21, 2100043. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Li, Y.; Jin, R.; Shrestha, T.; Choi, J.S.; Lee, W.J.; Moon, M.J.; Ju, H.T.; Choi, W.; Yoon, K.C. The efficiency of cyclosporine a-eluting contact lenses for the treatment of dry eye. Curr. Eye Res. 2019, 44, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Peng, C.-C.; Chauhan, A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef]
- Peng, C.-C.; Chauhan, A. Extended cyclosporine delivery by silicone–hydrogel contact lenses. J. Control. Release 2011, 154, 267–274. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shaikh, A.A.; Lakdawala, D.H.; Desai, A.R.; Pandya, M.M.; Singhania, S.S.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Design and optimization of a novel implantation technology in contact lenses for the treatment of dry eye syndrome: In vitro and in vivo evaluation. Acta Biomater. 2017, 53, 211–221. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Patel, P.J.; Soni, P.D.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Ranch, K.M.; Shah, S.A.; Shah, D.O. Novel poly (vinylpyrrolidone)-coated silicone contact lenses to improve tear volume during lens wear: In vitro and in vivo studies. ACS Omega 2020, 5, 18148–18154. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef]
- Jia, Z.; Lv, Y.; Zhang, W.; Zhang, X.; Li, F.; Lu, X.; Zhao, S. Mesenchymal stem cell derived exosomes-based immunological signature in a rat model of corneal allograft rejection therapy. Front. Biosci.-Landmark 2022, 27, 86. [Google Scholar] [CrossRef]
- Kymionis, G.D.; Bouzoukis, D.I.; Diakonis, V.F.; Siganos, C. Treatment of chronic dry eye: Focus on cyclosporine. Clin. Ophthalmol. 2008, 2, 829–836. [Google Scholar] [CrossRef]
- Mun, J.; won Mok, J.; Jeong, S.; Cho, S.; Joo, C.-K.; Hahn, S.K. Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.T.; Maulvi, F.A.; Desai, A.R.; Shukla, M.R.; Desai, B.V.; Khadela, A.D.; Shetty, K.H.; Shah, D.O.; Willcox, M.D. In vitro and in vivo evaluation of cyclosporine-graphene oxide laden hydrogel contact lenses. Int. J. Pharm. 2022, 613, 121414. [Google Scholar] [CrossRef] [PubMed]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Khalilian, A.; Khan, M.R.R.; Kang, S.-W. Highly sensitive and wide-dynamic-range side-polished fiber-optic taste sensor. Sens. Actuators B Chem. 2017, 249, 700–707. [Google Scholar] [CrossRef]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R. Smart contact lenses for biosensing applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Ferraz, M.P. Biomaterials for Ophthalmic Applications. Appl. Sci. 2022, 12, 5886. [Google Scholar] [CrossRef]
- Tighe, B.J. Contact lens materials. Contact Lenses 2006, 2, 18–31. [Google Scholar]
- Musgrave, C.S.A.; Fang, F. Contact lens materials: A materials science perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Rossos, A.; Banti, C.; Kalampounias, A.; Papachristodoulou, C.; Kordatos, K.; Zoumpoulakis, P.; Mavromoustakos, T.; Kourkoumelis, N.; Hadjikakou, S. pHEMA@ AGMNA-1: A novel material for the development of antibacterial contact lens. Mater. Sci. Eng. C 2020, 111, 110770. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact lenses as ophthalmic drug delivery systems: A review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Phadatare, S.P.; Momin, M.; Nighojkar, P.; Askarkar, S.; Singh, K.K. A comprehensive review on dry eye disease: Diagnosis, medical management, recent developments, and future challenges. Adv. Pharm. 2015, 2015, 704946. [Google Scholar] [CrossRef]
- Joshi, V.P.; Singh, S.; Thacker, M.; Pati, F.; Vemuganti, G.K.; Basu, S.; Singh, V. Newer approaches to dry eye therapy: Nanotechnology, regenerative medicine, and tissue engineering. Indian J. Ophthalmol. 2023, 71, 1292. [Google Scholar] [CrossRef]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, P.; Vaksh, U.K.S.; Chandra, R. Current approaches for the regeneration and reconstruction of ocular surface in dry eye. Front. Med. 2022, 9, 885780. [Google Scholar] [CrossRef]

| Approaches | Population (n) | Treatment Period | Results | References |
|---|---|---|---|---|
| Autologous serum | 48 | 6–10/day (6 months) |
| [62] |
| Autologous serum | 144 | 6/day (6 weeks) |
| [84] |
| Autologous serum | 240 | 4/day (12 weeks) |
| [46] |
| Autologous serum | 27 | 5/day (6 months) |
| [85] |
| Platelet-Rich Plasma | 30 | 5/0, 30, 60, and 90 days |
| [86] |
| Platelet-Rich Plasma | 47 | 4/day (3 months) |
| [87] |
| Platelet-Rich Plasma | 368 | 6/day (6 weeks) |
| [59] |
| Platelet-Rich Plasma | 3 | 6/day (3 months) |
| [88] |
| Platelet-Rich Plasma | 360 | 6/day (6 weeks) |
| [59] |
| Allogenic serum | 49 | 6/day (1 month) |
| [89] |
| Allogenic serum | 16 | 6–8/day |
| [90] |
| Umbilical cord blood derum | 20 | 8/day (2 months) |
| [91] |
| Umbilical cord blood derum | 60 | 8/day (1 month) |
| [92] |
| Type of Cells | Human/Animal | Number | Mode of Treatment | Results | References |
|---|---|---|---|---|---|
| Allogeneic bone marrow-derived MSCs (BM-MSCs) | Mice | 20 | Intraperitoneal injection |
| [105] |
| Human MSCs | Mice | N/A | Periorbital injection |
| [106] |
| Mesenchymal stromal cells-derived extracellular vesicles (MSC-EVS) | Mice | 6 | Topical drop |
| [107] |
| Allogenic adipose-derived bone marrow MSCs (AD BM-MSCs) | Rats | 16 | Tropical drop |
| [96] |
| Umbilical cord-derived -MSCs (UC-MSCs) | Rabbits | 36 | Intravenous injection |
| [108] |
| Allogenic adipose derived-MSCs (AD-MSCs) | Mice | 18 | Tropical drop |
| [101] |
| BM-MSCs | Humans | 20 | Intravenously |
| [109] |
| AD-MSCs | Humans | 7 | Transconjuctivital injection |
| [98] |
| AD-MSCs | Humans | 61 | Transconjuctivital injection |
| [110] |
| Nano-Systems | Method of Inducing DED | Animals | Treatment Period | Outcomes | References |
|---|---|---|---|---|---|
| Gelatin nanoparticle | 0.1% BAC | Rabbits | 21 days (twice daily) |
| [120] |
| Gold/poly(catechin) core-shell nanoparticle | 0.15% BAC | Rabbits | 4 days |
| [121] |
| Glycol chitosan nanoparticle | Subcutaneous injection of scopolamine hydrobromide in mice | Mice | 7 days |
| [129] |
| Cationized gelatin and chondroitin sulfate nanoparticles | Subcutaneous injection of scopolamine + desiccating stress | Mice | 5 days |
| [130] |
| Hydrogel | Method of Inducing DED | Animals | Treatment Period | Results | References |
|---|---|---|---|---|---|
| Crosslinked thiolated carboxymethyl HA | Diagnosed with DED | Dogs | 2 times each day for 2 weeks |
| [139] |
| Crosslinked thiolated carboxymethyl HA | Diagnosed with DED | Dogs | 3 weeks 3 times daily |
| [136] |
| Gelatin, poly(N-isopropylacrylamide), lectin helix pomatia agglutinin and EGCG drug | 0.1% BAC twice daily for 14 days in rabbits | Rabbits | One-time adminis-tration |
| [140] |
| FK506 loadedMPEP hydrogel | Scopolamine mice model | Mice | 5 days, twice daily |
| [141] |
| Hydroxybutyl chitosan as intracanalicular injection | 0.1% BAC for 5 weeks in rabbits | Rabbits | One-time intracan-alicular injection |
| [137] |
| Contact Lens Material | Drug/Molecule | Method of Drug Loading | Animals | Treatment Period | Outcomes | References |
|---|---|---|---|---|---|---|
| HEMA hydrogel | HA |
| Rabbits | 15 days |
| [144] |
| HEMA hydrogel | HA |
| R RRabbits | 15 days |
| [149] |
| HEMA hydrogel | PVP-K90 |
| Rabbits | - |
| [150] |
| Silicone hydrogel | Flurbiprofen sodium diclofenac sodium ketorolactromethamine |
| - | - |
| [151] |
| Silicone hydrogel | Pirfenidone |
| Rabbits | 3 h |
| [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhujel, B.; Oh, S.-H.; Kim, C.-M.; Yoon, Y.-J.; Chung, H.-S.; Ye, E.-A.; Lee, H.; Kim, J.-Y. Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review. Bioengineering 2024, 11, 39. https://doi.org/10.3390/bioengineering11010039
Bhujel B, Oh S-H, Kim C-M, Yoon Y-J, Chung H-S, Ye E-A, Lee H, Kim J-Y. Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review. Bioengineering. 2024; 11(1):39. https://doi.org/10.3390/bioengineering11010039
Chicago/Turabian StyleBhujel, Basanta, Se-Heon Oh, Chang-Min Kim, Ye-Ji Yoon, Ho-Seok Chung, Eun-Ah Ye, Hun Lee, and Jae-Yong Kim. 2024. "Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review" Bioengineering 11, no. 1: 39. https://doi.org/10.3390/bioengineering11010039
APA StyleBhujel, B., Oh, S.-H., Kim, C.-M., Yoon, Y.-J., Chung, H.-S., Ye, E.-A., Lee, H., & Kim, J.-Y. (2024). Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review. Bioengineering, 11(1), 39. https://doi.org/10.3390/bioengineering11010039







