Association between Gray-Scale Ultrasound Imaging and Serological Creatine Kinase for Quantifying Exercise-Induced Muscle Damage: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Exercise-Induced Muscle Damage Procedure
2.4. Assessments
2.4.1. Demographic and Body Composition Features
2.4.2. Muscle Damage Serological Biomarkers
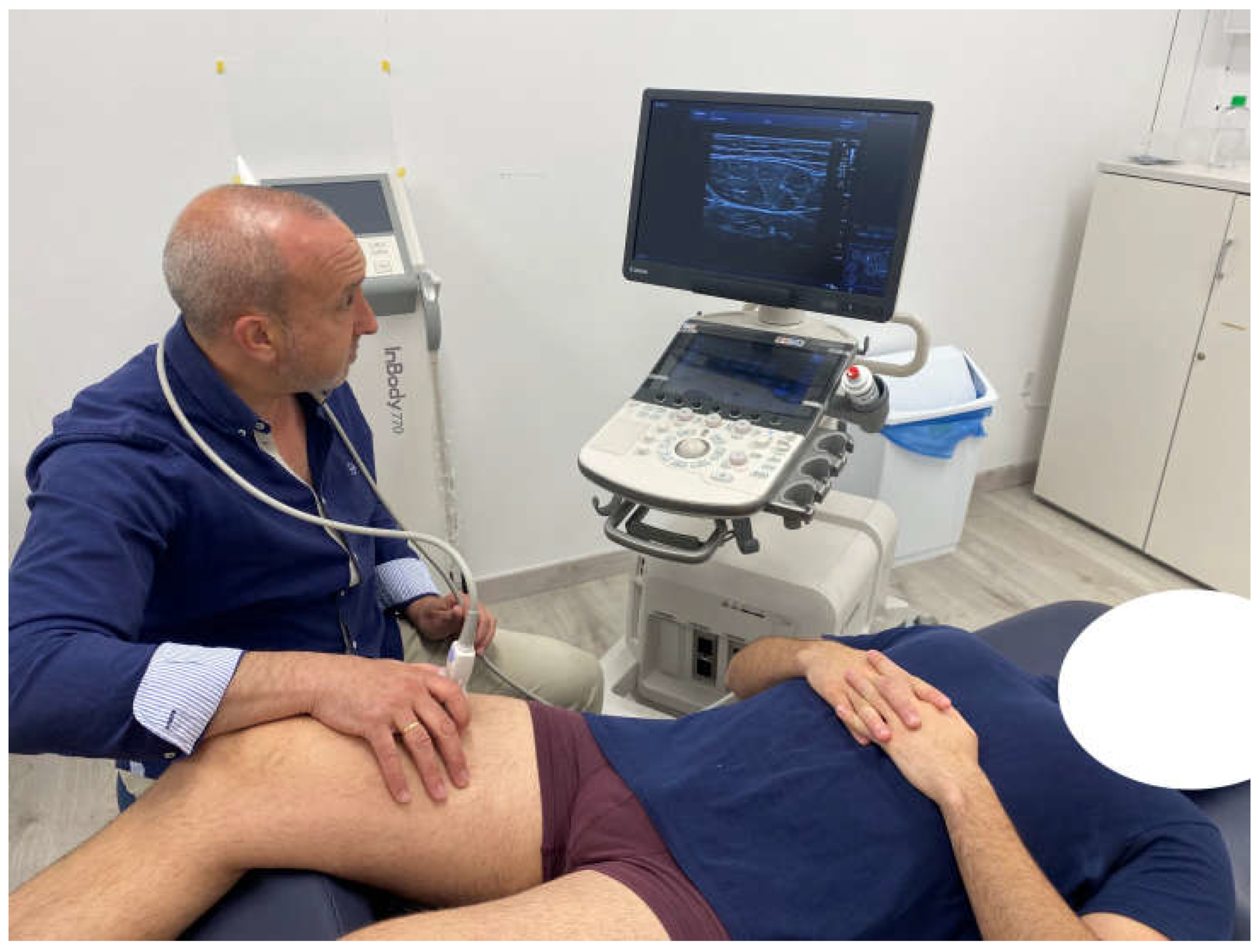
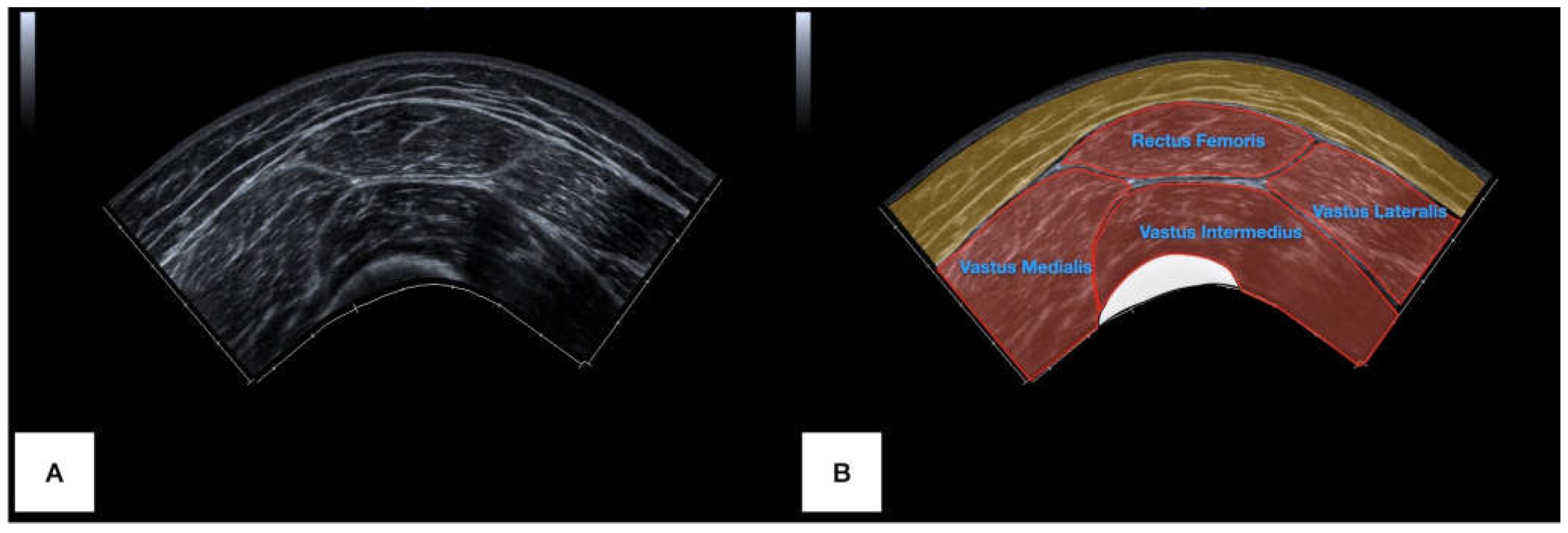
2.4.3. Ultrasound Imaging
- -
- At the middle distance between the origin of the rectus femoris muscle (anterior inferior iliac spine) and the insertion of the quadricipital tendon (upper limit of the patella base), both identified with US and marked with a skin marker pen.
- -
- At the middle distance between the anterior inferior iliac spine and the mid-point marked previously (25% of the thigh length).
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81 (Suppl. S11), S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Stožer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Kamandulis, S.; Skurvydas, A.; Snieckus, A.; Masiulis, N.; Aagaard, P.; Dargeviciute, G.; Brazaitis, M. Monitoring markers of muscle damage during a 3 week periodized drop-jump exercise programme. J. Sports Sci. 2011, 29, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.; Doma, K.; Sinclair, W.; Connor, J.; Leicht, A. Acute Effects of Training Loads on Muscle Damage Markers and Performance in Semi-elite and Elite Athletes: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 2181–2207. [Google Scholar] [CrossRef]
- Manfredi, T.G.; A Fielding, R.; O’Reilly, K.P.; Meredith, C.N.; Lee, H.Y.; Evans, W.J. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med. Sci. Sports Exerc. 1991, 23, 1028–1034. [Google Scholar] [CrossRef]
- Komulainen, J.; Takala, T.E.S.; Vihko, V. Does increased serum creatine kinase activity reflect exercise-induced muscle damage in rats? Int. J. Sports Med. 1995, 16, 150–154. [Google Scholar] [CrossRef]
- Magal, M.; Dumke, C.L.; Urbiztondo, Z.G.; Cavill, M.J.; Triplett, N.T.; Quindry, J.C.; McBride, J.M.; Epstein, Y. Relationship between serum creatine kinase activity following exercise-induced muscle damage and muscle fibre composition. J. Sports Sci. 2010, 28, 257–266. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Ojedo-Martín, C.; Fernández-De-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Hervás-Pérez, J.P. Reliability and Validity of Panoramic Ultrasound Imaging for Evaluating Muscular Quality and Morphology: A Systematic Review. Ultrasound Med. Biol. 2021, 47, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Plaza-Manzano, G.; Ortega-Santiago, R.; Fernández-De-Las-Peñas, C.; Varol, U. Panoramic ultrasound imaging does not produce muscle morphology deformation during imaging acquisition: A validity study. Phys. Medica 2023, 106, 102530. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Úbeda-D’Ocasar, E.; Caballero-Corella, M.; Fernández-De-Las-Peñas, C.; Sendarrubias, G.M.G.; Arias-Buría, J.L. Cervical Multifidus Morphology and Quality Are Not Associated with Clinical Variables in Women with Fibromyalgia: An Observational Study. Pain Med. 2022, 23, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Albayda, J.; van Alfen, N. Diagnostic Value of Muscle Ultrasound for Myopathies and Myositis. Curr. Rheumatol. Rep. 2020, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Finnoff, J.T.; Ray, J.; Corrado, G.; Kerkhof, D.; Hill, J. Sports Ultrasound: Applications Beyond the Musculoskeletal System. Sports Health 2016, 8, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.R. Ultrasound Imaging of the Musculoskeletal System. Veter Clin. N. Am. Small Anim. Pract. 2016, 46, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Al-Buqain-Ortega, A.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R. Echo-intensity, fatty infiltration, and morphology ultrasound imaging assessment in healthy and whiplash associated disorders populations: An observational study. Eur. Spine J. 2021, 30, 3059–3067. [Google Scholar] [CrossRef]
- Stock, M.S.; Thompson, B.J. Echo intensity as an indicator of skeletal muscle quality: Applications, methodology, and future directions. Eur. J. Appl. Physiol. 2021, 121, 369–380. [Google Scholar] [CrossRef]
- Rabe, K.G.; Jahanandish, M.H.; Fey, N.P. Ultrasound-Derived Features of Muscle Architecture Provide Unique Temporal Characterization of Volitional Knee Motion. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico, 1–5 November 2021; pp. 4828–4831. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), 31–34. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr. EQUATOR reporting guidelines should also be used by clinicians. J. Clin. Epidemiol. 2020, 117, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Exercise-Induced Muscle Damage and Recovery in Young and Middle-Aged Males with Different Resistance Training Experience. Sports 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Howatson, G.; Van Someren, K.; Gaze, D.; Legg, H.; Lineham, J.; Pedlar, C. The Effects of Compression-Garment Pressure on Recovery After Strenuous Exercise. Int. J. Sports Physiol. Perform. 2017, 12, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.N.; Krustrup, P.; Araújo Póvoas, S.C.; Castagna, C. Accuracy and reliability of the InBody 270 multi-frequency body composition analyser in 10-12-year-old children. PLoS ONE 2021, 16, e0247362. [Google Scholar] [CrossRef] [PubMed]
- Muanjai, P.; Mickevicius, M.; Kamandulis, S.; Snieckus, A.; Jones, D.A. The relationship between stiffness and pain following unaccustomed eccentric exercise: The effects of gentle stretch and repeated bout. Eur. J. Appl. Physiol. 2019, 119, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Belmonte, A.; Martínez-Cava, A.; Pallarés, J.G. Panoramic ultrasound requires a trained operator and specific evaluation sites to maximize its sensitivity: A comprehensive analysis of the measurement errors. Physiol. Behav. 2022, 248, 113737. [Google Scholar] [CrossRef] [PubMed]
- Cleary, C.J.; Nabavizadeh, O.; Young, K.L.; Herda, A.A. Skeletal muscle analysis of panoramic ultrasound is reliable across multiple raters. PLoS ONE 2022, 17, e0267641. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Martin, D.S.; Ploutz-Snyder, R.; Caine, T.; Matz, T.; Arzeno, N.M.; Buxton, R.; Ploutz-Snyder, L. Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med. Biol. 2012, 38, 1656–1661. [Google Scholar] [CrossRef]
- Jafari, M.; Ansari-Pour, N. Why, When and How to Adjust Your P Values? Cell J. 2019, 20, 604–607. [Google Scholar] [CrossRef]
- Morris, P.E.; Fritz, C.O. Effect sizes in memory research. Memory 2013, 21, 832–842. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Lee, J.; Clarkson, P.M. Plasma creatine kinase activity and glutathione after eccentric exercise. Med. Sci. Sports Exerc. 2003, 35, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Ličen, U.; Kozinc, Ž. Using Shear-Wave Elastography to Assess Exercise-Induced Muscle Damage: A Review. Sensors 2022, 22, 7574. [Google Scholar] [CrossRef] [PubMed]
- Kawama, R.; Yanase, K.; Hojo, T.; Wakahara, T. Acute changes in passive stiffness of the individual hamstring muscles induced by resistance exercise: Effects of contraction mode and range of motion. Eur. J. Appl. Physiol. 2022, 122, 2071–2083. [Google Scholar] [CrossRef]
- Lacourpaille, L.; Nordez, A.; Hug, F.; Couturier, A.; Dibie, C.; Guilhem, G. Time-course effect of exercise-induced muscle damage on localized muscle mechanical properties assessed using elastography. Acta Physiol. 2014, 211, 135–146. [Google Scholar] [CrossRef]
- Andonian, P.; Viallon, M.; Le Goff, C.; de Bourguignon, C.; Tourel, C.; Morel, J.; Giardini, G.; Gergelé, L.; Millet, G.P.; Croisille, P. Shear-Wave Elastography Assessments of Quadriceps Stiffness Changes prior to, during and after Prolonged Exercise: A Longitudinal Study during an Extreme Mountain Ultra-Marathon. PLoS ONE 2016, 11, e0161855. [Google Scholar] [CrossRef]
- Fouré, A.; Besson, T.; Stauffer, E.; Skinner, S.C.; Bouvier, J.; Féasson, L.; Connes, P.; Hautier, C.A.; Millet, G.Y. Sex-related differences and effects of short and long trail running races on resting muscle-tendon mechanical properties. Scand. J. Med. Sci. Sports 2022, 32, 1477–1492. [Google Scholar] [CrossRef]
- Chalchat, E.; Siracusa, J.; Bourrilhon, C.; Charlot, K.; Martin, V.; Garcia-Vicencio, S. Muscle Shear Elastic Modulus Provides an Indication of the Protection Conferred by the Repeated Bout Effect. Front. Physiol. 2022, 13, 877485. [Google Scholar] [CrossRef]
- Hicks, K.M.; Onambélé, G.L.; Winwood, K.; Morse, C.I. Muscle Damage following Maximal Eccentric Knee Extensions in Males and Females. PLoS ONE 2016, 11, e0150848. [Google Scholar] [CrossRef]
| Variable | Total Samples (n = 25) | Gender | ||
|---|---|---|---|---|
| Male (n = 11) | Female (n = 14) | Difference | ||
| Sociodemographic Characteristics | ||||
| Age (y) | 36.0 ± 9.7 | 37.5 ± 11.1 | 34.8 ± 8.8 | 2.6 (−6.4;11.7); p = 0.545 |
| Height (m) | 1.68 ± 0.09 | 1.76 ± 0.04 | 1.62 ± 0.07 | 0.13 (0.08; 0.18); p < 0.001 |
| Weight (kg) | 71.3 ± 19.4 | 85.2 ± 13.7 | 60.3 ± 16.0 | 24.9 (12.3; 37.4); p < 0.001 |
| Body mass index (kg/m2) | 24.7 ± 4.5 | 27.3 ± 3.4 | 22.6 ± 4.2 | 4.8 (1.5; 8.0); p = 0.006 |
| Body Composition Characteristics | ||||
| Body fat mass (kg) | 21.2 ± 8.8 | 23.7 ± 7.9 | 19.3 ± 9.3 | 4.4 (-2.8;11.7); p = 0.219 |
| Skeletal muscle mass (kg) | 27.9 ± 7.9 | 35.0 ± 5.0 | 22.4 ± 4.6 | 12.6 (8.6; 16.6); p < 0.001 |
| Water volume (kg) | 36.7 ± 9.5 | 45.1 ± 5.9 | 30.1 ± 5.7 | 15.0 (10.2; 19.8); p < 0.001 |
| Variable | Gender | Side | ||||
|---|---|---|---|---|---|---|
| Male | Female | Difference | Right Side | Left Side | Difference | |
| Proximal third (n = 50) | ||||||
| Area (cm2) | 12.1 ± 2.8 | 11.3 ± 2.6 | 0.8 (−0.8;2.3); p = 0.317 | 12.0 ± 2.8 | 11.2 ± 2.6 | 0.8 (−0.7;2.3); p = 0.292 |
| Mean echo intensity (0–255) | 50.5 ± 8.3 | 49.3 ± 11.7 | 1.2 (−4.8;7.1); p = 0.692 | 50.4 ± 9.0 | 49.3 ± 11.6 | 1.0 (−4.9;6.9); p = 0.732 |
| Aspect ratio | 2.8 ± 0.3 | 2.5 ± 0.5 | 0.3 (0.1;0.5); p = 0.016 | 2.6 ± 0.5 | 2.7 ± 0.4 | 0.1 (−0.1;0.4); p = 0.342 |
| Middle third (n = 50) | ||||||
| Area (cm2) | 14.8 ± 8.1 | 14.4 ± 2.9 | 0.5 (−2.8;3.8); p = 0.784 | 15.3 ± 2.7 | 13.8 ± 7.6 | 1.5 (−1.8;4.7); p = 0.369 |
| Mean echo intensity (0–255) | 43.2 ± 9.9 | 40.9 ± 11.5 | 2.3 (−3.9;8.5); p = 0.463 | 41.5 ± 10.2 | 42.3 ± 11.5 | 0.9 (−5.3;7.0); p = 0.777 |
| Aspect ratio | 2.6 ± 0.2 | 2.6 ± 0.5 | 0.0 (−0.2;0.3); p = 0.819 | 2.6 ± 0.4 | 2.6 ± 0.4 | 0.0 (−0.2;0.2); p = 0.815 |
| Measurement | Score | ANOVA Interaction Effect | Time Difference | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | |||||
| Ultrasound imaging—rectus femoris characteristics | ||||||
| Proximal third | Area (cm2) | Baseline (n = 50) | 11.6 ± 2.7 | F = 1.006 η2p = 0.014 p = 0.368 | −0.8 (−2.4;0.8); p = 0.702 | −0.8 (−2.4;0.8); p = 0.637 |
| After 24 h (n = 50) | 10.8 ± 3.3 | |||||
| After 48 h (n = 50) | 10.8 ± 3.8 | |||||
| Mean echo intensity (0–255) | Baseline (n = 50) | 49.8 ± 10.3 | F = 0.299 η2p = 0.004 p = 0.742 | −1.3 (−6.6;3.9); p = 1.000 | −1.5 (−6.8;3.7); p = 1.000 | |
| After 24 h (n = 50) | 48.5 ± 9.7 | |||||
| After 48 h (n = 50) | 48.3 ± 12.1 | |||||
| Aspect ratio | Baseline (n = 50) | 2.6 ± 0.4 | F = 1.509 η2p = 0.021 p = 0.225 | −0.2 (−0.4;0.1); p = 0.266 | −0.1 (−0.3;0.1); p = 0.838 | |
| After 24 h (n = 50) | 2.5 ± 0.5 | |||||
| After 48 h (n = 50) | 2.5 ± 0.4 | |||||
| Middle third | Area (cm2) | Baseline (n = 50) | 14.6 ± 5.7 | F = 1.706 η2p = 0.023 p = 0.185 | −1.7 (−3.9;0.5); p = 0.205 | −1.0 (−3.2;1.2); p = 0.852 |
| After 24 h (n = 50) | 12.9 ± 3.5 | |||||
| After 48 h (n = 50) | 13.6 ± 3.9 | |||||
| Mean echo intensity (0–255) | Baseline (n = 50) | 41.9 ± 10.8 | F = 0.096 η2p = 0.001 p = 0.909 | 0.5 (−4.1;5.1); p = 1.000 | −0.3 (−4.9;4.3); p = 1.000 | |
| After 24 h (n = 50) | 42.4 ± 9.3 | |||||
| After 48 h (n = 50) | 41.6 ± 8.1 | |||||
| Aspect ratio | Baseline (n = 50) | 2.6 ± 0.4 | F = 1.097 η2p = 0.015 p = 0.337 | −0.1 (−0.3;0.1); p = 0.621 | 0.0 (−0.2;0.2); p = 1.000 | |
| After 24 h (n = 50) | 2.5 ± 0.5 | |||||
| After 48 h (n = 50) | 2.6 ± 0.5 | |||||
| Serological biomarkers—creatine kinase (n = 25) | ||||||
| Concentration (U/L) | Baseline (n = 25) | 93.8 ± 52.5 | F = 11.319 η2p = 0.239 p < 0.001 | 130.3 (38.2;222.3); p = 0.003 | 171.0 (79.0;263.0); p < 0.001 | |
| After 24 h (n = 25) | 224.1 ± 136.1 | |||||
| After 48 h (n = 25) | 264.2 ± 150.1 | |||||
| Creatine Kinase Change | ||
|---|---|---|
| 24 h | 48 h | |
| 1. Proximal third area change | r = −0.087; p = 0.554 | r = −0.081; p = 0.580 |
| 2. Proximal third EI change | r = − 0.034; p = 0.817 | r = − 0.070; p = 0.633 |
| 3. Proximal third AR change | r = − 0.149; p = 0.308 | r = − 0.141; p = 0.333 |
| 4. Middle third area change | r = −0.124; p = 0.402 | r = −0.197; p = 0.171 |
| 5. Middle third EI change | r = 0.196; p = 0.183 | r = 0.113; p = 0.435 |
| 6. Middle third AR change | r = − 0.248; p = 0.089 | r = − 0.087; p = 0.549 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffet-García, J.; Vicente-Campos, D.; López-Redondo, M.; Sánchez-Jorge, S.; Álvarez-González, J.; Plaza-Manzano, G.; Seijas-Fernández, T.; Valera-Calero, J.A. Association between Gray-Scale Ultrasound Imaging and Serological Creatine Kinase for Quantifying Exercise-Induced Muscle Damage: An Observational Study. Bioengineering 2024, 11, 40. https://doi.org/10.3390/bioengineering11010040
Buffet-García J, Vicente-Campos D, López-Redondo M, Sánchez-Jorge S, Álvarez-González J, Plaza-Manzano G, Seijas-Fernández T, Valera-Calero JA. Association between Gray-Scale Ultrasound Imaging and Serological Creatine Kinase for Quantifying Exercise-Induced Muscle Damage: An Observational Study. Bioengineering. 2024; 11(1):40. https://doi.org/10.3390/bioengineering11010040
Chicago/Turabian StyleBuffet-García, Jorge, Davinia Vicente-Campos, Mónica López-Redondo, Sandra Sánchez-Jorge, Javier Álvarez-González, Gustavo Plaza-Manzano, Tamara Seijas-Fernández, and Juan Antonio Valera-Calero. 2024. "Association between Gray-Scale Ultrasound Imaging and Serological Creatine Kinase for Quantifying Exercise-Induced Muscle Damage: An Observational Study" Bioengineering 11, no. 1: 40. https://doi.org/10.3390/bioengineering11010040
APA StyleBuffet-García, J., Vicente-Campos, D., López-Redondo, M., Sánchez-Jorge, S., Álvarez-González, J., Plaza-Manzano, G., Seijas-Fernández, T., & Valera-Calero, J. A. (2024). Association between Gray-Scale Ultrasound Imaging and Serological Creatine Kinase for Quantifying Exercise-Induced Muscle Damage: An Observational Study. Bioengineering, 11(1), 40. https://doi.org/10.3390/bioengineering11010040






