Abstract
An effective approach to maintaining uninterrupted coolant flow in heat supply systems—and thereby reducing energy consumption—is to prevent the formation of corrosion-scale deposits on the inner surfaces of metal pipes. This is typically achieved by performing anti-corrosion treatment on the coolant. However, the efficiency of this method depends on several factors, including pipe conditions, water flow rate, and water composition. To inhibit corrosion and scale formation on the internal surfaces of pipelines, specific inhibitors are used to create protective films on the metal surface. For strong adhesion of these films, preliminary chemical cleaning of the metal surface with low-concentration acid solutions is essential. This cleaning is usually performed in circulation mode for several hours. The activated surface enhances inhibitor adhesion, leading to the formation of films with improved protective properties. The quality of the anticorrosive films was evaluated using a JSM-6490LV scanning electron microscope equipped with INCAEnergy energy-dispersive microanalysis systems, HKL-Basic structural analysis, ContrAA-300 atomic adsorption spectrometer, and potentiostat IPC-Pro MF.
1. Introduction
The main factors determining the thermodynamic possibility of the corrosion process are the rate of the electrochemical reaction, as well as the mechanism of its occurrence. Diffusion processes delivering reagents to the surface of the corroding metal, with subsequent removal of corrosion products, are also important. In heat supply systems, corrosion-scale compounds may form on the inner surface of pipelines, the composition of which may depend on the presence of ions of various compounds in the coolant, such as OH−, Cl−, SO42−, Ca2+, Mg2+, Cu2+, Mn2+, and others. Competition for a more advantageous place on the surface of a metal pipe in terms of energy will depend on the value of its electrode potential. But if in this case an inhibitor is found in a corrosive environment, then it is also able to participate in this competition, taking its place on the surface of the metal and simultaneously protecting the surface of the pipe from corrosion destruction with a protective film, thereby facilitating the free passage of the coolant [1,2,3,4,5,6]. The authors [7,8,9] describe the processes of corrosion of the inner surface of metal water pipes, determined by the corrosive activity of the coolant (water) and the peculiarities of its operation mode, as well as methods of anti-corrosion protection of metal equipment, and give recommendations on the application of new methods of protection, including the use of environmentally safe inhibitors.
Some nitrogen-containing compounds, such as imidozalines, pyridine derivatives, aliphatic amines and their derivatives, as well as quaternary ammonium compounds, can be used as inhibitors for corrosion protection of heat engineering equipment. The authors’ studies [10,11,12] have shown the prospects for the formation of a protective film by the proposed inhibitors.
Corrosion of metal equipment significantly depends on the composition of the corrosive medium. For example, in the oil and gas industry, there are such aggressive gases as sulfur dioxide and hydrogen sulfide, but chlorine can also cause destruction typical of this corrosive environment.
In such a corrosive environment, it is very difficult to approach the selection of inhibitors capable of protecting equipment from corrosion. The authors of the studies used gravimetric methods to select the mixtures, establishing the multifunctional action of the inhibitor mixture. For example, gossypol resin, a natural polyphenol, was dissolved in kerosene or another solvent. A significant reduction in corrosion of steel equipment was established when using this mixture in the oil industry [13]. A number of other researchers used nucleophilic compounds as inhibitors to protect oilfield equipment from acid corrosion, in addition to aminotriazole or its derivatives, but also in a mixture with halides, thiocyanates, alkali metal sulfides, thiourea, and urotropine. A significant positive effect of such mixed compositions for corrosion protection was recorded [3].
To obtain the quality index values of the films obtained on the metal surface of a pipe in a heat supply system, mixtures of inhibitors of silicate and sodium tripolyphosphate were studied, capable of forming a complex composition of ferrosilicate and ferrophosphate, capable of adsorbing on the metal surface of a steel sample and forming an effective ferrosilicate-phosphate protective film. The authors formulate the mechanism of formation of a film with high protective properties as follows: when iron is ionized from the surface of a metal pipe in water, compounds of divalent iron are formed, while the inhibitor components tend to take their place on the surface of the metal sample, actively clinging to its surface and forming a film. High protective properties of the film are confirmed by calculations of corrosion rate indices. The efficiency of a mixture of two inhibitors related in composition is shown [14].
The authors of [15,16] conducted studies on the effect of a number of inhibitors against hydrogen sulfide and carbon dioxide corrosion of metal oilfield equipment and oil pipelines. As inhibitors, the authors studied the effect of nitrogen-containing compounds with a long hydrocarbon chain—imidazolines, pyridine derivatives, aliphatic amines and their derivatives, quaternary ammonium compounds on the corrosion rate in an acidic medium of 0.1 N HCl and in water with 5 g/L of NaCl. The calculated corrosion rate and the effectiveness of the protective properties of inhibitors allow the recommendation of their use. Polyfluoride compounds are used as inhibitors and corrosion inhibitors.
It is considered that the economic effect depends on corrosion destruction, since it increases the material costs of repair, start-up, and adjustment of equipment. The purpose of reducing such costs and increasing the reliability of equipment is the use of various inhibitors in the inhibition of the corrosion process. For example, benzotriazole, as an inhibitor, is able to inhibit the anodic dissolution of metal, reducing the rate of oxygen ionisation due to the formation of a thin, dense adherent phase film of a complex compound of benzotriazole with Me2+ ions on the metal surface [17,18].
Currently, such surfactants as adducts of dialkyl phosphates: thiourea derivatives of dialkyphosphorous acids containing sulfur and phosphorus in their composition, with a complex formula ([(RO)2POH∙C(S)(NH3)2], can be used as inhibitors and adsorbed at the expense of sulfide sulfur with its high reducing properties on the metal surface of equipment, creating films with protective properties [19].
Components of the coolant (water) play an important role in the formation of scale compounds on the inner surface of steel equipment in heat supply systems, preventing the passage of the coolant through the system.
Water with a complex chemical composition with gases and microorganisms dissolved in it is capable of forming not only scale, but also of depositing corrosion-scale deposits consisting of carbonates, silicates, sulfates, and sulfides of alkali metals as a consequence of disturbance of the carbon dioxide equilibrium. Due to the imbalance in water, dense or loose deposits are formed on the internal metal surface, which have a negative effect on the circulation of the coolant and cause a slowdown in the passage of the coolant. By investigating the indicators of various water compositions, the authors concluded with a high probability that the main reason for the absolute corrosiveness of water is the high concentration of sulfate, silicate, chloride, and phosphate ions of calcium and magnesium, capable of forming corrosion-scale compounds. By studying the indicators of water composition in detail, the authors have shown their clear dependence on the formation of corrosion-scale deposits and an increase in the corrosion rate on the metal surface [20].
It is known that the modern classification of inhibitors with adsorption and complexing properties requires a very careful approach to the selection of inhibitors as components that prevent corrosion processes in metal equipment in heat supply systems. The protection levels and inhibition coefficients on background solutions of 5% sulfuric acid and 5% sulfate solution were determined by chronopotentiometry and gravimetry for inhibitors based on phosphorus-containing compounds in the oil and gas industry. Multicomponent mixtures were composed of pyrophosphates with gelatin, which showed high mobility in the range of different temperatures and pH [21].
The authors’ work [22] is devoted to the study of the properties of two-component inhibitors. As noted by the authors, the formation of highly effective films with protective properties on the metal surface of equipment, reducing metal corrosion, can be achieved only with the use of phosphorus-containing inhibitors at various concentrations (30–70 mg/L) in a wide temperature range (50 °C, 90 °C). Studies have shown that the calculated degree of protection increases slightly at high concentrations and high temperatures. Forming, at a concentration of inhibitors Ca2P2O7 −50 mg/L in a mixture with (NaPO3)n at a temperature of 50 °C, dense, stable chemisorption films in background media of weakly acidic solutions can be recommended for implementation.
In the heat power engineering system, the researchers used a heat supply scheme to protect the internal surfaces of pipelines from corrosion with a mixture of inhibitors. The selected mixture of inhibitors helps to create a protective film on the internal surface of steel pipelines. The authors, in order to obtain a highly effective film on the metal surface, pre-activated the metal surface by treating it with a dilute solution of sulfuric acid (3%), intended for washing solutions. Activation treatment of the pipe surface was carried out in circulation mode for more than 5 h; after such treatment, the metal surface was immediately treated with a mixture of inhibitor solution (sodium silicate 2000 mg/L and sodium tripolyphosphate 30 mg/L) for 3–5 days [23].
The aim of the study is to select a mixture of inhibitors for the formation of films with high protective properties on a metal surface, with an assessment of their effectiveness and subsequent recommendation for implementation. The originality of this research lies in the use of a dynamic circulation-based deposition system with optimization of exposure time, pre-activation conditions, and evaluation of inhibitor modulus effect—elements not extensively addressed together in previous studies.
2. Methodology of the Experiment
Figure 1 shows the scheme of the process for obtaining films on the metal surface of steel samples.
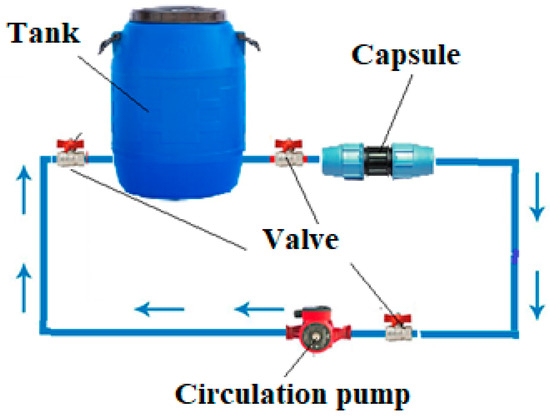
Figure 1.
General view of the scheme of the process of obtaining protective films on steel samples.
The container containing an inhibitor solution was connected by hoses to a capsule in which a steel pipe sample was placed. Using a circulation pump, the inhibitor solution was pumped through the capsule for a predetermined time. After the set time, a pipe sample was removed from the capsule and sent for energy-dispersive analysis using a scanning electron microscope JSM-6490LV (JEOL Ltd., Tokyo, Japan) with an INCAPentaFET-x3 energy dispersive X-ray microanalysis system (Oxford Instruments PLC, Abingdon, UK).
Sodium silicates with different modulus (m = 1,2,3) were studied as inhibitors both separately and in mixture with sodium tripolyphosphate. Oxalic, succinic, sulphamic, and hydrochloric acids were used as activators of the steel surface of the samples. Steel composition is presented in Table 1.

Table 1.
Steel composition according to GOST 380-2005.
The inhibitor solution was prepared using water from the Mayatal well. The water is used in the Shymkent heating network. Concentrations of elements in the water were measured by the ContrAA-300 atomic absorption spectrometer (Analytik Jena GmbH+Co, Jena, Germany) and are presented in Table 2.

Table 2.
Composition of the water used in the research.
The corrosion rate indicators on the metal surface of steel samples, unprotected and protected by an inhibitor film, were measured by GOST 9.908-85 and were calculated according to the formula:
where ∆m represents the change in sample mass, S is the sample area subject to corrosion, and τ is corrosion time.
Polarization curves were measured in water similar to that used for the inhibitor solution. A sample with an area of 0.25 cm2 made from the pipe was used as the working electrode. Silver chloride electrodes were used as an auxiliary electrode and a reference electrode. The scan rate was 3 mV/s.
3. Results and Discussion
A steel sample of the tube, cleaned of grease, washed with water, and weighed on an electronic scale, was immersed in a capsule. Sodium silicate solution with modulus of m = 1 was added to the vessel, containing the coolant. A circulation pump with a circulation speed of 1.5 m/s was switched on, and the solution containing sodium silicate was pumped through the capsule with a steel sample for 12 h at room temperature. At the end of the experiment, the sample was removed from the capsule, washed with water, dried, and weighed. The experiment was repeated with sodium silicate, where the modulus m = 2 and m = 3.
Table 3 shows the indicators of the obtained film mass on the surface of the steel tube, inhibited by sodium silicate of different modulus.

Table 3.
Mass of the deposited film on a steel sample, inhibited by sodium silicate of different modulus for 12 h.
Figure 2 shows a picture of the film on the steel surface of the sample, inhibited by sodium silicate with a modulus of m = 2 for 12 h.
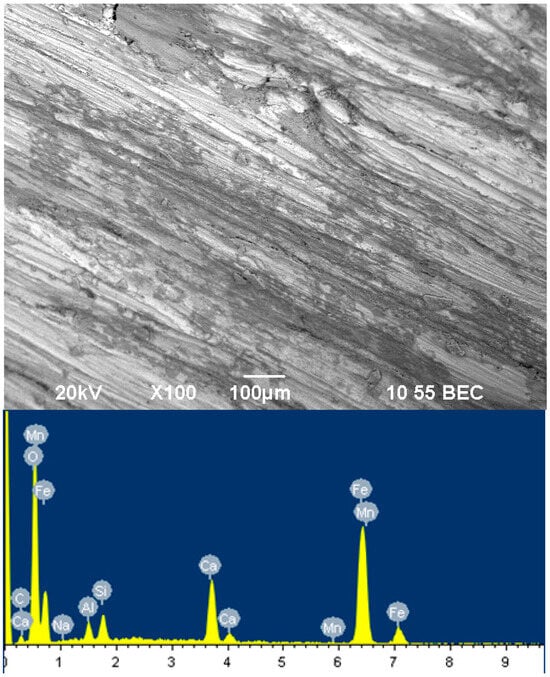
Figure 2.
Surface of film inhibited by sodium silicate with modulus m = 2 for 12 h.
The experiment was repeated at the same circulation rate, with the same modules of sodium silicate, only for 24 h. The mass values of the deposited film on the steel sample are given in Table 4.

Table 4.
Mass of the deposited film on the steel sample, inhibited by sodium silicate of different modulus for 24 h.
Figure 3 shows a picture of the film on the steel surface of the sample, inhibited by sodium silicate with a modulus of m = 2 for 24 h.
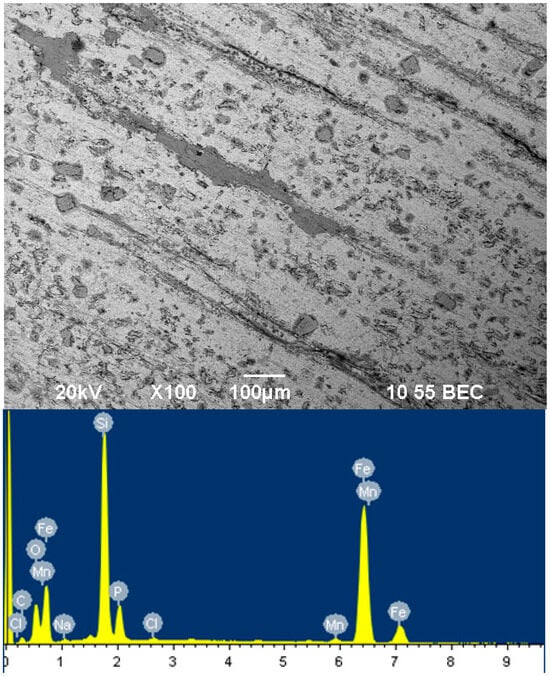
Figure 3.
Surface of film inhibited by sodium silicate with modulus m = 2 for 24 h.
Table 5 and Table 6 summarize the data affecting the film formation by a mixed inhibitor under the same conditions as given and described above.

Table 5.
Mass of the deposited film on a steel sample, inhibited by a mixture of sodium silicate and sodium tripolyphosphate within 24 h.

Table 6.
Mass of deposited film on the steel sample was inhibited by a mixture of sodium silicate and sodium tripolyphosphate for 12 h.
Figure 4 shows the surface of a film inhibited by a mixture of sodium silicate m = 3 with sodium tripolyphosphate for 24 h.
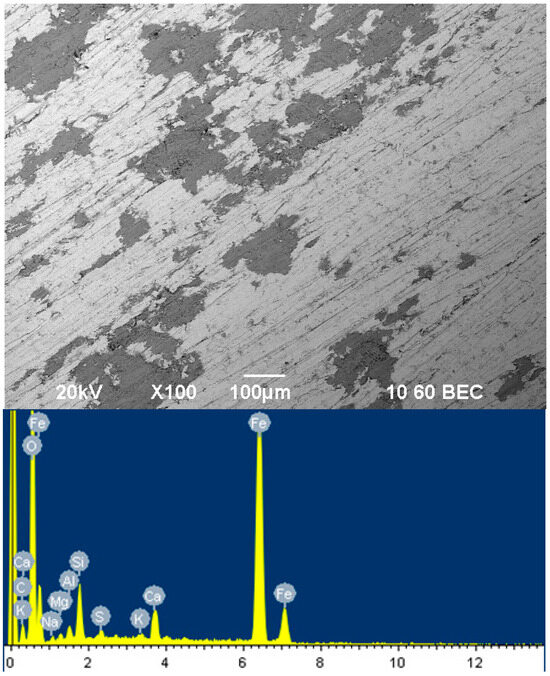
Figure 4.
Surface of film inhibited by sodium silicate with modulus m = 3 mixed with tripolyphosphate for 24 h.
Figure 5 shows the surface of the film inhibited by a mixture of sodium silicate with modulus m = 3 mixed with sodium tripolyphosphate for 12 h.
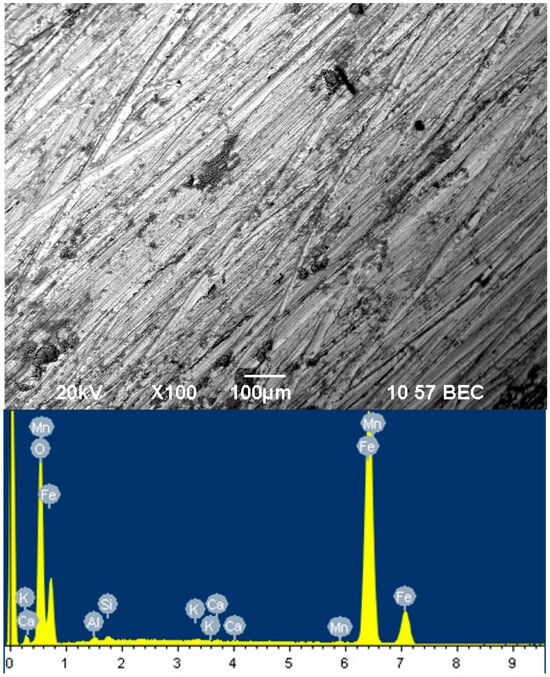
Figure 5.
Surface of film inhibited by sodium silicate of modulus m = 3 with tripolyphosphate for 12 h.
Figure 6, Figure 7 and Figure 8 show the graphical dependence of the film mass on the effect of both individual and mixed inhibitors, obtained in 12 h.
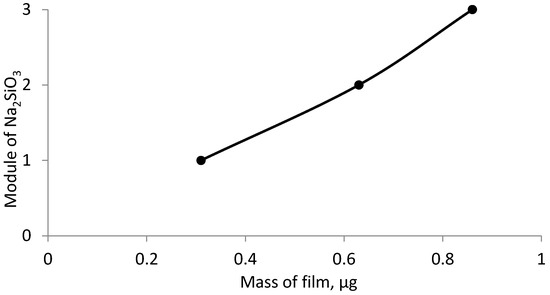
Figure 6.
Dependence of the deposited film mass on the steel sample, inhibited by sodium silicate of different modulus over a period of 12 h.
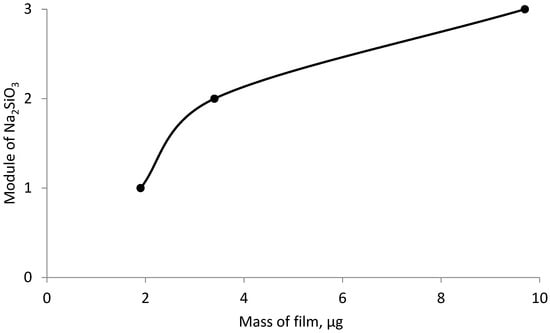
Figure 7.
Dependence of the deposited film mass on the steel sample, inhibited by sodium silicate of different modulus over a period of 24 h.
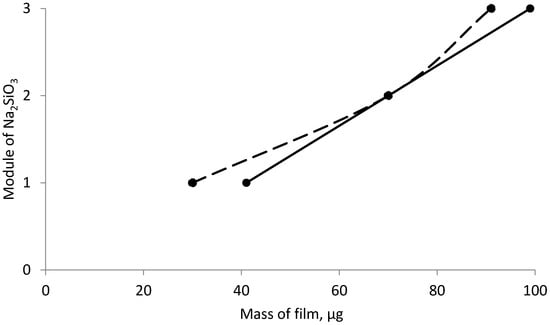
Figure 8.
Dependence of film mass on steel sample inhibited by a mixture of sodium silicate and sodium tripolyphosphate for 24 h (dashed line) and 12 h (solid line).
As can be seen from the figure, the mass of the deposited film over a period of 24 h is significantly higher than the mass of the film obtained for 12 h.
Figure 8 shows the dependence of the deposited film mass on the steel sample, inhibited by sodium silicate of different modulus in a mixture with sodium tripolyphosphate, for different periods of time.
Table 7 shows corrosion rate indicators for steel tube samples activated by acids for 24 h and subsequently treated with sodium silicate of modulus m = 3.

Table 7.
Corrosion rate indicators for steel tubes activated by acids for 24 h and treated with sodium silicate of modulus m = 3.
Table 8 shows the corrosion rate indicators for steel tubes activated by acids for 24 h and treated by a mixture of sodium silicate of modulus m = 3 and sodium tripolyphosphate. These acids are the most accessible to industry in our region.

Table 8.
Corrosion rate indicators for steel tubes activated by acids for 24 h and treated by a mixture of sodium silicate of modulus of m = 3 and sodium tripolyphosphate.
Figure 9 shows a graphical dependence of the corrosion rate of a steel tube activated by acids and subsequently treated by sodium silicate of modulus of 3 (white column) and a mixture of sodium silicate of modulus 3 with sodium tripolyphosphate (black column).
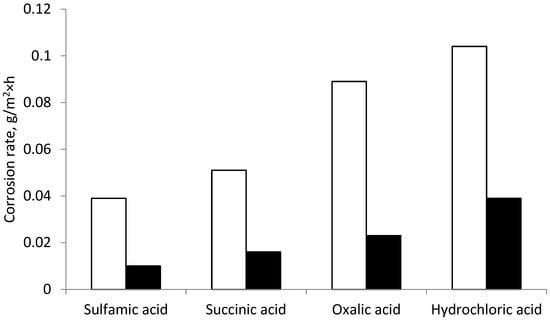
Figure 9.
Corrosion rates of a steel sample from the action of sodium silicate of modulus of 3 (white) and a mixed composition of inhibitors (black).
As we can see from the figure, the corrosion rate on the steel sample treated by the sodium silicate inhibitor (white) is significantly higher than the rate on the steel sample treated by the mixture of sodium silicate with tripolyphosphate (black).
Steel tubes were placed in a capsule through which a solution containing a mixed composition of an inhibitor of sodium silicate of m = 3 with sodium tripolyphosphate was passed for 24 h at a circulation rate of 1.5 m/s, then the tube was weighed, and the mass of the deposited film was determined. Subsequent tube experiments were repeated with holding times of 2 days, 4 days, 6 days, and 8 days. The mass of the deposited film was weighed and determined (Table 9).

Table 9.
Mass of the deposited film on the steel sample was inhibited by a mixture of sodium silicate of modulus of m = 3 mixed with sodium tripolyphosphate for different time intervals.
Table 9 shows the mass of the deposited film on steel samples inhibited by a mixture of sodium silicate of modulus of m = 3 mixed with sodium tripolyphosphate for different time intervals.
Based on the data in Table 7, a graphical dependence of the corrosion rate on samples activated by various acids and treated with a mixture of sodium silicate inhibitor of modulus of m = 3 in a mixture with sodium tripolyphosphate for different times of the day was plotted.
The analysis of the data in Table 9 and Figure 10 allows us to suggest that increasing the duration of sample treatment with a mixture of inhibitors contributes to a significant increase in the mass of the deposited film on the surface of the steel tube, but only up to a certain time.
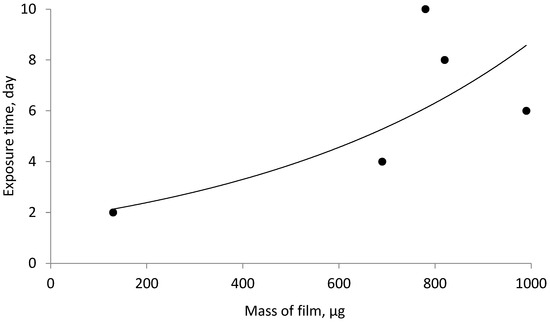
Figure 10.
Dependence of the film mass on the steel sample inhibited by a mixture of sodium silicate of modulus of m = 3 with sodium tripolyphosphate during different times of day.
Figure 11 shows the surface of a film inhibited by a mixture of sodium silicate m = 3 with sodium tripolyphosphate for 6 days.
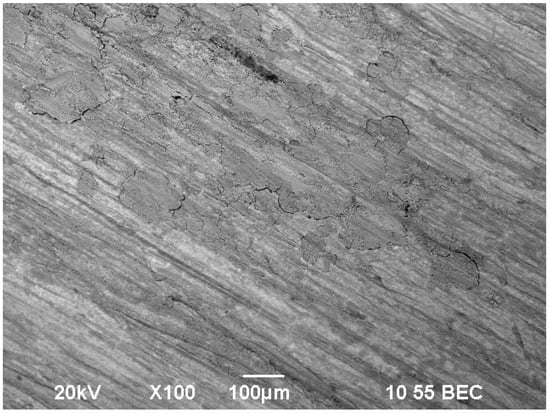
Figure 11.
Surface of film inhibited by the inhibitor mixture of sodium silicate of modulus m = 3 with tripolyphosphate for 6 days.
The protective characteristics of the obtained film are estimated by the polarization curves shown in Figure 12. The cathode current values for the same potentials for the sample inhibited for 144 h are lower than for the sample inhibited for 24 h. This means that the surface inhibited for a longer period interacts more passively with metal ions in water, leading us to conclude that the protective properties of the coating increase with increasing inhibition time.
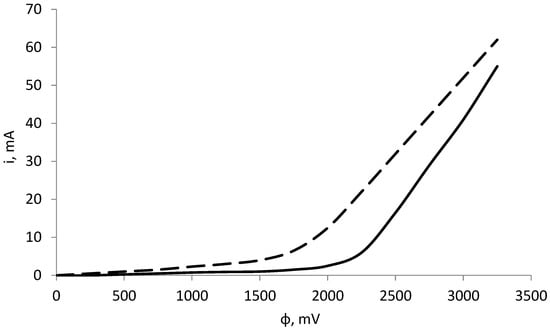
Figure 12.
Polarization curves of inhibited samples at 24 h (dashed line) and 144 h (solid line).
Based on the conducted experiments and the data obtained, it can be concluded that high-modulus sodium silicate in a mixture with sodium tripolyphosphate is adsorbed on the acid-activated surface much more strongly than on the non-activated surface, forming a film, while the mass of the deposited film depends on the duration of the deposition process.
The mechanism of formation of a protective film on the activated surface by a mixed inhibitor can be described in two stages. At the first stage, iron is ionized with the formation of iron hydroxide, which is capable of interacting with sodium silicate and forming a ferrosilicate of complex composition. At the second stage, these ferrosilicates form the initial ferrosilicate protective film.
4. Conclusions
The analysis and comparison of corrosion rate indicators with iron loss in mm/year on samples activated by various acids, subsequently treated with a mixed inhibitor with different sample treatment times (Table 7), allows us to draw the following conclusions:
- The highest corrosion rate and corrosion losses were obtained if the surface is activated with hydrochloric acid (corrosion rate = 0.104 g/m2 × h and corrosion losses = 0.13).
- The mixed inhibitor of sodium silicate with tripolyphosphate is more effective than the mono inhibitor of sodium silicate.
- The sodium silicate modulus for the formation of a protective film with the highest quality indicators on the surface of a steel sample is m = 3.
- The effective time for the formation of a protective film on the surface of a steel sample from a mixture of inhibitors is 6 days.
Author Contributions
B.K.—Project administration, Conceptualization, Data curation, Writing—original draft, Writing—review and editing. N.V.—Investigation, Data curation, Writing—original draft. A.A.—Formal analysis, Supervision, Methodology. R.S.—Methodology, Investigation, Writing—original draft. K.K.—Investigation, Visualization, Writing—original draft. G.K.—Investigation, Writing—original draft. Z.K.—Software, Visualization, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant No.: AP19679027).
Data Availability Statement
No new data were used for the study described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- Zhuk, N.P. Course in the Theory of Corrosion and Protection of Metals; Alliance: Moscow, Russia, 2016; 472p. [Google Scholar]
- Kozlova, L.S.; Sibileva, S.V.; Chesnokov, D.V.; Kutyrev, A.E. Corrosion inhibitors (Overview). Aviat. Mater. Technol. 2015, 2, 67–75. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Avdeev, Y.G.; Zel, O.O. Inhibitor of Acid Corrosion of Metals. Patent RF 2539129, 1 October 2015. [Google Scholar]
- Avdeev, Y.G.; Kuznetsov, Y.I. Organic inhibitors of metal corrosion in acid solutions. Features of the mechanism of protective action. J. Phys. Chem. 2023, 97, 305–321. [Google Scholar] [CrossRef]
- Reyzin, B.L.; Strizhevsky, I.V.; Shevelev, F.A. Corrosion and Protection of Municipal Water Pipelines; Stroyizdat: Moscow, Russia, 1979; 398p. [Google Scholar]
- Zhuk, N.P. Corrosion and Protection of Metals: Calculations; Alliance: Moscow, Russia, 2015; 332p. [Google Scholar]
- Sabanov, S.V.; Nikulin, S.A.; Karnavsky, E.L. Determination of the priority of withdrawal to repair of equipment of anti-corrosive protection on the section of the gas pipeline in the conditions of restriction of financing. Pract. Anti-Corros. Prot. 2020, 25, 7–17. [Google Scholar] [CrossRef]
- Aliyeva, L.I.; Afandiyeva, L.M.; Abbasov, V.M.; Rustamly, G.Y.; Akhmedbekova, S.F. The complexes of synthetic and petroleum acids n-derivatives as steel acidic corrosion inhibitors. Pract. Anti-Corros. Prot. 2020, 25, 19–25. [Google Scholar] [CrossRef]
- Teryusheva, S.A. Organic inhibitors of steel corrosion (analytical review of publications). Pract. Anti-Corros. Prot. 2020, 25, 60–65. [Google Scholar] [CrossRef]
- Vigdorovich, V.I.; Tsygaekova, L.E. Inhibition of Hydrogen Sulfide and Carbon Dioxide Corrosion of Metals. Universality of Inhibitors: Monograph; KARTEK: Moscow, Russia, 2011; 244p. [Google Scholar]
- Silin, M.A.; Magadova, L.A.; Davletshina, L.F.; Pakhomov, M.D.; Timerbulatova, Y.M.; Samsonenko, E.A. Research of current corrosion inhibitors action in acid systems. Pract. Anti-Corros. Prot. 2016, 4, 22–30. [Google Scholar]
- Shaker, N.O.; Badr, E.E.; Kandell, E.M. Adsorption and inhibitive properties of fatty imidazoline surfactants on mild steel. Chem. Sin. 2011, 2, 26–35. [Google Scholar]
- Gurbanov, G.R.; Adygezalova, M.B.; Pashaeva, S.M. Study of universal combined inhibitor for oil and gas industry. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. [Russ. J. Chem. Chem. Tech.] 2020, 63, 78–89. [Google Scholar] [CrossRef]
- Rozenfeld, I.L. Corrosion Inhibitors; McGraw Hill Inc.: New York, NY, USA, 1982; 327p. [Google Scholar]
- Fomenkov, O.A. Study of the Polyfunctionality of a Number of Inhibitors of Hydrogen Sulfide and Carbon Dioxide Corrosion. Ph.D Thesis, Tambov State Technical University, Tambov, Russia, 15 October 2009. [Google Scholar]
- Tsygankova, L.E.; Fomenkov, O.A.; Esina, M.N. Protective properties of inhibitors of hydrogen sulfide and carbon dioxide corrosion. Chem. Chem. Technol. 2009, 52, 66–69. [Google Scholar]
- Ostroukhova, O.I.; Prokin, E.V.; Bezmaternykh, M.A.; Mokrushin, V.S.; Ivanov, M.G. Synthesis and application of polyfluorinated corrosion inhibitors. In Proceedings of the XIV Youth Conference on Organic Chemistry, May 10-14, Ekaterinburg, Russia, 10–14 May 2011; Federal State Autonomous Educational Institution of Higher Professional Education “Ural Federal University Named After the First President of Russia B.N. Yeltsin”: Ekaterinburg, Russia, 2011; pp. 443–445. [Google Scholar]
- Rajabov, Y.N.; Eshmamatova, N.B.; Akbarov, H.I. Defense mechanisms and gravimetric estimation of the effectiveness of inhibitors on the base amino compounds. Univ. Chem. Biol. 2020, 12. Available online: https://7universum.com/ru/nature/archive/item/10939 (accessed on 8 May 2025).
- Kabylbekova, B.; Vysotskaya, N.; Anarbaev, A.; Spabekova, R.; Kurbanbekov, K.; Khussanov, Z.; Kaldybekova, G. Role of Heat Carrier Components in the Formation of Corrosion and Scale Deposits in the Pipes of Heat Supply Systems. Math. Model. Eng. Probl. 2024, 11, 2731–2739. [Google Scholar] [CrossRef]
- Kabylbekova, B.N.; Vysotskaya, N.; Anarbaev, A.; Spabekova, R.; Kurbanbekov, K.; Kaldybekova, G.; Anarbaev, N. The role of inhibitors in the process of forming a protective anti-corrosion film on the steel surface of equipment in heat supply systems. RASĀYAN J. Chem. 2025, 18, 77–85. [Google Scholar] [CrossRef]
- Kholikov, A.Z. Steel Corrosion Inhibitors Based on Phosphorus-Containing Compounds and Polyelectrolytes. Ph.D. Thesis, National Universityn of Uzbekistan, Tashkent, Uzbekistan, 2010. [Google Scholar]
- Atakulova, N.A.; Kholikov, A.Z.; Akbarov, H.I. Anti-corrosion properties of water-soluble two-component inhibitors. Eurasian Union Sci. Chem. Sci. 2014, 10, 81–83. [Google Scholar]
- Kabylbekova, B.N.; Vysotskaya, N.A.; Anarbaev, A.A.; Spabekova, R.S.; Kurbanbekov, K.T.; Kaldybekova, G.M.; Nurman, E. Method of Protection Against Corrosion of the Inner Surface of Metal Pipes in Heat Supply Systems. Patent 9384 RK, 2 April 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).