Abstract
Renewable natural and synthetic basic substances can be used to produce biodegradable polymers. Several methods of the polymerization of terpene limonene have been evaluated. The polymerization methods evaluated are radical polymerization, cationic polymerization and thiol-ene polymerization. The free-radical polymerization of limonene with azobisisobutyronitrile (AIBN) as an initiator was carried out. The cationic polymerization of limonene was carried out using AlCl3 as a catalyst. The copolymerization of limonene with mercaptoethanol, 2-mercaptoethyl ether without an initiator and with an AIBN initiator was studied and it was also shown that polymerization can proceed spontaneously. The resulting compounds were investigated by NMR and FTIR spectroscopy. The values of the molecular weight characteristics of the samples obtained are presented, such as: number-average molecular weight, hydrodynamic radius and characteristic viscosity, depending on the method of production. The coefficients α (molecular shape) in the Mark–Kuhn–Houwink equation are determined according to the established values of the characteristic viscosity. According to the values obtained, the AC molecules in solution have parameters α 0.14 to 0.26, which corresponds to a good solvent and the molecular shape-dense coil.
1. Introduction
Polymers are used in everyday life due to their wide range of chemical, mechanical, thermal and electro-physical properties [1,2]. By now, the problem of using renewable resources to meet needs without creating harmful effects on human health and the environment is quite acute. Nature provides mankind with a rich polymer material on the basis of which useful products can be obtained. Their production requires a non-renewable, depleting petroleum feedstock, which has significant energy costs during processing and produces toxic waste. Renewable natural and synthetic basic substances can be used to produce biodegradable polymers. There are principles for the selection of suitable monomers and methods for modifying natural origin polymers in order to impart the desired properties. One of these substances is limonene [3,4,5]. Limonene is found in many essential oils and is an adaptable chemical raw material [6]. As a component of turpentine, limonene can be obtained as a by-product from over 300 different plants [7,8,9]. Limonene makes up 92–97% of citrus rind oils and its global production, around 400,000 tons per year, is growing [10,11,12,13].
One of the promising areas for the application of limonene is its copolymerization to obtain new functional polymers [14,15,16].
Many scientific studies have been devoted to the polymerization of limonene [17,18,19]. It was shown in [20] that D-limonene faces difficulties during polymerization, which lead to low monomer conversion and molecular weight. To obtain poly (limonene) with a higher molecular weight, it is necessary to simultaneously reduce the concentrations of the monomer and initiator.
To increase the degree of polymerization, it is possible to use the photochemical radical polymerization of limonene at low temperatures using a combination of type II photoinitiators and alkyl halide initiators [21].
One of the main problems hindering the extension of the scope of application of modified polymers from renewable sources of biological origin is the low efficiency and selectivity of condensation reactions. In this context, there has been genuine interest in the development of new conjugation methods, collectively called click chemistry. Among the attractive features of click-chemistry methods are soft condensation conditions, high efficiency and selectivity. A special place among these processes is occupied by radical polymerization with reversible chain transfer (RCT) according to the addition–fragmentation mechanism due to tolerance to the functional groups of monomers and soft conditions [22,23,24]. This process is based on the use of sulphur-containing compounds of the general structure Z-C (=S) S-R. Under the right conditions of synthesis, macromolecules are formed whose α- and ω-end groups are determined by the chemical nature of the RCT agent used (R- and Z-C (=S) S-, respectively). The use of RCT agents, in which the R SH and C=C groups, allows such macromolecules to be further used as blanks for click reactions. Terpene-based monomers can then be used to synthesize various polymers.
This type of reaction can be carried out under soft conditions by simply mixing thiol and olefin substrates with terminal olefins. It has previously been demonstrated that thiol-ene systems can lead to extremely homogeneous glasses, elastomers and adhesives [25].
This article presents the results of a study of terpene limonene polymerization using radical polymerization, cationic polymerization and thiol-ene polymerization methods.
2. Materials and Methods
The following reagents were used to carry out the polymerization: toluene (99.5%, for synthesis), methanol (for analysis, Sigma Aldrich, Saint Louis, MO, USA), Limonene (b.p. = 176–177 °C, [α]20 = + 113 ± 2, d = 0.8411, Sigma Aldrich), Azobisisobutyronitrile (98%, Fluka), twice reprecipitated from methanol, AlCl3 (anhydrous, 99.5%, Sigma Aldrich), 2-mercaptoethanol (99.0%, Sigma Aldrich), 2-Mercaptoethyl ether (95%, Sigma Aldrich).
The results for determining the structure of the resulting compounds are obtained using measuring instruments: VERTEX 70 FTIR spectrometer (Bruker, Ettlingen, Germany); AVANCE III HD NMR spectrometer (400 MHz, Bruker, Ettlingen, Germany).
Molecular mass characteristics: weight-average molecular weight (Mw), number-average molecular weight (Mn) and polydispersity of the samples were determined by gel permeation chromatography using an Agilent 1260 Infinity II Multi-Detector GPC/SEC System (Agilent Technologies, Santa Clara, CA, USA) chromatograph with triple detection. Separation was carried out on a PLgel Mixed-E column designed for the analysis of oligomers and low molecular weight polymers using tetrahydrofuran stabilized with 250 ppm ionol as mobile phase. The column was calibrated using polydisperse polystyrene standards (Agilent, USA). The eluent feed rate is 1 mL/min; the sample volume is 100 µL. The alkyd resin samples are dissolved in tetrahydrofuran at a concentration of 5 mg/mL and held for 12 h until completely dissolved. All samples are filtered through a 0.22 µm polytetrafluoroethylene membrane filter Millipore (Merck KGaA, Darmstadt, Germany) after complete dissolution. Data are collected and processed using Agilent GPC/SEC MDS software.
3. Results and Discussion
3.1. Radical Polymerization
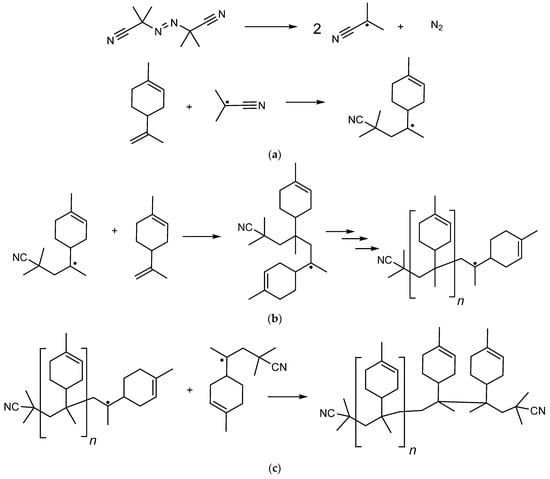
The free-radical polymerization of limonene with azobisisobutyronitrile (AIBN) as an initiator was carried out. The process of the radical polymerization of limonene involves the following steps:
No polymer precipitation was observed when the crude reaction mixture was precipitated with cooled methanol. Figure 2 shows the 1H NMR spectrum of the resulting product; there are no characteristic peaks of the polylimonene, and there is no decrease in the peaks of the inner double bond a (5.4 ppm) and the outer double bond b (4.7 ppm); i.e., the spectrum is identical to that of the initial monomer. The peak around 1.4–1.7 ppm corresponds to six protons in the limonene methyl groups (C–H).
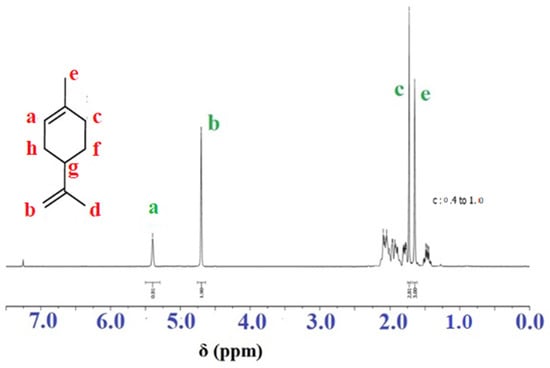
Figure 2.
1H-NMR spectrum of the free radical polymerization reaction mixture of limonene with AIBN as initiator.
The homopolymerization of monoterpenes by the free-radical mechanism is difficult due to the presence of allylic C-H bonds. The reason for this effect is that the chain transfer to the allylic monomer leads not only to the decay of the growing macroradical, but also to a complete interruption of the kinetic chain due to the abstraction of a hydrogen atom in the α-position to the allylic bond with the formation of a resonantly stabilized allylic radical. Limonene has two unconjugated double bonds due to steric effects and different radical stability [26,27]; it is difficult to homopolymerize limonene during free-radical polymerization due to destructive chain transfer caused by more reactive allyl hydrogen [28] (Figure 3).
Figure 3.
Alyl carbons in the structure of limonene.
This so-called degradation chain transfer to monomer or autoinhibition is the main reason for the low molecular weight of polymers and their inhibitory effect in polymerization processes derived from allyl monomer products.
3.2. Cationic Polymerization
The cationic polymerization of limonene using AlCl3 as a catalyst was carried out according to the following scheme. Equal parts by weight of limonene and dried toluene were cooled down to 0 °C or −10 °C in a nitrogen atmosphere in a round bottom flask. A 5% wt. % AlCl3 equivalent to limonene was added to the solution. Then, the temperature was slowly increased from 0 °C to 50 °C in intervals of 20 min. 1H-NMR analysis was used to monitor the reactions.
The AlCl3 catalyst was removed by stirring with 0.1 M HCl until the orange color disappeared and the organic phase was washed four times with 0.1 M NaOH and twice with deionized water. The organic phase was then dried with magnesium sulphate and the toluene was evaporated. The resulting reaction products were dissolved in tetrahydrofuran and precipitated with cold methanol and dried.
Cationic polymerization, as with radical polymerization, is a chain reaction consisting of four stages: preinitiation, initiation, propagation and interruption (Figure 4).
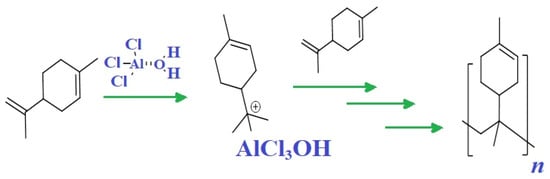
Figure 4.
Cationic polymerization of limonene.
The 1H NMR spectrum of the resulting product is shown in Figure 5. It can be seen that the peak of about 1.4–1.7 ppm corresponds to six protons in the methyl groups of limonene (C–H). It is noted that this peak also appears at about 0.8 m.d. for polylimonene.
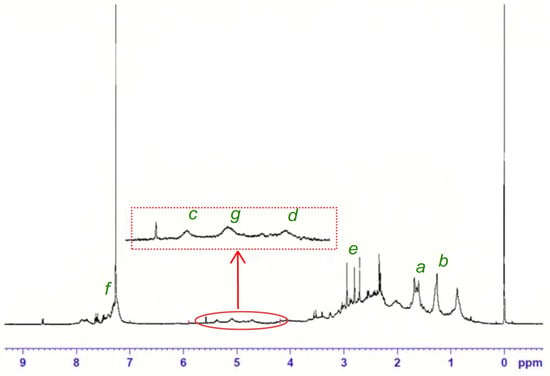
Figure 5.
1H-NMR spectrum of the product of cationic polymerization of limonene with AlCl3 as initiator.
In addition, in the poly (limonene) spectrum, a peak is observed at ~1.2 m.d. corresponding to the protons of the methylene group (-CH2-), which is not observed in the monomer spectrum, which indicates the break of the double bond and is an indication of the polymerization of limonene. It is also noted that the peaks related to the endocyclic and exocyclic double bonds of limonene are located at 5.4 (one proton) and 4.7 ppm (two protons), respectively. The ratio of peak intensities of exocyclic/endocyclic double bonds is 2:1, which is lower, indicating that the exocyclic double bonds of limonene mainly react through an additional radical reaction, confirming the result previously mentioned regarding the polymerization process.
It is expected that only these exocyclic double bonds will participate in polymerization, whereas endocyclic double bonds will remain in the polymer chain. However, a small amount of unreacted exocyclic double bond of limonene was also observed, with a peak of about 4.7 ppm, indicating that in addition to the limonene link included as a result of propagation, a different type of limonene link that contains endocyclic and exocyclic alkene groups is formed in the process. This result indicates that there was a case of destructive chain transfer caused by allyl hydrogen. Limonene contains more than one allyl hydrogen, and all of them can become reactive centers for this parallel reaction. Although the highly substituted tertiary allyl radical usually represents the highest degree of stability, in the case of limonene, the formation of a primary allyl radical is more likely due to less steric difficulties, and it is the dominant compound formed during the chain transfer reaction. Given that these allyl radicals are not prone to propagate, they eventually reproduce with each other or, more likely, with radicals in the propagation chain near the end of the process. Despite this expected behavior during the polymerization of limonene, it is extremely important to make sure that the intensity of these peaks is significantly reduced, which may indicate a decrease in the frequency of these chain transfer reactions. It is necessary to highlight some new peaks that have appeared in the spectrum. The peak is about 3 ppm. It is characteristic of a proton bound to an alcohol bond of the initiator (R-OH), which indicates the presence of part of its structure in the polymer. It is worth noting that the peak at 5.0 ppm refers to the myrcene links included in the polymer. Myrcene is the main impurity found in limonene; due to similar boiling points, limonene and myrcene are not easily separated.
Based on this analysis, it was possible to estimate the conversion of the monomer at about 10%.
The FTIR spectra of limonene (Figure 6) show an absorption band of valence vibrations at 1640 cm−1 corresponding to the exocyclic double bond, which disappears during the polymerization process.
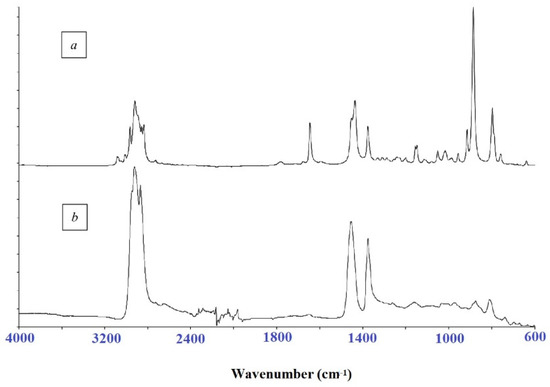
Figure 6.
The FTIR spectrum of limonene (a) and cationic homopolymerization product (b).
3.3. Thiol-Ene Polymerization
The copolymerization of limonene with mercaptoethanol, 2-mercaptoethyl ether without an initiator and with an AIBN initiator was studied and it was also shown that the polymerization could proceed spontaneously.
The reactivity of the endocyclic and exocyclic double bond in the limonene structure is different, resulting in different monomers depending on the reaction time (Figure 7). The reaction of 2-mercaptoethanol and limonene, obtained at room temperature after 24 h, gives the addition products at the exocyclic bond. After 48 h, both the double bonds of limonene react with thiol to form a difunctional monomer with end hydroxyl groups, most suitable for polycondensation. Increasing the reaction temperature to 60 °C results in a polymer.
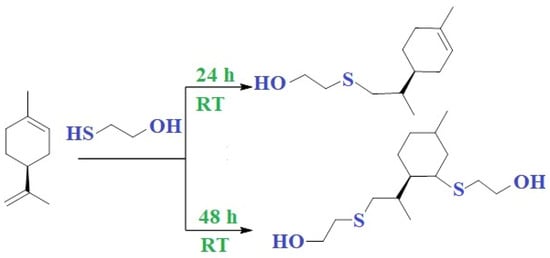
Figure 7.
Reaction of 2-mercaptoethanol and limonene for 24 and 48 h at room temperature.
A similar situation was observed with 2-mercaptoethyl ether; polymerization could not be achieved (Figure 8).
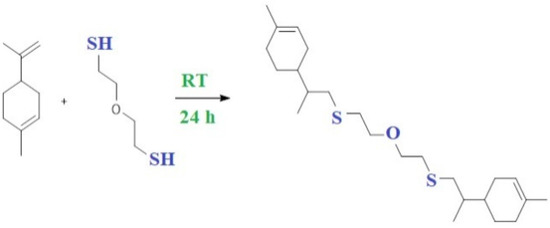
Figure 8.
Scheme of addition of mercaptoethyl ether to limonene at room temperature.
The copolymerization of limonene with 2-mercaptoethyl ether using AIBN as an initiator was carried out to obtain high molecular weight products. These reactions follow a free-radical chain mechanism and generally lead to anti-Markovnik products. The initially formed thiol radical attacks limonene, forming a carbon radical. The carbon radical then reacts with the thiol molecule to form the final product and a new thiol radical, thereby lengthening the radical chain (Figure 9). Since this involves the cleavage of the S-H bond, the overall rate of reaction will strongly depend on the thiol structure as well as the lifetime of the thiol. The unpaired electron on the sulphur atom is in the highest binding orbital. This determines their electrophilic properties, and the electron affinity of thiol radicals is higher than that of hydroxyl HO-. As a result, thiol radicals recombine with each other more easily than hydroxyl radicals.
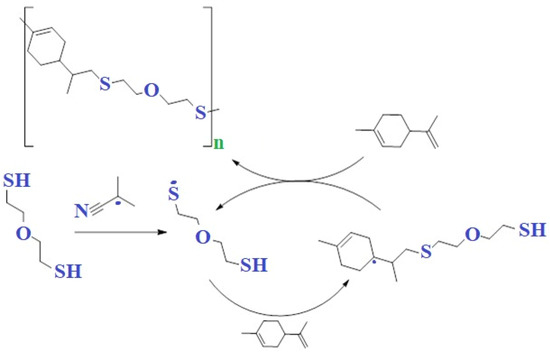
Figure 9.
Scheme of the reaction of copolymerization of limonene with 2-mercaptoethyl ether using AIBN as an initiator.
Limonene and 2-mercaptoethyl ether were mixed and stirred in a nitrogen atmosphere for 15 min. After 15 min, AIBN, 1 wt. % was added. (per monomer). The mixture was lowered into a preheated oil bath and left to polymerize for three hours. 1H-NMR analysis was carried out after polymerization. The mixture was then dissolved in tetrahydrofuran and added drop by drop to the cold methanol. The polymer was collected by decanting the solvent.
The 1H-NMR spectra of the sample after precipitation are shown in Figure 10. It can be seen that the exocyclic double bond signals have disappeared.
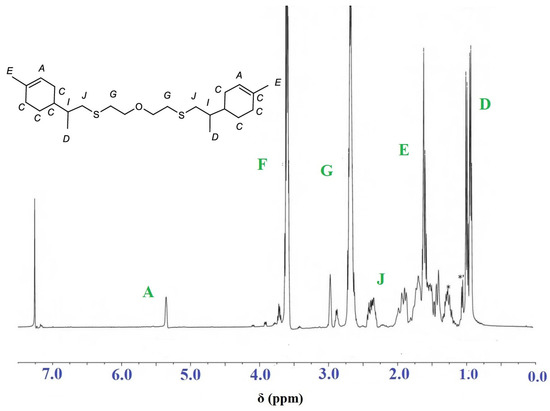
Figure 10.
1H-NMR spectrum of the polymerization product of limonene and 2-mercaptoethyl ether with AIBN as initiator.
The number-average molecular weight (Mn) of the synthesized samples was determined using an Agilent 1260 Infinity II Multi-Detector GPC/SEC System chromatograph. The data obtained are presented in Table 1.

Table 1.
The value of the molecular-weight characteristics of the samples depending on the synthesis.
Two main regions can be distinguished on the curves of the molecular mass distribution of the synthesized samples (Figure 11): a range of about 1000 g/mol, in which a mixture of monomeric and primary dimeric condensation products is identified, and with an increase in the duration of the process, the intensity of this region decreases significantly; a high-molecular region of 10,000–10,000,000 g/mol corresponding to oligomeric compounds. The coefficients α (molecular shape) in the Mark–Kuhn–Houwink equation are determined according to the established values of the characteristic viscosity. According to the values obtained, the AC molecules in solution have parameters α 0.14 to 0.26, which correspond to a good solvent and the molecular shape-dense coil.
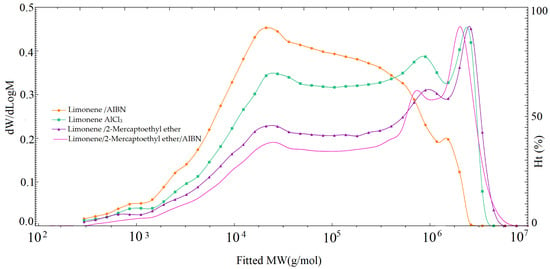
Figure 11.
Molecular mass distribution of the synthesized samples.
4. Conclusions
The samples obtained by thiol-ene polymerization have the highest molecular weight; homopolymerization in the presence of aluminum chloride gives a product with a low molecular weight. The free radical polymerization of limonene with AIBN does not give the possibility to produce polylimonene; however, as a general conclusion it is shown that limonene can polymerize and thus has the properties necessary for the subsequent creation of materials based on it.
Further research will be aimed at increasing the yield of the limonene polymerization reaction and effective synthesis methods, as well as the search for practically important copolymers of limonene.
Author Contributions
Conceptualization, R.A.L. and N.I.C.; methodology, validation, D.V.P. and D.S.M.; formal analysis, N.I.C.; investigation, A.S.S. and Y.S.R.; writing—original draft preparation, R.A.L.; writing—review and editing, R.A.L. and Y.S.R.; supervision, N.I.C.; funding acquisition, N.I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was realized using the equipment of the High Technology Center at BSTU named after V.G. Shukhov, the framework of the State Assignment of the Ministry of Education and Science of the Russian Federation, project No. FZWN-2021-0015.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bakshi, P.R.; Londhe, V.Y. Widespread applications of host-guest interactive cyclodextrin functionalized polymer nanocomposites: Its meta-analysis and review. Carbohydr. Polym. 2020, 242, 116430. [Google Scholar] [CrossRef] [PubMed]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Ishmuratov, G.Y.; Legostaeva, Y.V.; Botsman, L.P.; Nasibullina, G.V.; Muslukhov, R.R.; Kazakov, D.V.; Tolstikov, G.A. Ozonolytic transformations of (S)-(−)-limonene. Russ. J. Org. Chem. 2012, 48, 18–24. [Google Scholar] [CrossRef]
- Martínez, Q.H.; Paez-Mozo, E.A.; Martínez, O.F. Selective Photo-epoxidation of (R)-(+)- and (S)-(−)-Limonene by Chiral and Non-Chiral Dioxo-Mo(VI) Complexes Anchored on TiO2-Nanotubes. Top Catal. 2021, 64, 36–50. [Google Scholar] [CrossRef]
- Alexandrino, T.D.; Madureira de Medeiros, T.D.; Ruiz, A.L.T.G.; Favaro, D.C.; Pastore, G.M.; Bicas, J.L. Structural properties and evaluation of the antiproliferative activity of limonene-1,2-diol obtained by the fungal biotransformation of R-(+)- and S-(−)-limonene. Chirality 2022, 34, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavour Ingredients, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Oliveira, R.M.A.; Henriques, J.D.O.; Sartoratto, A.; Maciel, M.R.W.; Martinez, P.F.M. Evaluation of Limonene in sugarcane wax extraction. Sustain. Chem. Pharm. 2022, 27, 100657. [Google Scholar] [CrossRef]
- Santiago, B.; Moreira, M.T.; Feijoo, G.; González-García, S. Identification of environmental aspects of citrus waste valorization into D-limonene from a biorefinery approach. Biomass Bioenergy 2020, 143, 105844. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Braddock, R.J. Handbook of Citrus By-Products and Processing Technology; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Wulf, G.; Mayer, B.; Lommatzsch, U. Plasma Co-Polymerization of HMDSO and Limonene with an Atmospheric Pressure Plasma Jet. Plasma 2022, 5, 44–59. [Google Scholar] [CrossRef]
- Ren, S.; Trevino, E.; Dubé, M.A. Copolymerization of Limonene with n-Butyl Acrylate. Macromol. React. Eng. 2015, 9, 339–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A. Copolymerization of 2-Ethylhexyl Acrylate and D-Limonene. Polym. Technol. Eng. 2015, 54, 499–505. [Google Scholar] [CrossRef]
- Singh, A.; Kamal, M. Synthesis and characterization of polylimonene: Polymer of an optically active terpene. J. Appl. Polym. Sci. 2012, 125, 1456–1469. [Google Scholar] [CrossRef]
- Mija, A.; Louisy, E.; Lachegur, S.; Khodyrieva, V.; Martinaux, P.; Olivero, S.; Michelet, V. Limonene dioxide as a building block for 100% bio-based thermosets. Green. Chem. 2021, 23, 9855–9859. [Google Scholar] [CrossRef]
- Louisy, E.; Khodyrieva, V.; Olivero, S.; Michelet, V.; Mija, A. Use of Limonene Epoxides and Derivatives as Promising Monomers for Biobased Polymers. ChemPlusChem 2022, 87, e202200190. [Google Scholar] [CrossRef]
- Coelho, F.M.; Vieira, R.P. Synthesis of Renewable Poly(limonene): A Kinetic Modeling Study to Improve the Polymerization. Braz. Arch. Biol. Technol. 2020, 63, e20200022. [Google Scholar] [CrossRef]
- de Oliveira, E.R.M.; Vieira, R.P. Synthesis and Characterization of Poly(limonene) by Photoinduced Controlled Radical Polymerization. J. Polym. Environ. 2020, 28, 2931–2938. [Google Scholar] [CrossRef]
- Moad, G.; Chiefari, J.; Chong, Y.K.; Krstina, J.; Mayadunne, R.T.A.; Postma, A.; Rizzardo, E.; Thang, S.H. Living free radical polymerization with reversible addition—Fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49, 993–1001. [Google Scholar] [CrossRef]
- Moad, C.L.; Moad, G. Fundamentals of reversible addition–fragmentation chain transfer (RAFT). Chem. Teach. Int. 2021, 3, 3–17. [Google Scholar] [CrossRef]
- Destarac, M. On the Critical Role of RAFT Agent Design in Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization. Polym. Rev. 2011, 51, 163–187. [Google Scholar] [CrossRef]
- Carrodeguas, L.P.; Chen, T.T.D.; Gregory, G.L.; Sulley, G.S.; Williams, C.K. High elasticity, chemically recyclable, thermoplastics from bio-based monomers: Carbon dioxide, limonene oxide and ε-decalactone. Green Chem. 2020, 22, 8298–8307. [Google Scholar] [CrossRef]
- Killops, K.L.; Campos, L.M.; Hawker, C.J. Robust, Efficient, and Orthogonal Synthesis of Dendrimers via Thiol-ene “Click” Chemistry. J. Am. Chem. Soc. 2008, 130, 5062–5064. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide) bioplastics by ring-opening polymerization in the presence of chain extende. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).