Abstract
The first reported infections from COVID-19 were in 2019 and, since then, an outbreak has spread rapidly to other parts of the world, resulting in many deaths. As a result, governments began to implement border restrictions and quarantine measures, bringing the travel industry to a halt and plunging the global economy into a severe contraction. Many regions chose to coexist with COVID-19 and gradually eased their border restrictions with certain conditions, such as using personal health status certificates, vaccination certificates, etc. Digital certificates are becoming a global trend, and Taiwan has invested in developing related tools. This paper presents a technical evaluation from the government’s point of view. Taiwan uses the European Union (EU) Digital COVID Certificate as a basis to build a digital certificate that can fully meet the residents’ current international business and tourism needs. The government hopes that this digital proof will promote the public’s return to normal life and overcome the inconveniences brought about by the COVID-19 pandemic. In the post-pandemic era, finding a way to coexist with the virus while gradually relaxing border and community epidemic-prevention policies without impacting our Taiwan’s medical capacity is a significant challenge. Providing key technological solutions to assist in risk stratification is essential in addressing this issue.
1. Introduction
In 2019, COVID-19 posed a significant threat to public health. It then spread rapidly to many countries in early 2020, becoming a pandemic. Urgent research from the academic and medical communities regarding the clinical features used to identify and treat severe and non-severe COVID-19 patients has been ongoing. These studies have been successively published in academic journals [1,2,3], providing global references. As of 31 July 2022, there have been more than 577 million confirmed cases of COVID-19 worldwide, including more than 6.4 million deaths, with a mortality rate of about 1.1%, making it one of the worst pandemics in human history [4].
To develop specific measures and facilitate rapid mobilization against the pandemic, the “Central Epidemic Command Center (CECC)” was established on 20 January 2020. After CECC announced the first confirmed case of COVID-19 in Taiwan on 21 January 2020, Taiwan reported the case to World Health Organization (WHO) through the International Health Regulations (IHR). In response to the continued severity of COVID-19 and increasing level of community transmission, CECC raised a national epidemic alert level 3 on 19 May 2021. Furthermore, all regions of Taiwan concurrently intensified their epidemic prevention measures to prevent a continuous outbreak of the virus. Through the efforts of CECC and the Taiwanese government, using the Entry Quarantine System and Home Quarantine Tracking System developed by the Ministry of Health and Welfare (MOHW), CECC announced that they were lifting the Level 3 Alert on 28 February 2022 [5]. As the pandemic has slowed and the symptoms of COVID-19 patients have become less severe, many regions have chosen to coexist with the pandemic and gradually ease their border restrictions with conditions, such as requirements for COVID-related certificates, etc [6,7].
In the 1600s, various Italian states issued an Italian health pass [8] to exempt their bearers from quarantine. In 1959, the WHO created an International Certification of Vaccination, the Carte Jaune, an official record that some regions require for entrance. This can document vaccination against diseases ranging from cholera and yellow fever to childhood illnesses such as rubella. During the pre-pandemic period, many governments began to implement border restrictions and quarantine measures, bringing international travel to a standstill with a massive impact on the global economy. To restore international travel and the international economy as quickly as possible, some governments came up with the idea of “Immunity Passports” [9,10,11]. At present, many regions, regional alliances, and global organizations are planning for paper and digital certificates on COVID-19 health information. Paper certificates are usually the first version to be used. Still, their vulnerability to counterfeiting, tampering, and loss means the trend of using digital certificates is now growing internationally [12,13].
Since March 2021, MOHW has been studying various issuance standards, trust mechanisms, and information technologies for digital certificates, comparing both their advantages and disadvantages, and choosing the most suitable solution for Taiwan. This paper presents a technical evaluation from the government’s perspective, aiming to build a digital certification system that follows international standards within this time frame. Our government expects that this digital certificate could help people resume their lives, overcome the inconvenience caused by the pandemic, and support global economic recovery.
2. Digital Certificate Framework and Technology
2.1. FHIR
Fast Healthcare Interoperability Resources (FHIR) [14,15] represents the most recent medical information exchange standard released by HL7® International. It is primarily designed to focus on data interchanges within the medical information domain. One of its salient features is its ability to access various FHIR resources via RESTful API [16]. This standard has already found extensive application in several European and American nations.
FHIR integrates the advantages of its predecessors in international medical data exchange standards. It is versatile enough to support a range of formats, including XML, JSON, and Turtle. Simultaneously, it facilitates the storage of both clinical and non-clinical data while ensuring data interoperability across platforms and devices. This means that mobile phones, tablets, computers, medical instruments, and Internet of Medical Things (IoMT) devices can all exchange data utilizing the FHIR standard. This rectifies the previously encountered issues of data interchange uniformity among medical units.
2.2. Digital Certificate Framework
There are two main usage scenarios for digital proofs in international planning. The first is for the public to hold a certificate that must be checked and confirmed by the agency. Three actors will be involved in the use of these certificates: the certificate issuer, the certificate holder, and the certificate verifier, as shown in Figure 1. The second is that the public can present this certificate (including vaccination, test results, and confirmed recovery status) to medical institutions. Medical personnel can provide better medical decisions and services through this certification to provide continuity of care. This constitutes a part of their Personal Health Records (PHR).
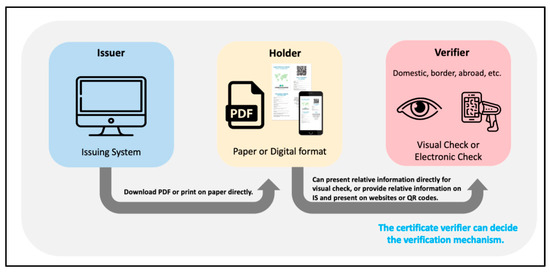
Figure 1.
Framework diagram of digital certificate.
2.3. Major International Digital COVID-19 Certificate
In the following, the authors will introduce several international digital certificates that are currently in use, and select the most suitable digital certificate development standard and format for Taiwan by detailing the advantages and disadvantages of each digital certificate.
2.3.1. Digital Documentation of COVID-19 Certificates
On 19 March 2021, the World Health Organization (WHO) released the “Interim guidance for developing a Smart Vaccination Certificate (SVC)” [17], which contains the following three points:
- The certificate needs to ensure continuity of care and proof of vaccination.
- A Global Health Trust Framework should be proposed.
- The core data set of SVC adopted Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR).
On 4 June 2021, the WHO released the “Revised scope and direction for the Smart Vaccination Certificate and WHO’s role in the Global Health Trust Framework”. In addition, the Digital Documentation of COVID-19 Certificates (DDCC) specification was renamed SVC [18]. This specification does not include a section on the Global Health Trust Framework; members and regional networks can build trust through bilateral or multilateral agreements with other members.
2.3.2. EU Digital COVID Certificates
On 17 March 2021, the EU Executive Committee proposed the Digital Green Certificate (DGC), later renamed the Digital COVID Certificate (EU-DCC) [19], which applies to all EU members and is open to applications from other regions (as of 31 July 2022, 27 EU members and 48 non-EU members have joined).
The EU-DCC complies with the European Union’s General Data Protection Regulation (GDPR): it uses a JSON minimum data set and contains only limited personal data, such as name, date of birth, date of issue, vaccine, test, rehabilitation-related data, and a single identifier. These data are only used to verify and certify the authenticity and suitability of this certificate. The following criteria are presented:
- The data format is consistent and provides a digital signature.
- QR code displays the information and digital signature.
- It can be verified offline.
2.3.3. Smart Health Cards
The Vaccination Credential Initiative (VCI) [20] was announced on 14 January 2021, by 12 health care and technology organizations to enable people to obtain encrypted digital certificates for COVID-19 vaccinations and store them in an electronic wallet. VCI is guided by a commitment to the following core design principles:
- Openness and interoperability: based on international standards and open technologies and interoperable across regions.
- Possibility of offline verification and transparency: operated in an open and transparent manner.
- Privacy by design: upholds and protects the privacy of individual health data and is designed to comply with applicable data privacy regulations.
- Flexible and equitable: designed to adapt over time as the pandemic and science evolve. Accessible and usable by anyone worldwide, in any language, regardless of level of wealth and economic development, including availability in digital and paper (QR code) forms.
VCI uses Smart Health Cards (SHC) [21] to provide vaccination records that conform to FHIR standards. SHC provides a platform to store healthcare information and other vital medical data, which achieves the following three points:
- Improves the privacy and security of patient information.
- Makes medical records portable.
- Reduces healthcare fraud.
SHC takes the user’s privacy into account. It does not use a central database, private information is stored and encrypted on the user’s device, and only essential information is displayed.
2.3.4. ICAO Visible Digital Seal
In 1944, 52 regions signed the Convention on International Civil Aviation in Chicago and established the International Civil Aviation Organization (ICAO) [22] to develop the principles and techniques of international aviation and promote international air transport planning and development. To achieve consistent acceptance and convenience for customs’ clearance inspection operations worldwide, the ICAO recommends that regions develop a new generation of IC chip passports that comply with the organization’s standards for the non-contact reading of built-in biometric features. This passport is also known as an Electronic Passport. The authenticator will use a public key infrastructure (PKI) to check the digital signature. To pass authentication, ICAO members have agreed to establish a central repository for the information needed to authenticate an electronic passport, called a Public Key Directory (PKD).
In 2018, ICAO members approved a technology that makes it easier to build the same trust or authentication model for electronic passports, called a Visible Digital Seal (VDS). The intended use of VDS is to add a similar level of digital security to non-chip documents [23]. In addition, on 9 June 2021, ICAO stored vaccination and test result data in the VDS as a digital certificate.
2.3.5. Selection Result
In this paper, the authors evaluated various digital certificates and organized them in Table 1. Based on the experience of other regions, the MOHW adopted the international standard instead of creating their own standard. Although systems that adopt national standards can quickly achieve application purposes, they cannot be connected to other systems or have more difficulties in these connections. Initially, the MOHW prioritized the WHO format as a reference, but in June 2021, WHO announced the suspension of the Global Health Trust Framework, which stores the digital public keys of member regions, and instead encouraged member regions and regional networks to establish mutual trust through bilateral or multilateral agreements.

Table 1.
Comparison table of digital certificates.
Therefore, MOHW refers to other larger-scale digital certificate issuing organizations with a broader scope of application, such as the EU, VCI, and ICAO. The EU is one of the world’s most influential organizations dedicated to standards, cooperation, and regional development with members. The EU launched the EU Digital COVID Certificate on 1 July 2021, and opened it to non-EU regions, which means that certificates issued by these regions may apply to the European Commission for an “equivalence decision” and would be directly acceptable under the same conditions as the EU-DCC.
Through several discussions and a confirmation of each specification, the MOHW adopted the EU-DCC format as a basis to establish its digital certificate system: “Digital COVID-19 Certificate.” In addition, since many regions (the United States, Canada, Japan, etc.) that have frequent contact with Taiwan have adopted the SHC format, MOHW added the SHC format to the “Digital COVID-19 Certificate” on 14 July 2022.
3. System Design and Implementation
3.1. System Framework
3.1.1. Data Flow
The data flow for digital certification can be divided into two parts: vaccination records and test results. Within 24 h of vaccination, medical facilities must upload primary and vaccination data, vaccination time, medical facility, and physician information to the CDC through the National Immunization Information System (NIIS). Finally, the CDC stores the data in the Digital Evidence Data Center, as shown in the top half of Figure 2. In addition, when a person undergoes a PCR test at a medical facility, the facility uploads the test results to the Laboratory Information Management System (LIMS) and the Health Insurance Agency and then stores them in the Data Warehouse System (DW). Finally, the CDC also stores the data in the Digital Certification Data Center, as shown in the bottom half of Figure 2.
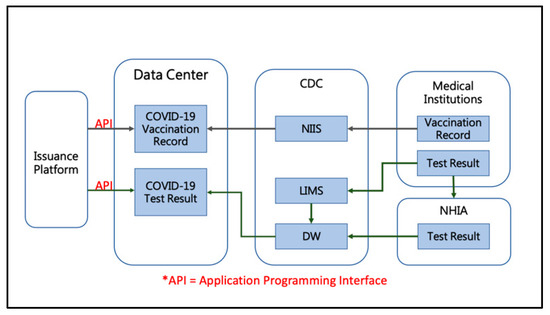
Figure 2.
Data flow diagram of “Digital COVID-19 Certificate”.
3.1.2. System Design Framework
MOHW put the system in the cloud instead of a local server because the cloud has a higher performance and can avoid system crashes due to too many users being online at the same time. In addition, the system is developed by a web app instead of a mobile app because mobile apps need to create multiple apps for different platforms (Android, iOS), while web apps can be used through browsers. Citizens and residents can apply for their digital certificate with a digital signature through the issuance platform, and Taiwan produces the following certificates, following EU regulations:
- Country Signing Certificate Authority (CSCA): The certificate issued by the National Certificate Center.
- Document Signer Certificate (DSC): The DSC is a certificate that must be signed with the private key corresponding to a CSCA certificate used by the Member State and will be added to the QR code.
- Upload Certificate: The required certificate when the system back-end platform upload data (CSCA, DSC) to the DCC gateway.
- Transport Layer Security (TLS) Certificate: Certificate for using the network channel between the system back-end platform and DCC Gateway.
Among them, the CSCA, DSC, and Upload Certificates are issued by Healthcare Certification Authority (HCA), and the TLS Certificate is issued by the Government Certificate Authority (GCA), as shown in Table 2. Since the EU-DCC standard signature certificate also meets the requirements of the SHC standard, our signature certificate for uploading to SHC Server and issuing digital certificate adopts the same format as EU-DCC.

Table 2.
Certificate Specifications.
When the system creates digital certificates, the information and the signature with the private key are issued in JSON format as QR codes. EU-DCC and SHC formats have different verification procedures according to the regulations.
- EU-DCC format: After each region uploads a signature certificate to DCC Gateway, the verification application will download them to the verification device for offline use.
- SHC format: The verification application needs to connect to SHC Server to read the certificate in each digital certificate verification.
When the system verifies the digital certificate, the signature with the private key in the QR code will be decrypted by matching the public key of the inspection program to identify its authenticity, as shown in Figure 3.
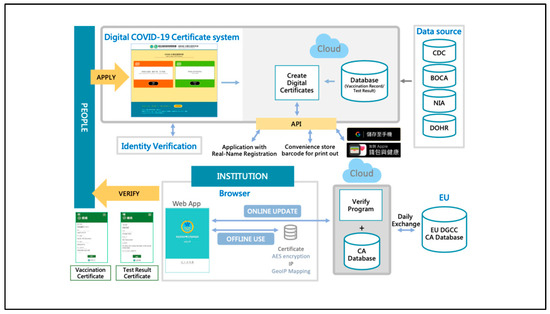
Figure 3.
Frame diagram of “Digital COVID-19 Certificate”.
3.2. System Function
The “Digital COVID-19 Certificate” was available on 28 December 2021, and the Web version verification application was available on 21 January 2022. This system has the following features:
- The system can produce digital certificates in EU-DCC and SHC formats.
- The verification application can verify digital certificates in the EU-DCC and SHC format without switching.
- The public can download or print online or use the cloud printing code function of the convenience store.
- A cross-carrier web technology is adopted to develop digital certificates rather than a more prolonged, costly, and difficult-to-maintain mobile App.
- Flexible cloud resources are used to ensure service is not interrupted.
- The digital certificate is available for international demand and uses the same version in all regions.
- The public can add digital certificates to their mobile devices with one click to quickly obtain and present the digital certificates.
The national digital certificate is centrally issued by the CECC and is available online. The public can apply online for a digital certificate with certificate signature, as shown in Figure 4, at least two days after receiving the vaccine or the next day after obtaining the paper report of the nucleic acid test.

Figure 4.
Template of “Digital COVID-19 Certificate”.
Applicants can download the digital certificate PDF file from the platform and obtain a cloud printing code to print at the convenience store, print out a paper copy by themselves, or present the certificate through relevant carriers according to their personal needs.
According to the information and networking capabilities of the verifier, digital certificates can be verified through visual or QR codes. The verifier is required to satisfy the current EU and VCI standards issued by the International Trust Mechanism Agreement to check the certificates issued by all participating regions of the EU-DCC and SHC. The verification results are shown in Figure 5.
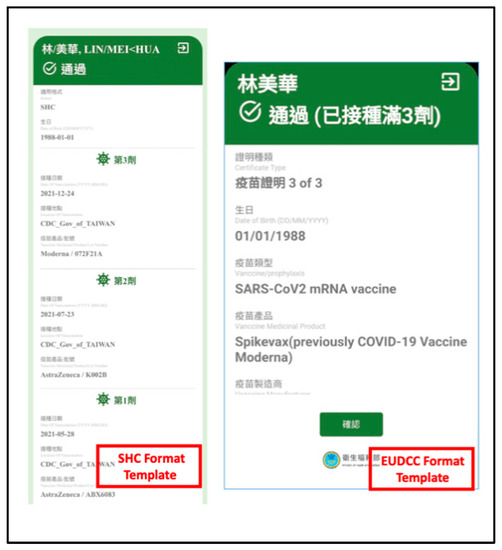
Figure 5.
Verification results.
4. Results
4.1. Equivalent to EU-DCC
To apply for EU-DCC, the MOHW went through a kick-off meeting, written and account applications, and three stages of technical tests in different EU environments, was mutually verified by more than 50 members, and passed the technical stage test on 9 December 2021, taking only 10 days to meet the EU technical team requirements successfully. Finally, on 21 December 2021, the Executive Committee announced Taiwan’s accession to the European Union system, becoming the third Asian region to join the system after Singapore and Israel and to be recognized on a reciprocal basis with other members in the system. This will help our reidents to travel smoothly around the world with our digital certificates and to obtain international recognition for our epidemic prevention work.
The verification mechanism of the EU-DCC typically employs encryption technologies and a decentralized architecture to ensure data security among regions. Here are some key security measures:
- Encryption technologies: EU-DCC uses encryption technologies to ensure the security of data. This includes using public and private keys to encrypt and decrypt data, and only the recipient who holds the corresponding private key can decrypt the data. This helps protect the data from unauthorized access or tampering during transmission.
- Decentralized architecture: EU-DCC adopts a decentralized architecture, with each member generating and storing its certificate public key. This helps reduce the risk of a single data center becoming a target for attacks.
- Identity authentication: The EU-DCC gateway needs to authenticate the identity of the entities communicating with it to ensure that only legitimate entities can access and manage the certificate public key.
- Compliance with GDPR: The decentralized data environment of the EU-DCC also complies with the General Data Protection Regulation (GDPR) of the European Union, requiring adherence to strict data protection and privacy standards.
Through these measures, the EU-DCC can ensure the security of data among different regions.
4.2. “Digital COVID-19 Certificate” Launch
The “Digital COVID-19 Certificate” was made available on 28 December 2021. The application covers vaccination records and test results and was initially available only for residents traveling across borders. On 21 January 2022, the system was fully open for inspection in all establishments of Taiwan and is expected to distinguish the risk of infecting people and effectively prevent community clusters of infections. As of 31 July 2022, there are more than 9,256,000 requests on the issuance platform, including more than 7,022,000 vaccination certificates and more than 2,234,000 PCR certificates. Figure 6 shows the number of requests for “Digital COVID-19 Certificate” for each month.
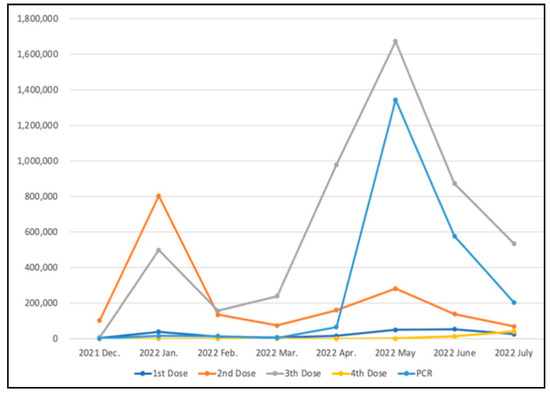
Figure 6.
Number of requests for “Digital COVID-19 Certificate”.
4.3. Improve Portable Function with One Click
Google also provides the “Save to Mobile” button in traditional Chinese, which is currently only available in the United States, the United Kingdom, Canada, Australia, New Zealand, Spain, and Singapore. From 24 March 2022 to 31 July 2022, there were 9.72 million users in iOS and 3.48 million in Android, totaling 13.2 million. Table 3 shows the difference between the various operating systems that add the digital certificate to the wallet with one click.

Table 3.
One-click wallet addition for all operating systems.
4.4. Value-Added Application for COVID-19 Prevention
Since May 2022, the number of confirmed cases has been increasing daily due to the increasing severity of the new crown epidemic in Taiwan and the influx of foreigners. Therefore, the “Digital COVID-19 Certificate” adds positive rapid test results. In addition, to reduce the administrative burden of epidemic investigation on local health officials, the “Isolation Certificate” and “Reissuing Designated Residence Isolation Notices” have been added to the digital certificate application.
4.5. Adding Smart Health Cards Standard
Considering that regions like the United States, Canada, and Japan, which frequently interact with Taiwan, all use the Smart Health Card, and there is a practical need for regions to inspect information on each dose of vaccine, the Ministry of Health and Welfare officially joined the VCI on 20 May 2022, thereby gaining the ability to issue digital proofs in SHC format and obtaining certification. Subsequently, it made this available for the public to download on 14 July 2022.
4.6. Benefits
In the past, it was necessary to apply for an International Certificate of Vaccination or Prophylaxis (commonly known as a yellow card) at a designated medical institution, which was checked manually at the time of entry. At present, it is possible to enter many regions quickly with a digital certificate downloaded from a mobile device, which shortens the time for entry inspection and solves the shortcomings of paper documents, which are not easy to keep and can make the manual review process more time-consuming. In addition, as regions adopt conditional border-opening policies, those who meet the requirements can present digital certificates to reduce the number of days of quarantine at home or be exempted from quarantine.
The “Digital COVID-19 Certificate” is installed in the cloud, considering the advantages of the cloud, such as stability, personal data protection, fast access, and the ability to handle many users. Since the system’s opening, there have been no traffic jams or crashes. Figure 7 and Figure 8 show the number of regions using the issuing platform and the verification application, respectively.
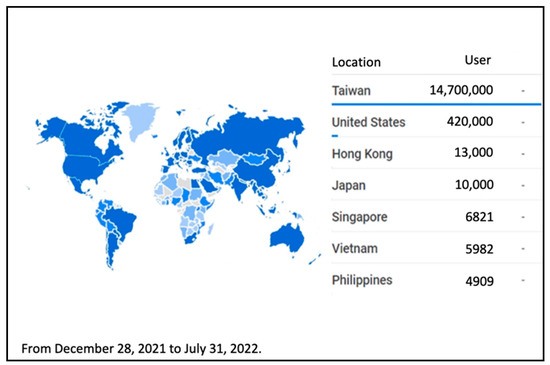
Figure 7.
“Digital COVID-19 Certificate” Issuance Platform Usage by Region and Quantity.
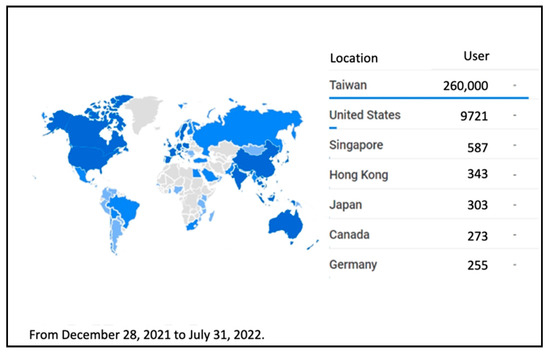
Figure 8.
“Digital COVID-19 Certificate” Verification Program Usage by Region and Quantity.
5. Discussion and Conclusions
The impact of the COVID-19 pandemic and the changes to our lives brought about by the new epidemic prevention measures mean that various epidemic prevention policies and actions need to be urgently implemented and adjusted in real-time, and can have significant effects. Manual and paper-based processes often fall short and can be inefficient in such urgent situations. However, Taiwan has used information and communications technology well to assist in pandemic prevention. For example, we already have successful cases, such as the Entry Quarantine System, Home Quarantine Tracking System, and Digital Fencing Tracking System [24], of the use of technology during the early stages of pandemic prevention. In addition, through the mature health information infrastructure and data-sharing mechanism, Taiwan successfully created the “Digital COVID-19 Certificate” to meet the international standards in a short period of time.
While developing the digital certificate, this study found that the verification mechanism of EU-DCC manages the national-level certificate public key produced by all members with the EU-DCC Gateway. The verifier could verify data offline and cross platforms. This kind of decentralized data environment built by the EU also complies with GDPR. With the development of technology and the increased awareness of individual privacy, this approach is a future development trend. The “Digital COVID-19 Certificate” is one of the few success stories in Taiwan with a digital signature that can cross platforms. Through the platform, Taiwanese residents can obtain their digital COVID-19 certificate, positive rapid test results, and isolation certificate, which can be used for pandemic prevention and as relevant documents for claiming pandemic insurance. With this successful experience, we believe that the government could take this platform as a reference when issuing other, similar certificates in the future, and move toward a decentralized data environment.
In addition, considering that our residents might need to travel to the United States, Japan, Canada, etc., regions with close ties, the MOHW added the SHC format based on the FHIR standard to the “Digital COVID-19 Certificate”. FHIR is an international standard to enhance the interoperability and collaboration of data in medical informatics, allowing for the exchange of data among medical institutions and cross-system, cross-device, and cross-platform interconnection functions. Through the interoperability of medical records, medical professionals can better understand their patient’s health status and provide continuity of care. In this study, the following aspects should be considered when adopting the standard [25]:
- Data Sharing: The interconnection function of data exchange across systems can use different techniques to plan various services and develop applications based on the acquired data. When systems have to exchange data, system construction costs are bound to increase; another problem is the interconnection cost for diverse development. Through the experience of building the “Digital COVID-19 Certificate”, this study concluded that if the means of data exchange could adopt the same standard, this would ease the problem mentioned above.
- Standard Application: In the past, we usually developed a standard format, without planning the applications, making implementation a challenge. The experience of creating a “Digital COVID-19 Certificate” in SHC format also provides a successful model for adopting FHIR in Taiwan. We hope to implement FHIR in various medical institutions by stacking successful applications such as ventilator settings.
In the post-pandemic era, many regions decided to coexist with the pandemic. With critical technical policies assisting in risk classification and effectively preventing coronavirus from spreading, Taiwan can coexist with the pandemic without impacting medical capacity and help people get back into the swing of things.
Author Contributions
Conceptualization, T.-C.Y. and F.-C.W.; software, G.-W.C., J.-S.Y., F.-C.W. and I.-M.P.; validation, G.-W.C.; formal analysis, T.-C.Y.; resources, G.-W.C. and J.-S.Y.; writing—original draft preparation, T.-C.Y.; writing—review and editing, F.-C.W.; visualization, T.-C.Y. and F.-C.W.; supervision, I.-M.P.; project administration, I.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable. (The contents inside Figure 4, Figure 5, and Table 3 are templates and do not contain any real personal data. Therefore, there will not be any issues related to personal privacy.)
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Habas, K.; Nganwuchu, C.; Shahzad, F.; Gopalan, R.; Haque, M.; Rahman, S.; Majumder, A.A.; Nasim, T. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev. Anti-Infect. Ther. 2020, 18, 1201–1211. [Google Scholar] [CrossRef]
- Gutiérrez, P.; Clarke, S.; Kirk, A. COVID World Map: Which Countries Have the Most Coronavirus Vaccinations, Cases and Deaths? The Guardian. Available online: https://www.theguardian.com/world/2021/dec/01/covid-world-map-which-countries-have-the-most-coronavirus-vaccinations-cases-and-deaths (accessed on 17 June 2022).
- Ministry of Health and Welfare. Timeline of Key Decisions in COVID-19 Prevention and Control. Available online: https://covid19.mohw.gov.tw/ch/sp-timeline0-205.html (accessed on 17 June 2022).
- Kissi, J.; Achampong, E.K.; Mensah, N.K.; Annobil, C.; Lamptey, J.N. Moving towards Digitising COVID-19 Vaccination Certificate: A Systematic Review of Literature. Vaccines 2022, 10, 2040. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, D.; Jocic, L.T. Accelerated Digitalization of the Epidemiological Measures: Overcoming the Technological and Process Complexities of Establishing the EU Digital COVID Certificate in Slovenia. Int. J. Environ. Res. Public Health 2022, 19, 14322. [Google Scholar] [CrossRef]
- Fedi’ di Sanita Archivi. Passaporto-Collezionismo-Scripofilia. Available online: http://www.passaporto-collezionismo-scripofilia.com/category/fedi-di-sanita/ (accessed on 17 June 2022).
- Chotani, R.A.; Ashraf, S.S.; Mize, C.; Clark, T. ‘Immunity Passport’ Key to Containing Spread of Coronavirus. UPI, 30 April 2020. Available online: https://www.upi.com/Top_News/Voices/2020/04/30/Immunity-passport-key-to-containing-spread-of-coronavirus/1961588246960/ (accessed on 2 September 2023).
- Chen, L.H.; Freedman, D.O.; Visser, L.G. COVID-19 Immunity Passport to Ease Travel Restrictions? J. Travel Med. 2020, 27, taaa085. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.H.; Kelly, D.; Wilkinson, D.; Savulescu, J. The scientific and ethical feasibility of immunity passports. Lancet Infect. Dis. 2021, 21, e58–e63. [Google Scholar] [CrossRef] [PubMed]
- Cascini, F.; Causio, F.A.; Failla, G.; Melnyk, A.; Puleo, V.; Regazzi, L.; Ricciardi, W. Emerging Issues from a Global Overview of Digital COVID-19 Certificate Initiatives. Front. Public Health 2021, 9, 744356. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Kaiser, L.; Gillet, P.; Thevoz, P.; Stringhini, S.; Guessous, I. Digital COVID Credentials: An Implementation Process. Front. Digit. Health 2021, 3, 594124. [Google Scholar] [CrossRef] [PubMed]
- HL7 FHIR Release 4.3.0. Available online: https://www.hl7.org/fhir/ (accessed on 17 June 2022).
- Sharma, M.; Aggarwal, H. HL-7 Based Middleware Standard for Healthcare Information System: FHIR. In Proceedings of the 2nd International Conference on Communication, Computing and Networking, Haldia, India, 14–15 March 2019; Springer: Singapore, 2019; pp. 889–899. [Google Scholar]
- Bender, D.; Sartipi, K. HL7 FHIR: An Agile and RESTful Approach to Healthcare Information Exchange. In Proceedings of the 2013 26th International Symposium on Computer-Based Medical Systems, Porto, Portugal, 20–22 June 2013; pp. 326–331. [Google Scholar]
- Interim Guidance for Developing a Smart Vaccination Certificate—Release Candidate 1, 19 March 2021. WHO. Available online: https://cdn.who.int/media/docs/default-source/documents/interim-guidance-svc_20210319_final.pdf (accessed on 17 June 2022).
- Digital Documentation of COVID-19 Certificates: Vaccination Status: Technical Specifications and Implementation Guidance, 27 August 2021. WHO. Available online: https://apps.who.int/iris/rest/bitstreams/1359417/retrieve (accessed on 19 June 2022).
- EU Digital COVID Certificate|European Commission. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans/eu-digital-covid-certificate_en (accessed on 17 June 2022).
- VCI. Available online: https://vci.org/ (accessed on 17 June 2022).
- Smart Health Cards. Available online: https://smarthealth.cards/en/ (accessed on 17 June 2022).
- Mahoro, J.C.G. ICAO’s role in environmental protection and its shortcomings under rapid growth of aviation industry. Diponegoro Law Rev. 2019, 4, 136–151. [Google Scholar] [CrossRef]
- “GUIDELINES—Visible Digital Seals (“VDS-NC”) for Travel-Related Public Health Proofs”, ICAO. Available online: https://www.icao.int/Security/FAL/TRIP/PublishingImages/Pages/Publications/Guidelines%20-%20VDS%20for%20Travel-Related%20Public%20Health%20Proofs.pdf (accessed on 17 June 2022).
- Lo, W.-C.; Wang, F.-C.; Lin, L.-Y.; Jyan, H.-W.; Wu, H.-C.; Huang, Y.-L.; Parng, I.-M.; Chiou, H.-Y. Enhancing Data Linkage to Break the Chain of COVID-19 Spread: The Taiwan Experience. J. Med. Internet Res. 2021, 23, e24294. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.; Cnossen, R.; Schnell, M.; Simons, D. Continua: An Interoperable Personal Healthcare Ecosystem. IEEE Pervasive Comput. 2007, 6, 90–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





