The Effect of the Supplementation of a Diet Low in Calcium and Phosphorus with Either Sheep Milk or Cow Milk on the Physical and Mechanical Characteristics of Bone using A Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets and Procedures
2.3. Diet and Milk Composition
2.4. Rat Femur Dimensions
2.5. µ-CT Analysis of Femora
2.6. Three-Point Bend Testing of Femur Mechanical Properties
2.7. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS) Analysis of Femur Mineral Composition
2.7.1. Femur Preparation
2.7.2. Femur Digestion
2.7.3. Statistical Analyses
3. Results
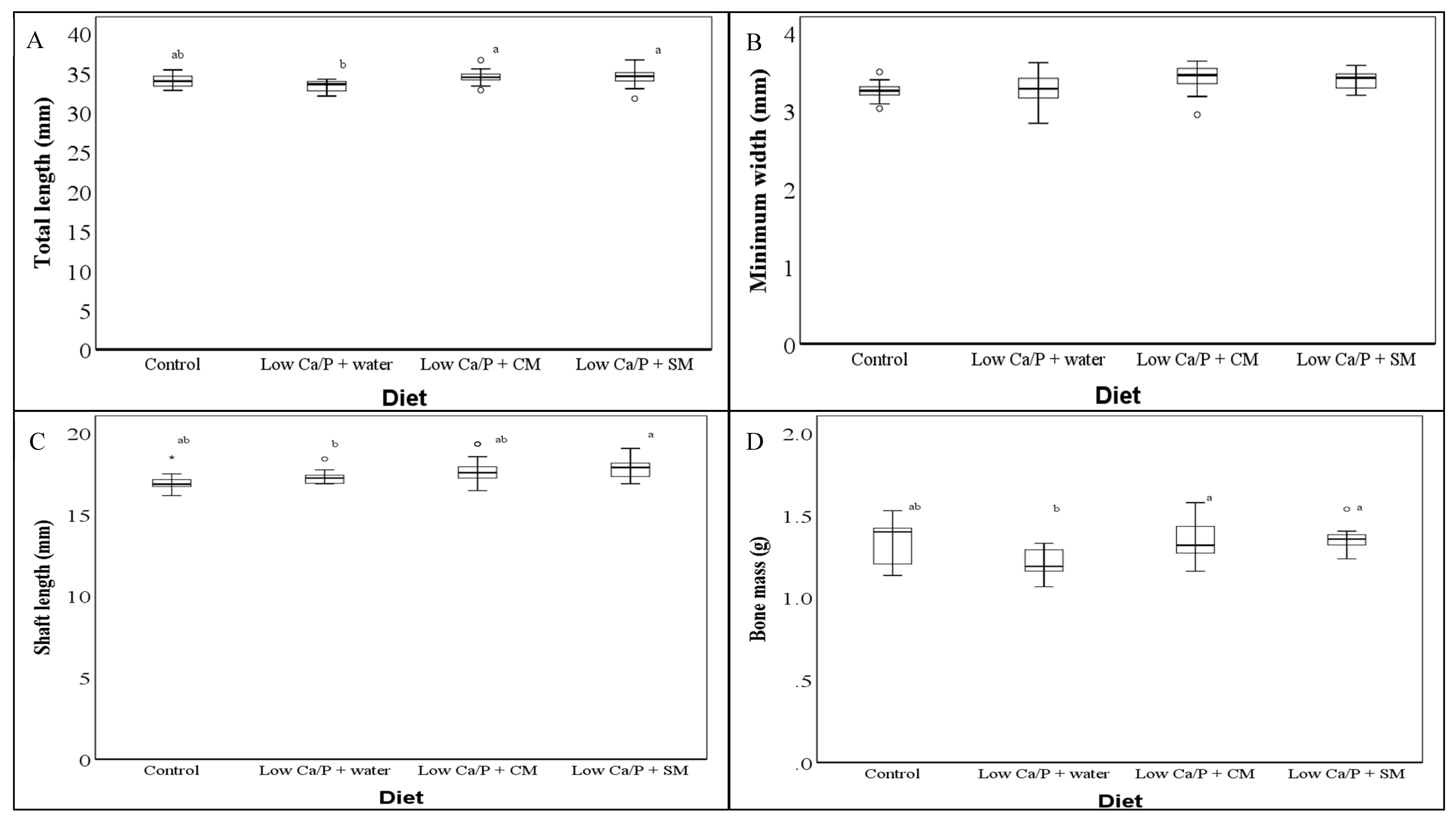
3.1. Rat Femur Dimensions
3.2. µ-CT Analysis of Femur Micro-Structure
3.3. Three-Point Bending Testing of Femur Mechanical Properties
3.4. ICP-MS Analysis of the Femur Mineral Composition
4. Discussion
4.1. Ca/P Deficient Diet Resulted in Significant Reductions in Structural and Mechanical Characteristics
4.2. Impact of Sheep Milk and Cow Milk on Bone Structural and Mechanical Characteristics
4.3. Impact of Diet on Femur Mineral Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobo, A.R.; Cocato, M.L.; Jorgetti, V.; de Sá, L.R.M.; Nakano, E.Y.; Colli, C. Changes in bone mass, biomechanical properties, and microarchitecture of calcium-and iron-deficient rats fed diets supplemented with inulin-type fructans. Nutr. Res. 2009, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; McClung, J.P. Homeostatic regulation of trace mineral transport by ubiquitination of membrane transporters. Nutr. Rev. 2016, 74, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.; Gordon, C.; Janz, K.; Kalkwarf, H.; Lappe, J.; Lewis, R.; O’Karma, M.; Wallace, T.; Zemel, B. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Gaddi, A.; Cicero, A.F.G.; Odo Wani, F.O.; Dormi, A.; Pasquarelli, V.; D’Addato, S. The realization of a project aimed at reducing the plasmatic lipid level in a large Italian population improves the mean calcium daily intake: The Brisighella Study. Eur. J. Clin. Nutr. 2001, 55, 97–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Govindarajan, P.; Khassawna, T.; Kampschulte, M.; Böcker, W.; Huerter, B.; Dürselen, L.; Faulenbach, M.; Heiss, C. Implications of combined ovariectomy and glucocorticoid (dexamethasone) treatment on mineral, microarchitectural, biomechanical and matrix properties of rat bone. Int. J. Exp. Pathol. 2013, 94, 387–398. [Google Scholar] [CrossRef]
- Michaëlsson, K. Calcium supplements do not prevent fractures. BMJ Publ. Group 2015, 351, h4825. [Google Scholar] [CrossRef]
- Kakigi, C.L.M.; Singh, K.; Wang, S.Y.; Enanoria, W.T.; Lin, S.C. Self-reported calcium supplementation and age-related macular degeneration. JAMA Ophthalmol. 2015, 133, 746–754. [Google Scholar] [CrossRef]
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef]
- López, A.I.; Alférez, M.J.M.; Barrionuevo, M.; Lisbona, F.; Campos, M.S. Influence of goat and cow milk on the digestive and metabolic utilization of calcium and iron. J. Physiol. Biochem. 2000, 56, 201–208. [Google Scholar] [CrossRef]
- Burrow, K.; Young, W.; McConnell, M.; Carne, A.; Bekhit, A.E.-D. Do Dairy Minerals Have a Positive Effect on Bone Health? Compr. Rev. Food Sci. Food Saf. 2018, 17, 989–1005. [Google Scholar] [CrossRef]
- Burrow, K.; Young, W.; McConnell, M.; Hammer, N.; Scholze, M.; Carne, A.; Bekhit, A.E.-D. Consumption of sheep milk compared to cow milk can affect trabecular bone ultrastructure in a rat model. Food Funct. 2019, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W.; Williams, L.R.; Melo, D.R.; Lipsztein, J.L. A physiologically based biokinetic model for cesium in the human body. Sci. Total Environ. 2003, 317, 235–255. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Nordberg, M.; Fowler, B.A.; Friberg, L.T. Handbook on the Toxicology of Metals, 3rd ed.; Elsevier: Burlington, ON, Canada, 2007. [Google Scholar]
- Burrow, K.; Young, W.; McConnell, M.; Carne, A.; Barr, D.; Reid, M.; Bekhit, A.E.-D. The distribution of essential, trace, and non-essential minerals in weanling male rats fed sheep or cow milk. Mol. Nutr. Food Res. 2018, 62, 1800482. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Animal Welfare Act 1999; New Zealand Statutes: Wellington, New Zealand, 1999.
- Burrow, K.; Young, W.; McConnell, M.; Carne, A.; Barr, D.; Reid, M.; Bekhit, A.E.-D. The Effect of Sheep and Cow Milk Supplementation of a Low Calcium Diet on the Distribution of Macro and Trace Minerals in the Organs of Weanling Rats. Nutrients 2020, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, H. Effect of Goat Milk on Bone Mass, Morphology and Biomechanics; Massey University: Palmerston North, New Zealand, 2012. [Google Scholar]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Leppänen, O.; Sievänen, H.; Jokihaara, J.; Pajamäki, I.; Järvinen, T.L. Three-point bending of rat femur in the mediolateral direction: Introduction and validation of a novel biomechanical testing protocol. J. Bone Miner. Res. 2006, 21, 1231–1237. [Google Scholar] [CrossRef]
- Raffalt, A.C.; Andersen, J.E.T.; Christgau, S. Application of inductively coupled plasma–mass spectrometry (ICP–MS) and quality assurance to study the incorporation of strontium into bone, bone marrow, and teeth of dogs after one month of treatment with strontium malonate. Anal. Bioanal. Chem. 2008, 391, 2199–2207. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Hoppler, M.; Membrez Scalfo, F.; Vuichoud, J.; Vigo, M.; Offord, E.A.; Ammann, P. Peak bone strength is influenced by calcium intake in growing rats. Bone 2014, 68, 85–91. [Google Scholar] [CrossRef]
- Agata, U.; Park, J.; Hattori, S.; Aikawa, Y.; Kakutani, Y.; Ezawa, I.; Akimoto, T.; Omi, N. The impact of different amounts of calcium intake on bone mass and arterial calcification in ovariectomized rats. J. Nutr. Sci. Vitaminol. 2015, 61, 391–399. [Google Scholar] [CrossRef]
- Aspray, T.J. Chapter 5-Calcium: Basic Nutritional Aspects. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 45–57. [Google Scholar] [CrossRef]
- Bonjour, J.-P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef]
- Masuyama, R.; Nakaya, Y.; Katsumata, S.; Kajita, Y.; Uehara, M.; Tanaka, S.; Sakai, A.; Kato, S.; Nakamura, T.; Suzuki, K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J. Bone Miner. Res. 2003, 18, 1217–1226. [Google Scholar] [CrossRef]
- Johnston, C.C.; Slemenda, C.W. Determinants of peak bone mass. Osteoporos. Int. 1993, 3, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.L.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The Determinants of Peak Bone Mass. J. Pediatr. 2017, 180, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Fried, A.; Manske, S.L.; Eller, L.K.; Lorincz, C.; Reimer, R.A.; Zernicke, R.F. Skim milk powder enhances trabecular bone architecture compared with casein or whey in diet-induced obese rats. Nutrition 2012, 28, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.-I.; Tsuboi, R.; Uehara, M.; Suzuki, K. Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci. Biotechnol. Biochem. 2006, 70, 2547–2550. [Google Scholar] [CrossRef]
- Maciejewska, K.; Drzazga, Z.; Kaszuba, M. Role of trace elements (Zn, Sr, Fe) in bone development: Energy dispersive X-ray fluorescence study of rat bone and tooth tissue. BioFactors 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Campos, M.S.; Barrionuevo, M.; Alférez, M.J.M.; Nestares, T.; Díaz-Castro, J.; Ros, P.B.; Ortega, E.; López-Aliaga, I. Consumption of caprine milk improves metabolism of calcium and phosphorus in rats with nutritional ferropenic anaemia. Int. Dairy J. 2007, 17, 412–419. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in Bone Health Revisited: A Story Beyond Calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Ovesen, J.; Møller-Madsen, B.; Thomsen, J.S.; Danscher, G.; Mosekilde, L. The Positive Effects of Zinc on Skeletal Strength in Growing Rats. Bone 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Ammann, P.; Shen, V.; Robin, B.; Mauras, Y.; Bonjour, J.P.; Rizzoli, R. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J. Bone Miner. Res. 2004, 19, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Langford, J.R.; Liporace, F.A. Essential Nutrients for Bone Health and a Review of their Availability in the Average North American Diet. Open Orthop. J. 2012, 6, 143–149. [Google Scholar] [CrossRef]
| Diet | Modified-AIN-93M(g/kg) | Low Ca/P Modified-AIN-93M (g/kg) |
|---|---|---|
| Ingredient | ||
| Corn starch | 495.69 | 499.27 |
| Beef protein extract | 140 | 141 |
| Maltodextrin | 125 | 125.9 |
| Sucrose | 106.69 | 107.5 |
| Cellulose | 50 | 50 |
| Soybean oil | 40 | 40 |
| CaCO3 a | 12.495 | 6.293 |
| Vitamin mix b | 10 | 10 |
| KH2PO4 c | 8.75 | 4.407 |
| Mineral mix d | 3.5 | 3.5 |
| NaCl | 2.59 | 2.61 |
| Choline bitartrate | 2.5 | 2.5 |
| L-cystine | 1.8 | 1.8 |
| K₃C₆H₅O e | 0.98 | 4.41 |
| t-butylhydroquinone | 0.008 | 0.008 |
| Yellow dye f | 0 | 0.05 |
| Diet | Control | Low Ca/P + water | Low Ca/P + CM | Low Ca/P + SM | |
|---|---|---|---|---|---|
| Parameter | Unit | ||||
| BV/TV | % | 45.2 ± 3.81 a | 41.6 ± 2.78 b | 45.2 ± 3.03 a | 44.7 ± 3.63 a |
| BS/BV | mm2/mm3 | 33.6 ± 2.33 | 34.0 ± 2.10 | 33.2 ± 1.65 | 33.8 ± 1.99 |
| BS/TV | mm2/mm3 | 15.1 ± 1.53 a | 14.1 ± 0.88 b | 15.0 ± 0.90 a | 15.1 ± 0.97 a |
| Tb.Th | mm | 0.09 ± 0.004 | 0.09 ± 0.003 | 0.09 ± 0.003 | 0.09 ± 0.003 |
| Tb.Ts | mm | 0.27 ± 0.06 ab | 0.30 ± 0.04 a | 0.26 ± 0.03 b | 0.26 ± 0.03 b |
| Tb.N. | 1/mm | 5.15 ± 0.47 a | 4.75 ± 0.27 b | 5.09 ± 0.32 a | 5.10 ± 0.36 a |
| Tb.Pf | N/A | −19.9 ± 3.34 b | −16.0 ± 2.86 a | −18.9 ± 2.65b | −18.9 ± 3.25 b |
| Diet | Control | Low Ca/P + water | Low Ca/P + CM | Low Ca/P + SM | |
|---|---|---|---|---|---|
| Parameter | Unit | ||||
| BV | mm3 | 14.2 ± 1.83 a | 12.7 ± 1.24 b | 14.7 ± 1.32 a | 14.8 ± 1.56 a |
| BS | mm2 | 95.3 ± 20.8 | 93.8 ± 13.4 | 96.6 ± 9.08 | 98.6 ± 12.3 |
| BS/BV | mm2/mm3 | 6.70 ± 0.97 b | 7.42 ± 1.03 a | 6.59 ± 0.51 b | 6.69 ± 0.75 b |
| PV | mm3 | 0.03 ± 0.03 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| BSD | mm2/mm3 | 0.33 ± 0.10 | 0.32 ± 0.07 | 0.32 ± 0.07 | 0.32 ± 0.07 |
| P% | % | 0.19 ± 0.16 | 0.15 ± 0.12 | 0.13 ± 0.08 | 0.17 ± 0.09 |
| Diet | Control | Low Ca/P + water | Low Ca/P + CM | Low Ca/P + SM | |
|---|---|---|---|---|---|
| Parameter | Unit | ||||
| Emod | GPa | 2.26 ± 0.65 | 1.93 ± 0.65 | 1.82 ± 0.90 | 1.87 ± 0.42 |
| Fmax | N | 103 ± 17.6 a | 93.1 ± 12.9 b | 103 ± 13.1 a | 107 ± 17.0 a |
| Fbreak | N | 65.2 ± 25.6 | 61.1 ± 33.1 | 57.6 ± 25.6 | 64.2 ± 27.5 |
| σmax | N/mm² | 97.7 ± 22.3 | 86.9 ± 23.2 | 84.4 ± 34.3 | 89.9 ± 14.7 |
| dl at Fmax | mm | 0.67 ± 0.14 | 0.71 ± 0.12 | 0.66 ± 0.10 | 0.73 ± 0.18 |
| dl at break | mm | 1.02 ± 0.20 | 1.17 ± 0.24 | 1.19 ± 0.20 | 1.11 ± 0.20 |
| ε at Fmax | % | 9.06 ± 1.95 b | 9.66 ± 1.73 ab | 9.42 ± 1.44 a | 10.3 ± 2.73 a |
| Diet | Control (mg/kg) | Low Ca/P + water (mg/kg) | Low Ca/P + CM (mg/kg) | Low Ca/P + SM (mg/kg) |
|---|---|---|---|---|
| Element | ||||
| Al | 45.8 ± 16.9 | 40.1 ± 31.6 | 33.6 ± 19.5 | 31.2 ± 17.8 |
| Ca # | 227 ± 4.20 a | 218 ± 7.69 b | 221 ± 7.80 ab | 225 ± 8.14 a |
| Cu | 0.78 ± 0.07 | 0.86 ± 0.23 | 0.82 ± 0.08 | 0.79 ± 0.11 |
| Fe | 35.0 ± 9.82 ab | 40.5 ± 10.6 a | 31.4 ± 7.34 ab | 29.8 ± 10.2 b |
| K | 224 ± 104 | 256 ± 42.3 | 289 ± 47.6 | 273 ± 47.0 |
| Mg # | 3.90 ± 0.33 | 3.76 ± 0.33 | 3.77 ± 0.21 | 3.80 ± 0.24 |
| Mn | 0.54 ± 0.25 | 0.68 ± 0.04 | 0.63 ± 0.05 | 0.63 ± 0.06 |
| Na # | 5.03 ± 0.08 | 4.83 ± 0.22 | 5.08 ± 0.21 | 4.99 ± 0.33 |
| P # | 116 ± 2.93 ab | 112 ± 3.82 b | 114 ± 4.09 ab | 116 ± 3.75 a |
| Pb | 0.31 ± 0.28 | 0.75 ± 0.37 | 0.67 ± 0.79 | 0.95 ± 0.67 |
| Rb | 0.15 ± 0.08 b | 0.19 ± 0.03 b | 0.29 ± 0.06 a | 0.27 ± 0.056 a |
| Sr | 53.3 ± 7.19 c | 82.5 ± 17.4 a | 67.2 ± 13.7 b | 85.0 ± 9.72 a |
| Zn | 152 ± 67.8 b | 176 ± 18.6 b | 184 ± 15.9 ab | 195 ± 12.4 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burrow, K.; Young, W.; Hammer, N.; Safavi, S.; Scholze, M.; McConnell, M.; Carne, A.; Barr, D.; Reid, M.; Bekhit, A.E.-D. The Effect of the Supplementation of a Diet Low in Calcium and Phosphorus with Either Sheep Milk or Cow Milk on the Physical and Mechanical Characteristics of Bone using A Rat Model. Foods 2020, 9, 1070. https://doi.org/10.3390/foods9081070
Burrow K, Young W, Hammer N, Safavi S, Scholze M, McConnell M, Carne A, Barr D, Reid M, Bekhit AE-D. The Effect of the Supplementation of a Diet Low in Calcium and Phosphorus with Either Sheep Milk or Cow Milk on the Physical and Mechanical Characteristics of Bone using A Rat Model. Foods. 2020; 9(8):1070. https://doi.org/10.3390/foods9081070
Chicago/Turabian StyleBurrow, Keegan, Wayne Young, Niels Hammer, Sarah Safavi, Mario Scholze, Michelle McConnell, Alan Carne, David Barr, Malcolm Reid, and Alaa El-Din Bekhit. 2020. "The Effect of the Supplementation of a Diet Low in Calcium and Phosphorus with Either Sheep Milk or Cow Milk on the Physical and Mechanical Characteristics of Bone using A Rat Model" Foods 9, no. 8: 1070. https://doi.org/10.3390/foods9081070
APA StyleBurrow, K., Young, W., Hammer, N., Safavi, S., Scholze, M., McConnell, M., Carne, A., Barr, D., Reid, M., & Bekhit, A. E.-D. (2020). The Effect of the Supplementation of a Diet Low in Calcium and Phosphorus with Either Sheep Milk or Cow Milk on the Physical and Mechanical Characteristics of Bone using A Rat Model. Foods, 9(8), 1070. https://doi.org/10.3390/foods9081070