The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition
2.3. Mineral Analysis
2.4. Physicochemical Parameters
2.5. Techno-Functional Properties
2.5.1. Water-Holding Capacity, Oil-Holding Capacity and Water Solubility Index
2.5.2. Swelling Capacity
2.5.3. Foaming Capacity
2.5.4. Gel Formation
2.5.5. Emulsifying Activity and Its Stability
2.6. Statistical Analysis
3. Results
3.1. Proximate Composition
3.2. Mineral Content
3.3. Techno-Functional Properties
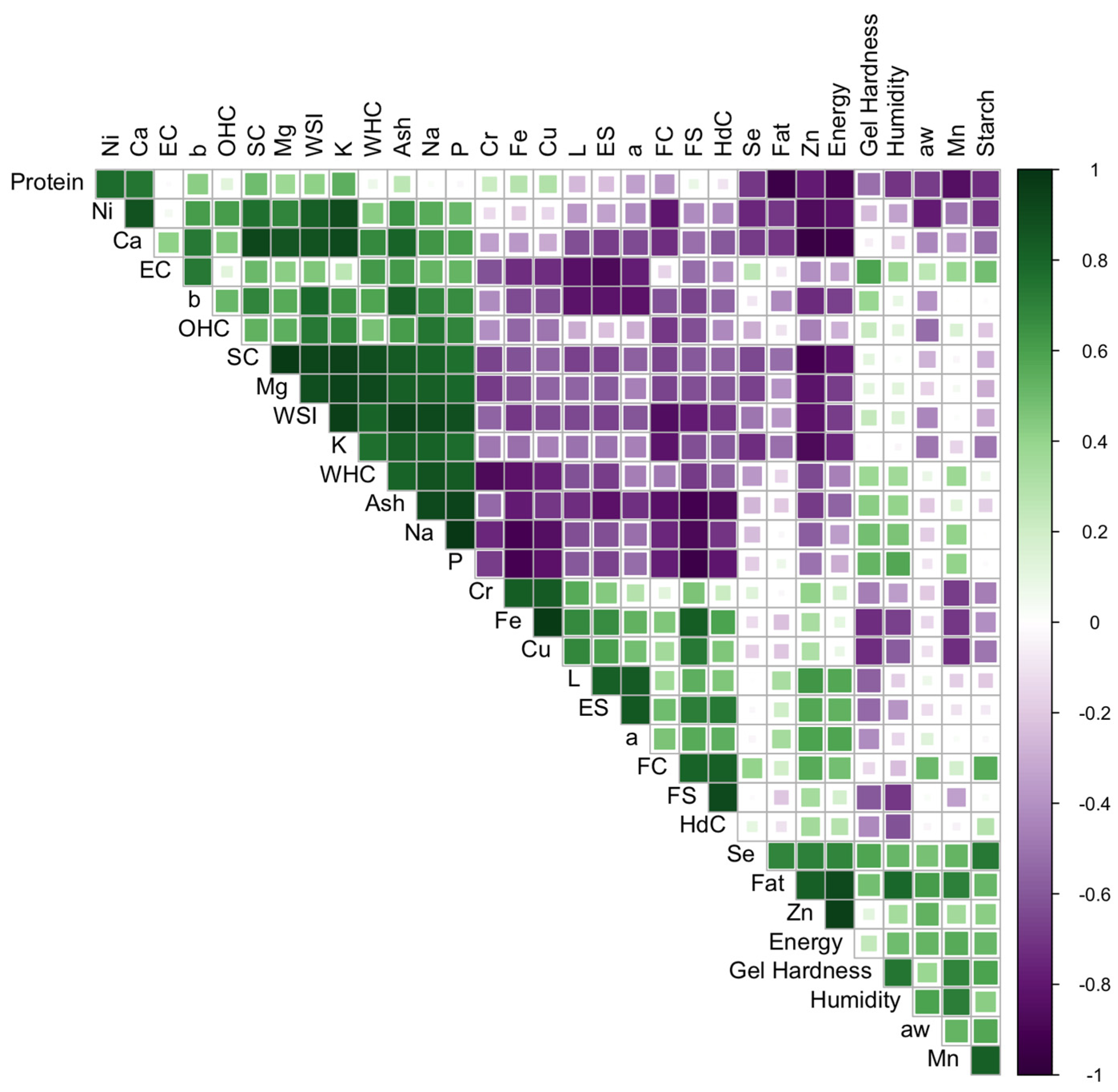
3.4. Correlations Between Physicochemical and Functional Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Reetu; Punia, S.; Dhakane-Lad, J.; Singh, S.; Dhumal, S.; Pradhan, P.C.; Bhushan, B.; et al. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Altschul, A.M.; Wilcke, H.L. (Eds.) New Protein Foods: Seed Storage Proteins; Academic Press: Orlando, FL, USA, 2013; Volume 5. [Google Scholar]
- Padalkar, G.; Mandlik, R.; Sudhakaran, S.; Vats, S.; Kumawat, S.; Kumar, V.; Kumar, V.; Rani, A.; Ratnaparkhe, M.B.; Jadhav, P.; et al. Necessity and challenges for exploration of nutritional potential of staple-food grade soybean. J. Food Compos. Anal. 2023, 117, 105093. [Google Scholar] [CrossRef]
- Amaya-Guerra, C.A.; Alanis-Guzman, M.G.; Serna Saldívar, S.O. Effects of soybean fortification on protein quality of tortilla-based diets produced from regular and quality protein maize. Plant Foods Hum. Nutr. 2004, 59, 45–50. [Google Scholar] [CrossRef]
- Tian, T.; Tong, X.; Ren, K.; Cao, J.; Yuan, Y.; Yang, J.; Zhu, J.; Miao, L.; Yang, S.; Yu, A.; et al. Influence of protein ratios on the structure and gel properties of soybean-wheat co-precipitated proteins. LWT 2022, 170, 114045. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Yang, M.; Li, N.; Tong, L.; Fan, B.; Wang, L.; Wang, F.; Liu, L. Comparison of physicochemical properties and volatile flavor compounds of pea protein and mung bean protein-based yogurt. LWT 2021, 152, 112390. [Google Scholar] [CrossRef]
- Poutanen, K.S.; O Kårlund, A.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains–a major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Jayaprakash, G.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. A narrative review on rice proteins: Current scenario and food industrial application. Polymers 2022, 14, 3003. [Google Scholar] [CrossRef]
- Cruz Solorio, A.; Garín Aguilar, M.; Valencia del Toro, G. Nutritional and functional properties of protein concentrate and protein isolates of foods. Science within food: Up-to-date advances on research and educational ideas. Formatex Res. Cent. 2020, 117, 1–229. [Google Scholar]
- Zhao, J.; Boatright, W.L. Static headspace analysis of odorants in commercial rice proteins. Food Chem. 2017, 221, 345–350. [Google Scholar] [CrossRef]
- Absi, Y.; Revilla, I.; Vivar-Quintana, A.M. Commercial Hemp (Cannabis sativa Subsp. sativa) Proteins and Flours: Nutritional and Techno-Functional Properties. Appl. Sci. 2023, 13, 10130. [Google Scholar] [CrossRef]
- Morr, C.V. Recalculated nitrogen conversion factors for several soybean protein products. J. Food Sci. 1982, 47, 1751–1752. [Google Scholar] [CrossRef]
- Krul, E.S. Calculation of nitrogen-to-protein conversion factors: A review with a focus on soy protein. J. Am. Oil Chem. Soc. 2019, 96, 339–364. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Gaithersbg, MD, USA, 2016. [Google Scholar]
- Rodríguez-Miranda, J.; Hernández-Santos, B.; Herman-Lara, E.; Gómez-Aldapa, C.A.; Garcia, H.S.; Martínez-Sánchez, C.E. Effect of some variables on oil extraction yield from Mexican pumpkin seeds. CyTA J. Food 2014, 12, 9–15. [Google Scholar] [CrossRef]
- Akpata, M.I.; Miachi, O.E. Proximate composition and selected functional properties of Detarium microcarpum. Plant Foods Hum. Nutr. 2001, 56, 297–302. [Google Scholar] [CrossRef]
- Chau, C.F.; Cheung, P.C.K. Functional properties of flours prepared from three Chinese indigenous legume seeds. Food Chem. 1998, 61, 429–433. [Google Scholar] [CrossRef]
- Tomé, A.S.; Pires, C.; Batista, I.; Sousa, I.; Raymundo, A. Protein gels and emulsions from mixtures of Cape hake and pea proteins. J. Sci. Food Agric. 2015, 95, 289–298. [Google Scholar] [CrossRef]
- Bora, P.S. Functional properties of native and succinylated lentil (Lens culinaris) globulins. Food Chem. 2002, 77, 171–176. [Google Scholar] [CrossRef]
- Galindo, M.P. Una alternativa de representacion simultanea: HJ-Biplot. Questiio 1986, 10, 13–23. [Google Scholar]
- Vicente-Villardon, J.L. MultBiplotR: Multivariate Analysis Using Biplots; R Package Version 19.11.19; R Foundation: Vienna, Austria, 2019. [Google Scholar]
- Güroy, D.; Şahin, İ.; Güroy, B.; Merrifield, D.L.; Bulut, M.; Tekinay, A.A. Replacement of fishmeal with rice protein concentrate in practical diets for European sea bass Dicentrarchus labrax reared at winter temperatures. Aquac. Res. 2013, 44, 462–471. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Gouvêa, L.; Caldeira, R.; de Lima Azevedo, T.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Mellinger, C.G. Physical and techno-functional properties of a common bean protein concentrate compared to commercial legume ingredients for the plant-based market. Food Hydrocoll. 2023, 137, 108351. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Processing technologies to produce plant protein concentrates and isolates. In Plant Protein Foods; Springer International Publishing: Guelph, ON, Canada, 2022; pp. 61–108. [Google Scholar] [CrossRef]
- Reda, R.M.; Maricchiolo, G.; Quero, G.M.; Basili, M.; Aarestrup, F.M.; Pansera, L.; Mirto, S.; El-Fattah, A.H.A.; Alagawany, M.; Rahman, A.N.A. Rice protein concentrate as a fish meal substitute in Oreochromis niloticus: Effects on immune response, intestinal cytokines, Aeromonas veronii resistance, and gut microbiota composition. Fish Shellfish Immunol. 2022, 126, 237–250. [Google Scholar] [CrossRef]
- Tömösközi, S.; Lásztity, R.; Haraszi, R.; Baticz, O. Isolation and study of the functional properties of pea proteins. Food/Nahr. 2001, 45, 399–401. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, X.; Wei, D.; Lin, X.; Qiu, S.; Ciampitti, I.; He, P. Soybean yield, nutrient uptake and stoichiometry under different climate regions of northeast China. Sci. Rep. 2020, 10, 8431. [Google Scholar] [CrossRef]
- Foh, M.B.K.; Wenshui, X.; Amadou, I.; Jiang, Q. Influence of pH shift on functional properties of protein isolated of tilapia (Oreochromis niloticus) muscles and of soy protein isolate. Food Bioprocess Technol. 2012, 5, 2192–2200. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Huang, S.; Franczyk, A.; Arganosa, G.C.; Warkentin, T.D.; House, J.D. Effect of genotype, year, and location on the proximate composition and in vitro protein quality of select pea cultivars. ACS Food Sci. Technol. 2021, 1, 1670–1676. [Google Scholar] [CrossRef]
- Mondor, M.; Aksay, S.; Drolet, H.; Roufik, S.; Farnworth, E.; Boye, J.I. Influence of processing on composition and antinutritional factors of chickpea protein concentrates produced by isoelectric precipitation and ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 342–347. [Google Scholar] [CrossRef]
- Awadalkareem, A.M.; Mustafa, A.I.; El Tinay, A.H. Protein, mineral content and amino acid profile of sorghum flour as influenced by soybean protein concentrate supplementation. Pak. J. Nutr. 2008, 7, 475–479. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Merchen, N.R.; Mahan, D.C.; Fahey, G.C. Chemical composition and protein quality comparisons of soybeans and soybean meals from five leading soybean-producing countries. J. Agric. Food Chem. 2004, 52, 6193–6199. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- de Paiva Gouvêa, L.; Caldeira, R.; de Lima Azevedo, T.; Antoniassi, R.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Mellinger, C.G. Nutritional properties of common bean protein concentrate compared to commercial legume ingredients for the plant-based market. Curr. Res. Food Sci. 2024, 9, 100937. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Allegretta, I.; Porfido, C.; Terzano, R.; Squeo, G.; Summo, C. Antinutritional factors, mineral composition and functional properties of dry fractionated flours as influenced by the type of pulse. Heliyon 2021, 7, e06177. [Google Scholar] [CrossRef]
- Kornet, R.; Shek, C.; Venema, P.; van der Goot, A.J.; Meinders, M.; van der Linden, E. Substitution of whey protein by pea protein is facilitated by specific fractionation routes. Food Hydrocoll. 2021, 117, 106691. [Google Scholar] [CrossRef]
- Anjum, F.M.; Pasha, I.; Bugti, M.A.; Butt, M.S. Mineral composition of different rice varieties and their milling fractions. Pak. J. Agri. Sci. 2007, 44, 332–336. [Google Scholar]
- Nasiroleslami, E.; Mozafari, H.; Sadeghi-Shoae, M.; Habibi, D.; Sani, B. Changes in yield, protein, minerals, and fatty acid profile of wheat (Triticum aestivum L.) under fertilizer management involving application of nitrogen, humic acid, and seaweed extract. J. Soil Sci. Plant Nutr. 2021, 21, 2642–2651. [Google Scholar] [CrossRef]
- Kolpakova, V.V.; Ulanova, R.V.; Kulikov, D.S.; Gulakova, V.A.; Semenov, G.V.; Shevjakova, L.V. Pea and chickpea protein concentrates: Quality indicators. Food Process. Tech. Technol. 2022, 52, 649–664. [Google Scholar] [CrossRef]
- do Carmo, C.S.; Silventoinen-Veijalainen, P.; Zobel, H.; Holopainen-Mantila, U.; Sahlstrøm, S.; Knutsen, S.H. The effect of dehulling of yellow peas and faba beans on the distribution of carbohydrates upon dry fractionation. LWT 2022, 163, 113509. [Google Scholar] [CrossRef]
- Xie, L.; Tang, S.; Wei, X.; Shao, G.; Jiao, G.; Sheng, Z.; Luo, J.; Hu, P. The cadmium and lead content of the grain produced by leading Chinese rice cultivars. Food Chem. 2017, 217, 217–224. [Google Scholar] [CrossRef]
- Li, P.C.; Jiang, S.J. Electrothermal vaporization inductively coupled plasma-mass spectrometry for the determination of Cr, Cu, Cd, Hg and Pb in rice flour. Anal. Chim. Acta 2003, 495, 143–150. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmed, M.; Ramzan, M.; Fatima, M.; Aftab, F.; Sanaullah, M.; Qamar, S.; Iftikhar, Z.; Wani, T.A.; Zargar, S. Appraisal of potentially toxic metals contamination in protein supplements for muscle growth: A chemometric approach and associated human health risks. J. Trace Elem. Med. Biol. 2024, 85, 127481. [Google Scholar] [CrossRef]
- Cabrera, C.; Lloris, F.; Giménez, R.; Olalla, M.; Lopez, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Squeo, G.; Caponio, F.; Summo, C. Amino acid and fatty acid compositions of texturized vegetable proteins: Composizione di proteine vegetali testurizzate. Ital. J. Food Sci. 2023, 35, 19–25. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Bivolarevic, V. Comparative study of the functional properties of three legume seed isolates: Adzuki, pea and soy bean. J. Food Sci. Technol. 2015, 52, 2779–2787. [Google Scholar] [CrossRef]
- Bou, R.; Navarro-Vozmediano, P.; Domínguez, R.; López-Gómez, M.; Pinent, M.; Ribas-Agustí, A.; Benedito, J.J.; Lorenzo, J.M.; Terra, X.; García-Pérez, J.V.; et al. Application of emerging technologies to obtain legume protein isolates with improved techno-functional properties and health effects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2200–2232. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Echeta, C.K. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019, 5, 139–160. [Google Scholar]
- Adebowale, K.O.; Lawal, O.S. Foaming, gelation and electrophoretic characteristics of mucuna bean (Mucuna pruriens) protein concentrates. Food Chem. 2003, 83, 237–246. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Protein and lipid enrichment of quinoa (cv. Titicaca) by dry fractionation. Techno-functional, thermal and rheological properties of milling fractions. Food Hydrocoll. 2020, 105, 105770. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Silventoinen, P.; Kortekangas, A.; Ercili-Cura, D.; Nordlund, E. Impact of ultra-fine milling and air classification on biochemical and techno-functional characteristics of wheat and rye bran. Food Res. Int. 2021, 139, 109971. [Google Scholar] [CrossRef] [PubMed]
- Bogahawaththa, D.; Chau, N.H.B.; Trivedi, J.; Dissanayake, M.; Vasiljevic, T. Impact of selected process parameters on solubility and heat stability of pea protein isolate. LWT 2019, 102, 246–253. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein-and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- Wang, M.; Hettiarachchy, N.S.; Qi, M.; Burks, W.; Siebenmorgen, T. Preparation and functional properties of rice bran protein isolate. J. Agric. Food Chem. 1999, 47, 411–416. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Toews, R.; Wang, N. Physicochemical and functional properties of protein concentrate from pulses. Food Res. Int. 2013, 52, 445–451. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; Abd El-Aty, A.M. Impact of high moisture contents on the structure and functional properties of pea protein isolate during extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Structure, gelation mechanism of plant proteins versus dairy proteins and evolving modification strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Luo, J.; Low, M.Y.; Shi, Z.; Tang, J.; Zhang, Z.; Peng, B.; Tam, K.C. Pickering emulsions stabilized by hydrophobically modified nanocellulose containing various structural characteristics. Cellulose 2019, 26, 7753–7767. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Konakbayeva, D.; Rajabzadeh, A.R.; Legge, R.L. Functional properties of navy bean (Phaseolus vulgaris) protein concentrates obtained by pneumatic tribo-electrostatic separation. Food Chem. 2019, 283, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, T.A.; Mjøs, S.A.; Oterhals, Å. Impact of variability in fishmeal physicochemical properties on the extrusion process, starch gelatinization and pellet durability and hardness. Anim. Feed Sci. Technol. 2013, 179, 77–84. [Google Scholar] [CrossRef]
- Vogelsang-O’dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’mahony, J.A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, F.; Roman, L.; Swaraj, V.S.; Ragavan, K.V.; Vidal, N.P.; Dutcher, J.R.; Martinez, M.M. Hemp (Cannabis sativa L.) protein concentrates from wet and dry industrial fractionation: Molecular properties, nutritional composition, and anisotropic structuring. Food Hydrocoll. 2022, 131, 107755. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Yu, G.; Li, X.; Liu, H.; Liu, T.; Zhu, J. Effect of enzymatic hydrolysis on the physicochemical and emulsification properties of rice bran albumin and globulin fractions. LWT 2022, 156, 113005. [Google Scholar] [CrossRef]
- Siddiquy, M.; JiaoJiao, Y.; Rahman, M.H.; Iqbal, M.W.; Al-Maqtari, Q.A.; Easdani, M.D.; Yiasmin, M.N.; Ashraf, W.; Hussain, A.; Zhang, L. Advances of protein functionalities through conjugation of protein and polysaccharide. Food Bioprocess Technol. 2024, 17, 2077–2097. [Google Scholar] [CrossRef]
- Vojdani, F. Solubility. In Methods of Testing Protein Functionality, 1st ed.; GM Hall. Blackie Academic & Professional: London, UK, 1996; pp. 11–60. [Google Scholar]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Proteins from pseudocereal seeds: Solubility, extraction, and modifications of the physicochemical and techno-functional properties. J. Sci. Food Agric. 2022, 102, 2630–2639. [Google Scholar] [CrossRef]
- Hernández Suárez, M.; Molina Pérez, D.; Rodríguez-Rodríguez, E.M.; Díaz Romero, C.; Espinosa Borreguero, F.; Galindo-Villardón, P. The compositional HJ-biplot—A new approach to identifying the links among bioactive compounds of tomatoes. Int. J. Mol. Sci. 2016, 17, 1828. [Google Scholar] [CrossRef]
- Cubilla-Montilla, M.; Nieto-Librero, A.B.; Galindo-Villardón, M.P.; Torres-Cubilla, C.A. Sparse HJ biplot: A new methodology via elastic net. Mathematics 2021, 9, 1298. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, F.; Saito, M.; Tatsumi, E.; Yin, L. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204. [Google Scholar] [CrossRef]
- Tan, H.L.; Tan, T.C.; Easa, A.M. Effects of sodium chloride or salt substitutes on rheological properties and water-holding capacity of flour and hardness of noodles. Food Struct. 2020, 26, 100154. [Google Scholar] [CrossRef]
- Lee, Y.S.; Zhekov, Z.G.; Owens, C.M.; Kim, M.; Meullenet, J.F. Effects of partial and complete replacement of sodium chloride with potassium chloride on the texture, flavor and water-holding capacity of marinated broiler breast fillets. J. Texture Stud. 2012, 43, 124–132. [Google Scholar] [CrossRef]
- Fatima, A.; Singh, P.; Pandey, V.K.; Singh, R.; Rustagi, S. Exploring the significance of protein concentrate: A review on sources, extraction methods, and applications. Food Chem. Adv. 2024, 5, 100771. [Google Scholar] [CrossRef]
- Langendörfer, L.J.; Avdylaj, B.; Hensel, O.; Diakité, M. Design of Plant-Based Food: Influences of Macronutrients and Amino Acid Composition on the Techno-Functional Properties of Legume Proteins. Foods 2023, 12, 3787. [Google Scholar] [CrossRef]
- Kohnhorst, A.L.; Uebersax, M.A.; Zabik, M.E. Production and functional characteristics of protein concentrates. J. Am. Oil Chem. Soc. 1990, 67, 285–292. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]

| Rice Proteins | Pea Proteins | Soybean Proteins | ||||
|---|---|---|---|---|---|---|
| Brand 1 | Brand 2 | Brand 1 | Brand 2 | Brand 1 | Brand 2 | |
| Moisture (%) | 4.35 ± 0.08 a | 5.43 ± 0.24 c | 7.15 ± 0.05 d | 7.19 ± 0.04 d | 5.71 ± 0.26 c | 4.86 ± 0.03 b |
| Proteins (g/100 g dw) | 87.00 ± 0.05 b | 87.77 ± 0.23 c | 84.35 ± 0.07 a | 84.85 ± 0.14 a | 89.60 ± 0.39 d | 89.80 ± 0.24 d |
| Fat (g/100 g dw) | 4.40 ± 0.01 b | 6.35 ± 0.02 c | 9.37 ± 0.18 e | 8.31 ± 0.13 d | 2.65 ±0.01 a | 2.63 ± 0.02 a |
| Carbohydrates (g/100 g dw) | 7.36 ± 0.05 e | 3.41 ± 0.02 d | 2.38 ± 0.02 b | 2.41 ± 0.12 b | 1.29 ±0.01 a | 2.76 ± 0.03 c |
| Starch (g/100 g dw) | 1.72 ± 0.01 d | 1.01 ± 0.05 a | 1.46 ± 0.10 c | 2.06 ± 0.02 e | 1.18 ± 0.02 b | 0.99 ± 0.01 a |
| Total sugar (g/100 g dw) | <1 | <1 | <1 | <1 | <1 | <1 |
| Fibre (g/100 g dw) | <1 | <1 | <1 | <1 | <1 | <1 |
| Ash (g/100 g dw) | 1.27 ± 0.02 a | 2.17 ± 0.05 b | 3.70 ± 0.22 c | 4.29 ± 0.08 d | 5.12 ± 0.02 e | 4.86 ± 0.02 e |
| Energy (Kcal/100 g) | 417.04 ± 0.09 c | 421.86 ± 0.99 d | 431.25 ± 1.85 e | 423.89 ± 1.40 d | 387.46± 1.58 a | 393.85 ± 1.11 b |
| Rice Proteins | Pea Proteins | Soybean Proteins | ||||
|---|---|---|---|---|---|---|
| Brand 1 | Brand 2 | Brand 1 | Brand 2 | Brand 1 | Brand 2 | |
| ppm | ||||||
| Na | 1861.82 ± 31.01 b | 816.24 ± 16.36 a | 11,530.00 ± 153.61 d | 10,719.10 ± 7.88 c | 10,944.44 ± 67.13 c | 13,307.24 ± 10.41 e |
| Mg | 330.50 ± 1.47 b | 179.52 ± 2.71 a | 707.86 ± 7.79 d | 429.35 ± 16.69 c | 971.99 ± 11.79 f | 799.06 ± 0.76 e |
| P | 2650.93 ± 2.94 a | 3270.61 ± 26.44 b | 8083.31 ± 106.50 d | 7611.91 ± 17.50 c | 7730.74 ± 30.45 c | 8176.00 ± 26.03 d |
| K | 78.65 ± 1.36 b | 42.20 ± 0.01 a | 1224.59 ± 0.65 d | 447.74 ± 4.93 c | 2027.68 ± 1.38 e | 2410.37 ± 4.19 f |
| Ca | 688.41 ± 4.37 a | 1043.30 ± 20.86 b | 1421.15 ± 11.52 c | 1423.29 ± 14.57 c | 5225.22 ± 11.55 e | 4418.93 ± 22.91 d |
| Mn | 25.51 ± 0.11 d | 31.48 ± 0.27 e | 10.98 ± 0.15 b | 5.84 ± 0.04 a | 13.15 ± 0.47 c | 13.81 ± 0.16 c |
| Fe | 180.00 ± 0.35 c | 87.08 ± 1.30 a | 256.15 ± 3.46 d | 267.77 ± 3.74 e | 132.34 ± 2.81 b | 133.14 ± 0.94 b |
| Cu | 14.64 ± 2.23 b,c | 17.69 ± 0.24 c | 11.18 ± 0.20 a,b | 8.95 ± 0.00 a | 12.00 ± 0.00 a,b | 11.59 ± 0.16 a,b |
| Zn | 59.49 ± 0.21 b | 73.95 ± 0.70 e | 67.77 ± 0.95 d | 63.07 ± 0.11 c | 20.42 ± 0.04 a | 21.75 ± 0.25 a |
| ppb | ||||||
| Se | 496.21 ± 22.08 b,c | 613.30 ± 45.04 c | 436.15 ± 2.53 b | 1039.83 ± 35.33 d | 200.95 ± 60.32 a | 185.51 ± 23.46 a |
| Ni | 240.22 ± 19.88 a | 604.54 ± 65.39 b | 585.49 ± 47.94 b | 268.27 ± 35.21 a | 1447.33 ± 35.89 c | 2282.92 ± 30.11 d |
| Cr | 208.98 ± 4.31 a | 627.11 ± 2.34 b | 78.97 ± 11.24 a | 114.11 ± 31.83 a | 103.74 ± 12.28 a | 189.71 ± 88.97 a |
| Cd * | nd | nd | nd | nd | nd | nd |
| Pb ** | nd | 175.40 ± 3.15 | nd | nd | nd | nd |
| Rice Proteins | Pea Proteins | Soybean Proteins | ||||
|---|---|---|---|---|---|---|
| Brand 1 | Brand 2 | Brand 1 | Brand 2 | Brand 1 | Brand 2 | |
| WHC (g/g) | 3.46 ± 0.06 b | 2.13 ± 0.03 a | 5.18 ± 0.13 d | 4.56 ± 0.32 c | 5.94 ± 0.27 e | 4.84 ± 0.26 c,d |
| OHC (g/g) | 0.99 ± 0.09 a | 1.00 ± 0.11 a | 1.16 ± 0.03 b | 1.12 ± 0.09 a,b | 1.08 ± 0.05 a,b | 1.29 ± 0.03 c |
| WSI (°Brix) | 1.0 ± 0.00 a | 1.7 ± 0.06 a | 11.7 ± 0.06 b | 11.0 ± 0.10 b | 18.3 ± 0.06 c | 22.0 ± 0.10 d |
| SC (%) | 0.74 ± 0.01 b | 0.25 ± 0.07 a | 1.35 ± 0.04 d | 1.05 ± 0.03 c | 2.43 ± 0.08 f | 2.08 ± 0.06 e |
| FC (%) | 474.60 ± 2.75 e | 294.66 ± 8.39 d | 257.57 ± 10.50 c | 296.82 ± 5.50 d | 228.61 ± 3.37 b | 136.36 ± 31.8 a |
| FS (%) | 465.08 ± 2.75 d | 284.33 ± 17.21 c | 87.88 ± 5.25 b | 49.20 ± 5.50 a | 101.39 ± 2.41 b | 92.42 ± 2.62 b |
| EC (%) | 43.54 ± 2.12 b | 35.62 ± 0.74 a | 41.61 ± 2.06 a,b | 63.25 ± 3.84 c | 59.88 ± 1.45 c | 45.59 ± 1.90 b |
| ES (%) | 82.54 ± 1.15 e | 77.13 ± 0.65 d | 75.05 ± 2.57 d | 55.19 ± 2.77 b | 48.75 ± 0.86 a | 67.83 ± 0.37 c |
| GF (N) | 85.39 ± 24.66 b | 59.79 ± 1.14 a | 639.90 ± 1015.93 c | 619.08 ± 158.51 c | 2067.33 ± 1870.70 d | 2482.80 ± 218.24 d |
| aw | 0.344 ± 0.001 c | 0.318 ± 0.004 b | 0.404 ± 0.001 f | 0.389 ± 0.001 e | 0.371 ± 0.002 d | 0.134 ± 0.002 a |
| L* | 82.03 ± 27.38 d | 78.08 ± 1.17 c | 76.36 ± 2.42 b,c | 73.52 ± 1.83 a | 74.69 ± 2.14 a,b | 83.53 ± 2.14 d |
| a* | 2.71 ± 0.07 d | 2.46 ± 0.07 c | 5.06 ± 0.01 e | 5.39 ± 0.01 f | 0.37 ± 0.07 a | 0.51 ± 0.02 b |
| b* | 15.91 ± 0.16 a | 19.16 ± 0.35 b | 25.00 ± 0.61 e | 23.23 ± 0.47 d | 19.96 ± 0.48 c | 18.77 ± 0.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Calabozo, R.; Martínez-Martín, I.; Rodríguez-Fernández, M.; Absi, Y.; Vivar-Quintana, A.M.; Revilla, I. The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties. Foods 2025, 14, 509. https://doi.org/10.3390/foods14030509
López-Calabozo R, Martínez-Martín I, Rodríguez-Fernández M, Absi Y, Vivar-Quintana AM, Revilla I. The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties. Foods. 2025; 14(3):509. https://doi.org/10.3390/foods14030509
Chicago/Turabian StyleLópez-Calabozo, Rocío, Iván Martínez-Martín, Marta Rodríguez-Fernández, Yamina Absi, Ana María Vivar-Quintana, and Isabel Revilla. 2025. "The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties" Foods 14, no. 3: 509. https://doi.org/10.3390/foods14030509
APA StyleLópez-Calabozo, R., Martínez-Martín, I., Rodríguez-Fernández, M., Absi, Y., Vivar-Quintana, A. M., & Revilla, I. (2025). The Influence of the Nutritional and Mineral Composition of Vegetable Protein Concentrates on Their Functional Properties. Foods, 14(3), 509. https://doi.org/10.3390/foods14030509







