Evaluation of Litchi Honey Quality in Southern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Sampling
2.2. Reagents
2.3. Melissopalynological Analysis
2.4. Determination of Physicochemical Indicators
2.4.1. Sensory Inspection Method
2.4.2. Measurement of Moisture Content
2.4.3. Carbohydrate Analysis Method
2.4.4. Determination of Amylase Activity
2.4.5. Conductivity Measurement
2.4.6. Isotope Detection
2.4.7. Determination of HMF Content
2.5. Volatile Substances Analysis
2.5.1. Extraction of Volatile Substances
2.5.2. GC-MS Conditions
2.5.3. Qualitative Analysis of Volatile Substances
3. Results and Discussion
3.1. Characterization of Pollen Types in Honey
3.2. Analysis of Basic Physicochemical Indicators
3.2.1. Sensory Data and Interpretations
3.2.2. Moisture Content
3.2.3. Conductivity
3.2.4. Amylase Value
3.2.5. Quantitative Analysis of Carbohydrate Composition
3.2.6. HMF
3.2.7. The Stable Carbon Isotope Ratio Analysis
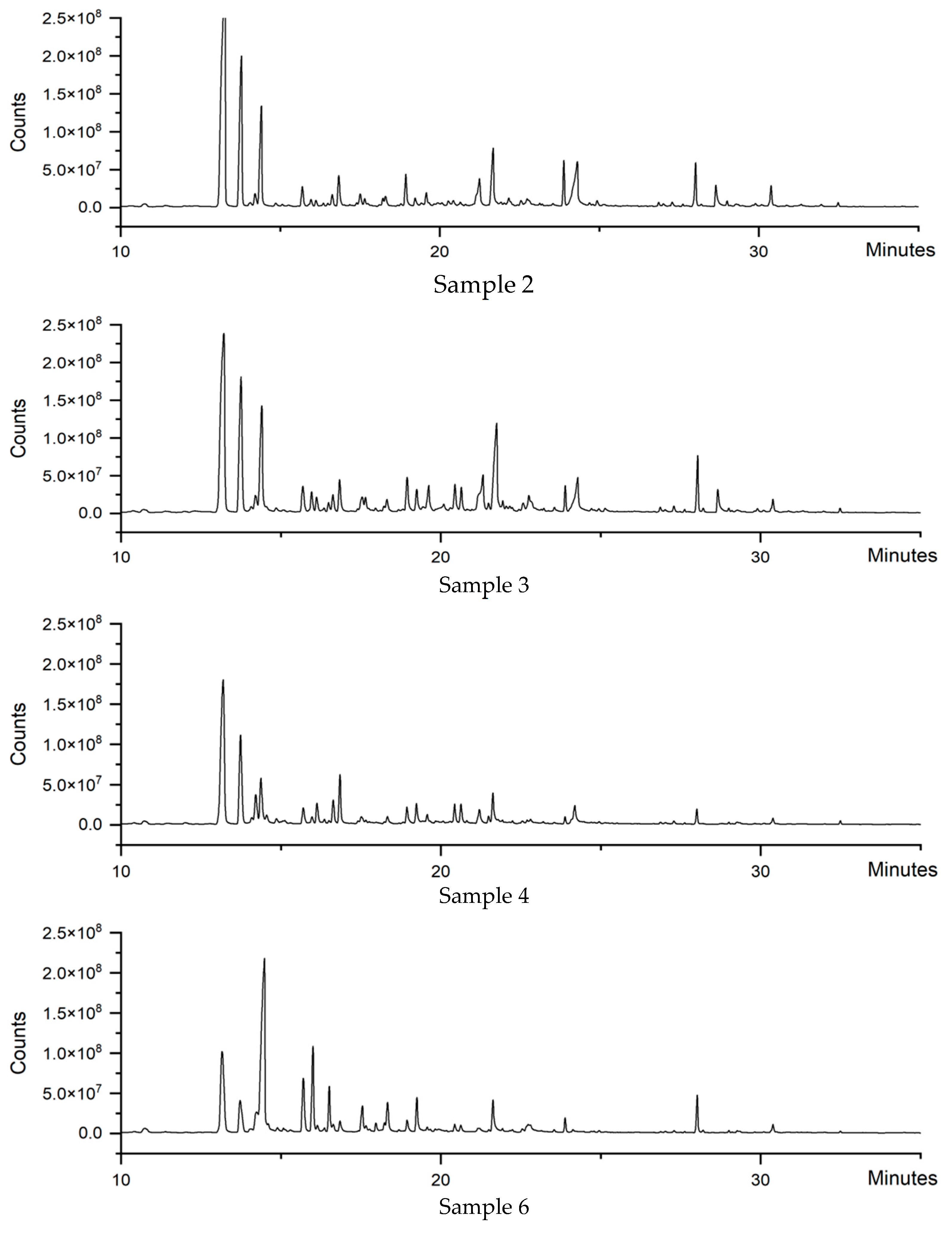
3.3. Analysis of Volatile Components
3.4. Variations in Honey Quality Among Bee Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Akanda, M.K.M.; Mehjabin, S.; Parvez, G.M.M. Honey for Nutrition and Health Benefits: An Overview. In Honey in Food Science and Physiology; Kumar, R., Hajam, Y.A., Bala Dhull, S., Giri, A., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 33–56. ISBN 978-981-9735-64-8. [Google Scholar]
- Wu, S. China Fruit Trees Record: Litchi; China Agriculture Publishing House: Beijing, China, 1998. [Google Scholar]
- The Ministry of Agriculture. China Agriculture Encyclopedia; China Agriculture Publishing House: Beijing, China, 1997. [Google Scholar]
- Yao, P.; Gao, Y.; Simal-Gandara, J.; Farag, M.A.; Chen, W.; Yao, D.; Delmas, D.; Chen, Z.; Liu, K.; Hu, H.; et al. Litchi (Litchi Chinensis Sonn.): A Comprehensive Review of Phytochemistry, Medicinal Properties, and Product Development. Food Funct. 2021, 12, 9527–9548. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Jothi, J.S.; Islam, M. Antioxidant and Physico-Chemical Properties of Litchi Honey Procured from Gazipur and Tangail District, Bangladesh. J. Entomol. Zool. Stud. 2014, 2, 207–211. [Google Scholar]
- Qi, L.-K.; Lin, L.; Chen, D.-L.; Tang, D.-S. Fingerprint of the Litchi Honey by Raman Spectroscopic. Mod. Food Sci. Technol. 2014, 30, 201–205. [Google Scholar]
- Lin, J.; Zheng, H.; Zhou, W.; Tan, D. Effect of Ultrafiltration on Characteristic and the Nutrition Value of Litchi Honey. Mod. Food Sci. Technol. 2009, 25, 741–744. [Google Scholar]
- Manickavasagam, G.; Saaid, M.; Osman, R. The Trend in Established Analytical Techniques in the Investigation of Physicochemical Properties and Various Constituents of Honey: A Review. Food Anal. Methods 2022, 15, 3116–3152. [Google Scholar] [CrossRef]
- Tasić, A.; Pezo, L.; Lončar, B.; Pešić, M.B.; Tešić, Ž.; Kalaba, M. Assessing the Impact of Botanical Origins, Harvest Years, and Geographical Variability on the Physicochemical Quality of Serbian Honey. Foods 2024, 13, 1530. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Magalhães, L.; Moreira, M.M.; Rede, D.; Fernandes, V.C.; Viegas, O.; Pinto, E.; Almeida, A.; Azevedo, R.; Delerue-Matos, C. Exploring Geographical Influences on Physicochemical Characteristics of Honey: The Montesinho Natural Park Scenario. Food Qual. Saf. 2024, 8, fyae015. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, J.; Zhao, H.; Wu, F.; Xue, X.; Wu, L.; Cao, W. Volatile Compounds of Five Types of Unifloral Honey in Northwest China: Correlation with Aroma and Floral Origin Based on HS-SPME/GC–MS Combined with Chemometrics. Food Chem. 2022, 384, 132461. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontominas, M.G. A Decisive Strategy for Monofloral Honey Authentication Using Analysis of Volatile Compounds and Pattern Recognition Techniques. Microchem. J. 2020, 152, 104263. [Google Scholar] [CrossRef]
- Liang, D.; Wen, H.; Zhou, Y.; Wang, T.; Jia, G.; Cui, Z.; Li, A. Simultaneous Qualitative and Quantitative Analyses of Volatile Components in Chinese Honey of Six Botanical Origins Using Headspace Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. J. Sci. Food Agric. 2023, 103, 7631–7642. [Google Scholar] [CrossRef]
- Evahelda; Kusmiadi, R.; Helmi, H.; Sumarwan, A. Identification of Volatile Compounds in Kelulut Honey (Trigona Sp.) from the Nectar of Pelawan Trees in the Pelawan Forest, Central Bangka Regency, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2024, 1419, 12022. [Google Scholar] [CrossRef]
- Wang, H.-T.; Huang, W.-Z.; Zhu, Y.-Y.; Zhang, X.-F.; Zhao, H.-X. Study on the Chromatographic Fingerprint of Volatile Components from Litchi Honey. J. Environ. Entomol. 2018, 40, 1437–1444. [Google Scholar]
- Zhu, H.; Du, Y.; Lin, X.; Huang, Z.; Dong, J.; Qiao, J.; Zhang, H. Terpenoids Identification and Authenticity Evaluation of Longan, Litchi and Schefflera Honey. Food Sci. Hum. Wellness 2025, 14. [Google Scholar] [CrossRef]
- SN/T 0852-2012; Detection Method for Salmonella in Foods. General Administration of Customs of the People’s Republic of China: Beijing, China, 2012.
- GB/T 18932.16-2003; Determination of Lead, Cadmium, Mercury, Arsenic, Selenium and Antimony in Foods—Method of Hydride-Atomic Fluorescence Spectrometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GB/T 18932.15-2003; Determination of Tin in Foods—Method of Graphite Furnace Atomic Absorption Spectrometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GB/T 18932.1-2002; Determination of Cadmium in Foods—Method of Electrothermal Atomic Absorption Spectrometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GB/T 18932.18; Method for the Determination of Hydroxymethylfurfural in Honey—HPLC-UV Detection Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GH/T 18796-2012; Supply and Marketing Corporatives Standard of the People’s Republic of China: Beijing, China. All-China Federation of Supply and Marketing Cooperatives: Beijing, China, 2012.
- AOAC Official Method 998.12 C-4; Plant Sugars in Honey. AOAC International: Rockville, MD, USA, 1998.
- CXS 12-1981, Rev. 1; Codex Standard for Honey. Codex Alimentarius Commission: Rome, Italy, 2019.
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two Aspects of Honeydew Honey Authenticity: Application of Advance Analytical Methods and Chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef]
- Chen, H.; Fan, C.; Wang, Z.; Chang, Q.; Wang, W.; Li, X.; Pang, G. Evaluation of Measurement Uncertainty in EA–IRMS: For Determination of δ13C Value and C-4 Plant Sugar Content in Adulterated Honey. Accredit. Qual. Assur. 2013, 18, 351–358. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Wu, L.; Xue, X.; Zhao, J.; Li, Y.; Ye, Z.; Lin, G. Classification of Chinese Honeys According to Their Floral Origins Using Elemental and Stable Isotopic Compositions. J. Agric. Food Chem. 2015, 63, 5388–5394. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Estimating the Geographical Origin of Food Products by Stable Isotope and Trace Element Analyses. Bunseki Kagaku 2019, 68, 671–682. [Google Scholar] [CrossRef]
- Wang, T.; Cui, Z.; Zhang, S.; Jia, G.; Wei, H.; Li, X.; Zhang, J.; Wei, Y.; Wang, X. Determination and Comparative Analysis of Volatile Components in Chinese and Italian Bee Lychee Honey. Food Res. Dev. 2020, 41, 159–166. [Google Scholar]

| Number | Honeybee Species | Geographical Origin | Plant Species | Harvest Time | Pollen Grain Counted (%) |
|---|---|---|---|---|---|
| 1 | Apismellifera | Guangdong province | Litchi chinensis ’Nuo Mi Ci’ | April 2022 | 61.25 |
| 2 | Apis cerana | Guangdong province | Litchi chinensis ’Nuo Mi Ci’ | April 2022 | 71.01 |
| 3 | Apis cerana | Fujian province | Litchi chinensis ’Chen Zi’ | May 2022 | 72.88 |
| 4 | Apis cerana | Hainan province | Litchi chinensis ’Feizixiao’ | March 2022 | 53.87 |
| 5 | Apis cerana | Guangxi province | Litchi chinensis ’Gui Wei’ | April 2022 | 82.38 |
| 6 | Apis mellifera | Guangxi province | Litchi chinensis ’Gui Wei’ | April 2022 | 76.79 |
| Parameter | Sample Code | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Moisture content/% | 17.18 ± 0.06 E | 20.09 ± 0.16 D | 22.13 ± 0.10 B | 22.70 ± 0.08 A | 21.66 ± 0.16 C | 22.00 ± 0.14 B |
| Color/mm | 46.0 ± 1.0 A | 47.3 ± 0.6 A | 35.3 ± 0.6 B | 31.0 ± 1.0 C | 35.7 ± 2.1 B | 33.7 ± 0.6 BC |
| Fructose/% | 39.60 ± 0.25 A | 38.41 ± 0.27 ABC | 39.25 ± 0.27 AB | 37.46 ± 0.34 ABC | 36.66 ± 2.22 BC | 36.50 ± 0.46 C |
| Glucose/% | 37.63 ± 0.35 A | 36.29 ± 0.47 AB | 30.57 ± 0.42 D | 34.01 ± 0.62 BC | 32.97 ± 2.01 CD | 33.14 ± 0.14 C |
| Sucrose/% | 1.15 ± 0.06 A | 0.72 ± 0.03 C | 1.11 ± 0.08 A | 0.59 ± 0.01 D | 0.97 ± 0.03 B | 0.90 ± 0.02 B |
| Maltose/% | 1.44 ± 0.18 A | 1.09 ± 0.03 B | 1.51 ± 0.08 A | 1.32 ± 0.04 AB | 1.35 ± 0.09 A | 1.38 ± 0.05 A |
| Reducing Sugar/% | 77.23 ± 0.57 A | 74.70 ± 0.69 AB | 69.83 ± 0.68 BC | 71.47 ± 0.94 BC | 69.63 ± 4.22 C | 69.64 ± 0.55 C |
| Fructose/Reducing Sugar/% | 51.28 ± 0.13 C | 51.43 ± 0.23 C | 56.22 ± 0.18 A | 52.41 ± 0.27 B | 52.65 ± 0.16 B | 52.42 ± 0.27 B |
| F/G | 1.05 ± 0.01 C | 1.06 ± 0.01 C | 1.28 ± 0.01 A | 1.10 ± 0.01 B | 1.11 ± 0.01 B | 1.10 ± 0.01 B |
| HMF/mg/kg | 1.04 ± 0.05E | 1.33 ± 0.09 DE | 3.49 ± 0.13 A | 2.75 ± 0.16 B | 1.65 ± 0.03 CD | 1.79 ± 0.19 C |
| Amylase value/mL/g·h | 14.09 ± 0.53 A | 8.22 ± 0.76 BC | 7.75 ± 0.52 C | 7.72 ± 0.60 C | 8.07 ± 0.68 BC | 9.60 ± 0.65 B |
| Conductivity mS/cm | 0.21 ± 0.01 C | 0.25 ± 0.01 B | 0.28 ± 0.01 A | 0.21 ± 0.01 C | 0.16 ± 0.01 D | 0.15 ± 0.01 D |
| Protein δ13C‰ | −24.28 ± 0.14 A | −24.36 ± 0.09 A | −25.20 ± 0.06 B | −25.66 ± 0.06 C | −24.95 ± 0.13 B | −24.97 ± 0.13 B |
| Honey δ13C‰ | −25.53 ± 0.09 B | −25.51 ± 0.18 B | −25.19 ± 0.04 A | −25.96 ± 0.12 C | −25.64 ± 0.05 B | −25.51 ± 0.07 B |
| Glucose δ13C | −25.28 ± 0.29 A | −25.52 ± 0.28 AB | −25.24 ± 0.13 A | −25.88 ± 0.08 B | −25.55 ± 0.20 AB | −25.35 ± 0.16 AB |
| Fructose δ13C | −25.75 ± 0.13 BC | −25.52 ± 0.09 AB | −25.24 ± 0.19 A | −26.08 ± 0.22 C | −25.71 ± 0.23 BC | −25.64 ± 0.09 ABC |
| Disaccharide δ13C | −26.10 ± 0.33 AB | −26.51 ± 0.23 BC | −25.44 ± 0.22 A | −27.17 ± 0.43 C | −27.19 ± 0.18 C | −27.17 ± 0.09 C |
| Disaccharide/% | 3.28 ± 0.07 A | 2.28 ± 0.05 C | 2.92 ± 0.14 B | 2.39 ± 0.09 C | 3.53 ± 0.05 A | 3.41 ± 0.13 A |
| Trisaccharide/% | ND | ND | ND | ND | ND | ND |
| Oligosaccharide/% | ND | ND | ND | ND | ND | ND |
| RT(min) | Chemical Compound | Confidence(%) | Molecular Formula | CAS | Peak Area (×106) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 6 | |||||
| 9.6569 | 1-heptanol | 91.8 | C7H16O | 111-70-6 | 20.53 | |||
| 10.7005 | octamethyl-cyclotetrasiloxane | 93.3 | C8H24O4Si4 | 556-67-2 | 0.80 | 14.43 | 14.51 | 14.00 |
| 11.9884 | 3,5,5-trimethyl-3-cyclohexen-1-one | 90.4 | C9H14O | 471-01-2 | 0.11 | 0.14 | 0.05 | |
| 13.1558 | trans-Linalool oxide | 96.9 | C10H18O2 | 34995-77-2 | 249.92 | 33.59 | 234.66 | 133.27 |
| 13.2223 | Ethyl-2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate | 97.9 | C13H22O4 | 1000373-80-3 | 107.93 | 111.29 | 111.42 | 45.79 |
| 14.1985 | Linalool | 96.3 | C10H18O | 78-70-6 | 9.91 | 14.28 | 28.11 | 27.49 |
| 14.4065 | 3,7-dimethyl-1,5,7-octatrien-3-ol | 94.8 | C10H16O | 29957-43-5 | 142.91 | 150.82 | 49.92 | 368.06 |
| 14.5553 | Isophorone | 88.9 | C9H14O | 78-59-1 | 7.47 | 8.18 | 7.78 | |
| 15.1222 | tetrahydro-4-methyl-2-(2-methyl-1-propenyl)-2H-pyran | 91.7 | C10H18O | 16409-43-1 | 0.84 | 2.12 | ||
| 15.2483 | 1,3,8-p-Menthatriene | 90.8 | C10H14 | 18368-95-1 | 0.96 | 0.08 | ||
| 15.6926 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 92.2 | C9H12O2 | 1125-21-9 | 26.34 | 36.36 | 23.44 | 27.81 |
| 15.9586 | Lilac aldehyde B | 95.2 | C10H16O2 | 53447-45-3 | 5.38 | 16.3 | 2.43 | 62.85 |
| 16.1124 | 3,6-dihydro-4-methyl-2-(2-methyl-1-propenyl)-2H-Pyran | 96 | C10H16O | 1786-08-9 | 6.28 | 18.21 | 20.05 | 6.41 |
| 16.3478 | Cyclopentasiloxane, decamethyl- | 98.1 | C10H30O5Si5 | 541-02-6 | 2.13 | 3.21 | 3.34 | 2.26 |
| 16.488 | Lilac aldehyde D | 95.2 | C10H16O2 | 53447-47-5 | 3.26 | 8.26 | 2.45 | 34.06 |
| 16.8330 | (3R,6S)-2,2,6-Trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | 97.1 | C10H18O2 | 39028-58-5 | 25.67 | 16.09 | 43.00 | 7.32 |
| 17.3901 | α-Terpineol | 88.4 | C10H18O | 98-55-5 | 5.48 | 8.64 | 6.74 | 1.86 |
| 17.5468 | 2,6-dimethyl-3,7-Octadiene-2,6-diol | 94.8 | C10H18O2 | 13741-21-4 | 23.14 | 36.00 | 49.78 | |
| 17.6443 | 2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde | 98.3 | C10H14O | 116-26-7 | 4.83 | 9.22 | 2.62 | 4.42 |
| 17.9552 | Decanal | 97.1 | C10H20O | 112-31-2 | 1.83 | 0.15 | 4.76 | |
| 18.2195 | alpha,4-dimethyl-3-cyclohexene-1-acetaldehyde | 92 | C10H16O | 29548-14-9 | 18.67 | 10.64 | 3.06 | 15.97 |
| 18.3247 | 4-Methyleneisophorone | 92.9 | C10H14O | 20548-00-9 | 12.84 | 7.47 | 5.65 | 18.33 |
| 18.9364 | 2-Hydroxy-3,5,5-trimethylcyclohex-2-ene-1,4-dione | 97.3 | C9H12O3 | 35692-98-9 | 15.88 | 20.21 | 4.78 | 9.12 |
| 20.0034 | Diethylene glycol dibutyrate | 84.8 | C12H22O5 | 1000458-46-7 | 1.30 | 2.41 | 1.56 | 0.075 |
| 20.6409 | Epoxy-linalooloxide | 85.8 | C10H18O3 | 1000007-96-5 | 19.57 | 3.10 | 12.89 | 5.39 |
| 22.7249 | 6-Methyl-5-octen-2-one | 80 | C9H16O | 24199-46-0 | 0.82 | |||
| 23.5352 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | 92.9 | C12H24O3 | 77-68-9 | 1.40 | 3.03 | 1.03 | 1.82 |
| 23.8828 | (E)-2-Buten-1-one,1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl) | 96.5 | C13H18O | 23726-93-4 | 53.3 | 6.61 | 7.92 | 16.25 |
| 24.936 | 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one | 89.2 | C13H18O | 1000187-51-9 | 0.71 | |||
| 25.1318 | 4-Hydroxy-2,6,6-trimethylcyclohex-1-enecarbaldehyde | 93.3 | C10H16O2 | 35692-94-5 | 0.15 | 0.14 | 0.12 | |
| 27.014 | 2’,6’-Dimethyl-4’-propoxyacetophenone | 90.2 | C13H18O2 | 1000195-98-1 | 1.87 | 1.86 | 1.11 | 0.57 |
| 28.6623 | 1,2,3,4-Tetramethoxybenzene | 90.2 | C10H14O4 | 21450-56-6 | 42.98 | 50.05 | ||
| 29.0038 | (E)-Benzene, 4-(2-butenyl)-1,2-dimethyl | 87.4 | C12H16 | 54340-86-2 | 4.91 | 3.42 | 2.80 | 1.33 |
| 29.27 | dodecamethyl-Cyclohexasiloxane | 80.3 | C12H36O6Si6 | 540-97-6 | 1.55 | 0.71 | 0.73 | 1.35 |
| 30.3222 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 88.4 | C16H30O4 | 6846-50-0 | 0.36 | 0.39 | 3.54 | 0.20 |
| 30.3855 | Cedrol | 93.8 | C15H26O | 77-53-2 | 6.25 | 3.04 | 2.24 | 2.90 |
| 32.4849 | hexadecamethyl-Cyclooctasiloxane | 87 | C16H48O8Si8 | 556-68-3 | 1.30 | 3.55 | 2.80 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhou, S.; Wu, C.; Xu, X.; Zhu, X. Evaluation of Litchi Honey Quality in Southern China. Foods 2025, 14, 510. https://doi.org/10.3390/foods14030510
Zhang C, Zhou S, Wu C, Xu X, Zhu X. Evaluation of Litchi Honey Quality in Southern China. Foods. 2025; 14(3):510. https://doi.org/10.3390/foods14030510
Chicago/Turabian StyleZhang, Cuiping, Shujing Zhou, Chenxinzi Wu, Xinjian Xu, and Xiangjie Zhu. 2025. "Evaluation of Litchi Honey Quality in Southern China" Foods 14, no. 3: 510. https://doi.org/10.3390/foods14030510
APA StyleZhang, C., Zhou, S., Wu, C., Xu, X., & Zhu, X. (2025). Evaluation of Litchi Honey Quality in Southern China. Foods, 14(3), 510. https://doi.org/10.3390/foods14030510






