Influence of Jackfruit Wood Barrels and Chips During Aging on the Quality and Phenolic Compounds of Cachaça
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
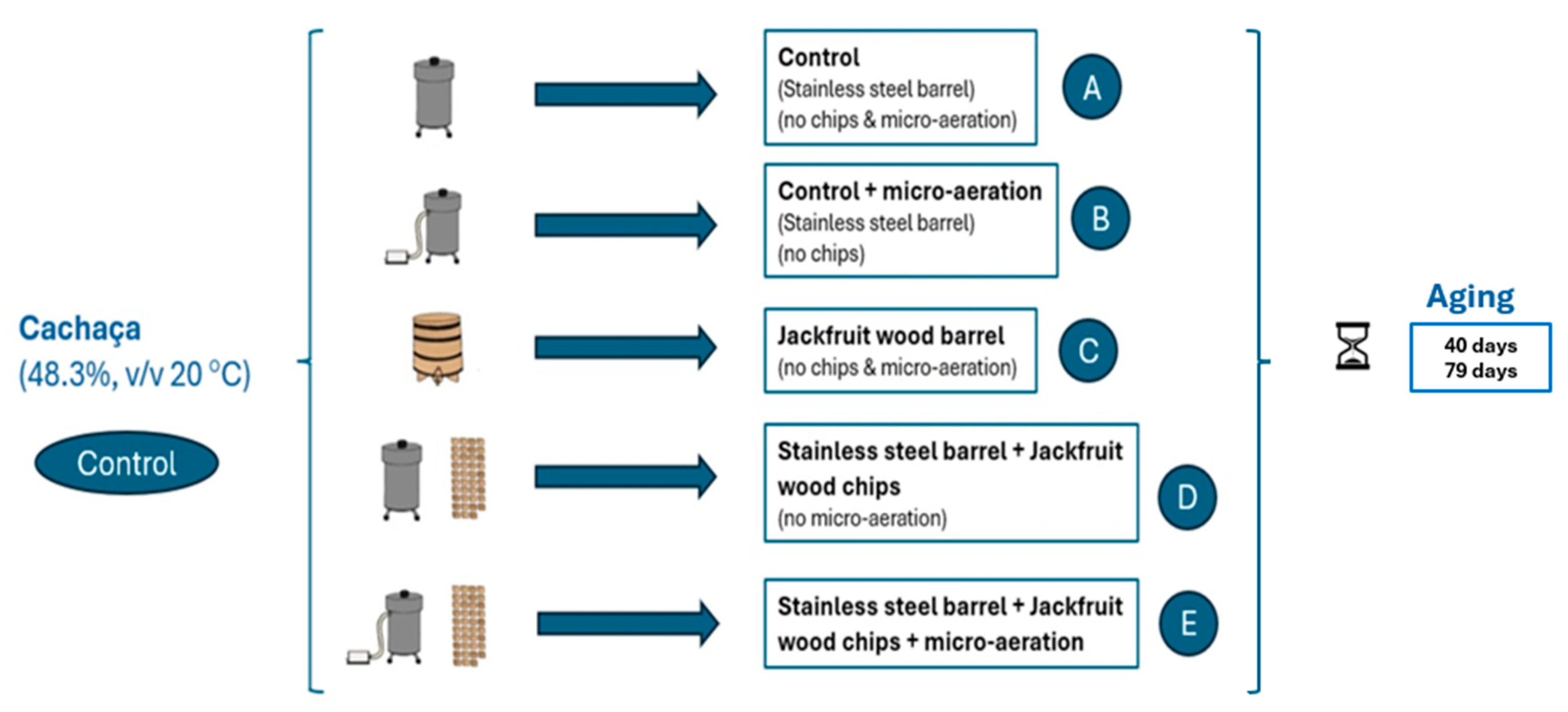
2.2. Experimental Design
2.3. Physicochemical Characterization
2.4. CIE L*a*b* Color Characteristics
2.5. Inorganic Contaminants
2.6. Analysis of Phenolic Compounds by HPLC
2.7. Statistical Analysis
3. Results and Discussion
3.1. Impact of the Treatments on the Physicochemical Characteristics
3.2. Impact of the Treatments on Inorganic Contaminants
3.3. Impact of the Treatments on Higher and Butyl and Methyl Alcohols
3.4. Impact of the Treatments on the CIE L*a*b* Color Characteristics
3.5. Total Phenolic Contents
3.6. Multivariate Analysis of Physicochemical and Color Parameters
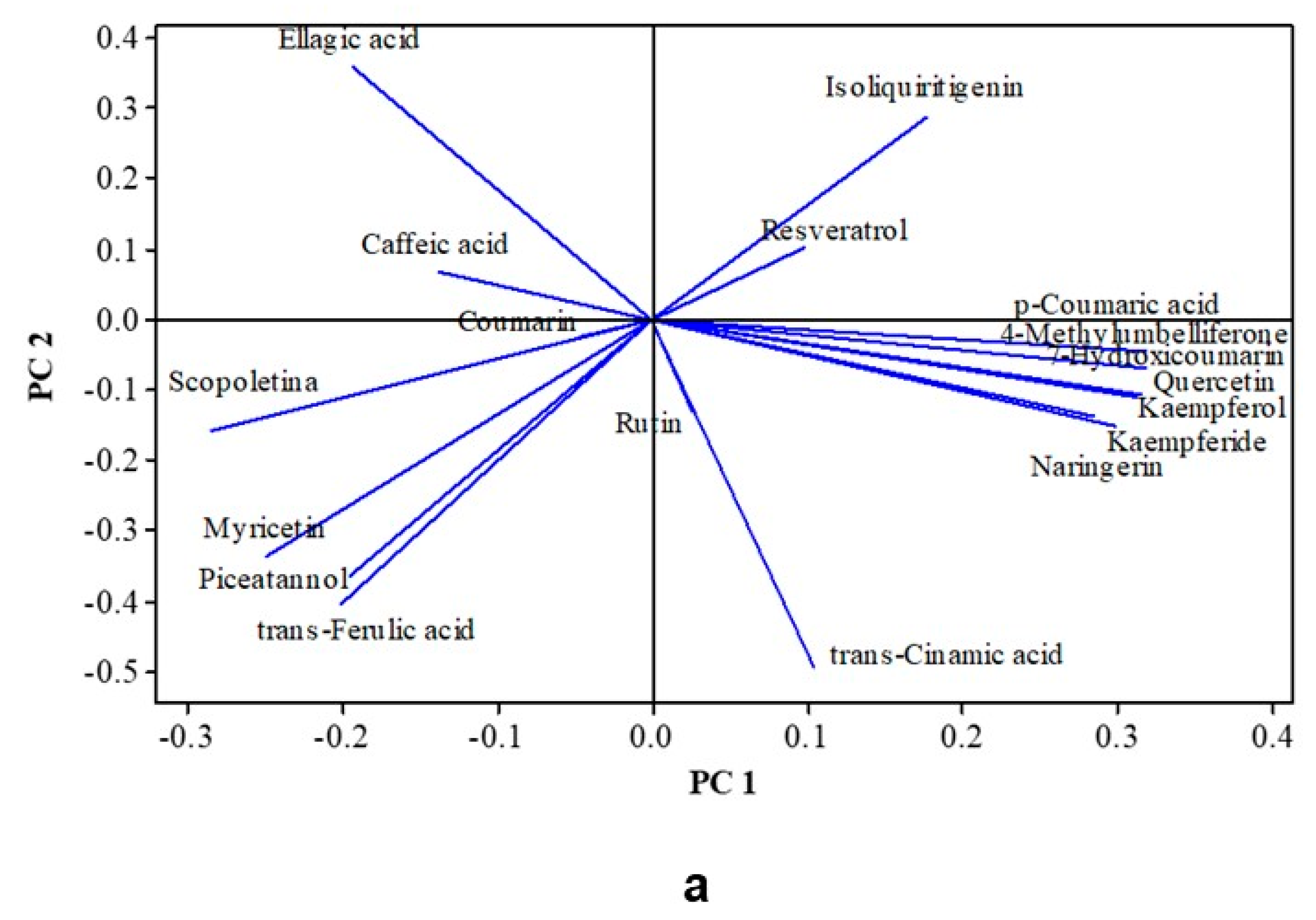
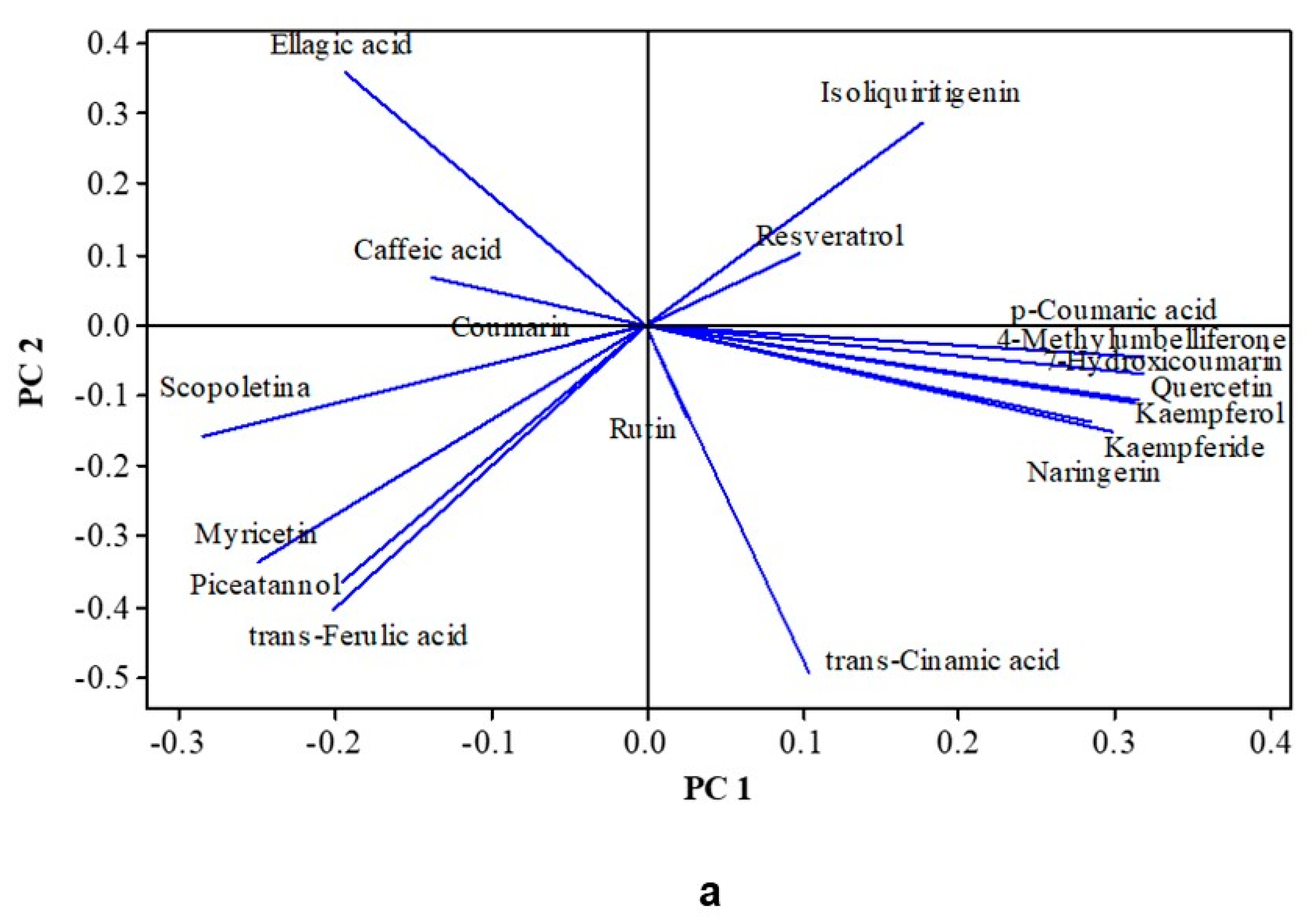
3.7. Impact of the Treatments on Some Specific Phenolic Compounds and Coumarins
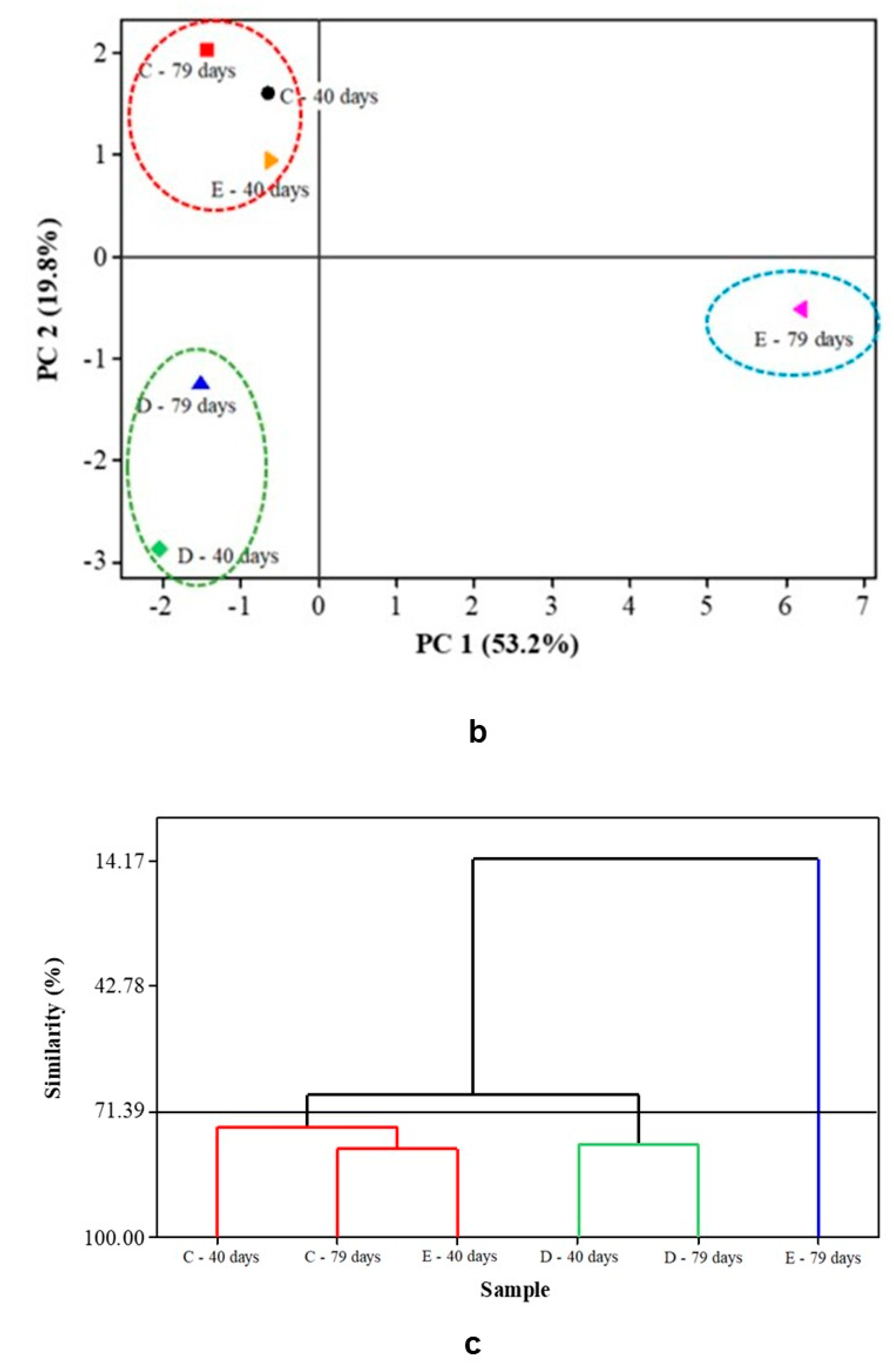
3.8. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratkovich, N.; Esser, C.; de Resende Machado, A.M.; Mendes, B.d.A.; Cardoso, M.d.G. The Spirit of Cachaça Production: An Umbrella Review of Processes, Flavour, Contaminants and Quality Improvement. Foods 2023, 12, 3325. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.B.; Santiago, W.D.; Alvarenga, G.F.; Oliveira, R.E.S.; Ferreira, V.R.F.; Nelson, D.L.; Cardoso, M.G. Physical–chemical profile and quantification of phenolic compounds and polycyclic aromatic hydrocarbons in cachaça samples aged in oak (Quercus sp.) barrels with different heat treatments. Food Bioprocess. Technol. 2022, 15, 1977–1987. [Google Scholar] [CrossRef]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Portaria nº 539, de 26 de Dezembro 2022. Available online: https://www.in.gov.br/en/web/dou/-/portaria-mapa-n-539-de-26-de-dezembro-de-2022-453828778 (accessed on 27 September 2023).
- IBRAC. Instituto Brasileiro da Cachaça. Mercado Interno. 2022. Available online: https://ibrac.net/servicos/mercado-interno (accessed on 23 July 2022).
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Portaria nº 90, de 23 de Agosto de 2016. Available online: https://www.gov.br/agricultura/pt-br/acesso-a-informacao/participacao-social/consultas-publicas/documentos/portaria-90-2016-envelhecimento-de-bebidas.pdf (accessed on 27 September 2023).
- Maia, A.B.; Marinho, L.S.; Tonidandel, L.O.; Conceição, E.C.; Bárbara Dias Machado, B.D. Wood chips and cachaça aging. Int. J. Dev. Res. 2023, 13, 64230–64234. [Google Scholar] [CrossRef]
- Cardoso, M.G.; Machado, A.M.R.; Caetano, A.R.S.; Campolina, G.A.; Nelson, D.L. Volatile compounds formation in cachaça. In Volatile Compounds Formation in Specialty Beverages; Reis, F.R., dos Santos, C.M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 1–17. [Google Scholar] [CrossRef]
- Castro, M.C.; Silvello, G.C.; Corniani, L.S.; Acevedo, M.S.M.S.F.; Pereira, A.A.M.; Alcarde, A.R. Maturation-related phenolic compounds in cachaça aged in oak barrels: Influence of reuses. Wood Sci. Technol. 2023, 57, 781–795. [Google Scholar] [CrossRef]
- Rodrigues, L.M.A.; Cardoso, M.G.; Santiago, W.D.; Barbosa, R.B.; Santiago, J.D.A.; Lima, L.M.Z.; Nelson, D.L. Organic contaminants in distilled sugar cane spirits produced by column and copper alembic distillation. Res. Soc. Dev. 2020, 9, e930974879. [Google Scholar] [CrossRef]
- Feng, Z.; Martínez-Lapuente, L.; Palacios, A.; Ayestarán, B.; Guadalupe, Z. Influence of Quercus alba oak geographical origin on the colour characteristics and phenolic composition of Tempranillo wines. Eur. Food Res. Technol. 2024, 250, 1587–1609. [Google Scholar] [CrossRef]
- Silva, F.A.; Morais, K.C.R.C.; Ribeiro, K.O.; Garcia, L.G.C.; Caliari, M. Evolution of the content of phenolic compounds, antioxidant activity and color in organic sugarcane spirit aged in barrels of different woods. Res. Soc. Dev. 2020, 9, e138953302. [Google Scholar] [CrossRef]
- Jordão, A.J.; Correia, A.C.; Botelho, R.V.; Ortega-Heras, M.; González-San José, M.L. Potential of the enological use of several Brazilian wood species on the phenolic composition and sensory quality of different wines. BIO Web Conf. 2023, 68, 02016. [Google Scholar] [CrossRef]
- Bortoletto, A.M.; Correa, A.C.; Alcarde, A.R. Aging practices influence chemical and sensory quality of cachaça. Food Res. Int. 2016, 86, 46–53. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Anjos, O.; Belchior, A.P. Phenolic profile and colour acquired by the wine spirit in the beginning of ageing: Alternative technology using micro-oxygenation vs. traditional technology. LWT—Food Sci. Technol. 2019, 111, 260–269. [Google Scholar] [CrossRef]
- Sanches-Gómez, R.; Del Álamo-Sanza, M.; Nevares, I. Volatile composition of oak wood from different customized oxygenation wine barrels: Effect on red wine. Food Chem. 2020, 329, 127181. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Xu, Y. Distilled beverage aging: A review on aroma characteristics, maturation mechanisms, and artificial aging techniques. Compr. Rev. Food Sci. Food Saf. 2022, 22, 502–534. [Google Scholar] [CrossRef] [PubMed]
- Granja-Soares, J.; Roque, R.; Cabrita, M.J.; Anjos, O.; Belchior, A.P.; Caldeira, I.; Canas, S. Effect of innovative technology using staves and micro-oxygenation on the odorant and sensory profile of aged wine spirit. Food Chem. 2020, 333, 127450. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Vitória, C.; Anjos, O.; Fernandes, T.A.; Gallardo, E.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Wine spirit ageing with chestnut staves under different micro-oxygenation strategies: Effects on the volatile compounds and sensory profile. Appl. Sci. 2021, 11, 3991. [Google Scholar] [CrossRef]
- Gollihue, J.; Pook, V.G.; DeBolt, S. Sources of variation in bourbon whiskey barrels: A review. J. Inst. Brew. 2021, 127, 210–223. [Google Scholar] [CrossRef]
- Mahanta, C.L.; Kalita, D. Chapter 47—Jackfruit processing and utilization of jackfruit seeds. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 395–400. [Google Scholar] [CrossRef]
- Castro, M.C.; Bortoletto, A.M.; Silvello, G.C.; Alcarde, A.R. Lignin-derived phenolic compounds in cachaça aged in new barrels made from two oak species. Heliyon 2020, 6, e05586. [Google Scholar] [CrossRef]
- Brasil. Ministério da Agricultura, Pecuária e do Abastecimento. Instrução Normativa nº 13, de 29 de Junho de 2005. Aprova o Regulamento Técnico para Fixação dos Padrões de Identidade e Qualidade para Aguardente de Cana e para Cachaça. Diário Oficial da União, 30 June 2005; Section 1, number 124. [Google Scholar]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Saito, M.S.; Santos, W.A.; Mamede, M.E.O. Coffee flavoured kombucha: Development, physicochemical characterization and sensory analysis. Fermentation 2024, 10, 334. [Google Scholar] [CrossRef]
- Lima, D.A.R.; Alves, E.C.; Anjos, J.P. Greener method proposal for the determination of phenolic compounds and coumarins in aged sugarcane spirits using green bio-based solvents in HPLC-DAD-FLD system. Green. Anal. Chem. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Silvello, G.C.; Bortoletto, A.M.; Castro, M.C.; Alcarde, A.R. New approach for barrel-aged distillates classification based on maturation level and machine learning: A study of cachaça. LWT—Food Sci. Technol. 2021, 140, 110836. [Google Scholar] [CrossRef]
- Morais, K.C.R.C.; Jesus, L.S.; Ribeiro, G.O.; Caliari, M.; Silva, F.A.; Lião, L.M. Chemical evaluation of cachaça aged in different Brazilian woods. Beb Ferment. Destil Pesq. Aplic. 2022, 1, 1–15. [Google Scholar] [CrossRef]
- García-Moreno, M.V.; Sánchez-Guillén, M.M.; Delgado-González, M.J.; Durán-Guerrero, E.; Rodríguez-Dodero, M.C.; García-Barroso, C.; Guillén-Sánchez, D.A. Chemical content and sensory changes of oloroso sherry wine when aged with four different wood types. LWT—Food Sci. Technol. 2021, 140, 110706. [Google Scholar] [CrossRef]
- Santiago, W.D.; Cardoso, M.G.; Nelson, D.L. Cachaça stored in casks newly constructed of oak (Quercus sp.), amburana (Amburana cearensis), jatoba (Hymenaeae carbouril), balsam (Myroxylon peruiferum) and peroba (Paratecoma peroba): Alcohol content, phenol composition, colour intensity and dry extract. J. Inst. Brew. 2017, 123, 232–241. [Google Scholar] [CrossRef]
- Biagioni, M.A. Papel da madeira no envelhecimento da cachaça. Rev. Cient. Multidiscip. 2021, 2, e28682. [Google Scholar] [CrossRef]
- Zacaroni, L.M.; Cardoso, M.G.; Santiago, W.D.; Mendonça, J.G.P.; Nunes, C.A.; Duarte, F.C. Avaliação multivariada de composição fenólica de cachaças envelhecidas em diferentes barris de madeira. Científica 2014, 42, 101–107. [Google Scholar] [CrossRef]
- ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Copper (Draft for Public Comment); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2023.
- Clark, A.C.; Wilkes, E.N.; Scollary, G.R. Copper in white wine. Aust. J. Grape Wine Res. 2015, 21, 339–350. [Google Scholar] [CrossRef]
- ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Zinc; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2021.
- ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Aluminum; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2015.
- Bortoletto, A.M. Rum and cachaça. In Distilled Spirits; Hill, A., Jack, F., Eds.; Academic Press: London, UK, 2023; pp. 61–74. [Google Scholar]
- Lima, C.M.G.; Benoso, P.; Pierezan, M.D.; Santana, R.F.; Hassemer, G.S.; Rocha, R.A.; Nora, F.M.D.; Verruck, S.; Caetano, D.; Simal-Gandara, J. A state-of-the-art review of the chemical composition of sugarcane spirits and current advances in quality control. J. Food Compos. Anal. 2022, 106, 104338. [Google Scholar] [CrossRef]
- Nekoukar, Z.; Zakariaei, Z.; Taghizadeh, F.; Musavi, F.; Banimostafavi, E.S.; Sharifpour, A.; Ebrahim Ghuchi, N.; Fakhar, M.; Tabaripour, R.; Safanavaei, S. Methanol poisoning as a new world challenge: A review. Ann. Med. Surg. 2021, 66, 102445. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.G.; Ranzan, L.; Trierweiler, L.F.; Trierweiler, J.O. Determination of the concentration of total phenolic compounds in aged cachaça using two-dimensional fluorescence and mid-infrared spectroscopy. Food Chem. 2020, 329, 127142. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Wang, X.; Shi, T.; Lv, P.; Li, Q.X.; Hua, R. Myricetin inhibits photodegradation of profenofos in water: Pathways and mechanisms. Agronomy 2024, 14, 399. [Google Scholar] [CrossRef]
- Chavez-Santiago, J.O.; Rodríguez-Castillejos, G.C.; Montenegro, G.; Bridi, R.; Valdés-Gómez, H.; Alvarado-Reyna, S.; Castillo-Ruiz, O.; Santiago-Adame, R. Phenolic content, antioxidant, and antifungal activity of jackfruit extracts (Artocarpus heterophyllus Lam.). Food Sci. Technol. 2022, 42, 02221. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutri. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Rezaeiroshan, A.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Hedayatizadeh-Omran, A.; Goli, H.; Nokhodchi, A. Vesicular formation of trans-ferulic acid: An efficient approach to improve the radical scavenging and antimicrobial properties. J. Pharm. Innov. 2022, 17, 652–661. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rokonuzzman, M.; Hossain, M.I.; Ansari, S.A.; Ansari, I.A.; Islam, T.; Al Hasan, M.S.; Mubarak, M.S.; Islam, M.T. Anxiolytic-like effects by trans-ferulic acid possibly occur through GABAergic interaction pathways. Pharmaceuticals 2023, 16, 1271. [Google Scholar] [CrossRef] [PubMed]
- Mude, H.; Balapure, A.; Thakur, A.; Ganesan, R.; Ray Dutta, J. Enhanced antibacterial, antioxidant and anticancer activity of caffeic acid by simple acid-base complexation with spermine/spermidine. Nat. Prod. Res. 2022, 36, 6453–6458. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Bolerazska, B.; Marekova, M.; Birkova, A. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Golmei, P.; Kasna, S.; Roy, K.P.; Kumar, S. A review on pharmacological advancement of ellagic acid. J. Pharmacol. Pharmacother. 2024, 15, 93–104. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Pibuel, M.A.; Poodts, D.; Sias, S.A.; Byrne, A.; Hajos, S.E.; Franco, P.G.; Lompardía, S.L. 4-Methylumbelliferone enhances the effects of chemotherapy on both temozolomide-sensitive and resistant glioblastoma cells. Sci. Rep. 2023, 13, 9356. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Al-Qudah, M.A.; Barhoumi, L.M.; Abu-Sal, H.E.; Hasan, H.S.; Al-Bataineh, N.; Abu-Orabi, S.; Mubarak, M.S. Piceatannol, a comprehensive review of health perspectives and pharmacological aspects. Arab. J. Chemi. 2024, 17, 105939. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive oxygen species in biological systems, pathways, associated diseases, and potential inhibitors: A review. Food Sci. Nutri. 2024, 12, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Codex Alimentarius: General Requirements for Natural Flavourings (CAC/GL 66-2008). 2008. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/de/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-714-44%252FCRDs%252Ffl44_crd02x.pdf (accessed on 20 November 2023).
- AFC. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to coumarin. EFSA J. 2004, 104, 1–36. [Google Scholar] [CrossRef]

| Sample/Aging Time (days) | Alcoholic Degree (% v/v) | Density at 20 °C (g/L) | Total Acidity † | Volatile Acidity † | Total Esters ‡ | Dry Extract (g/L) | Total Sugars (g/L) | Cu (mg/L) | Zn (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 48.30 ± 0.01 a | 0.935 ± 0.001 e | 16.95 ± 0.00 e | 32.39 ± 1.81 e | 27.5 ± 0.55 d | 0.04 ± 0.06 e | 0.17 ± 0.01 c | 1.17 ± 0.06 a | 0.11 ± 0.01 c |
| A—40 | 47.00 ± 0.06 ab | 0.938 ± 0.001 d | 16.95 ± 0.00 e | 32.21 ± 0.04 e | 27.4 ± 0.84 d | 0.04 ± 0.01 e | 0.17 ± 0.01 c | 1.07 ± 0.06 a | 0.12 ± 0.01 abc |
| A—79 | 48.17 ± 1.17 a | 0.940 ± 0.001 c | 17.22 ± 0.00 e | 33.38 ± 0.11 e | 27.4 ± 0.09 d | 0.04 ± 0.01 e | 0.20 ± 0.04 c | 1.20 ± 0.17 a | 0.12 ± 0.01 bc |
| B—40 | 46.01 ± 0.30 bc | 0.939 ± 0.001 c | 16.95 ± 0.25 e | 37.29 ± 2.00 cde | 32.5 ± 2.05 cd | 0.03 ± 0.01 e | 0.18 ± 0.01 c | 1.20 ± 0.01 a | 0.16 ± 0.04 a |
| B—79 | 43.76 ± 0.66 ef | 0.944 ± 0.001 b | 17.90 ± 0.59 e | 39.47 ± 1.93 bcd | 36.5 ± 1.56 bc | 0.04 ± 0.01 e | 0.21 ± 0.02 c | 1.23 ± 0.06 a | 0.15 ± 0.01 ab |
| C—40 | 45.71 ± 0.57 bc | 0.941 ± 0.001 c | 68.63 ± 3.49 d | 42.31 ± 2.02 abc | 39.3 ± 2.20 b | 5.48 ± 0.99 d | 0.18 ± 0.01 c | 0.62 ± 0.03 b | nd |
| C—79 | 45.24 ± 0.64 cde | 0.943 ± 0.001 b | 112.1 ± 13.7 bc | 46.06 ± 2.01 a | 42.6 ± 1.23 b | 7.45 ± 1.23 c | 0.49 ± 0.08 b | 0.53 ± 0.05 b | nd |
| D—40 | 45.48 ± 0.11 bcd | 0.940 ± 0.001 c | 96.88 ± 6.06 c | 43.39 ± 0.11 ab | 54.9 ± 1.90 a | 8.40 ± 0.69 bc | 0.56 ± 0.01 ab | 0.73 ± 0.04 b | nd |
| D—79 | 45.21 ± 0.65 cde | 0.944 ± 0.001 b | 129.6 ± 12.80 b | 46.70 ± 2.23 a | 32.5 ± 2.26 cd | 9.78 ± 0.87 ab | 0.61 ± 0.02 a | 0.63 ± 0.09 b | nd |
| E—40 | 43.93 ± 0.31 de | 0.943 ± 0.001 b | 107.0 ± 3.49 c | 35.61 ± 1.94 de | 52.9 ± 2.35 a | 9.08 ± 0.12 bc | 0.57 ± 0.02 ab | 0.71 ± 0.05 b | nd |
| E—79 | 42.24 ± 0.49 f | 0.948 ± 0.001 a | 158.1 ± 8.11 a | 46.90 ± 2.34 a | 32.5 ± 5.36 cd | 10.81 ± 0.13 a | 0.59 ± 0.02 a | 0.65 ± 0.10 b | nd |
| Sample/Aging | Level (mg/100 mL Anhydrous Alcohol) | |||
|---|---|---|---|---|
| Time (Days) | Isobutyl Alcohol | Isoamyl Alcohol | Propyl Alcohol | ∑ Higher Alcohols |
| Control | 5.62 ± 0.04 a | 9.31 ± 0.03 ab | 8.97 ± 0.12 c | 23.9 ± 0.00 ab |
| A—40 | 4.37 ± 0.18 bc | 8.98 ± 0.83 ab | 10.4 ± 0.41 abc | 23.8 ± 1.38 ab |
| A—79 | 3.78 ± 0.20 c | 8.18 ± 1.19 ab | 9.42 ± 0.66 bc | 21.4 ± 2.05 b |
| B—40 | 4.26 ± 0.34 bc | 9.49 ± 0.90 ab | 10.2 ± 0.79 abc | 24.0 ± 2.02 ab |
| B—79 | 4.69 ± 0.26 bc | 10.6 ± 0.99 ab | 11.4 ± 0.59 ab | 26.6 ± 1.74 ab |
| C—40 | 4.53 ± 0.15 bc | 9.25 ± 0.26 ab | 10.9 ± 0.50 abc | 24.7 ± 0.90 ab |
| C—79 | 4.14 ± 0.16 bc | 7.64 ± 0.91 b | 9.89 ± 0.35 bc | 21.7 ± 1.42 b |
| D—40 | 4.56 ± 0.61 bc | 9.54 ± 1.57 ab | 10.9 ± 1.30 abc | 25.0 ± 3.47 ab |
| D—79 | 4.6 ± 0.38 bc | 10.2 ± 0.72 ab | 11.0 ± 0.89 abc | 25.7 ± 1.98 ab |
| E—40 | 5.02 ± 0.25 ab | 10.8 ± 0.66 a | 12.3 ± 0.84 a | 28.2 ± 1.57 a |
| E—79 | 4.78 ± 0.47 ab | 10.2 ± 1.52 a | 11.5 ± 1.08 ab | 26.5 ± 3.06 ab |
| Sample/Aging | CIE L*a*b* Color Characteristics | ||||
|---|---|---|---|---|---|
| Time (Days) | L* | a* | b* | C* | h |
| Control | 96.6 ± 0.01 a | −0.28 ± 0.06 e | 1.50 ± 0.02 c | 1.52 ± 0.02 c | 100.8 ± 0.12 b |
| A—40 | 103 ± 0.09 a | −0.55 ± 0.01 e | −0.14 ± 0.06 c | 0.57 ± 0.01 c | 197.3 ± 3.50 a |
| A—79 | 96.7 ± 0.02 a | −0.09 ± 0.29 e | 1.40 ± 0.05 c | 1.44 ± 0.07 c | 100.4 ± 0.10 b |
| B—40 | 103 ± 0.06 a | −0.46 ± 0.05 e | −0.18 ± 0.05 c | 0.50 ± 0.04 c | 200.8 ± 4.78 a |
| B—79 | 96.5 ± 0.11 a | −0.25 ± 0.04 e | 1.58 ± 0.23 c | 1.60 ± 0.23 c | 99.07 ± 0.42 b |
| C—40 | 81.6 ± 2.27 b | 11.7 ± 2.59 d | 59.8 ± 9.53 b | 60.7 ± 9.65 b | 79.01 ± 0.88 c |
| C—79 | 75.3 ± 1.86 bc | 21.4 ± 1.89 cd | 74.9 ± 1.48 a | 78.0 ± 1.04 a | 74.04 ± 1.61 cd |
| D—40 | 68.0 ± 6.30 cd | 28.9 ± 8.51 abc | 61.2 ± 6.47 ab | 68.2 ± 3.40 ab | 64.55 ± 8.43 de |
| D—79 | 61.4 ± 7.20 d | 35.6 ± 7.29 a | 56.7 ± 11.2 b | 67.7 ± 6.12 ab | 57.32 ± 9.87 e |
| E—40 | 70.1 ± 0.37 cd | 25.1 ± 0.74 bc | 64.4 ± 1.1 ab | 69.1 ± 1.29 ab | 68.70 ± 0.24 cde |
| E—79 | 63.9 ± 0.40 d | 33.6 ± 0.16 ab | 62.8 ± 0.95 ab | 71.2 ± 0.91 ab | 61.86 ± 0.26 de |
| Phenolic Compounds | Mean Levels ± Standard Deviation (mg/L) | |||||
|---|---|---|---|---|---|---|
| C—40 | C—79 | D—40 | D—79 | E—40 | E—79 | |
| Phenolic acids | ||||||
| Caffeic acid | 3.33 ± 1.99 bc | 2.57 ± 0.98 bc | 3.93 ± 2.61 b | 3.11 ± 0.61 bc | 8.42 ± 2.15 a | 1.18 ± 0.01 bc |
| trans-Cinnamic acid | 0.76 ± 0.02 b | 0.83 ± 0.47 ab | 1.46 ± 0.40 a | 1.25 ± 0.89 a | 0.92 ± 0.18 ab | 1.38 ± 0.06 a |
| p-Coumaric acid | 0.56 ± 0.46 b | 0.87 ± 0.91 b | 0.31 ± 0.20 b | 0.63 ± 0.52 b | 0.48 ± 0.05 b | 7.43 ± 0.08 a |
| Ellagic acid | 2.20 ± 2.18 a | 3.55 ± 4.13 a | 0.88 ± 1.06 a | 2.49 ± 2.33 a | 2.31 ± 0.36 a | 0.48 ± 0.06 a |
| trans-Ferulic acid | 3.94 ± 1.98 a | 1.61 ± 1.11 a | 12.0 ± 8.67 a | 8.39 ± 11.7 a | 3.65 ± 0.47 a | 0.25 ± 0.01 a |
| Stilbene | ||||||
| Piceatannol | 1.48 ± 0.75 a | 2.43 ± 2.48 a | 4.44 ± 3.46 a | 3.01 ± 4.08 a | 1.17 ± 0.11 a | 1.03 ± 0.03 a |
| Resveratrol | 2.03 ± 0.92 a | 0.80 ± 0.20 abc | 1.17 ± 0.80 abc | 0.64 ± 0.47 bc | 0.90 ± 0.51 abc | 1.40 ± 0.20 ab |
| Flavonoids | ||||||
| iso-Liquiritigenin | 0.77 ± 0.92 b | 2.13 ± 0.57 a | 0.19 ± 0.01 b | 0.28 ± 0.13 b | 0.55 ± 0.54 b | 1.89 ± 0.15 a |
| Kaempferol | 1.08 ± 0.11 a | 0.92 ± 0.06 a | 1.18 ± 0.30 a | 1.03 ± 0.45 a | 1.28 ± 0.26 a | 2.26 ± 3.51 a |
| Kaempferide | 0.19 ± 0.02 b | 0.33 ± 0.18 b | 0.57 ± 0.29 b | 0.59 ± 0.40 b | 0.94 ± 1.02 ab | 1.74 ± 0.04 a |
| Myricetin | 6.32 ± 3.06 bc | 7.14 ± 5.25 bc | 18.2 ± 1.95 a | 14.7 ± 9.21 ab | 7.46 ± 1.01 bc | 0.15 ± 0.02 c |
| Naringerin | 0.76 ± 0.04 b | 0.59 ± 0.05 b | 0.78 ± 0.14 b | 0.72 ± 0.33 b | 0.81 ± 0.03 b | 1.16 ± 0.05 a |
| Quercetin | 0.44 ± 0.08 b | 0.43 ± 0.15 b | 0.79 ± 0.13 b | 1.10 ± 1.14 b | 1.14 ± 0.30 b | 5.79 ± 0.55 a |
| Rutin | 1.22 ± 1.56 a | 0.46 ± 0.66 a | 0.06 ± 0.15 a | 5.16 ± 8.81 a | 0.18 ± 0.04 a | 1.89 ± 0.13 a |
| Coumarins | ||||||
| Coumarin | 0.31 ± 0.40 a | 1.42 ± 1.21 a | 1.23 ± 0.07 a | 0.78 ± 0.77 a | 1.43 ± 0.80 a | 0.93 ± 0.01 a |
| 4-Methyl-umbelliferone | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.02 ± 0.01 b | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 3.80 ± 0.28 a |
| 7-Hydroxi-coumarin | 0.02 ± 0.01 b | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.00 ± 0.01 b | 0.01 ± 0.01 b | 1.28 ± 0.40 a |
| Scopoletin | 0.23 ± 0.04 ab | 0.33 ± 0.23 ab | 0.47 ± 0.27 a | 0.27 ± 0.34 ab | 0.24 ± 0.05 ab | 0.02 ± 0.01 b |
| Total phenolic-FC | 228.1 ± 17.29 d | 370.3 ± 48.27 abc | 292.5 ± 58.44 cd | 393.3 ± 65.69 ab | 301.5 ± 38.73 bc | 426.8 ± 10.76 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, W.A.; Bonfim, G.B.R.; Jesus, J.S.; Fonseca, R.F.S.; da Conceição, M.d.F.B.; Sousa, L.S.; Soares, S.A.R.; Mendes, B.A.; Anjos, J.P.; Dala-Paula, B.M.; et al. Influence of Jackfruit Wood Barrels and Chips During Aging on the Quality and Phenolic Compounds of Cachaça. Foods 2025, 14, 1812. https://doi.org/10.3390/foods14101812
Santos WA, Bonfim GBR, Jesus JS, Fonseca RFS, da Conceição MdFB, Sousa LS, Soares SAR, Mendes BA, Anjos JP, Dala-Paula BM, et al. Influence of Jackfruit Wood Barrels and Chips During Aging on the Quality and Phenolic Compounds of Cachaça. Foods. 2025; 14(10):1812. https://doi.org/10.3390/foods14101812
Chicago/Turabian StyleSantos, Wilton Amaral, Gabriel Benedito Rozendo Bonfim, Jaqueline Santos Jesus, Raimunda Fernandes Souza Fonseca, Maria de Fátima Bomfim da Conceição, Luciane Santos Sousa, Sarah Adriana Rocha Soares, Benjamim Almeida Mendes, Jeancarlo Pereira Anjos, Bruno Martins Dala-Paula, and et al. 2025. "Influence of Jackfruit Wood Barrels and Chips During Aging on the Quality and Phenolic Compounds of Cachaça" Foods 14, no. 10: 1812. https://doi.org/10.3390/foods14101812
APA StyleSantos, W. A., Bonfim, G. B. R., Jesus, J. S., Fonseca, R. F. S., da Conceição, M. d. F. B., Sousa, L. S., Soares, S. A. R., Mendes, B. A., Anjos, J. P., Dala-Paula, B. M., Gloria, M. B. A., & Mamede, M. E. O. (2025). Influence of Jackfruit Wood Barrels and Chips During Aging on the Quality and Phenolic Compounds of Cachaça. Foods, 14(10), 1812. https://doi.org/10.3390/foods14101812








