Efficiency of Ozonated Water Treatment with a Microbubble System for Sanitization and Preservation of Postharvest Quality of Acerolas
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Fruits and Initial Characterization
2.2. Ozone Treatment Settings
2.3. Acerola Storage After Ozonation
2.4. Microbiological Analyses
2.5. Physicochemical Quality Analysis
2.5.1. Fresh Mass Loss (%)
2.5.2. Firmness (Newton)
2.5.3. Total Soluble Solid Content
2.5.4. Potential of Hydrogen (pH)
2.5.5. Total Titratable Acidity (Citric Acid)
2.5.6. Vitamin C (Ascorbic Acid)
2.5.7. Pulp Color
2.5.8. Total Phenolic Compounds and Total Antioxidant Activity
Acerola Extract Preparation
Total Phenolic Compounds (Gallic Acid)
Total Antioxidant Activity According to the ABTS and DPPH Methods
2.6. Statistical Analysis
3. Results
3.1. Disinfection of Acerolas
3.2. Physicochemical Quality
3.2.1. Fresh Mass Loss, Firmness, Total Soluble Solids, Potential of Hydrogen, Total Titratable Acidity, and Vitamin C
3.2.2. Pulp Color, Total Phenolic Compounds, and Total Antioxidant Activity (ABTS and DPPH)
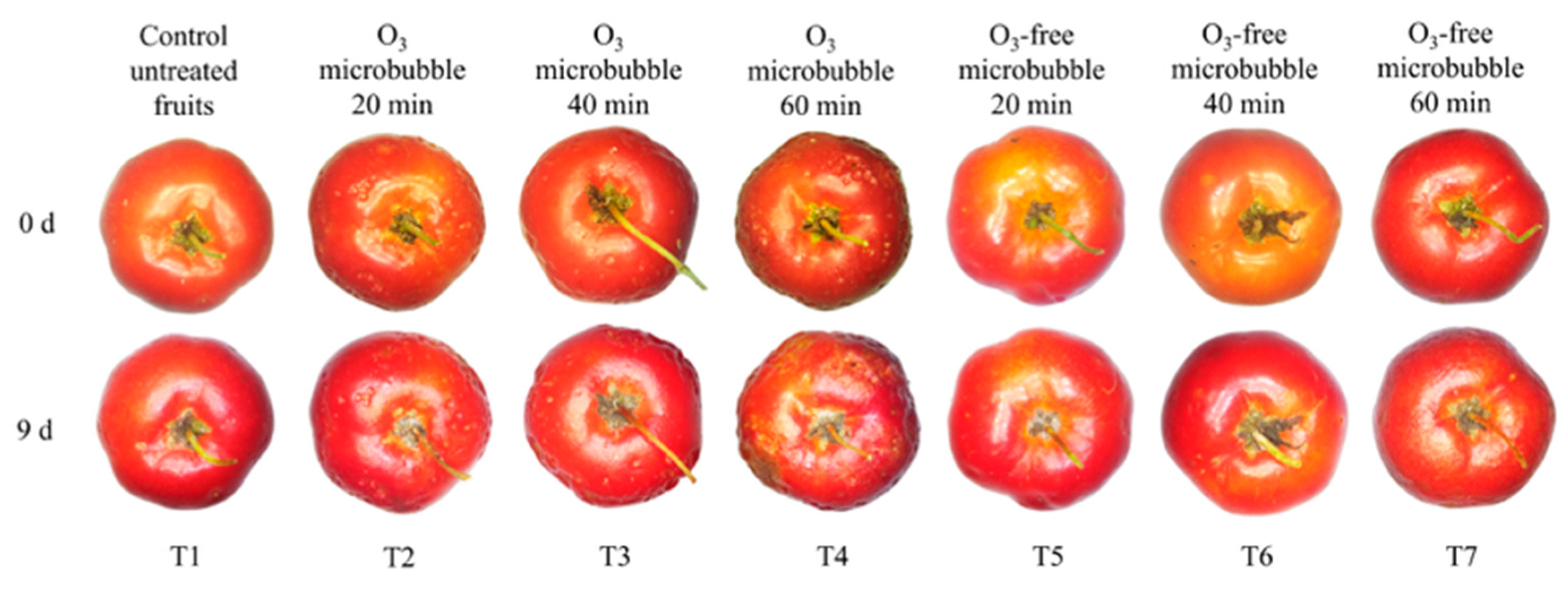
3.2.3. External Appearance of Acerolas During Storage
4. Discussion
4.1. Disinfection of Acerolas
4.2. Physicochemical Quality
4.2.1. Fresh Mass Loss, Firmness, Total Soluble Solids, Potential of Hydrogen, Total Titratable Acidity, and Vitamin C
4.2.2. Pulp Color, Total Phenolic Compounds, and Total Antioxidant Activity (ABTS and DPPH)
4.2.3. External Appearance of Acerolas During Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaeschke, D.P.; Marczak, L.D.F.; Mercali, G.D. Evaluation of non-thermal effects of electricity on ascorbic acid and carotenoid degradation in acerola pulp during ohmic heating. Food Chem. 2016, 199, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Baskaran, R. Acerola, an untapped functional superfruit: A review on latest frontiers. J. Food Sci. Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.S.; Souza, C.B.; Padilha, M.; Zoetendal, E.G.; Smidt, H.; Saad, S.M.I.; Koen Venema, K. Impact of a fermented soy beverage supplemented with acerola by-product on the gut microbiota from lean and obese subjects using an in vitro model of the human colon. Environ. Biotechnol. 2021, 105, 3771–3785. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.D.; Silva, J.; Petronilho, F.C.; Biélla, M.S.; Lopes, A.; Binatti, A.R.; Daumann, F.; Schuck, P.F.; Andrade, V.M. Acerola (Malpighia emarginata DC.) juice intake protects against oxidative damage in mice fed by cafeteria diet. Food Res. Int. 2015, 77, 649–656. [Google Scholar] [CrossRef]
- Horta, R.N.; Kahl, V.F.S.; Sarmento, M.S.; Nunes, M.F.S.; Porto, C.R.M.; Andrade, V.M.; Ferraz, A.B.F.; Silva, J. Protective effects of acerola juice on genotoxicity induced by iron in vivo. Genet. Mol. Biol. 2016, 39, 122–128. [Google Scholar] [CrossRef]
- Belwal, T.; Devkota, H.P.; Hassan, H.A.; Ahluwalia, S.; Ramadan, M.F.; Mocan, A.; Atanasov, A.G. Phytopharmacology of acerola (Malpighia spp.) and its potential as functional food. Trends Food Sci. Technol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; Leblanc, A.M.; Saad, S.M.I.; Leblanc, J.G. Tropical fruit by-products water extracts as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Carpentieri-Pípolo, V.; Prete, C.E.C.; Gonzalez, M.G.N.; Popper, I.O. Three new caribbean cherry (Malpighia emarginata DC) cultivars: UEL-3 Dominga, UEL 4-Ligia and UEL 5–Natalia. Rev. Bras. Frutic. 2002, 24, 124–126. [Google Scholar] [CrossRef]
- Mercali, G.D.; Schwartz, S.; Marczak, L.D.F.; Tessaro, I.C.; Sastry, S. Ascorbic acid degradation and color changes in acerola pulp during ohmic heating: Effect of electric field frequency. J. Food Eng. 2014, 123, 1–7. [Google Scholar] [CrossRef]
- Mazaro, S.M.; Borsatti, F.C.; Dalacosta, N.L.; Lewandowski, A.; Danner, M.A.; Busso, C.; Wagner-Junior, A. Qualidade pós-colheita de acerolas tratadas com ácido salicílico. Rev. Bras. Ciências Agrárias 2015, 10, 512–517. [Google Scholar] [CrossRef][Green Version]
- Araújo, M.N.T.; Castro, R.S.; Rodrigues, A.C.S.; Rêgo, J.F.; Uchôa, V.T. Evaluation of vitamin c content in acerola pulp marketed in supermarkets in Piripiri-PI. Ciência Agrícola 2017, 15, 59–68. [Google Scholar] [CrossRef]
- Delva, L.; Schneider, R.G. Acerola (Malpighia emarginata DC): Production, Postharvest Handling, Nutrition, and Biological Activity. Food Rev. Int. 2013, 29, 107–126. [Google Scholar] [CrossRef]
- Mendes, A.M.S.; Oliveira, A.R.; Teixeira, A.H.C.; Bastos, D.C.; Batista, D.C.; Angelotti, F.; Leal, I.M.; Oliveira, J.R.P.; Anjos, J.B.; Flori, J.E.; et al. A Cultura da Acerola, 3rd ed.; Coleção Plantar 69; Calgaro, M., Braga, M.B., Eds.; Embrapa: Brasília, Brazil, 2012; 144p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/128278/1/PLANTAR-Acerola-ed03-2012.pdf (accessed on 15 May 2023).
- Souza, K.O.; Moura, C.F.H.; Lopes, M.M.A.; Rabelo, M.C.; Miranda, M.R.A. Quality of acerola (Malpighia emarginata) treated with gibberelic acid and stored under refrigeration. Rev. Bras. Frutic. 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M. Acerola (Malpighia emarginata DC.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Limited.: Sawston, UK, 2011; Chapter 2; pp. 27–47. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Censo Agropecuário. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/acerola/br (accessed on 5 May 2025).
- Santos, P.H.; Villwock, A.P.S. Analysis of the economic feasibility of acerola cultivation on family property in the state of Sergipe. Extensão Rural 2024, 31, e71667. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2481–2508. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Simpson, A.M.A.; Mitch, W.A. Chlorine and ozone disinfection and disinfection byproducts in postharvest food processing facilities: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1825–1867. [Google Scholar] [CrossRef]
- Chinchkar, A.V.; Singh, A.; Singh, S.V.; Acharya, A.M.; Kamble, M.G. Potential sanitizers and disinfectants for fresh fruits and vegetables: A comprehensive review. J. Food Process. Preserv. 2022, 46, e16495. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT-Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Dubey, P.; Singh, A.; Yousuf, O. Ozonation: An evolving disinfectant technology for the food industry. Food Bioprocess Technol. 2022, 15, 2102–2113. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Singh, A.; Agriopoulou, S.; Sachadyn-Król, M.; Aslam, R.; Lima, C.M.G.; Khanashyam, A.C.; Kothakota, A.; Atakan, O.; Kuman, M.; et al. A comprehensive review of impacts of ozone treatment on textural properties in different food products. Trends Food Sci. Technol. 2022, 127, 74–86. [Google Scholar] [CrossRef]
- Zhou, Z.; Zuber, S.; Cantergiani, F.; Sampers, I.; Devlieghere, F.; Uyttendaele, M. Inactivation of foodborne pathogens and their surrogates on fresh and frozen strawberries using gaseous ozone. Front. Sustain. Food Syst. 2018, 2, 51. [Google Scholar] [CrossRef]
- Castro Medeiros, L.; Alencar, F.L.S.; Navoni, J.A.; Araujo, A.L.C.; Amaral, V.S. Toxicological aspects of trihalomethanes: A systematic review. Environ. Sci. Pollut. Res. 2019, 26, 5316–5332. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, B.; Li, W.; Li, B.; Han, Z.; Zhang, Y.; Ding, U.; Wang, S.; Ma, J.; Ele, X. The catalytic oxidation process of atrazine by ozone microbubbles: Bubble formation, ozone mass transfer and hydroxyl radical generation. Chemosphere 2023, 325, e138361. [Google Scholar] [CrossRef]
- John, A.; Mahato, L.K.; Devashish, K.; Nayak, S.; Javed, S.M.; Sinha, H. Comparison of misoprostol in conjunction with oxytocin and oxytocin alone for the prevention of postpartum hemorrhage during active management of the third stage of labor. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1682–1685. [Google Scholar] [CrossRef]
- Li, C.; Xie, Y.; Guo, Y.; Cheng, Y.; Yu, P.; Qian, E.; Yao, W. Effects of ozone-microbubble treatment on the removal of residual pesticides and the adsorption mechanism of pesticides onto the apple matrix. Food Control 2021, 120, e107548. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Liu, F.; Zhang, X.; Chen, X.; Peng, Q.; Wub, G.; Zhaob, Z. Substantial removal of four pesticide residues in three fruits with ozone microbubbles. Food Chem. 2024, 441, e138293. [Google Scholar] [CrossRef] [PubMed]
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, C.M.; Somiya, I. Guideline for measurement of ozone concentration in the process gas from an ozone generator. Ozone Sci. Eng. 1996, 18, 209–229. [Google Scholar] [CrossRef]
- ISO 6887-1; Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. International Organization for Standardization (ISO): Geneva, Switzerland, 1999. Available online: https://cdn.standards.iteh.ai/samples/26850/ede9dca9b457404388bb34d2ab341d68/ISO-6887-1-1999.pdf (accessed on 7 May 2025).
- Salfinger, Y.; Tortorello, M.L. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association-APHA: Washington, DC, USA, 2015. [Google Scholar]
- IAL. Métodos Físicos-Químicos Para Análise de Alimentos, 1st ed. 2008. Available online: https://wp.ufpel.edu.br/nutricaobromatologia/files/2013/07/NormasADOLFOLUTZ.pdf (accessed on 18 April 2023).
- CIE. Commission Internationale de L’éclairage, International Commission on Illumination, Internationaal Beleuchtungskommission. CIE Technical Report Colorimetry, 3rd ed. 2004. Available online: https://cielab.xyz/pdf/cie.15.2004%20colorimetry.pdf (accessed on 10 January 2024).
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Ji, L.; Pang, J.; Li, S.; Xiong, B.; Cai, L.-G. Application of new physical storage technology in fruit and vegetable industry. Afr. J. Biotechnol. 2012, 11, 6718–6722. [Google Scholar] [CrossRef]
- Manousaridis, G.; Nerantzaki, A.; Paleologos, E.K.; Tsiotsias, A.; Savvaidis, I.N.; Kontominas, M.G. Effect of ozone on microbial, chemical and sensory attributes of shucked mussels. Food Microbiol. 2005, 22, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Song, Y.; Li, M.; Yu, Q. Quality of vacuum packaged beef as affected by aqueous ozone and sodium citrate treatment. Int. J. Food Prop. 2020, 23, 1475–1489. [Google Scholar] [CrossRef]
- Lone, S.A.; Raghunathan, S.; Davoodbasha, M.; Srinivasan, H.; Lee, S. An investigation on the sterilization of berry fruit using ozone: An option to preservation and longterm storage. Biocatal. Agric. Biotechnol. 2019, 20, e101212. [Google Scholar] [CrossRef]
- Costa, A.R.; Faroni, L.R.A.; Salomão, L.C.C.; Cecon, P.R.; Alencar, E.R. Use of Ozonized Water to Control Anthracnose in Papaya (Carica papaya L.) and its effect on the Quality of the Fruits. Ozone Sci. Eng. 2020, 43, 384–393. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, G.M.; Contigiani, E.V.; Castro, M.A.; Hodara, K.; Alzamora, S.M.; Loredo, A.G.; Nieto, A.B. Freshness Maintenance of Blueberries (Vaccinium corymbosum L.) During Postharvest Using Ozone in Aqueous Phase: Microbiological, Structure, and Mechanical issues. Food Bioprocess Technol. 2019, 12, 2136–2147. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Jaramillo-Sánchez, G.; Castro, M.A.; Gómez, P.L.; Alzamora, S.M. Postharvest Quality of Strawberry Fruit (Fragaria x Ananassa Duch cv. Albion) as Affected by Ozone Washing: Fungal Spoilage, Mechanical Properties, and Structure. Food Bioprocess Technol. 2018, 11, 1639–1650. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Kronberg, M.F.; Sánchez, G.J.; Gómez, P.L.; Gárcia-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone washing decreases strawberry susceptibility to Botrytis cinerea while maintaining antioxidant, optical and sensory quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Santos-Pedro, D.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of aqueous ozone, blanching and combined treatments on microbial load of red bell peppers, strawberries and watercress. J. Food Eng. 2011, 105, 277–282. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Raheem, M.I.U.; Khan, A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined aqueous ozone and ultrasound application inhibits microbial spoilage, reduces pesticide residues and maintains storage quality of strawberry fruits. J. Food Meas. Charact. 2020, 15, 1437–1451. [Google Scholar] [CrossRef]
- Alves, H.; Alencar, E.R.; Ferreira, W.F.S.; Silva, C.R.; Ribeiro, J.L. Microbiological and physical-chemical aspects of strawberry exposed to ozone gas at different concentrations during storage. Braz. J. Food Technol. 2019, 22, e2018002. [Google Scholar] [CrossRef]
- Alencar, E.R.; Faroni, L.R.A.; Pinto, M.S.; Costa, A.R.; Silva, T.A. Postharvest quality of ozonized “nanicão” cv. Bananas. Rev. Ciência Agronômica 2013, 44, 107–114. [Google Scholar] [CrossRef]
- Cordeiro, Z.J.M.; Matos, A.P.; Meissner Filho, P.E. Doenças e métodos de controle. In O Cultivo da Bananeira; Borges, A.L., Souza, L.S., Eds.; Nova Civilização: Cruz das Almas, Brazil, 2004; pp. 146–182. [Google Scholar]
- Rodrigues, A.A.Z.; Queiroz, M.E.L.R.; Faroni, L.R.A.; Prates, L.H.F.; Neves, A.A.; Oliveira, A.F.; Freitas, J.F.; Heleno, F.F.; Zambolim, L. The efficacy of washing strategies in the elimination of fungicide residues and the alterations on the quality of bell peppers. Food Res. Int. 2021, 147, e110549. [Google Scholar] [CrossRef]
- Suslow, T.V.; Oria, M.P.; Beuchat, L.R.; Garrett, E.H.; Parish, M.E.; Harris, L.J.; Farber, J.N.; Busta, F.F. Production practices as risk factors in microbial food safety of fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 38–77. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Zhao, Q.; Liu, S.; Hu, W. Effects of ozonated water on microbial growth, quality retention and pesticide residue removal of fresh-cut onions. Ozone Sci. Eng. 2020, 42, 399–407. [Google Scholar] [CrossRef]
- Gonçalves-Magalhães, C.; Faroni, L.R.A.; Cecon, P.R.; Alencar, E.R.; Silva, M.V.A.; Rodrigues, A.A.Z.; Sitoe, E.P.E.; Massango, H.G.L.L. Potential of ozonated mist for microbiological disinfection and preservation of the physicochemical quality of strawberries. Ozone Sci. Eng. 2025, 1–15. [Google Scholar] [CrossRef]
- Shin, Y.; Ryu, J.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Wyrwisz, J.; Moczkowska, M.; Stelmasiak, A.; Lipińska, A.; Szpicer, A.; Zalewska, M.; Zaremba, R.; Kuboń, M.; et al. Impact of ozonisation on pro-health properties and antioxidant capacity of ‘Honeoye’ strawberry fruit. CyTA J. Food 2017, 15, 58–64. [Google Scholar] [CrossRef]
- Chitarra, M.I.F.; Chitarra, B.F. Tecnologia e Qualidade Pós-colheita de Frutas e Hortaliças, 2nd ed.; UFLA: Lavras, Brazil, 2005; 785p. [Google Scholar]
- Carboni, K.; Mencarelli, F. Influence of short-term postharvest ozone treatments in nitrogen or air atmosphere on the metabolic response of white wine grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- Duarte-Molina, F.; Gómez, P.L.; Agueda Castro, M.; Alzamora, S.M. Storage quality of strawberry fruit treated by pulsed light: Fungal decay, water loss and mechanical properties. Innov. Food Sci. Emerg. Technol. 2016, 34, 267–274. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef]
- Nayak, S.L.; Sethi, S.; Sharma, R.R.; Sharma, R.M. Aqueous ozone controls decay and maintains quality attributes of strawberry (Fragaria × ananassa Duch.). J. Food Sci. Technol. 2020, 57, 319–326. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Tao, J.; Wang, J.; Wu, Z. Monitoring quality parameters and antioxidant potential of fresh-cut red pitaya fruit treated with gaseous ozone using kinetic models. J. Food Meas. Charact. 2023, 17, 4208–4224. [Google Scholar] [CrossRef]
- Vlassi, E.; Vlachos, P.; Kornaros, M. Effect of ozonation on table grapes preservation in cold storage. J. Food Sci. Technol. 2018, 55, 2031–2038. [Google Scholar] [CrossRef]
- Pandit, P.S.; Shukla, S.P. Effect of ozonized reverse osmosis plant water washing on microbial load of lychee. Acta Hortic. 2018, 1211, 23–28. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Galactosidase during Fruit Development and Ripening in Two Different Texture Types of Apple Cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef]
- Piechowiak, T.; Migut, D.; Józefczyk, R.; Balawejder, M. Ozone Treatment Improves the Texture of Strawberry Fruit during Storage. Antioxidants 2022, 11, e821. [Google Scholar] [CrossRef]
- Toti, M.; Carboni, C.; Botondi, R. Postharvest gaseous ozone treatment enhances quality parameters and delays softening in cantaloupe melon during storage at 6 °C. J. Sci. Food Agric. 2018, 98, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Goffi, V.; Modesti, M.; Forniti, R.; Botondi, R. Quality of green (Actinidia chinensis var. deliciosa ‘Hayward’) and yellow (A. chinensis var. chinensis ‘Soreli’) kiwifruit during cold storage at 0 °C in normal atmosphere and with gaseous ozone. Acta Hortic. 2018, 1218, 473–480. [Google Scholar] [CrossRef]
- Brazil. Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa nº 37, de 01 de outubro de 2018 que Estabele os Parâmetros Analíticos de Suco e de Polpa de Frutas e a Listagem das Frutas e Demais Quesitos Complementares aos Padrões de Identidade e Qualidade já Fixados pelo Ministro da Agricultura, Pecuária e Abastecimento através da IN MAPA nº 49, de 26 de Setembro de 2018. Normas Brasil. 2018. Available online: https://www.normasbrasil.com.br/norma/instrucao-normativa-37-2018_368178.html (accessed on 18 January 2024).
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd ed.Artmed: Porto Alegre, Brazil, 2004. [Google Scholar]
- Moing, A.; Renaud, C.; Gaudillère, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar] [CrossRef]
- Barboni, T.; Cannac, M.; Chiaramonti, N. Effect of cold storage and ozone treatment on physicochemical parameters, soluble sugars and organic acids in Actinidia deliciosa. Food Chem. 2010, 121, 946–951. [Google Scholar] [CrossRef]
- Onopiuk, A.; Póltorak, A.; Moczkowska, M.; Szpicer, A.; Wierzbicka, A. The impact of ozone on health-promoting, microbiological, and colour properties of Rubus ideaus raspberries. CyTA J. Food 2017, 15, 563–573. [Google Scholar] [CrossRef]
- Chatzopoulou, F.; Sanmartin, M.; Mellidou, I.; Pateraki, I.; Koukounaras, A.; Tanou, G.; Kalamaki, M.S.; Veljović-Jovanović, S.; Antić, T.C.; Kostas, S.; et al. Silencing of ascorbate oxidase results in reduced growth, altered ascorbic acid levels and ripening pattern in melon fruit. Plant Physiol. Biochem. 2020, 156, 291–303. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Frutas-IBRAF. Soluções Fruta a Fruta: Acerola, 2nd ed.; IBRAF: São Paulo, Brazil, 1995; 61p. [Google Scholar]
- Tabakoglu, N.; Karaca, H. Effects of ozone-enriched storage atmosphere on postharvest quality of black mulberry fruits (Morus nigra L.). LWT-Food Sci. Technol. 2018, 92, 276–281. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Olmo, M.; García, J.M. Effect of ozone treatment on postharvest disease and quality of diferente citrus varieties at laboratory and at industrial facility. Postharvest Biol. Technol. 2018, 137, 77–85. [Google Scholar] [CrossRef]
- Araújo, P.G.L.; Figueiredo, R.W.; Alves, R.E.; Maia, G.A.; Moura, C.F.H.; Sousa, P.H.M. Physico-chemical and chemical quality of acerola fruit clones coated with PVC film and conserved under refrigeration. Semin. Ciências Agrárias 2009, 30, 867–880. Available online: http://www.alice.cnptia.embrapa.br/alice/handle/doc/631942 (accessed on 8 August 2023). [CrossRef]
- Lima, R.M.T.; Figueiredo, R.W.; Maia, G.A.; Sousa, P.H.M.; Figueiredo, E.A.T.; Rodrigues, C.S. Chemical, physicochemical and microbiological stability of pasteurized and non pasteurized acerola pulps from organic cultivation. Ciência Rural 2012, 42, 367–373. [Google Scholar] [CrossRef]
- Ong, M.K.; Ali, A.; Alderson, P.G.; Forney, C.F. Effect of different concentrations of ozone on physiological changes associated to gas exchange, fruit ripening, fruit surface quality and defense-related enzymes levels in papaya fruit during ambient storage. Sci. Hortic. 2014, 179, 163–169. [Google Scholar] [CrossRef]
- Gonçalves-Magalhães, C.; Faroni, L.R.A.; Alencar, E.R.; Rodrigues, A.A.Z.; Cecon, P.R.; Silva, M.V.A.; Sitoe, E.P.E.; Melo, C.E.L.D. Postharvest Quality of Lychee Treated with Ozone Gas or Ozonated Mist. Rev. Bras. Eng. Agrícola E Ambient. 2024, 28, e280838. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, G.M.; Loredo, A.G.; Goméz, P.L.; Alzamora, S.M. Ozone processing of peach Juice: Impact on physicochemical parameters, color, and viscosity. Ozone Sci. Eng. 2018, 40, 305–312. [Google Scholar] [CrossRef]
- Goffi, V.; Magri, A.; Botondi, R.; Petriccione, M. Response of antioxidant system to postharvest ozone treatment in ‘Soreli’ kiwifruit. Sci. Food Agric. 2020, 100, 961–968. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Balawejder, M. Ozone Treatment Induces Changes in Antioxidative Defense System in Blueberry Fruit During Storage. Food Bioprocess Technol. 2020, 13, 1240–1245. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT-Food Sci. Technol. 2019, 115, e108447. [Google Scholar] [CrossRef]
- Kaur, K.; Pandiselvam, R.; Kothakota, A.; Ishwarya, S.P.; Zalpouri, R.; Mahanti, N.K. Impact of ozone treatment on food polyphenols—A comprehensive review. Food Control 2022, 142, e109207. [Google Scholar] [CrossRef]
- Lv, Y.; Tahirb, I.I.; Olssonb, M.E. Effect of ozone application on bioactive compounds of apple fruit during short-term cold storage. Sci. Hortic. 2019, 253, 49–60. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, G.; Han, Z.; Li, Q.; Chen, Y.; Li, D. Effect of ozone on the antioxidant capacity of “Qiushui” pear (Pyrus pyrifolia Nakai cv. Qiushui) during postharvest storage. J. Food Qual. 2013, 36, 190–197. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, X.; Gao, H.; Zhang, Z.; Zhu, H.; Duan, X.; Qu, H.; Yun, Z.; Jiang, Y. Comparative profiling of primary metabolites and volatile compounds in Satsuma mandarin peel after ozone treatment. Postharvest Biol. Technol. 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Beltrán, D.; Selma, M.V.; Marín, A.; Gil, M.I. Ozonated water extends the shelf life of fresh-cut lettuce. J. Agric. Food Chem. 2005, 53, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Muzammil, R.; Saha, S.; Shabeer, A.; Oulkar, A.; Banerjee, K.; Singh, S.B. Evaluation of ozonation technique for pesticide residue removal and its effect on ascorbic acid, cyanidin-3-glucoside, and polyphenols in apple (Malus domesticus) fruits. Environ. Monit. Assess. 2016, 188, 301. [Google Scholar] [CrossRef] [PubMed]
- Agostini-Costa, T.S.; Abreu, L.N.; Rossetti, A.G. Effect of freezing and storing time of pulp of acerola on the carotenoid contents. Rev. Bras. Frutic. 2003, 25, 56–58. [Google Scholar] [CrossRef]
- Mezadri, T.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoid pigments in acerola fruits (Malpighia emarginata DC.) and derived products. Eur. Food Res. Technol. 2005, 220, 63–69. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Tremarin, A.; Garcia, E.; Brandão, T.R.S.; Silva, C.L.M. Quality assessment of Cantaloupe melon juice under ozone processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 461–466. [Google Scholar] [CrossRef]
- Santos, D.Y.A.C.; Egydio, A.P.M. Total wax and n-alkane profiles from fruit and leaf waxes of Malpighia glabra L. Bol. Botânica Da Univ. São Paulo 2010, 28, 1–7. Available online: https://www.jstor.org/stable/42871709 (accessed on 4 April 2023).
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Mukhtar, A.; Damerow, L.; Blanke, M. Non-invasive assessment of glossiness and polishing of the wax bloom of European plum. Postharvest Biol. Technol. 2014, 87, 144–151. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.J.; Contigiani, E.V.; Alzamora, S.M.; Santagapita, P.R. Changes on epicuticular waxes and colour induced by ozone in blueberries (Vaccinium corymbosum L.’ O’ Neal’). J. Food Compos. Anal. 2022, 108, e104404. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Saeed, P.A. Sanitization potential of ozone and its role in postharvest quality management of fruits and vegetables. Food Eng. Rev. 2020, 12, 48–67. [Google Scholar] [CrossRef]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro-and nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Jahan, B.N.; Li, L.; Pagilla, K.R. Fate and reduction of bromate formed in advanced water treatment ozonation systems: A critical review. Chemosphere 2021, 266, 128964. [Google Scholar] [CrossRef]
- Nuvolone, D.; Petri, D.; Voller, F. The effects of ozone on human health. Environ. Sci. Pollut. Res. 2018, 25, 8074–8088. [Google Scholar] [CrossRef]
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of ozone in water: A review. Ozone Sci. Eng. 2012, 34, 233–242. [Google Scholar] [CrossRef]

| Aerobic Mesophiles (log CFU g−1) | ||||
|---|---|---|---|---|
| Treatment | 0 | 3 | 6 | 9 |
| T1 | 4.42 | 4.03 | Uncountable | Uncountable |
| T2 | 3.65 | <2.0 log (CFU g−1) | 4.79 | 3.40 |
| T3 | 3.70 | 4.92 | 5.52 | 4.84 |
| T4 | 3.43 | 4.53 | 4.25 | 4.23 |
| T5 | 3.76 | 5.54 | Uncountable | Uncountable |
| T6 | 3.71 | 5.19 | Uncountable | Uncountable |
| T7 | 5.09 | 5.10 | Uncountable | Uncountable |
| Treatment | Filamentous Fungi and Yeasts (log CFU g−1) | |||
| T1 | <2.0 log (CFU g−1) | 5.04 | Uncountable | Uncountable |
| T2 | <2.0 log (CFU g−1) | <2.0 log (CFU g−1) | 5.76 | 6.77 |
| T3 | <2.0 log (CFU g−1) | 5.32 | 6.04 | 6.02 |
| T4 | 4.32 | 4.45 | 4.63 | 4.73 |
| T5 | <2.0 log (CFU g−1) | 5.36 | Uncountable | Uncountable |
| T6 | <2.0 log (CFU g−1) | 6.23 | Uncountable | Uncountable |
| T7 | 4.41 | 5.50 | Uncountable | Uncountable |
| Fresh Mass Loss (%) | ||||
|---|---|---|---|---|
| Storage (Days) | ||||
| Treatment | 0 | 3 | 6 | 9 |
| T1 | - | 1.25 a | 2.41 a | 3.91 a |
| T2 | - | 1.42 a | 2.67 a | 3.95 a |
| T3 | - | 1.37 a | 2.49 a | 3.96 a |
| T4 | - | 1.12 a | 2.78 a | 4.19 a |
| T5 | - | 1.38 a | 2.54 a | 4.15 a |
| T6 | - | 1.55 a | 2.54 a | 4.01 a |
| T7 | - | 1.21 a | 2.41 a | 3.97 a |
| Treatment | Firmness (N) | |||
| T1 | 0.51 a | 0.52 ab | 0.27 c | 0.11 c |
| T2 | 0.46 a | 0.46 abc | 0.46 ab* | 0.27 b* |
| T3 | 0.48 a | 0.48 abc | 0.35 bc | 0.25 b* |
| T4 | 0.55 a | 0.54 a | 0.52 a* | 0.37 a* |
| T5 | 0.51 a | 0.29 d* | 0.29 c | 0.11 c |
| T6 | 0.52 a | 0.38 bcd* | 0.30 c | 0.13 c |
| T7 | 0.41 a | 0.34 cd* | 0.35 bc | 0.14 c |
| Treatment | Total soluble solid content (%) | |||
| T1 | 10.60 a | 10.83 ab | 9.57 c | 9.30 c |
| T2 | 9.70 b* | 10.47 b | 9.77 bc | 9.33 c |
| T3 | 8.73 d* | 10.67 ab | 9.50 c | 10.10 b* |
| T4 | 9.20 c* | 10.80 ab | 10.10 b* | 10.17 ab* |
| T5 | 10.00 b* | 11.27 a | 10.57 a* | 10.67 a* |
| T6 | 9.83 b* | 10.63 ab | 10.07 b* | 10.63 ab* |
| T7 | 9.70 b* | 10.30 b | 9.10 d* | 9.10 c |
| Treatment | Potential of Hydrogen (pH) | |||
| T1 | 2.90 b | 2.70 c | 3.10 a | 3.10 a |
| T2 | 2.57 d* | 2.70 c | 2.90 b* | 2.90 c* |
| T3 | 2.77 c* | 2.80 b | 2.87 b* | 3.00 b |
| T4 | 2.80 c* | 2.60 d | 3.10 a | 3.00 b |
| T5 | 2.90 b | 2.90 a* | 3.10 a | 3.00 b |
| T6 | 2.90 b | 2.90 a* | 3.07 a | 3.00 b |
| T7 | 3.00 a* | 2.90 a* | 3.10 a | 3.00 b |
| Treatment | Total titratable acidity (citric acid) | |||
| T1 | 2.53 ab | 2.93 a | 2.11 a | 1.85 a |
| T2 | 2.55 a | 2.18 b* | 2.07 a | 1.81 a |
| T3 | 2.32 c* | 1.95 e* | 1.78 bc* | 1.58 c* |
| T4 | 2.53 ab | 2.09 c* | 1.83 b* | 1.69 b* |
| T5 | 2.44 b | 1.54 f* | 1.87 b* | 1.59 c* |
| T6 | 2.22 c* | 2.03 d* | 1.69 c* | 1.57 c* |
| T7 | 2.03 d* | 2.07 cd* | 1.88 b* | 1.87 a |
| Treatment | Vitamin C (ascorbic acid) | |||
| T1 | 1673.14 a | 1585.08 a | 1135.97 b | 951.05 b |
| T2 | 1617.37 ab | 1435.38 b* | 1241.65 a* | 1121.30 a* |
| T3 | 1661.40 a | 1100.75 d* | 1012.69 c* | 789.60 c* |
| T4 | 1602.69 ab | 1138.91 c* | 883.54 d* | 786.67 c* |
| T5 | 1361.99 bc* | 1091.94 d* | 821.89 e* | 636.97 d* |
| T6 | 1479.41 abc | 1015.63 e* | 774.93 e* | 545.97 e* |
| T7 | 1309.16 c* | 1162.39 c* | 983.34 c* | 968.66 b |
| Variable | Treatment | Adjusted Equations | R2/r2 |
|---|---|---|---|
| Fresh mass loss (%) | Control (untreated fruits) (T1) | −0.144442 + 0.4447 ** ST | 0.9422 |
| Ozone microbubble (T2, T3, and T4) | −0.07661 + 0.45513 ** ST | 0.9884 | |
| Ozone-free microbubble (T5, T6, and T7) | −0.02336 + 0.44007 ** ST | 0.9836 | |
| Variable | Treatment | Adjusted equations | R2/r2 |
| Firmness (N) | Control (untreated fruits) (T1) | 0.567 − 0.04766 ** ST | 0.8827 |
| Ozone microbubble (T2, T3, and T4) | 0.609611 + 0.01487 ns ST − 0.0040432 ** ST2 − 0.00977 * ET + 0.0001468 * ET2 | 0.9312 | |
| Ozone-free microbubble (T5, T6, and T7) | 0.4768 − 0.03622 ** ST | 0.8707 | |
| Variable | Treatment | Adjusted equations | R2/r2 |
| Total soluble solids content (%) | Control (untreated fruits) (T1) | 10.85 − 0.17222 * ST | 0.7806 |
| Ozone microbubble (T2, T3, and T4) | 9.87 | - | |
| Ozone-free microbubble (T5, T6, and T7) | 10.15 | - | |
| Variable | Treatment | Adjusted equations | R2/r2 |
| Potential of Hydrogen (pH) | Control (untreated fruits) (T1) | 2.95 | - |
| Ozone microbubble (T2, T3, and T4) | 2.68 + 0.03407 ** ST | 0.5467 | |
| Ozone-free microbubble (T5, T6, and T7) | 2.98 | - | |
| Variable | Treatment | Adjusted equations | R2/r2 |
| Total titratable acidity (citric acid) | Control (untreated fruits) (T1) | 2.35 | - |
| Ozone microbubble (T2, T3, and T4) | 2.41 − 0.08377 ** ST | 0.8503 | |
| Ozone-free microbubble (T5, T6, and T7) | 2.1587 − 0.05754 ** ST | 0.5380 | |
| Variable | Treatment | Adjusted equations | R2/r2 |
| Vitamin C (ascorbic acid) | Control (untreated fruits) (T1) | 1728.62 − 87.1794 * ST | 0.9398 |
| Ozone microbubble (T2, T3, and T4) | 1804.74 − 62.742 ** ST − 7.8764 ** ET | 0.8753 | |
| Ozone-free microbubble (T5, T6, and T7) | 1347.02 − 74.296 ** ST | 0.8178 |
| Color Difference (Dif*) | ||||
|---|---|---|---|---|
| Storage (Days) | ||||
| Treatment | 0 | 3 | 6 | 9 |
| T1 | 0.83 d | 2.27 b | 4.83 b | 10.17 c |
| T2 | 4.19 a* | 2.21 b | 2.96 cde* | 12.41 b* |
| T3 | 3.23 b* | 2.72 ab | 2.14 e* | 14.11 a* |
| T4 | 4.06 ab* | 3.57 ab | 4.00 bc | 14.16 a* |
| T5 | 1.85 c* | 2.42 b | 2.36 de* | 10.92 c |
| T6 | 0.94 d | 3.51 ab | 3.29 cd* | 11.01 c |
| T7 | 1.12 cd | 4.07 a* | 6.60 a* | 14.23 a* |
| Treatment | Color hue (h*) | |||
| T1 | 22.43 e | 26.06 c | 26.96 e | 33.04 de |
| T2 | 24.92 bc* | 28.21 b* | 28.72 cd* | 40.44 b* |
| T3 | 27.75 a* | 28.24 b* | 30.04 b* | 42.36 a* |
| T4 | 23.95 d* | 30.41 a* | 32.22 a* | 40.25 b* |
| T5 | 24.55 bcd* | 29.73 ab* | 28.39 d* | 34.49 c* |
| T6 | 25.26 b* | 28.40 b* | 28.98 cd* | 33.74 cd |
| T7 | 24.28 cd* | 29.03 ab* | 29.58 bc* | 32.48 e |
| Treatment | Color saturation (C*) | |||
| T1 | 11.90 cd | 13.55 d | 15.61 a | 17.56 c |
| T2 | 15.09 a* | 15.71 b* | 15.56 a | 22.57 a* |
| T3 | 13.80 b* | 15.70 b* | 12.47 c* | 22.96 a* |
| T4 | 14.83 a* | 17.09 a* | 15.93 a | 23.17 a* |
| T5 | 13.38 b* | 13.14 d | 13.50 bc* | 19.34 b* |
| T6 | 12.11 c | 14.78 bc* | 13.51 b* | 17.98 c |
| T7 | 11.16 d | 14.15 cd | 15.86 a | 20.65 b* |
| Treatment | Total phenolic compounds (gallic acid) | |||
| T1 | 2392.98 ab | 1727.81 a | 1481.28 ab | 1412.35 ab |
| T2 | 2672.61 a | 1686.00 a | 1503.89 a | 1487.73 a |
| T3 | 2154.00 b | 1363.68 c* | 1268.81 c* | 1214.07 bc |
| T4 | 2537.98 ab | 1547.42 b* | 1415.32 b | 1292.37 ab |
| T5 | 1617.19 c* | 1525.00 b* | 1475.75 ab | 1041.18 c* |
| T6 | 1604.04 c* | 1520.04 b* | 1451.89 ab | 1365.30 ab |
| T7 | 1517.11 c* | 1492.39 b* | 1481.05 ab | 1415.84 ab |
| Treatment | Total antioxidant activity ABTS (Trolox) | |||
| T1 | 9814.48 a | 7719.56 abc | 7433.29 ab | 6943.30 ab |
| T2 | 8656.72 b* | 8536.92 a | 8199.30 a | 6665.47 b |
| T3 | 7206.77 c* | 7016.03 bc | 4136.04 d* | 4062.47 e* |
| T4 | 9204.84 ab* | 7672.56 abc | 7150.03 abc | 6138.27 c* |
| T5 | 9181.46 ab* | 8074.13 ab | 7067.67 abc | 7045.56 a |
| T6 | 6954.81 c* | 6855.22 c | 6754.25 bc | 6064.99 c* |
| T7 | 9323.78 a | 7453.67 abc | 6139.41 c* | 4645.77 d* |
| Treatment | Total antioxidant activity DPPH (Trolox) | |||
| T1 | 429.74 d | 245.06 e | 217.86 d | 170.68 c |
| T2 | 893.39 a* | 575.17 a* | 429.33 a* | 392.19 a* |
| T3 | 782.04 b* | 269.34 de | 266.45 c* | 144.69 cd* |
| T4 | 669.19 c* | 493.10 b* | 365.96 b* | 108.19 e* |
| T5 | 635.94 c* | 504.06 b* | 372.18 b* | 153.69 c |
| T6 | 773.11 b* | 319.82 d* | 218.48 d | 117.14 de* |
| T7 | 870.78 a* | 424.28 c* | 356.45 b* | 236.04 b* |
| Variable | Treatment | Adjusted Equations | R2/r2 | |
|---|---|---|---|---|
| Pulp color difference (Dif*) | Control (untreated fruits) (T1) | −0.05879 + 1.01923 * ST | 0.9223 | |
| Ozone microbubble (T2, T3, and T4) | 4.2844 − 1.9016 ** ST + 0.320179 ** ST2 | 0.9283 | ||
| Ozone-free microbubble (T5, T6, and T7) | 0.4418 − 0.38436 ns ST + 0.16498 ** ST2 + 0.0006872 * ET | 0.9096 | ||
| Variable | Treatment | Adjusted equations | R2/r2 | |
| Pulp color hue (h*) | Control (untreated fruits) (T1) | 22.2174 + 1.09059 * ST | 0.9203 | |
| Ozone microbubble (T2, T3, and T4) | 26.107 − 0.2245 ns ST + 0.20199 ** ST2 | 0.9068 | ||
| Ozone-free microbubble (T5, T6, and T7) | 25.094 + 0.8848 ** ST | 0.8644 | ||
| Variable | Treatment | Adjusted equations | R2/r2 | |
| Pulp color saturation (C*) | Control (untreated fruits) (T1) | 11.7931 + 0.63554 ** ST | 0.9979 | |
| Ozone microbubble (T2, T3, and T4) | 15.2178 − 0.8811 ns ST + 0.184778 ** ST2 | 0.7702 | ||
| Ozone-free microbubble (T5, T6, and T7) | 12.5312 − 0.0866 ns ST + 0.08958 * ST2 | 0.8201 | ||
| Variable | Treatment | Adjusted equations | R2/r2 | |
| Total phenolic compounds (gallic acid) | Control (untreated fruits) (T1) | 2231.87 − 106.281 * ST | 0.8470 | |
| Ozone microbubble (T2, T3, and T4) | 2419.15 − 331.364 ** ST + 23.83 ** ST2 | 0.8752 | ||
| Ozone-free microbubble (T5, T6, and T7) | 1458.89 | - | ||
| Variable | Treatment | Adjusted equations | R2/r2 | |
| Total antioxidant activity ABTS | Control (untreated fruits) (T1) | 9312.63 − 296.66 * ST | 0.8239 | |
| Ozone microbubble (T2, T3, and T4) | 16186.6 − 314.961 ** ST − 446.367 ** ET + 5.43172 ** ET2 | 0.8937 | ||
| Ozone-free microbubble (T5, T6, and T7) | 8406.7 − 283.699 ** ST | 0.5853 | ||
| Variable | Treatment | Adjusted equations | R2/r2 | |
| Total antioxidant activity DPPH | Control (untreated fruits) (T1) | 386.493 − 26.8124 * ST | 0.8369 | |
| Ozone microbubble (T2, T3, and T4) | 881.22 − 59.7168 ** ST + 4.0851 * ET | 0.8108 | ||
| Ozone-free microbubble (T5, T6, and T7) | 696.164 − 62.4442 * ST | 0.8292 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-Magalhães, C.; Faroni, L.R.D.; Cecon, P.R.; Alencar, E.R.d.; Silva, M.V.d.A.; Rodrigues, A.A.Z.; Massango, H.d.G.L.L.; Silva, M.J.d. Efficiency of Ozonated Water Treatment with a Microbubble System for Sanitization and Preservation of Postharvest Quality of Acerolas. Foods 2025, 14, 1814. https://doi.org/10.3390/foods14101814
Gonçalves-Magalhães C, Faroni LRD, Cecon PR, Alencar ERd, Silva MVdA, Rodrigues AAZ, Massango HdGLL, Silva MJd. Efficiency of Ozonated Water Treatment with a Microbubble System for Sanitization and Preservation of Postharvest Quality of Acerolas. Foods. 2025; 14(10):1814. https://doi.org/10.3390/foods14101814
Chicago/Turabian StyleGonçalves-Magalhães, Carollayne, Lêda Rita D’Antonino Faroni, Paulo Roberto Cecon, Ernandes Rodrigues de Alencar, Marcus Vinícius de Assis Silva, Alessandra Aparecida Zinato Rodrigues, Handina da Graça Lurdes Langa Massango, and Marcia Joaquim da Silva. 2025. "Efficiency of Ozonated Water Treatment with a Microbubble System for Sanitization and Preservation of Postharvest Quality of Acerolas" Foods 14, no. 10: 1814. https://doi.org/10.3390/foods14101814
APA StyleGonçalves-Magalhães, C., Faroni, L. R. D., Cecon, P. R., Alencar, E. R. d., Silva, M. V. d. A., Rodrigues, A. A. Z., Massango, H. d. G. L. L., & Silva, M. J. d. (2025). Efficiency of Ozonated Water Treatment with a Microbubble System for Sanitization and Preservation of Postharvest Quality of Acerolas. Foods, 14(10), 1814. https://doi.org/10.3390/foods14101814








