Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Proteins
2.2.1. pH Shift Alkaline Extraction–Isoelectric Precipitation Protocol
2.2.2. Pretreatments of the Selected Alkaline Extraction–Isoelectric Precipitation Protocol
Dephenolization
Enzyme-Assisted Extraction
Ultrasound-Assisted Extraction
Dephenolization with Ultrasound
2.3. Determination of Protein Content and Calculation of Yield
2.4. Optimization of Method for Dephenolization of RSC
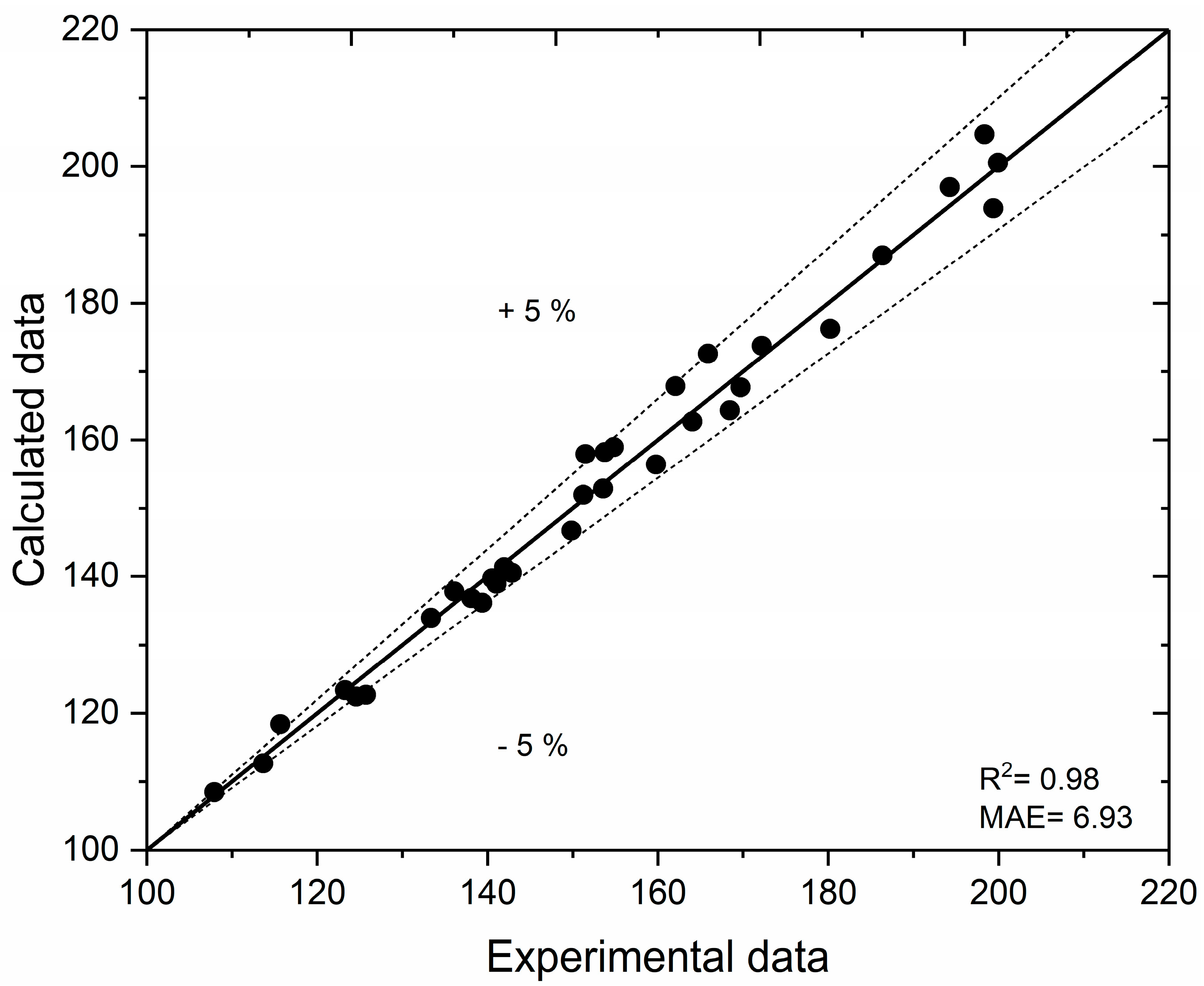
2.4.1. Artificial Neural Network
2.4.2. Particle Swarm Optimization
2.4.3. Alkaline Extraction of Proteins from RSC Under Optimized Pretreatment Conditions
2.4.4. Determination of Total Phenolic Content
2.5. Characterization of Protein Isolates
2.5.1. Sodium Dodecyl-Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5.2. Fourier-Transform Infrared Spectrum (FTIR)
2.5.3. Determination of Amino Acid Composition
2.5.4. Determination of Color
2.5.5. Determination of Protein Solubility
- Abs is the measured absorbance at 595 nm;
- 1.9899 is the calculated factor based on the BSA calibration curve;
- dilution factor accounts for any pre-measurement dilutions;
- V is the aliquot volume (mL) used in the assay.
2.6. Digestibility of Proteins
2.7. Statistical Analysis
3. Results and Discussion
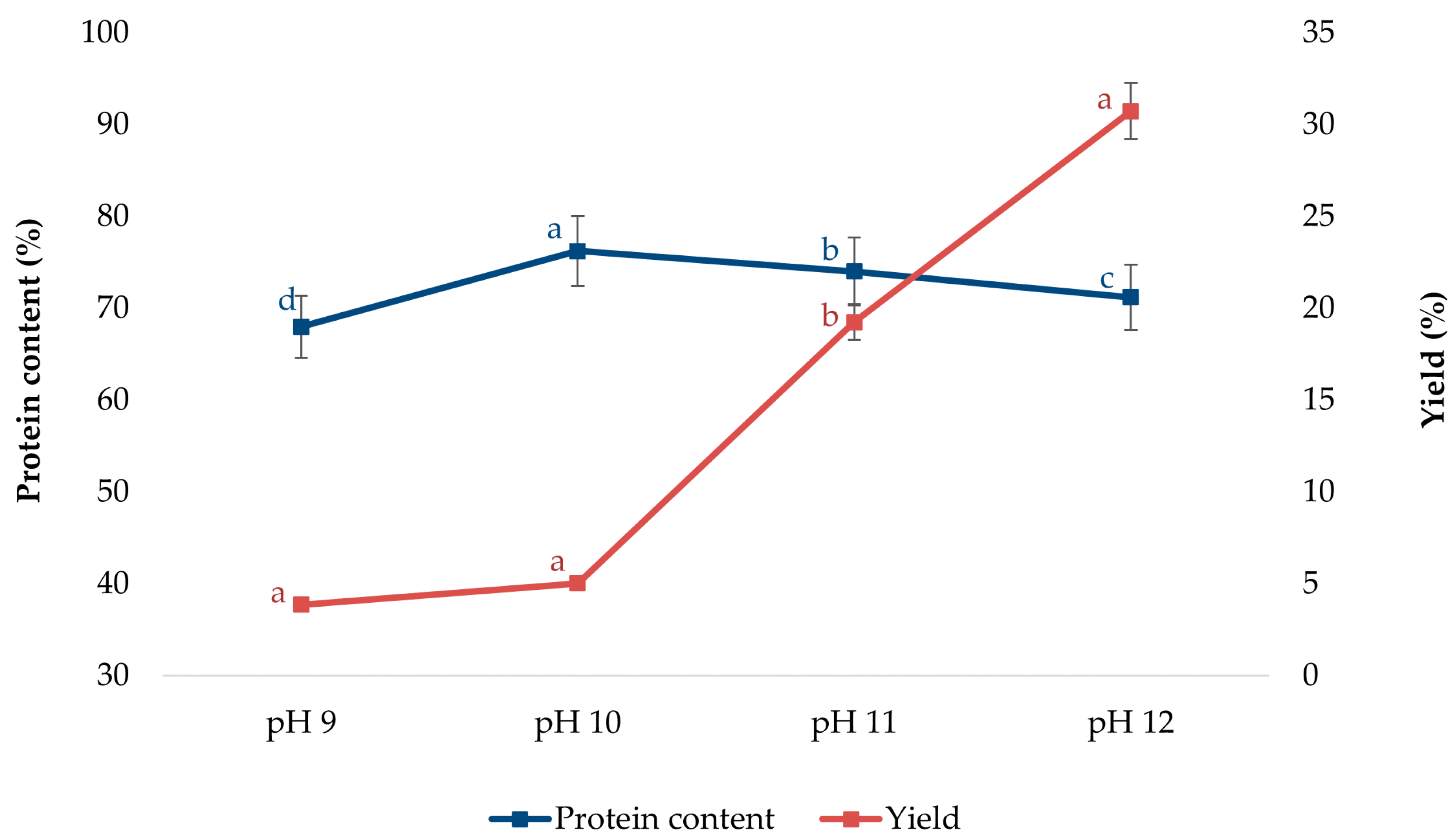
3.1. Effect of pH on the Extraction Yield and Protein Content
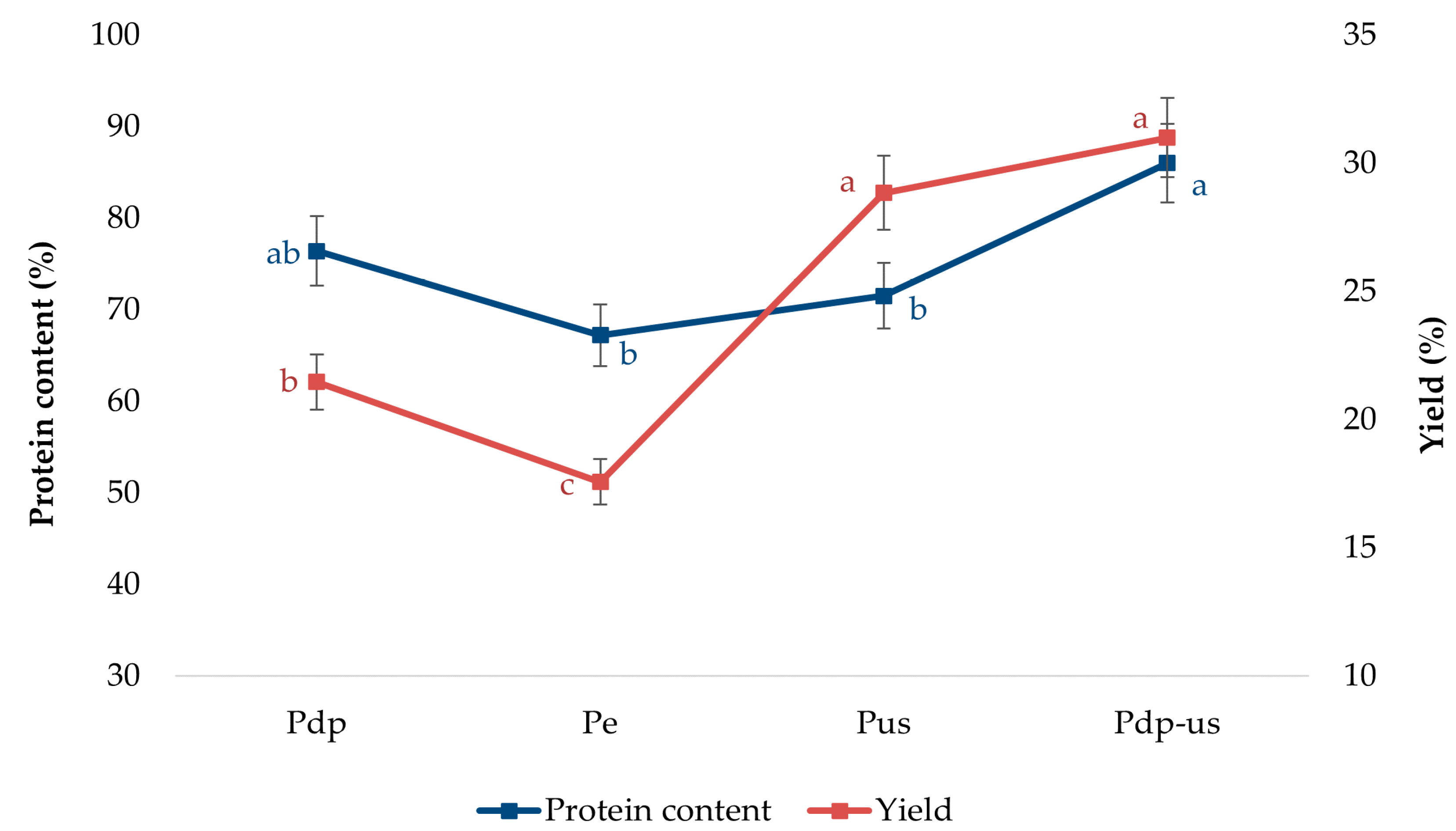
3.2. Effects of Different Extraction Methods on the Extraction Yield and Protein Content
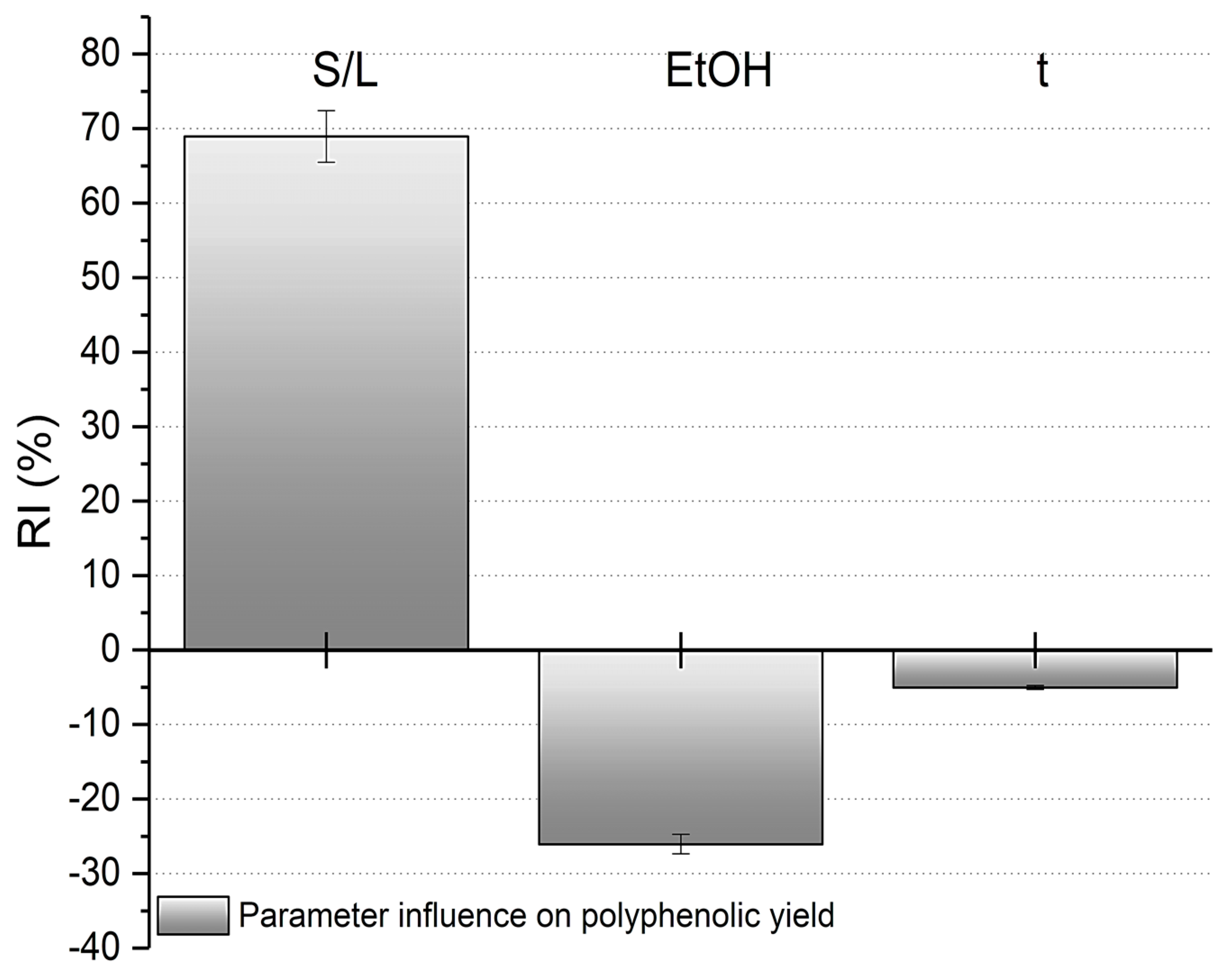
3.3. Impact of Process Parameters on RSC Dephenolization
3.4. Results of Protein Isolate Characterization
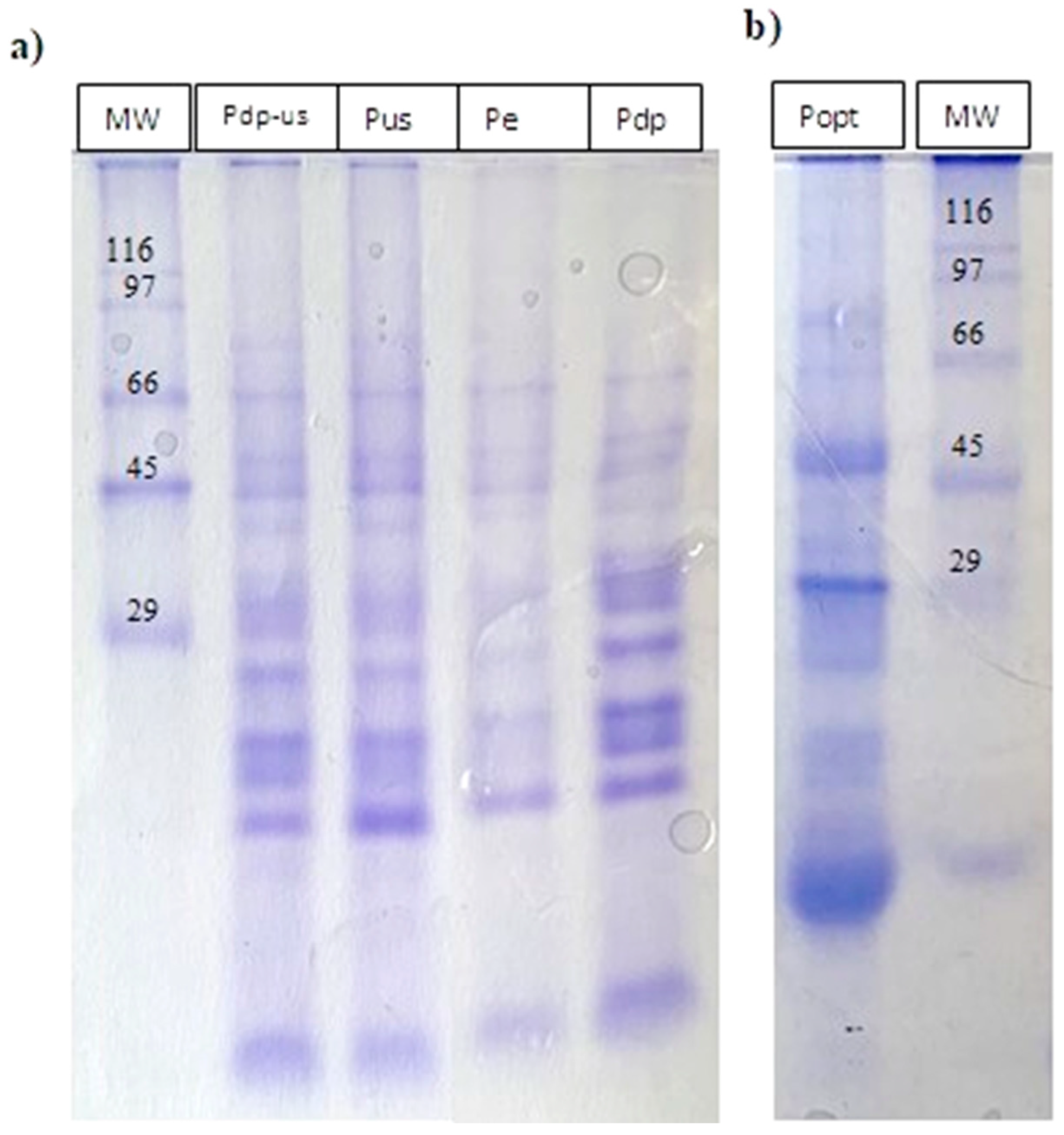
3.4.1. Protein Profile SDS-PAGE
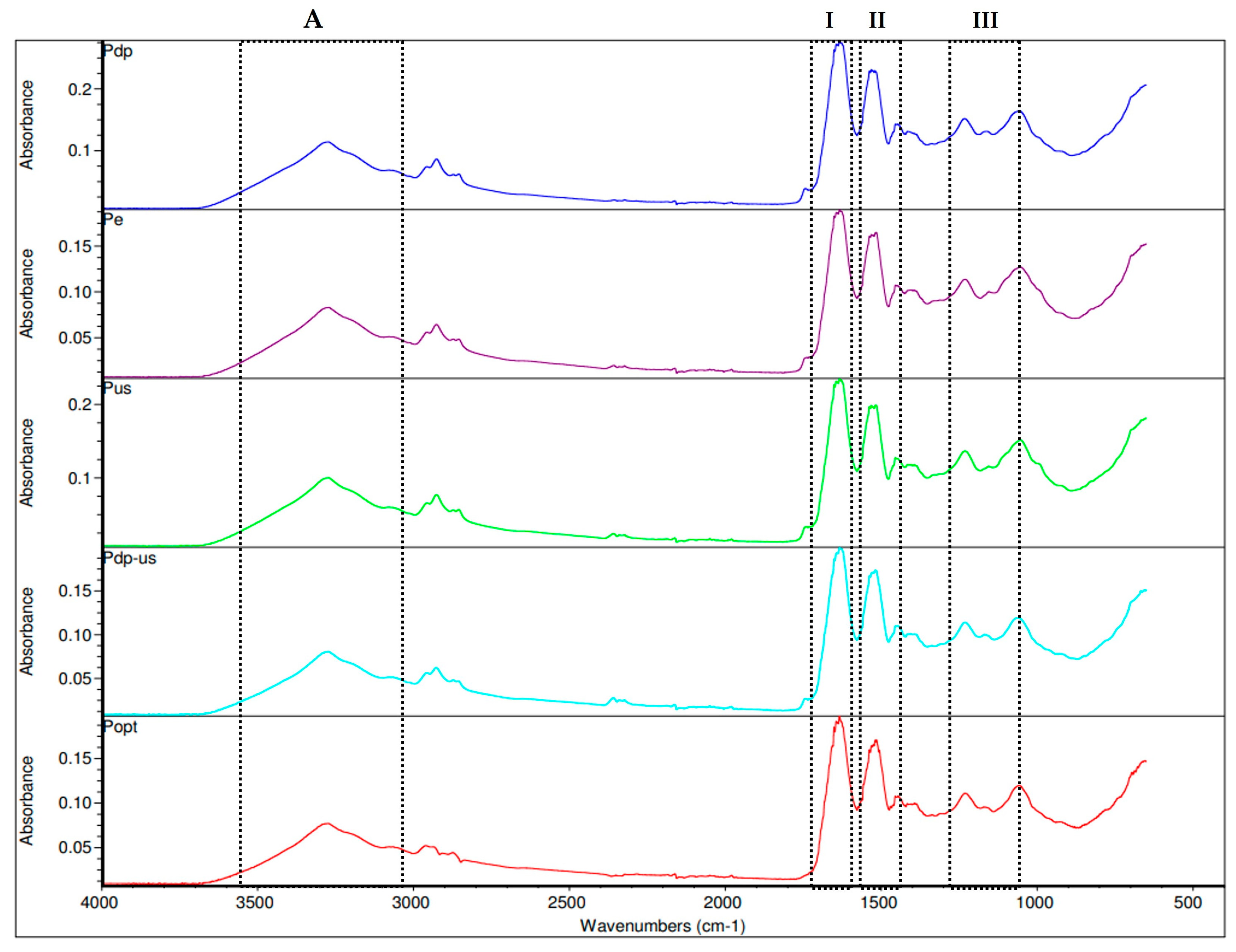
3.4.2. FTIR
3.4.3. Amino Acid Composition
3.4.4. Color Analysis
3.4.5. Protein Solubility
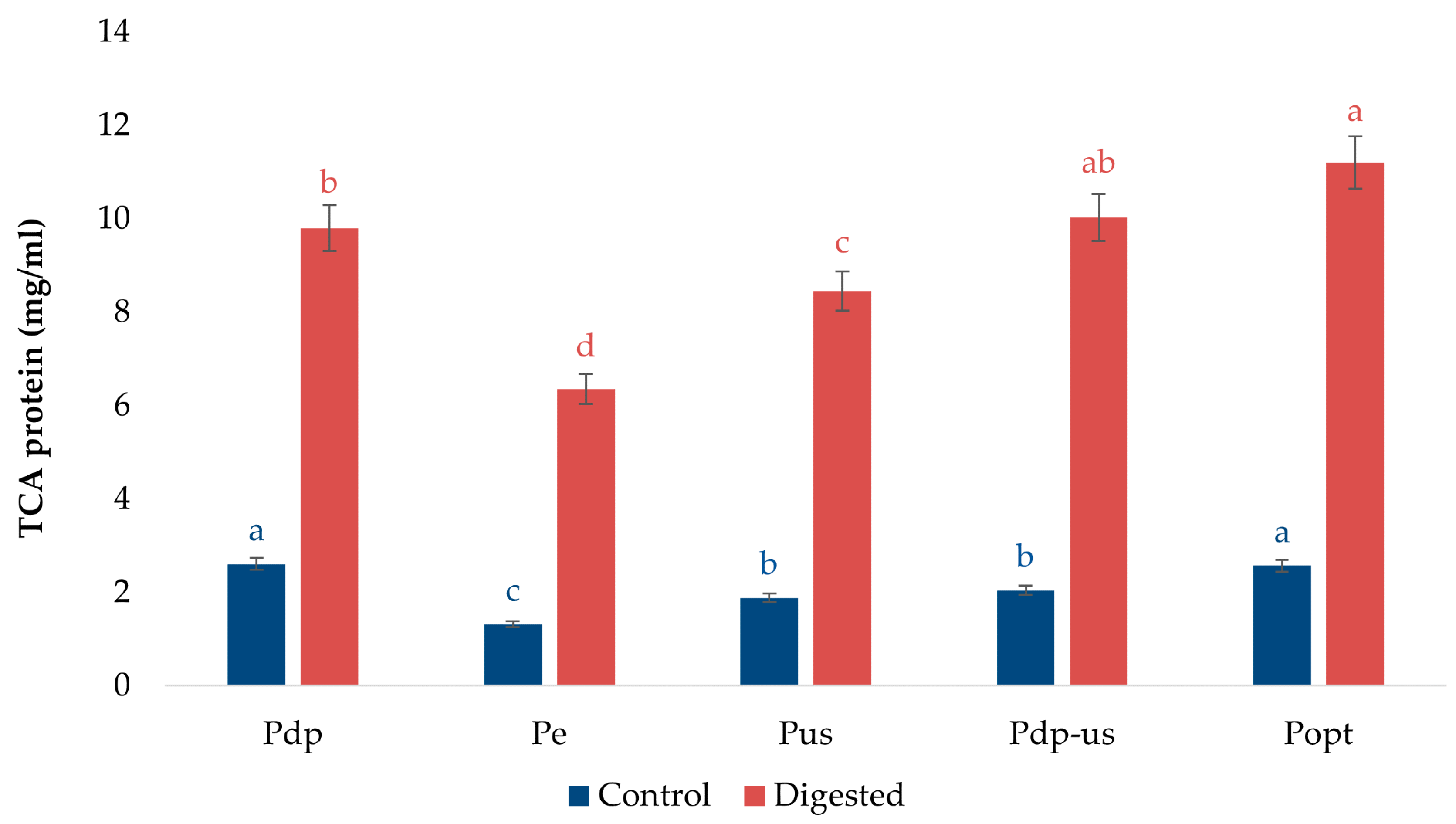
3.5. Protein Digestibility Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Circular Economy for the SDGs: From Concept to Practice. Available online: https://www.un.org/en/ga/second/73/circular_economy.pdf (accessed on 10 February 2025).
- Bryant, C.; Szejda, K.; Parekh, N.; Desphande, V.; Tse, B. A Survey of Consumer Perceptions of Plant-Based and Clean Meat in the USA, India, and China. Front. Sustain. Food Syst. 2019, 3, 11. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Database: Value of Agricultural Production; FAO: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/ (accessed on 10 February 2025).
- Östbring, K.; Tullberg, C.; Burri, S.; Malmqvist, E.; Rayner, M. Protein Recovery from Rapeseed Press Cake: Varietal and Processing Condition Effects on Yield, Emulsifying Capacity and Antioxidant Activity of the Protein Rich Extract. Foods 2019, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Subasi, B.G.; Ivanova, P.; Goksen, G.; Chalova, V.; Capanoglu, E. Towards Sustainable and Nutritional-Based Plant Protein Sources: A Review on the Role of Rapeseed. Food Res. Int. 2025, 202, 115553. [Google Scholar] [CrossRef] [PubMed]
- Beaubier, S.; Pineda-Vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the in Vitro Digestibility of Rapeseed Albumins Resistant to Gastrointestinal Proteolysis While Preserving the Functional Properties Using Enzymatic Hydrolysis. Food Chem. 2023, 407, 135132. [Google Scholar] [CrossRef]
- Chmielewska, A.; Kozłowska, M.; Rachwał, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/Rapeseed Protein–Nutritional Value, Functionality and Food Application: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3836–3856. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Liu, H.; Sun, X.; Ye, Y.; Cao, X.; Wu, Z.; Sun, H. Effect of PH Regulation on the Components and Functional Properties of Proteins Isolated from Cold-Pressed Rapeseed Meal through Alkaline Extraction and Acid Precipitation. Food Chem. 2020, 327, 126998. [Google Scholar] [CrossRef]
- Manamperi, W.A.R.; Wiesenborn, D.P.; Chang, S.K.C.; Pryor, S.W. Effects of Protein Separation Conditions on the Functional and Thermal Properties of Canola Protein Isolates. J. Food Sci. 2011, 76, E266–E273. [Google Scholar] [CrossRef]
- Gaber, M.A.F.M.; Trujillo, F.J.; Mansour, M.P.; Taylor, C.; Juliano, P. Megasonic-Assisted Aqueous Extraction of Canola Oil from Canola Cake. J. Food Eng. 2019, 252, 60–68. [Google Scholar] [CrossRef]
- Tanwar, B.; Goyal, A. Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2020; 516p. [Google Scholar] [CrossRef]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Canola Proteins for Human Consumption: Extraction, Profile, and Functional Properties. J. Food Sci. 2011, 76, R16–R28. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Brandão, T.R.S.; Silva, C.L.M.; Chalova, V.I. A Two-Step Factorial Design for Optimization of Protein Extraction from Industrial Rapeseed Meal after Ethanol-Assisted Reduction of Antinutrients. Int. Food Res. J. 2019, 26, 1155–1163. [Google Scholar]
- Kalaydzhiev, H.; Georgiev, R.; Ivanova, P.; Stoyanova, M.; Silva, C.L.M.; Chalova, V.I. Enhanced Solubility of Rapeseed Meal Protein Isolates Prepared by Sequential Isoelectric Precipitation. Foods 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Alpiger, S.B.; Corredig, M. Changes in the Physicochemical Properties of Rapeseed-derived Protein Complexes during Enzyme-assisted Wet Milling. Sustain. Food Proteins 2023, 1, 16–29. [Google Scholar] [CrossRef]
- Boukroufa, M.; Sicaire, A.G.; Fine, F.; Larré, C.; Le Goff, A.; Jamault, V.S.; Rakotomanomana, N.; Chemat, F. Green Sonoextraction of Protein from Oleaginous Press Rapeseed Cake. Molecules 2017, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. A Comparative Study between the Electro-Activation Technique and Conventional Extraction Method on the Extractability, Composition and Physicochemical Properties of Canola Protein Concentrates and Isolates. Food Biosci. 2015, 11, 56–71. [Google Scholar] [CrossRef]
- Ali, F.; Mondor, M.; Ippersiel, D.; Lamarche, F. Production of Low-Phytate Soy Protein Isolate by Membrane Technologies: Impact of Salt Addition to the Extract on the Purification Process. Innov. Food Sci. Emerg. Technol. 2011, 12, 171–177. [Google Scholar] [CrossRef]
- Golpour, I.; Ferrão, A.C.; Gonçalves, F.; Correia, P.M.R.; Blanco-Marigorta, A.M.; Guiné, R.P.F. Extraction of Phenolic Compounds with Antioxidant Activity from Strawberries: Modelling with Artificial Neural Networks (ANNs). Foods 2021, 10, 2228. [Google Scholar] [CrossRef]
- Eftekhari, M.; Yadollahi, A.; Ahmadi, H.; Shojaeiyan, A.; Ayyari, M. Development of an Artificial Neural Network as a Tool for Predicting the Targeted Phenolic Profile of Grapevine (Vitis vinifera) Foliar Wastes. Front. Plant Sci. 2018, 9, 837. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.S.; Singhal, R.S. Comparison of Artificial Neural Network (ANN) and Response Surface Methodology (RSM) in Fermentation Media Optimization: Case Study of Fermentative Production of Scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Teh, S.S.; El-Din Bekhit, A.; Birch, J. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Yoon, Y.; Swales, G.; Margavio, T.M. A Comparison of Discriminant Analysis versus Artificial Neural Networks. J. Oper. Res. Soc. 1993, 1, 51–60. [Google Scholar] [CrossRef]
- Kennedy, J.; Eberhart, R. Particle Swarm Optimization. In Proceedings of the Proceedings of ICNN’95—International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; pp. 1942–1948. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent6s, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Popović, L.; Peričin, D.; Vaštag, Ž.; Popović, S.; Krimer, V.; Torbica, A. Antioxidative and Functional Properties of Pumpkin Oil Cake Globulin Hydrolysates. JAOCS, Journal of the American Oil Chemists’ Society 2013, 90, 1157–1165. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein Digestion of Different Protein Sources Using the INFOGEST Static Digestion Model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Fair, A.L.; Randall, R.J. Rotein Measurement with the Folin-Phenol Reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sultan, Z.; Ashfaq, A.; Jahan, K.; Qadri, O.S.; Younis, K.; Yousuf, O. PH Shift Extraction Technique for Plant Proteins: A Promising Technique for Sustainable Development. Energy Nexus 2024, 16, 100329. [Google Scholar] [CrossRef]
- Jahan, K.; Ashfaq, A.; Islam, R.U.; Younis, K.; Yousuf, O. Optimization of Ultrasound-Assisted Protein Extraction from Defatted Mustard Meal and Determination of Its Physical, Structural, and Functional Properties. J. Food Process Preserv. 2022, 46, e16764. [Google Scholar] [CrossRef]
- Das, D.; Mir, N.A.; Chandla, N.K.; Singh, S. Combined Effect of PH Treatment and the Extraction PH on the Physicochemical, Functional and Rheological Characteristics of Amaranth (Amaranthus Hypochondriacus) Seed Protein Isolates. Food Chem. 2021, 353, 129466. [Google Scholar] [CrossRef]
- Hildebrand, G.; Poojary, M.M.; O’Donnell, C.; Lund, M.N.; Garcia-Vaquero, M.; Tiwari, B.K. Ultrasound-Assisted Processing of Chlorella Vulgaris for Enhanced Protein Extraction. J. Appl. Phycol. 2020, 32, 1709–1718. [Google Scholar] [CrossRef]
- Kumar, A.; Rani, P.; Purohit, S.R.; Rao, P.S. Effect of Ultraviolet Irradiation on Wheat (Triticum Aestivum) Flour: Study on Protein Modification and Changes in Quality Attributes. J. Cereal Sci. 2020, 96, 103094. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified Soy Protein Extraction by Ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Turan, S.; Capanoglu, E. Interaction of Lentil Protein and Onion Skin Phenolics: Effects on Functional Properties of Proteins and in Vitro Gastrointestinal Digestibility. Food Chem. 2022, 372, 130892. [Google Scholar] [CrossRef] [PubMed]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein Extracts from De-Oiled Sunflower Cake: Structural, Physico-Chemical and Functional Properties after Removal of Phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Haykin, S. Neural Networks and Learning Machines, 3/E; Pearson Education India: Noida, India, 2009. [Google Scholar]
- Zhang, M.; Zheng, C.; Yang, M.; Zhou, Q.; Li, W.; Liu, C.; Huang, F. Primary Metabolites and Polyphenols in Rapeseed (Brassica napus L.) Cultivars in China. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 303–317. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. Study of Total Dry Matter and Protein Extraction from Canola Meal as Affected by the PH, Salt Addition and Use of Zeta-Potential/Turbidimetry Analysis to Optimize the Extraction Conditions. Food Chem. 2016, 201, 243–252. [Google Scholar] [CrossRef]
- Karimi, A.; Bhowmik, P.; Yang, T.C.; Samaranayaka, A.; Chen, L. Extraction of Canola Protein via Natural Deep Eutectic Solvents Compared to Alkaline Treatments: Isolate Characteristics and Protein Structural and Functional Properties. Food Hydrocoll. 2024, 152, 109922. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S.; Mitra, P. Canola/Rapeseed Protein-Functionality and Nutrition. OCL Oilseeds Fats Crops Lipids 2016, 23, D407. [Google Scholar] [CrossRef]
- Akbari, A.; Wu, J. An Integrated Method of Isolating Napin and Cruciferin from Defatted Canola Meal. LWT 2015, 64, 308–315. [Google Scholar] [CrossRef]
- Albe-Slabi, S.; Defaix, C.; Beaubier, S.; Galet, O.; Kapel, R. Selective Extraction of Napins: Process Optimization and Impact on Structural and Functional Properties. Food Hydrocoll. 2022, 122, 107105. [Google Scholar] [CrossRef]
- Perera, S.P.; McIntosh, T.C.; Wanasundara, J.P.D. Structural Properties of Cruciferin and Napin of Brassica napus (Canola) Show Distinct Responses to Changes in PH and Temperature. Plants 2016, 5, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Nie, Y.; Sun, X.; Xiong, Y.L. Partial Removal of Phenolics Coupled with Alkaline Ph Shift Improves Canola Protein Interfacial Properties and Emulsion in in Vitro Digestibility. Foods 2021, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR Spectroscopic Imaging of Protein Aggregation in Living Cells. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent Applications of ATR FTIR Spectroscopy and Imaging to Proteins. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 2849–2858. [Google Scholar] [CrossRef]
- Das Purkayastha, M.; Gogoi, J.; Kalita, D.; Chattopadhyay, P.; Nakhuru, K.S.; Goyary, D.; Mahanta, C.L. Physicochemical and Functional Properties of Rapeseed Protein Isolate: Influence of Antinutrient Removal with Acidified Organic Solvents from Rapeseed Meal. J. Agric. Food Chem. 2014, 62, 7903–7914. [Google Scholar] [CrossRef]
- Fu, J.; Yang, W.; Huang, T.; Shi, H.; Zhao, Y.; Han, M.; Zhong, G. Effects of Ultrasound-Assisted Electrolyzed Water Treatment on the Structural and Functional Properties of Rapeseed Protein Isolate. ACS Food Sci. Technol. 2024, 4, 917–925. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary Protein-Phenolic Interactions: Characterization, Biochemical-Physiological Consequences, and Potential Food Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Fleddermann, M.; Fechner, A.; Rößler, A.; Bähr, M.; Pastor, A.; Liebert, F.; Jahreis, G. Nutritional Evaluation of Rapeseed Protein Compared to Soy Protein for Quality, Plasma Amino Acids, and Nitrogen Balance—A Randomized Cross-over Intervention Study in Humans. Clin. Nutr. 2013, 32, 519–526. [Google Scholar] [CrossRef]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Mnzava, N.A.; Olsson, K. Studies on Tropical Vegetables. Part 1: Seed Amino, Fatty Acid and Glucosinolate Profile of Ethiopian Mustards (Brassica Carinata Braun). Food Chem. 1990, 35, 229–235. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, X.; Feng, Z.; Chen, M.; Qiu, X.; Sun, J.; Wu, M.; He, J. Protein from Rapeseed for Food Applications: Extraction, Sensory Quality, Functional and Nutritional Properties. Food Chem. 2024, 439, 138109. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A Review of Methods Used for Investigation of Protein–Phenolic Compound Interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Vujetić, J.; Fraj, J.; Milinković Budinčić, J.; Popović, S.; Đorđević, T.; Stolić, Ž.; Popović, L. Plum Protein Isolate-Caffeic Acid Conjugate as Bioactive Emulsifier: Functional Properties and Bioavailability. Int. J. Food Sci. Technol. 2024, 59, 6183–6193. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double Emulsions Prepared by Two–Step Emulsification: History, State-of-the-Art and Perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Gawrys, O.; Roszkowska-Chojecka, M.M.; Badzynska, B.; Tymecka, D.; Olszynski, K.H.; Kompanowska-Jezierska, E. Chymase Dependent Pathway of Angiotensin II Generation and Rapeseed Derived Peptides for Antihypertensive Treatment of Spontaneously Hypertensive Rats. Front. Pharmacol. 2021, 12, 658805. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Chen, F. Prediction and Analysis of Antimicrobial Peptides from Rapeseed Protein Using in Silico Approach. J. Food Biochem. 2021, 45, e13598. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, X.; Zhang, X.; Wu, G.; Liu, F. A Review of Erucic Acid Production in Brassicaceae Oilseeds: Progress and Prospects for the Genetic Engineering of High and Low-Erucic Acid Rapeseeds (Brassica napus). Front. Plant Sci. 2022, 13, 899076. [Google Scholar] [CrossRef]

| Amino Acids (g/100 g Proteins) * | Pdp | Pe | Pus | Pdp-us | Popt |
|---|---|---|---|---|---|
| Thr | 4.55 ± 0.07 b | 5.20 ±0.04 a | 4.94 ± 0.08 a | 4.56 ± 0.07 b | 4.97 ± 0.11 a |
| Val | 5.14 ± 0.04 c | 5.27 ± 0.06 bc | 5.14 ± 0.08 c | 5.42 ± 0.08 b | 5.93 ± 0.06 a |
| Met | 3.10 ± 0.04 b | 2.58 ± 0.06 c | 2.58 ± 0.08 c | 3.02 ± 0.06 b | 3.35 ± 0.06 a |
| Ile | 4.31 ± 0.08 ab | 4.58 ± 0.07 a | 4.35 ± 0.04 ab | 4.21 ± 0.03 b | 4.44 ± 0.10 ab |
| Leu | 6.71 ± 0.03 c | 7.10 ± 0.07 b | 6.73 ± 0.08 c | 6.83 ± 0.04 c | 7.57 ± 0.08 a |
| Phe | 4.83 ± 0.04 bc | 5.14 ± 0.07 a | 4.96 ± 0.03 ab | 4.65 ± 0.04 c | 4.96 ± 0.07 ab |
| Lys | 6.95 ± 0.03 ab | 6.21 ± 0.10 c | 6.65 ± 0.13 b | 6.71 ± 0.03 b | 7.24 ± 0.11 a |
| His | 2.97 ± 0.04 a | 2.71 ± 0.06 bc | 2.76 ± 0.08 abc | 2.57 ± 0.04 c | 2.86 ± 0.07 ab |
| Ʃ EAA | 38.56 ± 0.01 bc | 38.79 ± 0.13 b | 38.11 ± 0.37 bc | 37.97 ± 0.17 c | 41.32 ± 0.05 a↑ |
| Asp | 8.42 ± 0.03 c | 9.21 ± 0.11 ab | 8.92 ± 0.10 b | 9.07 ± 0.03 b | 9.47 ± 0.14 a |
| Ser | 4.94 ± 0.11 b | 5.23 ± 0.03 b | 5.20 ± 0.10 b | 5.12 ± 0.07 b | 5.55 ± 0.04 a |
| Glu | 13.68 ± 0.07 c | 13.11 ± 0.11 d | 13.19 ± 0.14 d | 15.20 ± 0.07 b | 16.79 ± 0.14 a |
| Gly | 4.21 ± 0.07 c | 4.16 ± 0.03 c | 4.10 ± 0.06 c | 4.61 ± 0.03 b | 5.14 ± 0.10 a |
| Ala | 4.04 ± 0.04 c | 4.07 ± 0.06 c | 4.05 ± 0.03 c | 4.35 ± 0.08 b | 5.01 ± 0.06 a |
| Tyr | 3.30 ± 0.08 c | 3.95 ± 0.07 a | 3.70 ± 0.04 b | 3.18 ± 0.06 c | 3.16 ± 0.03 c |
| Arg | 8.30 ± 0.08 a | 7.55 ± 0.03 c | 8.14 ± 0.11 ab | 7.90± 0.03 b | 6.32 ± 0.04 d |
| Pro | 6.16 ± 0.03 a | 5.05 ± 0.03 e | 5.38 ± 0.04 d | 5.61 ± 0.07 c | 5.82 ± 0.06 b |
| Ʃ NEAA | 53.05 ± 0.13 c | 52.33 ± 0.07 c | 52.68 ± 0.14 c | 55.04 ± 0.04 b | 57.26 ± 0.35 a |
| Ʃ TAA | 91.61 ± 0.11 c | 91.12 ± 0.20 c | 90.79 ± 0.51 c | 93.01 ± 0.21 b | 98.58 ± 0.40 a↑ |
| EAA/NEAA (%) | 73.00 | 74.00 | 72.00 | 69.00 | 72.00 |
| EAA/TAA (%) | 42.00 | 43.00 | 42.00 | 41.00 | 42.00 |
| Samples | Pdp | Pe | Pus | Pdp-us | Popt |
|---|---|---|---|---|---|
| L* | 50.34 ± 0.1 b | 49.80 ± 0.30 b | 51.88 ± 0.18 a | 47.78 ± 0.26 c | 31.46 ± 0.15 d |
| a* | 6.02 ± 0.05 a | 5.08 ± 0.07 c | 5.21 ± 0.11 c | 5.83 ± 0.04 b | 3.43 ± 0.05 d |
| b* | 18.10 ± 0.22 b | 17.20 ± 0.17 c | 20.52 ± 0.19 a | 17.86 ± 0.09 b | 7.45 ± 0.08 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đermanovć, B.; Vujetić, J.; Sedlar, T.; Dragojlović, D.; Popović, L.; Kojić, P.; Jovanov, P.; Šarić, B. Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks. Foods 2025, 14, 1762. https://doi.org/10.3390/foods14101762
Đermanovć B, Vujetić J, Sedlar T, Dragojlović D, Popović L, Kojić P, Jovanov P, Šarić B. Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks. Foods. 2025; 14(10):1762. https://doi.org/10.3390/foods14101762
Chicago/Turabian StyleĐermanovć, Branislava, Jelena Vujetić, Tea Sedlar, Danka Dragojlović, Ljiljana Popović, Predrag Kojić, Pavle Jovanov, and Bojana Šarić. 2025. "Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks" Foods 14, no. 10: 1762. https://doi.org/10.3390/foods14101762
APA StyleĐermanovć, B., Vujetić, J., Sedlar, T., Dragojlović, D., Popović, L., Kojić, P., Jovanov, P., & Šarić, B. (2025). Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks. Foods, 14(10), 1762. https://doi.org/10.3390/foods14101762









