Physicochemical Properties and Structural Study of Heat Treatment-Modified Chinese Yam (Dioscorea opposita Thunb.) Starch–Ferulic Acid Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Preparation of Chinese Yam Starch–Ferulic Acid Complex
2.4. Determination of Swelling Power and Solubility
2.5. Determination of Water Holding Capacity (WHC)
2.6. X-Ray Diffraction (XRD) Analysis
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.8. Thermal Properties Analysis
2.9. Scanning Electron Microscopy (SEM) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Solubility and Swelling Power
3.2. Water-Holding Capacity
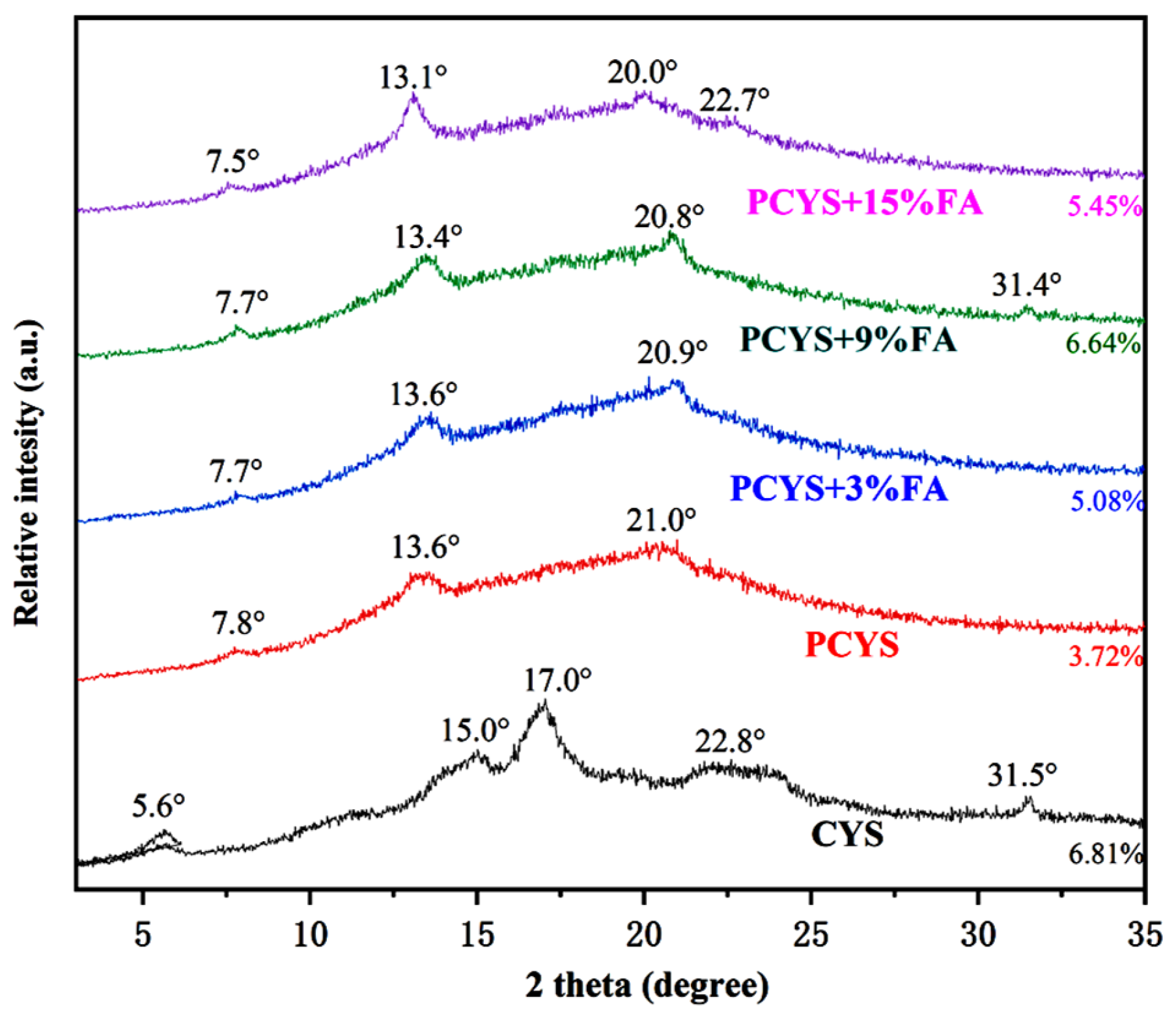
3.3. Long-Range Ordered Structure
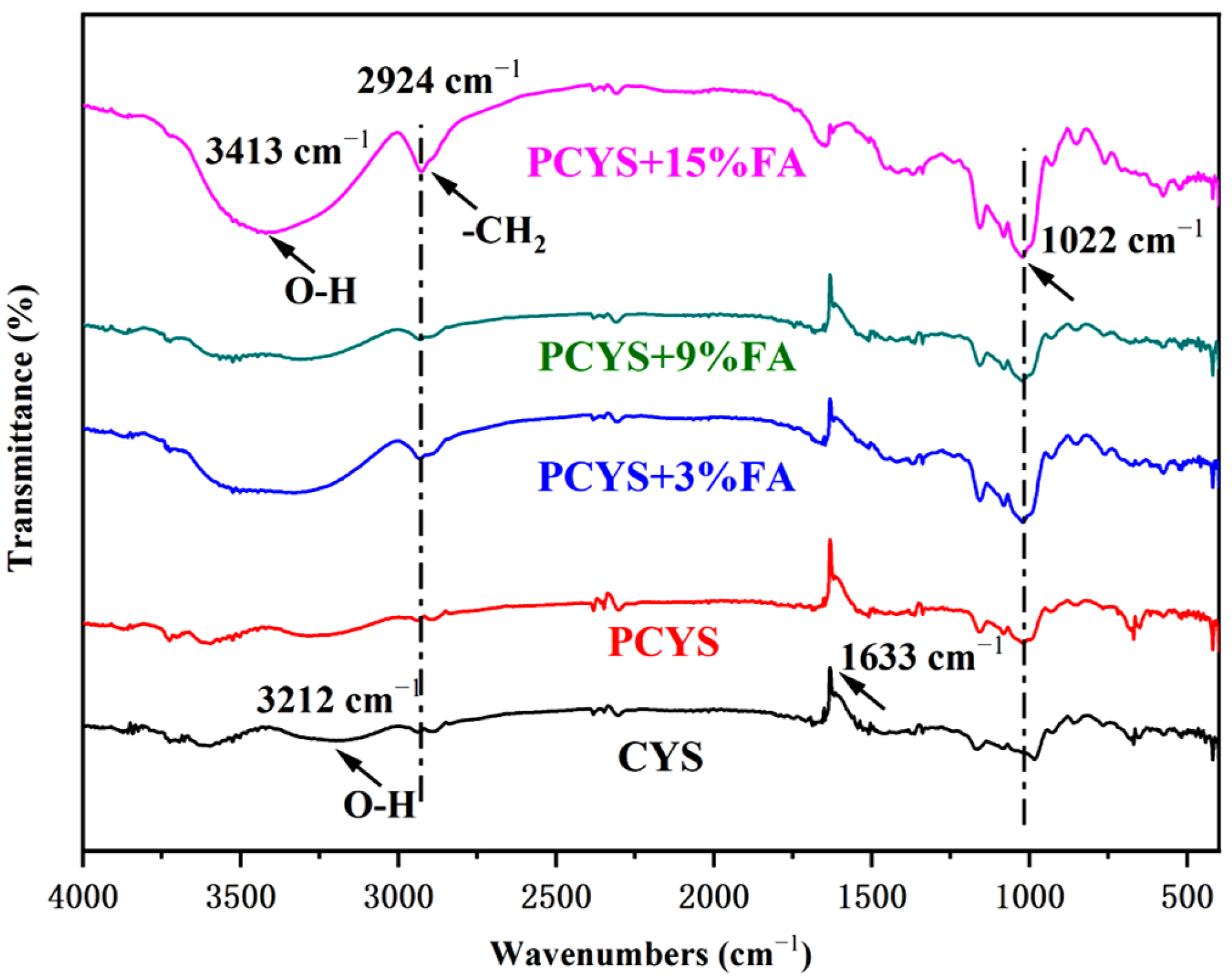
3.4. Short-Range Ordered Structure
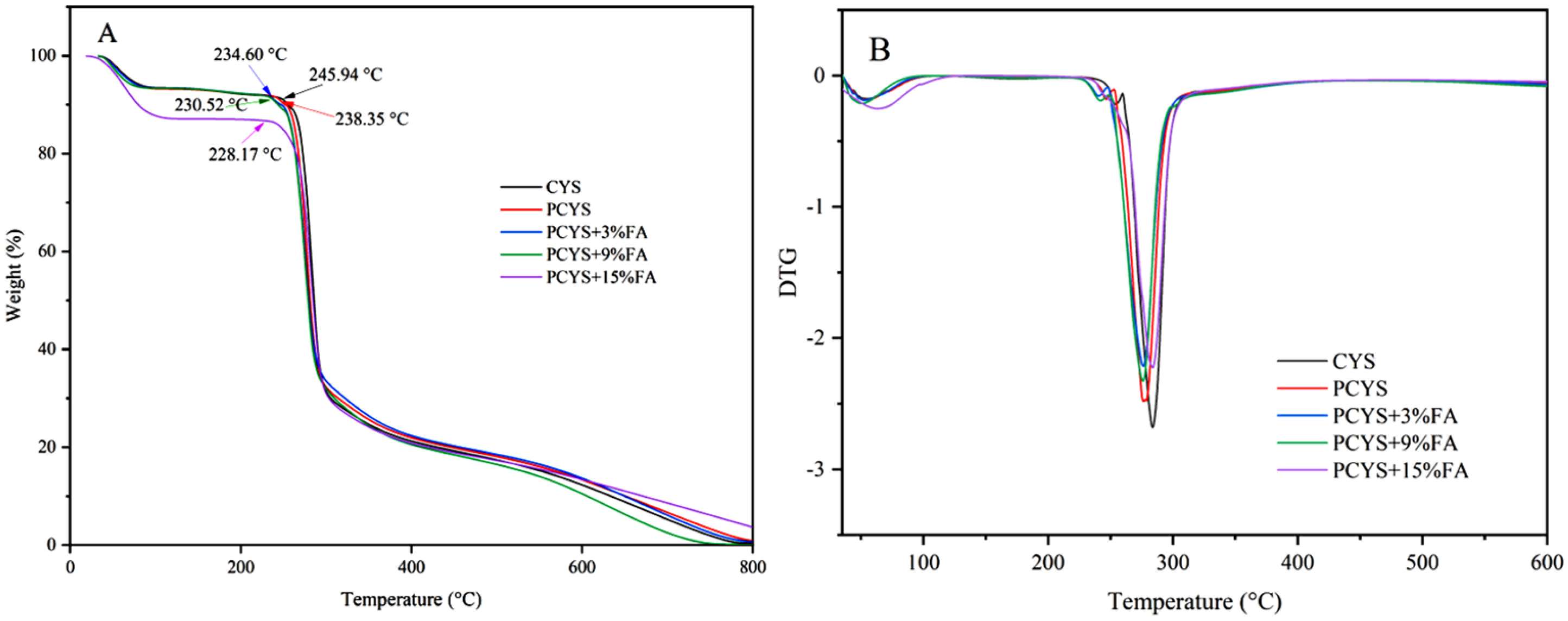
3.5. Thermal Properties
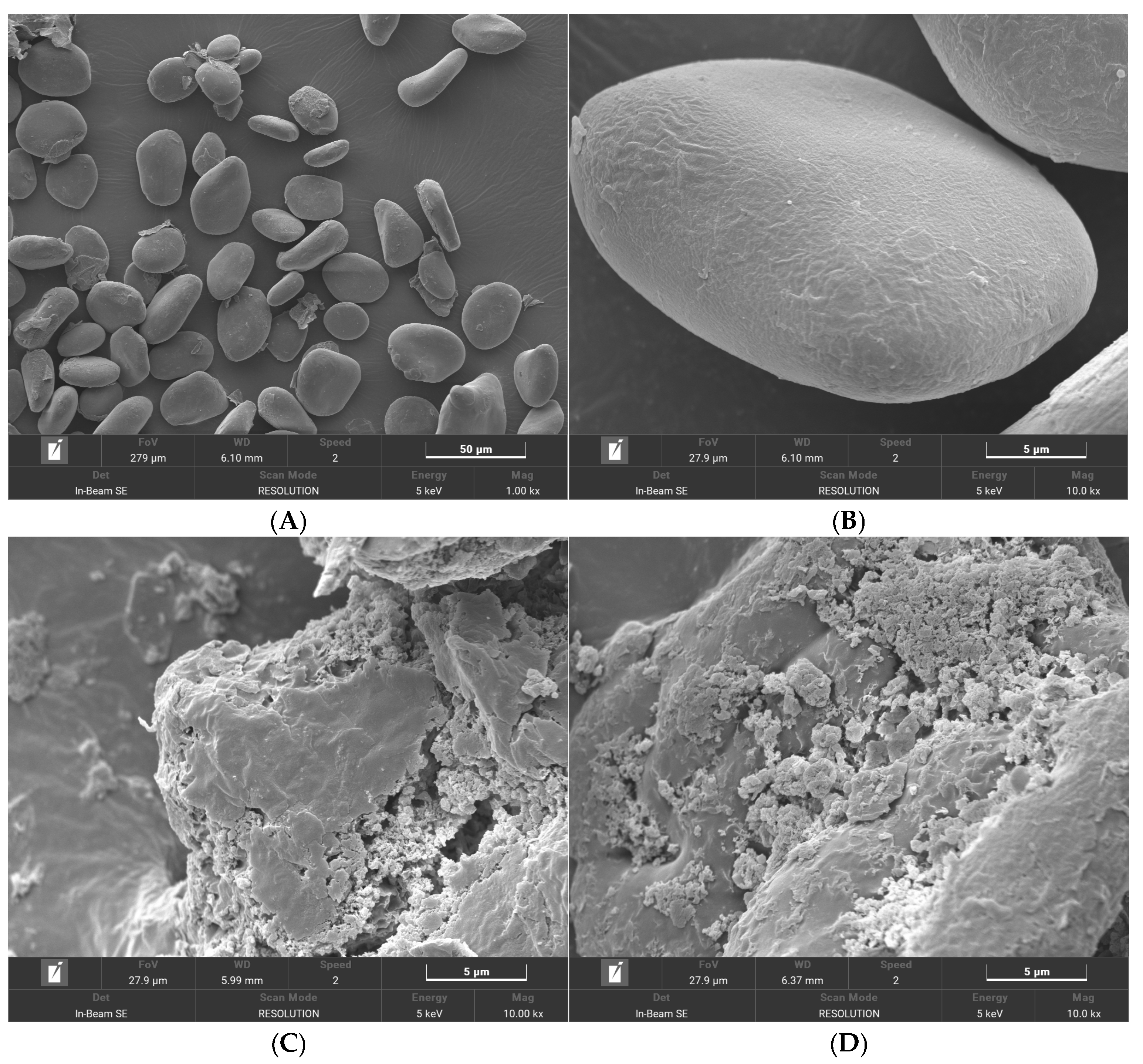
3.6. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, J.; Xu, M.; Wen, L.; Yang, B. Structure and physicochemical properties of native starch and resistant starch in Chinese yam (Dioscorea opposita Thunb.). Carbohydr. Polym. 2020, 237, 116188. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, J.; Tao, H.; Zhao, H.; Liu, P.; Cui, B. Physicochemical properties and in vitro digestibility of hydrothermal treated Chinese yam (Dioscorea opposita Thunb.) starch and flour. Int. J. Biol. Macromol. 2021, 176, 177–185. [Google Scholar] [CrossRef] [PubMed]
- He, Z.L.; Zeng, J.Y.; Hu, J.J.; Chen, J.H.; Peng, D.; Du, B.; Pan, L. Effects of cooking methods on the physical properties and in vitro digestibility of starch isolated from Chinese yam. Int. J. Biol. Macromol. 2024, 267, 131597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.X.; Lu, J.; Mao, X.H.; Wang, Y.; Feng, D.M.; Cao, J.G.; Huang, L.Q.; Gao, W.J. Complex formation, physicochemical properties of different concentrationof palmitic acid yam (Dioscorea pposita Thunb.) starch preparation mixtures. LWT 2019, 101, 130–137. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Carvalho, H.J.M.; Pereira, D.T.V.; Barcia, M.T.; Schmiele, M. Current advances in the interaction mechanisms, nutritional role and functional properties of phenolic compound-starch complexes. Food Res. Int. 2025, 202, 115744. [Google Scholar] [CrossRef]
- Yue, S.-J.; Zhang, P.-X.; Zhu, Y.; Li, N.-G.; Chen, Y.-Y.; Li, J.-J.; Zhang, S.; Jin, R.-Y.; Yan, H.; Shi, X.-Q.; et al. A Ferulic Acid Derivative FXS-3 Inhibits Proliferation and Metastasis of Human Lung Cancer A549 Cells via Positive JNK Signaling Pathway and Negative ERK/p38, AKT/mTOR and MEK/ERK Signaling Pathways. Molecules 2019, 24, 2165. [Google Scholar] [CrossRef]
- Xie, S.; Chen, H.; Jiang, X.; Zhou, B.; Guo, Z.; Zeng, H.; Zhang, Y. Structural and Physicochemical Properties of a Chinese Yam Starch–Tea Polyphenol Complex Prepared Using Autoclave-Assisted Pullulanase Treatment. Foods 2023, 12, 3763. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Lu, L.; Liu, L.; Wang, L. Effect of ferulic acid incorporation on structural, rheological, and digestive properties of hot-extrusion 3D-printed rice starch. Int. J. Biol. Macromol. 2024, 266, 131279. [Google Scholar] [CrossRef]
- Song, B.; Zheng, Q.; Xing, J.; Miao, Z.; Zheng, M.; Zhao, C.; Wu, Y.; Xu, X.; Liu, J. Understanding the multiscale structure and in vitro digestibility changes of corn starch-ferulic acid complexes induced by high hydrostatic pressure. Int. J. Biol. Macromol. 2024, 279, 135215. [Google Scholar] [CrossRef]
- Maibam, B.D.; Nickhil, C.; Deka, S.C. Preparation, physicochemical characterization, and in vitro starch digestibility on complex of Euryale ferox kernel starch with ferulic acid and quercetin. Int. J. Biol. Macromol. 2023, 250, 126178. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yu, Y.; Yang, X.; Wei, Z.; Han, L. Physicochemical properties of ultrasound-pretreated pea starch and its inclusion complexes with lauric acid. Food Chem. X 2023, 20, 100879. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Kang, Z.; Jiang, P.; Wu, D.; Bao, Y.; Chen, X. Effects of laminarin and ferulic acid on pasting, rheology, freeze-thaw stability and in vitro digestion of cassava starch. Int. J. Biol. Macromol. 2025, 300, 140248. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, X.; Chen, Z.; Zhou, Z.; Ji, S.; Xu, Y.; Zhang, C.; Shen, J.; Chen, Q.; Li, K.; et al. Insights into the improved cold-water solubility and digestibility of alkaline-alcohol modified cassava starch: A discussion from the perspective of fine structure. Int. J. Biol. Macromol. 2025, 305, 140952. [Google Scholar] [CrossRef]
- Sun, X.; Jin, R.; Ma, F.; Ma, W.; Pan, Y.; Liu, J.; Liu, X.; Zhu, J.; Zhang, J. Effects of different fatty acids on the structure, physicochemical properties, and in vitro digestibility of Chinese yam resistant starch-lipid complexes. Food Chem. 2025, 465, 142159. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Chen, W.; Jia, R.; Zheng, B.; Guo, Z. Insights into impact of chlorogenic acid on multi-scale structure and digestive properties of lotus seed starch under autoclaving treatment. Int. J. Biol. Macromol. 2024, 278, 134863. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Ou, Y.; Zheng, B. Effect of chlorogenic acid on the structural properties and digestibility of lotus seed starch during microwave gelatinization. Int. J. Biol. Macromol. 2021, 191, 474–482. [Google Scholar] [CrossRef]
- Li, J.; Shen, M.; Xiao, W.; Li, Y.; Pan, W.; Xie, J. Regulating the physicochemical and structural properties of different starches by complexation with tea polyphenols. Food Hydrocoll. 2023, 142, 108836. [Google Scholar] [CrossRef]
- Zhao, R.; Yao, J.; Li, C.; Liu, Q.; Liu, W.; Zhang, L.; Zhang, Z.; Zhao, R.; Hu, H. Multi-scale structural influence of starch on their interaction of caffeic acid and starch after freeze-thaw: Taking potato starch and lotus seed starch as examples. Int. J. Biol. Macromol. 2025, 284, 137997. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.Q.; Aziz, T.; Al-Asmari, F.; Alghamdi, S.; Bayahya, S.H.; Cui, H.Y.; Lin, L. Effects of cold plasma pretreatment before different drying process on the structural and functional properties of starch in Chinese yam. Int. J. Biol. Macromol. 2024, 274, 133307. [Google Scholar] [CrossRef]
- Gu, L.B.; Liu, X.X.; Li, S.Y.; Song, Q.Y.; Gu, R.; Wang, L.; Zhang, K.P.; Qin, Z.; Liu, H.M.; Hao, T.X.; et al. Effect of freeze-thawing treatment on the structural and physicochemical characteristics of starch isolated from Chinese yam. LWT 2025, 222, 117639. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Q.; Duan, H.; Zhou, S.Q.; Zhou, Y.X.; Li, W.H.; Yan, W.J. Understanding CaCl2 induces surface gelatinization to promote cold plasma modified maize starch: Structure-effect relations. Carbohydr. Polym. 2023, 320, 121200. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, W.; Jia, R.; Gong, H.; Zhang, Y.; Zheng, B.; Guo, Z. High hydrostatic pressure technology promotes the formation of lotus seed starch and epigallocatechin gallate complex: An evaluation of structure and digestive characteristics. LWT 2024, 213, 116939. [Google Scholar] [CrossRef]
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326. [Google Scholar] [CrossRef]
- Wang, W.; Ma, S.; Shao, Q.; Yi, S. Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein. Foods 2024, 13, 2920. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takahashi, K. Effects of Suwari Suppression and Enzyme Inhibitors on Water Holding Capacity of Alaska Pollock (Theragra chalcogramma) Surimi Gel and Its Improvement by Modified Grinding Method. Fishes 2024, 9, 504. [Google Scholar] [CrossRef]
- Xu, T.; Zhong, Y.H.; Chen, Q.; Wu, L.P.; Ji, S.Y.; Yang, B.W.; Zhang, Y.Z.; Shen, J.F.; Lu, B.Y. Modulating the digestibility of cassava starch by esterification with phenolic acids. Food Hydrocoll. 2022, 127, 107432. [Google Scholar] [CrossRef]
- Wang, X.; Reddy, C.K.; Xu, B. A systematic comparative study on morphological, crystallinity, pasting, thermal and functional characteristics of starches resources utilized in China. Food Chem. 2018, 259, 81–88. [Google Scholar] [CrossRef]
- Deng, N.; Bian, X.; Luo, S.; Liu, C.; Hu, X. The starch-polyphenol inclusion complex: Preparation, characterization and digestion. Food Biosci. 2023, 53, 102655. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, L.; Rao, C.; Zheng, B. Starch-guar gum-ferulic acid molecular interactions alter the ordered structure and ultimate retrogradation properties and in vitro digestibility of chestnut starch under extrusion treatment. Food Chem. 2023, 416, 135803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Y.; Chen, Y.; Liu, W.; Li, L.; Chen, J.; Ren, G.; Li, X.; Luo, Z.; Pan, L.; et al. Effects of chlorogenic acid on the physicochemical properties, 3D printing characteristics, and anti-digestive properties of sweet potato starch. Int. J. Biol. Macromol. 2025, 288, 138726. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Shuai, X.; Geng, Q.; Guo, X.; Chen, J.; Li, T.; Liu, C.; Dai, T. The interaction and physicochemical properties of the starch-polyphenol complex: Polymeric proanthocyanidins and maize starch with different amylose/amylopectin ratios. Int. J. Biol. Macromol. 2023, 253, 126617. [Google Scholar] [CrossRef]
- Li, H.; Zhai, F.; Li, J.; Zhu, X.; Guo, Y.; Zhao, B.; Xu, B. Physicochemical properties and structure of modified potato starch granules and their complex with tea polyphenols. Int. J. Biol. Macromol. 2021, 166, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Kang, Z.; Jiang, P.; Liu, Y.; Liu, M.; Li, Q. Effects of fucoidan and ferulic acid on potato starch: Pasting, rheological and retrogradation properties and their interactions. Food Hydrocoll. 2024, 150, 109635. [Google Scholar] [CrossRef]
- Raza, H.; Zhou, Q.; Cheng, K.-W.; He, J.; Wang, M. Synergistic impact of ultrasound-high pressure homogenization on the formation, structural properties, and slow digestion of the starch-phenolic acid complex. Food Chem. 2024, 445, 138785. [Google Scholar] [CrossRef]
- Hao, Z.; Han, S.; Zhao, Z.; Wu, Z.; Xu, H.; Li, C.; Zheng, M.; Zhou, Y.; Du, Y.; Yu, Z. Investigation of physicochemical properties and structure of ball milling pretreated modified starch-ferulic acid complexes. Food Chem. X 2024, 24, 101919. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Ye, X.; Kong, X.; Tian, J. Regulating the physicochemical, structural characteristics and digestibility of potato starch by complexing with different phenolic acids. Int. J. Biol. Macromol. 2023, 253, 127474, Corrigendum in Int. J. Biol. Macromol.2025, 306, 143291. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Hu, Y.; Ye, X.; Wang, S.; Tian, J. The cooperation of maize starch and ferulic acid under different treatments and its effect on postprandial blood glucose level. Food Hydrocoll. 2024, 157, 110361. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S.; Hao, Z.; Gu, Z.; Li, C.; Wu, Z.; Zhao, Z.; Xiao, Y.; Liu, Y.; Liu, K.; et al. Preparation of the black rice starch-gallic acid complexes by ultrasound treatment: Physicochemical properties, multiscale structure, and in vitro digestibility. Int. J. Biol. Macromol. 2024, 263, 130331. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A. Effect of the gelatinization level of normal maize starch under extrusion on ferulic acid–starch complex formation. J. Cereal Sci. 2024, 117, 103905. [Google Scholar] [CrossRef]

| Sample | Water Solubility Index (%) | ||||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| CYS | 1.67 ± 0.58 c | 2.00 ± 1.00 b | 2.00 ± 0.00 c | 13.67 ± 5.51 a | 14.67 ± 4.73 a |
| PCYS | 5.33 ± 2.31 b | 6.67 ± 1.15 a | 9.00 ± 2.00 ab | 8.67 ± 1.53 a | 10.67 ± 5.51 a |
| PCYS+3%FA | 5.67 ± 1.15 b | 5.67 ± 2.31 a | 7.33 ± 1.53 b | 11.00 ± 2.00 a | 11.33 ± 2.52 a |
| PCYS+9%FA | 7.00 ± 2.00 ab | 7.00 ± 1.00 a | 8.00 ± 1.00 ab | 13.33 ± 0.58 a | 15.33 ± 8.39 a |
| PCYS+15%FA | 10.00 ± 2.00 a | 8.33 ± 2.08 a | 10.67 ± 2.08 a | 14.33 ± 2.31 a | 18.00 ± 7.81 a |
| Sample | Swelling Power (g/g) | ||||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| CYS | 2.92 ± 0.17 c | 3.46 ± 0.20 b | 3.60 ± 1.16 b | 17.22 ± 2.31 b | 24.26 ± 2.57 a |
| PCYS | 13.96 ± 2.22 b | 19.70 ± 1.31 a | 22.29 ± 1.24 a | 22.70 ± 2.28 a | 25.60 ± 2.04 a |
| PCYS+3%FA | 15.05 ± 2.40 ab | 19.01 ± 2.19 a | 22.64 ± 1.60 a | 24.05 ± 0.72 a | 25.60 ± 0.43 a |
| PCYS+9%FA | 16.29 ± 2.03 ab | 18.95 ± 1.66 a | 21.30 ± 0.34 a | 23.83 ± 0.98 a | 26.38 ± 3.32 a |
| PCYS+15%FA | 17.76 ± 2.32 a | 19.53 ± 0.59 a | 22.31 ± 1.48 a | 24.1 ± 1.28 a | 26.49 ± 2.89 a |
| Sample | Water-Holding Capacity (%) | ||||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| CYS | 139.67 ± 26.27 c | 143.33 ± 14.98 b | 177.33 ± 48.19 c | 217.67 ± 2.31 b | 1073.67 ± 53.72 c |
| PCYS | 1144.67 ± 101.44 ab | 1322.00 ± 273.41 a | 1404.33 ± 100.01 ab | 1518.00 ± 91.79 a | 1631.33 ± 18.04 ab |
| PCYS+3%FA | 1111.00 ± 37.24 b | 1256.67 ± 93.39 a | 1544.00 ± 118.29 a | 1483.00 ± 177.79 a | 1710.67 ± 86.6 a |
| PCYS+9%FA | 1205.33 ± 112.52 ab | 1410.00 ± 182.05 a | 1461.67 ± 98.15 ab | 1574.33 ± 55.77 a | 1556.00 ± 37.00 b |
| PCYS+15%FA | 1261.67 ± 19.73 a | 1365.33 ± 125.09 a | 1367.33 ± 77.8 b | 1453.67 ± 56.54 a | 1545.33 ± 66.53 b |
| Samples | R1047/1022 | R995/1022 |
|---|---|---|
| CYS | 1.009 | 0.984 |
| PCYS | 1.021 | 1.014 |
| PCYS+3%FA | 1.057 | 1.052 |
| PCYS+9%FA | 1.031 | 1.030 |
| PCYS+15%FA | 1.031 | 1.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Lei, Y.; Chen, H.; Liu, S.; Lin, X.; Guo, Z.; Zhang, Y.; Zheng, B. Physicochemical Properties and Structural Study of Heat Treatment-Modified Chinese Yam (Dioscorea opposita Thunb.) Starch–Ferulic Acid Complexes. Foods 2025, 14, 1761. https://doi.org/10.3390/foods14101761
Xie S, Lei Y, Chen H, Liu S, Lin X, Guo Z, Zhang Y, Zheng B. Physicochemical Properties and Structural Study of Heat Treatment-Modified Chinese Yam (Dioscorea opposita Thunb.) Starch–Ferulic Acid Complexes. Foods. 2025; 14(10):1761. https://doi.org/10.3390/foods14101761
Chicago/Turabian StyleXie, Sandu, Yanping Lei, Huiqing Chen, Shuqi Liu, Xiaojuan Lin, Zebin Guo, Yi Zhang, and Baodong Zheng. 2025. "Physicochemical Properties and Structural Study of Heat Treatment-Modified Chinese Yam (Dioscorea opposita Thunb.) Starch–Ferulic Acid Complexes" Foods 14, no. 10: 1761. https://doi.org/10.3390/foods14101761
APA StyleXie, S., Lei, Y., Chen, H., Liu, S., Lin, X., Guo, Z., Zhang, Y., & Zheng, B. (2025). Physicochemical Properties and Structural Study of Heat Treatment-Modified Chinese Yam (Dioscorea opposita Thunb.) Starch–Ferulic Acid Complexes. Foods, 14(10), 1761. https://doi.org/10.3390/foods14101761








