Antioxidant and Antibacterial Activity of Four Tannins Isolated from Different Sources and Their Effect on the Shelf-Life Extension of Vacuum-Packed Minced Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Determination of Total Phenolic Content
2.3. Determination of Antioxidant Activities
2.3.1. 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH) Radical Scavenging Activity
2.3.2. The Ferric-Reducing Power Assay
2.3.3. Metal-Chelating Assay
2.3.4. Linoleic Acid Peroxidation Method
2.4. Antibacterial Analysis
2.5. Effect of TO and TVG on the Quality of Beef Mince during Refrigerated Storage
2.5.1. Preparation of Beef Mince Treated with TO and TVG
2.5.2. Chemical Analyses
pH Measurement
Analysis of Thiobarbituric Acid Reactive Substances (TBARS)
Determination of Metmyoglobin (Metmb)
2.5.3. Colour Measurement
2.5.4. Microbial Analyses
Total Viable Count
H2S-Producing Bacteria Count
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activities of Tannin Samples
3.1.1. Total Phenolic Contents
3.1.2. DPPH Radical Scavenging Activity
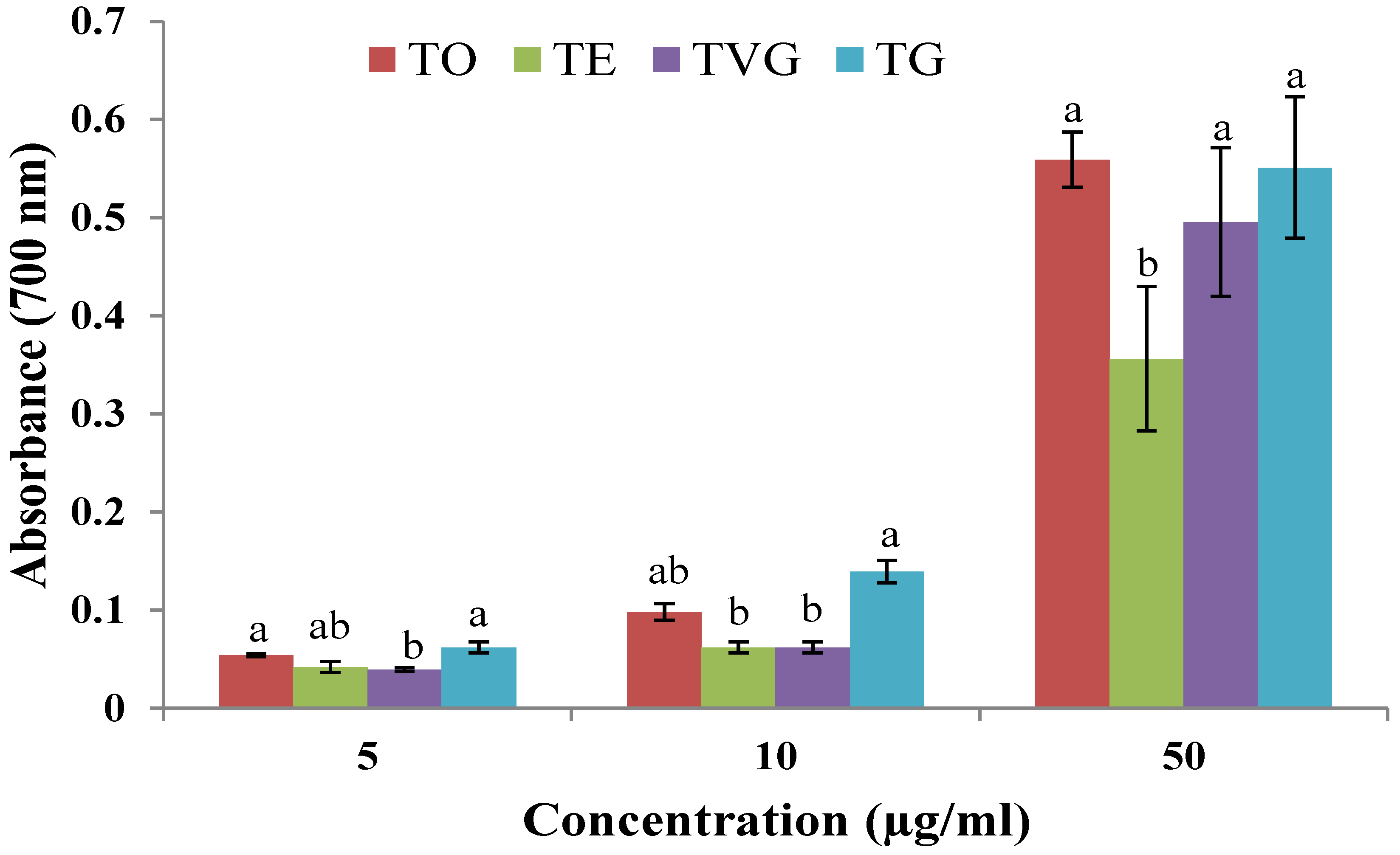
3.1.3. Ferric-Reducing Power
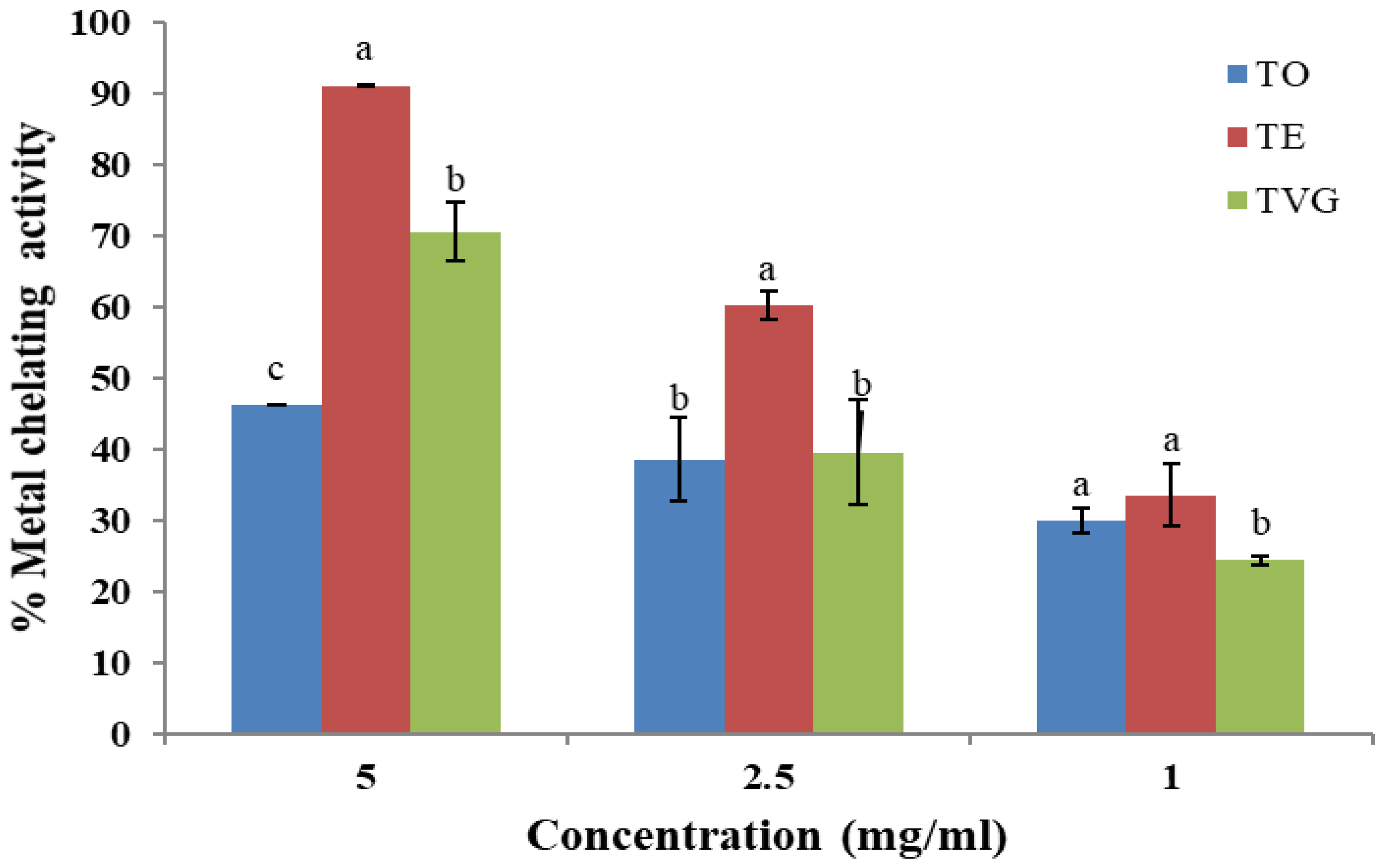
3.1.4. Metal-Chelating Activity
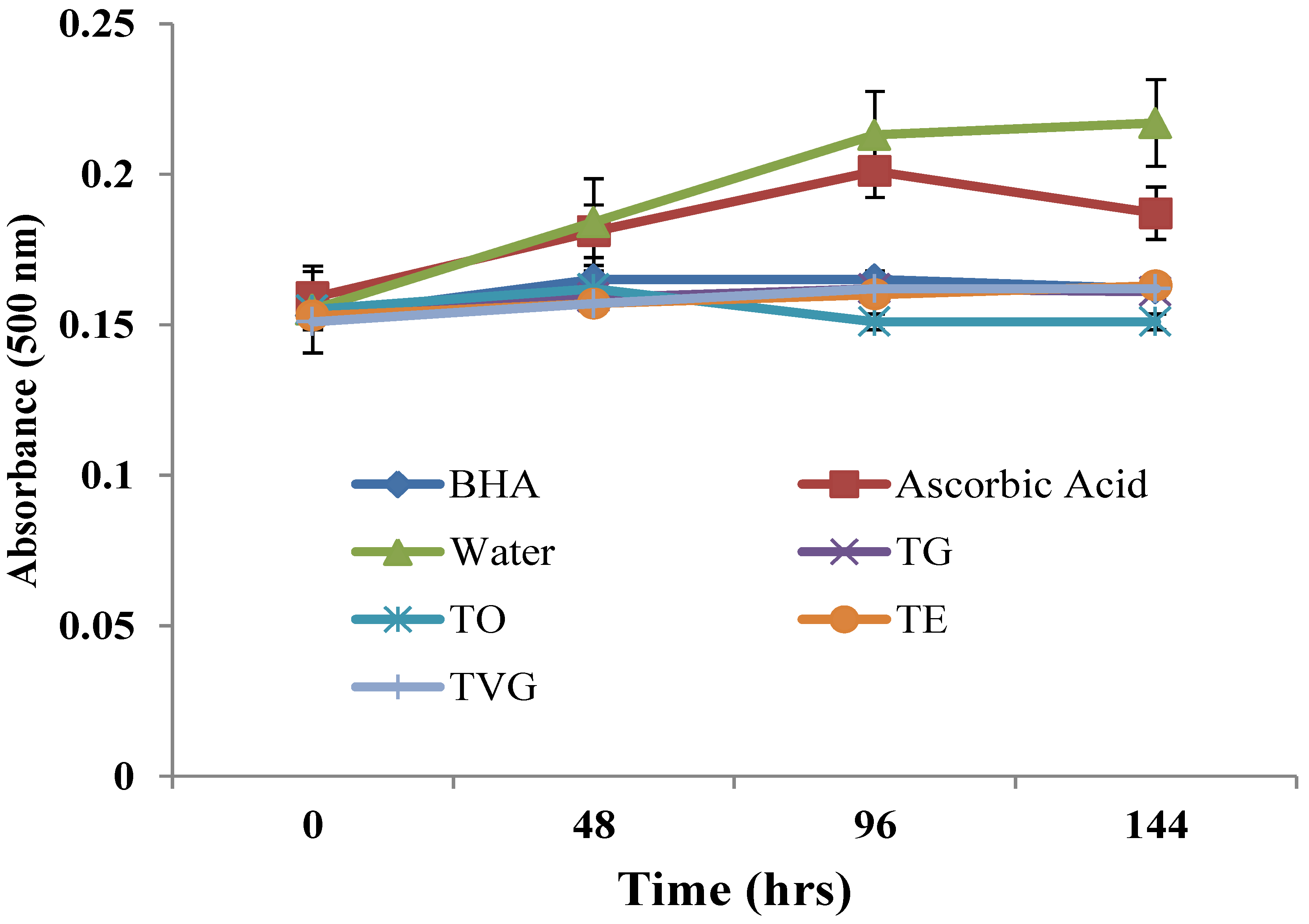
3.1.5. Linoleic Acid Peroxidation
3.2. Antibacterial Activity of Tannin Samples
3.3. Effect of TO and TVG on Vacuum-Packed Beef Mince during Refrigerated Storage
3.3.1. Chemical Analyses
3.3.2. Colour
3.3.3. Microbial Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pop, A.; Kiss, B.; Loghin, F. Endocrine Disrupting Effects of Butylated Hydroxyanisole (BHA-E320). Clujul Med. 2013, 86, 16–20. [Google Scholar] [PubMed]
- Singh, B.; Mense, S.M.; Remotti, F.; Liu, X.; Bhat, H.K. Antioxidant Butylated Hydroxyanisole Inhibits Estrogen-Induced Breast Carcinogenesis in Female ACI Rats. J. Biochem. Mol. Toxicol. 2009, 23, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.; Fung, D.Y.C. Inactivation of Listeria Monocytogenes Scott a 49594 in Apple Juice Supplemented with Cinnamon. J. Food Prot. 2002, 65, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.; Fung, D.Y.C. Evaluation of Salmonella Typhimurium, Yersinia Enterocolitica and Staphylococcus Aureus Counts in Apple Juice with Cinnamon, by Conventional Media and Thin Agar Layer Method. Food Microbiol. 2003, 20, 365–370. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; de Lampasona, M.; Catalan, C. Chemical Constituents, Antimicrobial Investigations and Antioxidative Potential of Volatile Oil and Acetone Extract of Star Anise Fruits. J. Sci. Food Agric. 2005, 86, 111–121. [Google Scholar] [CrossRef]
- De, M.; De, A.K.; Sen, P.; Banerjee, A.B. Antimicrobial Properties of Star Anise (Illicium verum Hook F). Phytother. Res. 2002, 16, 94–95. [Google Scholar] [CrossRef]
- ChemLer, J.A.; Koffas, M.A. Metabolic Engineering for Plant Natural Product Biosynthesis in Microbes. Curr. Opin. Biotechnol. 2008, 19, 597–605. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Grape Phytochemicals: A Bouquet of Old and New Nutraceuticals for Human Health. Med. Hypotheses 2006, 67, 833–838. [Google Scholar] [CrossRef]
- Forkmann, G.; Martens, S. Metabolic Engineering and Applications of Flavonoids. Curr. Opin. Biotechnol. 2001, 12, 155–160. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in Nutrient Dynamics of Forest Ecosystems-a Review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Häring, D.A.; Huber, M.J.; Suter, D.; Edwards, P.J.; Lüscher, A. Plant Enemy-Derived Elicitors Increase the Foliar Tannin Concentration of Onobrychis Viciifolia without a Trade-off to Growth. Ann. Bot. 2008, 102, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Vaquero, M.J.; Aredes Fernández, P.A.; Manca De Nadra, M.C.; Strasser De Saad, A.M. Effect of Phenolic Compounds from Argentinean Red Wines on Pathogenic Bacteria in A Meat Model System. J. Food Biochem. 2012, 37, 425–431. [Google Scholar] [CrossRef]
- Maisak, H.; Jantrakajorn, S.; Lukkana, M.; Wongtavatchai, J. Antibacterial Activity of Tannin from Sweet Chestnut Wood against Aeromonas and Streptococcal Pathogens of Tilapia (Oreochromis niloticus) Original Article. Thai. J. Vet. Med. 2013, 43, 105–111. [Google Scholar]
- Ricci, A.; Lagel, M.-C.; Parpinello, G.P.; Pizzi, A.; Kilmartin, P.A.; Versari, A. Spectroscopy Analysis of Phenolic and Sugar Patterns in a Food Grade Chestnut Tannin. Food Chem. 2016, 203, 425–429. [Google Scholar] [CrossRef]
- Downey, M.; Mazza, M.; Seddon, T.; Rochfort, S.; Millikan, M. Variation in Condensed Tannin Content, Composition and Polymer Length Distribution in the Skin of 36 Grape Cultivars. Curr. Bioact. Compd. 2012, 8, 200–217. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed Tannins from Grape Pomace: Characterization by FTIR and MALDI TOF and Production of Environment Friendly Wood Adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Hayward, A.; Stone, G.N. Oak Gall Wasp Communities: Evolution and Ecology. Basic Appl. Ecol. 2005, 6, 435–443. [Google Scholar] [CrossRef]
- Senvaitiene, J.; Beganskiene, A.; Kareiva, A. Spectroscopic Evaluation and Characterization of Different Historical Writing Inks. Vib. Spectrosc. 2005, 37, 61–67. [Google Scholar] [CrossRef]
- Paaver, U.; Matto, V.; Raal, A. Total Tannin Content in Distinct Quercus robur L. Galls. J. Med. Plants Res. 2010, 4, 702–705. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Analysis of Valonia Oak (Quercus aegylops) Acorn Tannin and Wood Adhesives Application. BioResources 2015, 10, 7165–7177. [Google Scholar] [CrossRef]
- Crowley, K.M.; Prendergast, D.M.; Sheridan, J.J.; McDowell, D.A. The Influence of Storing Beef Aerobically or in Vacuum Packs on the Shelf Life of Mince. J. Appl. Microbiol. 2010, 109, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Reyes, T.M.; Wagoner, M.P.; Zorn, V.E.; Coursen, M.M.; Wilborn, B.S.; Bonner, T.; Brandebourg, T.D.; Rodning, S.P.; Sawyer, J.T. Vacuum Packaging Can Extend Fresh Color Characteristics of Beef Steaks during Simulated Display Conditions. Foods 2022, 11, 520. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Webber, D.; Sultanbawa, Y. Biochemical, Antioxidant and Sensory Evaluation of Davidsonia Pruriens and Davidsoina Jerseyana Fruit Infusion. Food Chem. 2021, 342, 128349. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Melanosis and Quality Changes of Pacific White Shrimp (Litopenaeus vannamei) Treated with Catechin during Iced Storage. J. Agric. Food Chem. 2009, 57, 3578–3586. [Google Scholar] [CrossRef]
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluation of the Chemical Composition and Antioxidant Activity of the Peel Oil OfCitrus Nobilis. Int. J. Food Prop. 2010, 13, 983–991. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant Activity of Grape Seed (Vitis vinifera) Extracts on Peroxidation Models in Vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, Characterisation and Antibacterial Activity of Lemon Myrtle and Anise Myrtle Essential Oil in Water Nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Badr, H.M. Antioxidative Activity of Carnosine in Gamma Irradiated Ground Beef and Beef Patties. Food Chem. 2007, 104, 665–679. [Google Scholar] [CrossRef]
- Ahmad, M.; Hani, N.M.; Nirmal, N.P.; Fazial, F.F.; Mohtar, N.F.; Romli, S.R. Optical and Thermo-Mechanical Properties of Composite Films Based on Fish Gelatin/Rice Flour Fabricated by Casting Technique. Prog. Org. Coat. 2015, 84, 115–127. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Retardation of Quality Changes of Pacific White Shrimp by Green Tea Extract Treatment and Modified Atmosphere Packaging during Refrigerated Storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total Phenolic Content and Antioxidant Capacity of Extracts Obtained from Six Important Fruit Residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H. Characterisation and Comparison of Phenols, Flavonoids and Isoflavones of Soymilk and Their Correlations with Antioxidant Activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Griffiths, D.W. Condensed Tannins. In Toxic Substances in Crop Plants; D’Mello, J.P.F., Duffus, C.M., Duffus, J.H., Eds.; The Royal Society of Chemistry: London, UK, 1991. [Google Scholar]
- Aron, P.M.; Kennedy, J.A. Flavan-3-Ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant Properties of Catechins and Proanthocyanidins: Effect of Polymerisation, Galloylation and Glycosylation. Free. Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Keser, S.; Celik, S.; Turkoglu, S. Total Phenolic Contents and Free-Radical Scavenging Activities of Grape (Vitis vinifera L.) and Grape Products. Int. J. Food Sci. Nutr. 2012, 64, 210–216. [Google Scholar] [CrossRef]
- Karamać, M. Polish Journal of Food and Nutrition Sciences FE(II), CU(II) and ZN(II) Chelating Activity of Buckwheat and Buckwheat Groats Tannin Fractions. Pol. J. Food Nutr. Sci 2007, 57, 357–362. [Google Scholar]
- Mignone, C.F.; Donati, E.R. ATP Requirements for Growth and Maintenance of Iron-Oxidizing Bacteria. Biochem. Eng. J. 2004, 18, 211–216. [Google Scholar] [CrossRef]
- Reis, A.; Spickett, C.M. Chemistry of Phospholipid Oxidation. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 2374–2387. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, L.; Ominski, K.H.; He, M.; Xu, Z.; Krause, D.O.; Acharya, S.N.; Wittenberg, K.M.; Liu, X.L.; Stanford, K.; et al. Screening of Condensed Tannins from Canadian Prairie Forages for Anti–Escherichia Coli O157:H7 with an Emphasis on Purple Prairie Clover (Dalea purpurea Vent). J. Food Prot. 2013, 76, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R. Ferric Siderophore Transport via Outer Membrane Receptors of Escherichia Coli: Structural Advancement and a Tribute to Dr. Dick van Der Helm—An “Ironman” of Siderophore Biology. In Iron Uptake in Bacteria with Emphasis on E. coli and Pseudomonas; Springer: Dordrecht, The Netherlands, 2013; pp. 1–29. [Google Scholar] [CrossRef]
- Scalbert, A. Tannins in Woods and Their Contribution to Microbial Decay Prevention. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; pp. 935–952. [Google Scholar] [CrossRef]
- Yang, X.; Niu, L.; Zhu, L.; Liang, R.; Zhang, Y.; Luo, X. Shelf-Life Extension of Chill-Stored Beef Longissimus Steaks Packaged under Modified Atmospheres with 50% O2 and 40% CO2. J. Food Sci. 2016, 81, C1692–C1698. [Google Scholar] [CrossRef] [PubMed]
- Istrati, D.; Constantin, O.; Ionescu, A.; Vizireanu, C.; Dinică, R. Original Research Paper Study of The Combined Effect Of Spices and Margination on Beef Meat Vacuum Packaged. Food Technol. 2011, 35, 75–85. [Google Scholar]
- Aksu, M.I.; Özer, H. Effects of Lyophilized water extract of Satureja Hortensis on The Shelf Life and Quality Properties of Ground Beef. J. Food Process. Preserv. 2012, 37, 777–783. [Google Scholar] [CrossRef]
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha Piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef Meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf Life Extension of Lamb Meat Using Thyme or Oregano Essential Oils and Modified Atmosphere Packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Duffy, G.; Walsh, D.; Sheridan, J.J.; Logue, C.M.; Harrington, D.; Blair, I.S.; McDowell, D.A. Behaviour of Listeria Monocytogenes in the Presence of Listeria Innocua during Storage of Minced Beef under Vacuum or in Air at 0°c and 10°C. Food Microbiol. 2000, 17, 571–578. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of Catechin and Ferulic Acid on Melanosis and Quality of Pacific White Shrimp Subjected to Prior Freeze–Thawing during Refrigerated Storage. Food Control 2010, 21, 1263–1271. [Google Scholar] [CrossRef]
- Ahn, J.; Grun, I.U.; Fernando, L.N. Antioxidant Properties of Natural Plant Extracts Containing Polyphenolic Compounds in Cooked Ground Beef. J. Food Sci. 2002, 67, 1364–1369. [Google Scholar] [CrossRef]
- Ahn, J.; Grun, I.; Mustapha, A. Effects of Plant Extracts on Microbial Growth, Color Change, and Lipid Oxidation in Cooked Beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef]
- Amany, M.; Shaker, M.; Abeer, A. Antioxidant Activities of Date Pits in a Model Meat System. Int. Food Res. J. 2012, 19, 223–227. [Google Scholar]
- Kallel, F.; Driss, D.; Bouaziz, F.; Belghith, L.; Zouari-Ellouzi, S.; Chaari, F.; Haddar, A.; Chaabouni, S.E.; Ghorbel, R. Polysaccharide from Garlic Straw: Extraction, Structural Data, Biological Properties and Application to Beef Meat Preservation. RSC Adv. 2015, 5, 6728–6741. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant Activity of Bovine and Porcine Meat Treated with Extracts from Edible Lotus (Nelumbo nucifera) Rhizome Knot and Leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef]
- King, D.A.; Hunt, M.; Barbut, S.; Claus, J.; Cornforth, D.; Joseph, P.; Kim, B.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. AMSA Meat Color Measurement Guidelines. Meat Muscle Biol. 2022; in press. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yoon, S.K.; Song, Y.H.; Lee, S.K. Effect of Season on Color of Hanwoo (Korean Native Cattle) Beef. Meat Sci. 2003, 63, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Baublits, R.T.; Brown, A.H.; Pohlman, F.W.; Rule, D.C.; Johnson, Z.B.; Onks, D.O.; Murrieta, C.M.; Richards, C.J.; Loveday, H.D.; Sandelin, B.A.; et al. Fatty Acid and Sensory Characteristics of Beef from Three Biological Types of Cattle Grazing Cool-Season Forages Supplemented with Soyhulls. Meat Sci. 2006, 72, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.E.; Hsin, I.-M.; Zipser, M.Y.W. Retardation of oxidative color changes in raw ground beef. J. Food Sci. 1971, 36, 940–942. [Google Scholar] [CrossRef]
- Mohan, C.C.; Rakhavan, K.R.; Radha Krishnan, K.; Babuskin, S.; Sudharsan, K.; Azhagu Saravana Babu, P.; Sukumar, M. Development of Predictive Preservative Model for Shelf Life Parameters of Beef Using Response Surface Methodology. LWT-Food Sci. Technol. 2016, 72, 239–250. [Google Scholar] [CrossRef]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Effect of Lutein, Sesamol, Ellagic Acid and Olive Leaf Extract on the Quality and Shelf-Life Stability of Packaged Raw Minced Beef Patties. Meat Sci. 2010, 84, 613–620. [Google Scholar] [CrossRef]
- O’Grady, M.N.; Monahan, F.J.; Brunton, N.P. Oxymyoglobin Oxidation and Lipid Oxidation in Bovine Muscle—Mechanistic Studies. J. Food Sci. 2001, 66, 386–392. [Google Scholar] [CrossRef]
- Mitsumoto, M.; O’Grady, M.N.; Kerry, J.P.; Joe Buckley, D. Addition of Tea Catechins and Vitamin c on Sensory Evaluation, Colour and Lipid Stability during Chilled Storage in Cooked or Raw Beef and Chicken Patties. Meat Sci. 2005, 69, 773–779. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Geornaras, I. Microbiology of Land Muscle Foods. In Handbook of Food Science, Technology, and Engineering; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Compendium of Microbiological Criteria for Food; Food Standards Australia New Zealand: Canberra, Australia, 2016.
| Extract | TPC (g GAE/100 g Powder) | IC50 (µg/mL) |
|---|---|---|
| TO | 35.82 ± 1.91 d | 4.03 ± 0.08 d |
| TE | 51.75 ± 1.19 c | 20.25 ± 1.02 a |
| TVG | 71.25 ± 4.04 a | 15.26 ± 0.76 b |
| TG | 61.14 ± 0.53 b | 6.23 ± 0.06 c |
| Bacteria | TG (mg/mL) | TO (mg/mL) | TE (mg/mL) | TVG (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | |
| L. monocytogenes | 100 ± 4.0 a | 100 ± 9.1 a | 100 ± 2.8 a | 100 ± 5.6 a | 100 ± 3.7 a | 100 ± 0.9 a | 100 ± 0.6 a | 100 ± 9.2 a |
| S. aureus | 0.0 ± 0.0 c | 42.4 ± 3.6 b | 0.0 ± 0.0 a | 56.4 ± 2.0 b | 68.6 ± 0.8 c | 100 ± 6.2 a | 100 ± 3.4 a | 100 ± 4.1 a |
| E. coli | 99 ± 1.8 a | 100 ± 2.3 a | 100 ± 1.3 a | 100 ± 2.2 a | 0.0 ± 0.0 d | 34.9 ± 2.2 c | 43.4 ± 8.2 b | 54.4 ± 2.3 b |
| P. aeruginosa | 36.3 ± 9.6 b | 44.5 ± 5.3 b | 100 ± 3.7 a | 100 ± 4.1 a | 82.7 ± 7.6 b | 81.8 ± 2.3 b | 97.4 ± 4.6 a | 96.3 ± 5.6 a |
| Control | SMS (0.05%) | TO (0.25%) | TO (0.5%) | TVG (0.25%) | TVG (0.5%) | |
|---|---|---|---|---|---|---|
| pH | 5.37 ± 0.03 a | 5.65 ± 0.02 a | 5.26 ± 0.01 ab | 5.16 ± 0.00 b | 5.31 ± 0.01 a | 5.27 ± 0.01 ab |
| L* | 47.69 ± 1.5 a | 44.87 ± 0.6 a | 43.24 ± 1.9 ab | 43.05 ± 0.6 b | 47.48 ± 0.4 a | 46.45 ± 1.8 a |
| a* | 15.09 ± 0.7 a | 15.63 ± 0.8 a | 15.16 ± 1.4 a | 14.28 ± 0.5 a | 14.89 ± 0.4 a | 15.10 ± 0.8 a |
| b* | 6.29 ± 0.4 c | 5.92 ± 0.3 c | 7.92 ± 0.2 b | 9.17 ± 0.4 a | 7.96 ± 0.3 b | 9.01 ± 0.4 a |
| ΔE* | 4.82 ± 1.6 a | 2.24 ± 1.1 b | 3.40 ± 1.0 ab | 3.58 ± 0.6 ab | 3.14 ± 1.5 ab | 3.60 ± 1.5 ab |
| Samples | TBARS (mg Malonaldehyde/kg Sample) | Metmb (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0-Day | 7-Day | 14-Day | 21-Day | 0-Day | 7-Day | 14-Day | 21-Day | |
| Control | 1.71 ± 0.1 a | 0.98 ± 0.1 ab | 1.14 ± 0.3 a | 1.46 ± 0.1 a | 69.49 ± 3.5 ab | 44.54 ± 3.5 d | 1.58 ± 0.8 c | 2.97 ± 1.9 d |
| SMS (0.05%) | 1.04 ± 0.3 ab | 0.89 ± 0.1 ab | 0.68 ± 0.0 b | 0.85 ± 0.1 ab | 46.83 ± 1.4 c | 56.49 ± 2.1 c | 2.93 ± 0.9 c | 16.56 ± 0.6 c |
| TO (0.25%) | 1.02 ± 0.1 b | 0.49 ± 0.0 b | 0.98 ± 0.2 ab | 0.81 ± 0.0 ab | 78.08 ± 3.8 a | 118.38 ± 6.4 a | 65.00 ± 3.2 a | 66.19 ± 3.6 a |
| TO (0.5%) | 1.51 ± 0.1 a | 0.46 ± 0.1 b | 0.75 ± 0.0 b | 0.53 ± 0.0 b | 79.25 ± 3.9 a | 114.14 ± 2.9 a | 66.44 ± 1.5 a | 67.33 ± 3.5 a |
| TVG (0.25%) | 1.51 ± 0.1 a | 1.28 ± 0.0 a | 1.50 ± 0.1 a | 1.08 ± 0.0 a | 63.67 ± 4.1 b | 81.86 ± 0.5 b | 24.77 ± 0.5 b | 23.56 ± 2.3 b |
| TVG (0.5%) | 1.8 ± 0.0 a | 1.61 ± 0.3 a | 1.85 ± 0.2 a | 1.42 ± 0.3 a | 73.71 ± 5.6 a | 81.22 ± 3.2 b | 68.12 ± 2.2 a | 24.06 ± 0.1 b |
| Samples | TVC (CFU/g) | H2S-Producing Bacteria Count (CFU/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0-Day | 7-Day | 14-Day | 21-Day | 0-Day | 7-Day | 14-Day | 21-Day | |
| Control | 6.22 ± 0.71 a | 6.66 ± 0.15 a | 6.86 ± 0.36 a | 7.31 ± 0.08 a | 3.96 ± 0.11 a | 4.46 ± 0.11 a | 4.58 ± 0.24 a | 4.69 ± 0.55 a |
| SMS (0.05%) | 6.57 ± 0.01 ab | 6.57 ± 0.20 a | 6.69 ± 0.13 a | 6.39 ± 0.12 b | 3.74 ± 0.19 a | 3.80 ± 0.14 b | 3.30 ± 0.00 b | 3.30 ± 0.00 b |
| TO (0.25%) | 6.67 ± 0.12 a | 6.93 ± 0.09 a | 7.42 ± 0.11 a | 7.56 ± 0.20 a | 4.26 ± 0.08 a | 3.99 ± 0.12 b | 3.60 ± 0.00 b | 2.47 ± 0.19 c |
| TO (0.5%) | 6.76 ± 0.06 a | 6.81 ± 0.03 a | 7.30 ± 0.14 a | 7.40 ± 0.14 a | 4.11 ± 0.05 a | 4.00 ± 0.00 ab | 3.24 ± 0.34 b | 2.11 ± 0.22 c |
| TVG (0.25%) | 6.88 ± 0.13 a | 7.22 ± 0.60 a | 7.06 ± 0.03 a | 7.51 ± 0.09 a | 3.93 ± 0.08 a | 4.14 ±0.12 a | 4.03 ±0.23 a | 2.39 ± 0.05 c |
| TVG (0.5%) | 6.66 ± 0.13 a | 6.76 ± 0.07 a | 7.33 ± 0.01 a | 7.50 ± 0.04 a | 3.93 ± 0.39 a | 4.20 ± 0.11 | 4.10 ± 0.14 a | 2.21 ± 0.11 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.N.M.; Nirmal, N.P.; Sultanbawa, Y.; Ziora, Z.M. Antioxidant and Antibacterial Activity of Four Tannins Isolated from Different Sources and Their Effect on the Shelf-Life Extension of Vacuum-Packed Minced Meat. Foods 2023, 12, 354. https://doi.org/10.3390/foods12020354
Nguyen CNM, Nirmal NP, Sultanbawa Y, Ziora ZM. Antioxidant and Antibacterial Activity of Four Tannins Isolated from Different Sources and Their Effect on the Shelf-Life Extension of Vacuum-Packed Minced Meat. Foods. 2023; 12(2):354. https://doi.org/10.3390/foods12020354
Chicago/Turabian StyleNguyen, Chau Ngoc Minh, Nilesh Prakash Nirmal, Yasmina Sultanbawa, and Zyta M. Ziora. 2023. "Antioxidant and Antibacterial Activity of Four Tannins Isolated from Different Sources and Their Effect on the Shelf-Life Extension of Vacuum-Packed Minced Meat" Foods 12, no. 2: 354. https://doi.org/10.3390/foods12020354
APA StyleNguyen, C. N. M., Nirmal, N. P., Sultanbawa, Y., & Ziora, Z. M. (2023). Antioxidant and Antibacterial Activity of Four Tannins Isolated from Different Sources and Their Effect on the Shelf-Life Extension of Vacuum-Packed Minced Meat. Foods, 12(2), 354. https://doi.org/10.3390/foods12020354









